Summary
Pituitary adenomas can be classified as functioning or non-functioning adenomas. Approximately 64% of clinically non-functioning pituitary adenomas are found to be gonadotroph adenomas on immunohistochemistry. There are reported cases of gonadotroph adenomas causing clinical symptoms, but this is unusual. We present the case of a 36-year-old female with abdominal pain. Multiple large ovarian cysts were identified on ultrasound requiring bilateral cystectomy. Despite this, the cysts recurred resulting in further abdominal pain, ovarian torsion and right oophorectomy and salpingectomy. On her 3rd admission with abdominal pain, she was found to have a rectus sheath mass which was resected and histologically confirmed to be fibromatosis. Endocrine investigations revealed elevated oestradiol, follicle-stimulating hormone (FSH) at the upper limit of the normal range and a suppressed luteinising hormone (LH). Prolactin was mildly elevated. A diagnosis of an FSH-secreting pituitary adenoma was considered and a pituitary MRI revealed a 1.5 cm macroadenoma. She underwent transphenoidal surgery which led to resolution of her symptoms and normalisation of her biochemistry. Subsequent pelvic ultrasound showed normal ovarian follicular development. Clinically functioning gonadotroph adenomas are rare, but should be considered in women presenting with menstrual irregularities, large or recurrent ovarian cysts, ovarian hyperstimulation syndrome and fibromatosis. Transphenoidal surgery is the first-line treatment with the aim of achieving complete remission.
Learning points:
Pituitary gonadotroph adenomas are usually clinically non-functioning, but in rare cases can cause clinical symptoms.
A diagnosis of a functioning gonadotroph adenoma should be considered in women presenting with un-explained ovarian hyperstimulation and/or fibromatosis.
In women with functioning gonadotroph adenomas, the main biochemical finding is elevated oestradiol levels. Serum FSH levels can be normal or mildly elevated. Serum LH levels are usually suppressed.
Transphenoidal surgery is the first-line treatment for patients with functioning gonadotroph adenomas, with the aim of achieving complete remission.
Background
Pituitary adenomas are tumours arising from the pituitary gland. Prevalence estimates from radiological and autopsy studies range from <1% to over 30%, with the overall estimated prevalence of pituitary adenomas being 16.7% (1).
Pituitary adenomas can be classified as functioning or non-functioning depending on hormonal secretion. Approximately 64% of clinically non-functioning pituitary adenomas are found to be gonadotroph adenomas on immunohistochemistry (2).
Gonadotroph adenomas are usually clinically non-functioning or silent, but in rare cases can cause clinical symptoms. The prevalence of clinically functioning gonadotroph adenomas is not known, but there are only about 30 reported cases in the literature (3).
We present the case of a clinically functioning gonadotroph adenoma in a premenopausal woman with abdominal pain, ovarian hyperstimulation and fibromatosis. To our knowledge, this is the first case of fibromatosis associated with a functioning gonadotroph adenoma.
Case presentation
A 36-year-old female initially presented with acute abdominal pain in January 2016. She had no significant past medical history and had experienced two normal pregnancies, with no complications.
An ultrasound scan (USS) of the abdomen and pelvis showed large bilateral ovarian cysts. The right ovary measured 7.8 × 5.1 cm and contained several large simple cysts measuring 4–5 cm. In the left adnexa, there was a large 9 × 5.2 cm septated ovarian cyst extending across the midline. She underwent bilateral cystectomy and histology confirmed benign follicular cysts.
In May 2016, she presented for the second time. An USS pelvis showed a recurrence of bilateral ovarian cysts. She underwent a laparoscopy which revealed right ovarian torsion and therefore a right oophorectomy and salpingectomy was performed. Multiple cysts were seen on the left ovary and cyst aspiration was performed on these. The left ovary was preserved as the patient wanted further children. Histology confirmed torted and infarcted ovarian tissue with no evidence of malignancy.
She presented for a third time in September 2016 with severe right upper quadrant pain. USS abdomen showed a mass arising from the upper right rectus abdominis muscle. MRI of the abdomen confirmed a 3.2 × 1.6 cm rectus sheath mass. An ultrasound guided biopsy was performed, and histology was consistent with fibromatosis.
In April 2017, she underwent resection of the rectus sheath mass and histology confirmed fibromatosis (desmoid tumour), with no evidence of malignancy.
In May 2017, she was reviewed in the endocrine clinic. She reported persistent abdominal pain, slightly irregular periods, no galactorrhea and no headaches. Examination findings, including visual fields, were normal.
Investigation
Endocrine investigations (Table 1) were performed at a 2-week interval in her cycle. These showed an elevated oestradiol, FSH at the upper limit of the normal range and a suppressed LH. Prolactin was mildly elevated.
Table 1.
Endocrine investigations.
| Normal values | Pre-operative results | Post-operative results | ||
|---|---|---|---|---|
| 12/5/2017 | 26/5/2017 | 27/11/2017 | ||
| Oestradiol (pmol/L) | FP: <571; LP: 122–1094 | 2096 | 1111 | <100 |
| FSH (IU/L) | FP and LP: 1–9 | 8.7 | 9.0 | 3.2 |
| LH (IU/L) | FP: 3.2–8.0; LP 2.4–7.2 | 0.8 | 0.7 | 1.8 |
| Prolactin (mIU/L) | <700 | 740 | 191 | |
| Testosterone (nmol/L) | 0.2–1.7 | <0.3 | ||
| Free T4 (pmol/L) | 12–22 | 19.9 | 21.5 | |
| TSH (mIU/L) | 0.27–4.2 | 1.07 | 0.60 | |
| Cortisol (nmol/L) | 396 | 428 | ||
| IGF1 (nmol/L) | 7.4–31.3 | 17 | ||
FP, follicular phase; LP, luteal phase.
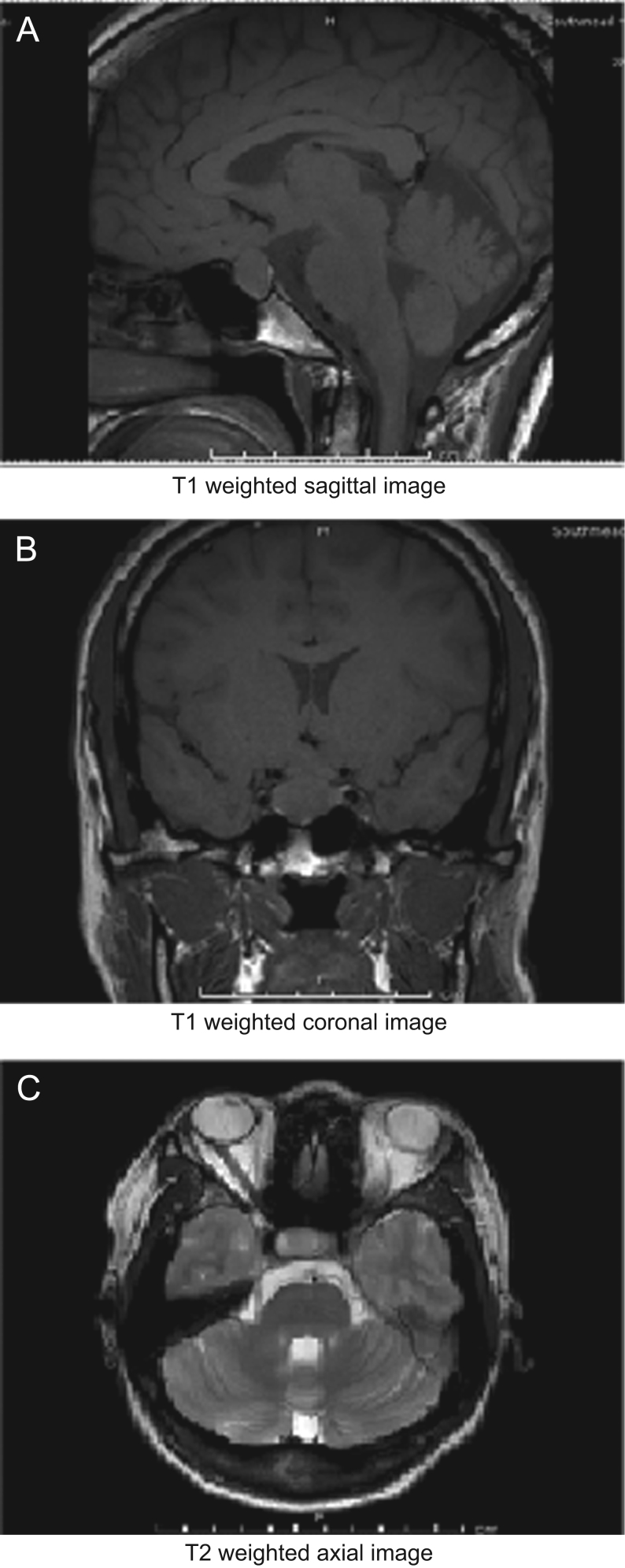
She underwent a pituitary MRI (Fig. 1), which revealed a 1.5 cm pituitary macroadenoma, with no evidence of chiasmatic compression. Pre-operative Humphrey visual field testing was normal.
Figure 1.
(A) T1-weighted sagittal image; (B) T1-weighted coronal image; (C) T2-weighted axial image. MRI pituitary showing a mass lesion within the pituitary gland, which is predominantly on the right side. It is 1.5 cm in maximum craniocaudal extent. There is mild contact with the under surface of the optic chiasm, but no clear chiasmatic compression or cavernous sinus invasion.
A diagnosis of follicle-stimulating hormone (FSH)-secreting pituitary adenoma was made, and the patient was referred to the joint pituitary multi-disciplinary meeting, where a decision was made to proceed with surgery.
Treatment
She underwent a transphenoidal hypophysectomy in November 2017. Histology confirmed a pituitary adenoma with FSH immunopositivity in keeping with a gonadotroph cell adenoma. No atypical features were identified.
Outcome and follow-up
Postoperatively she developed transient cranial diabetes insipidus which was managed with desmopressin.
Her abdominal pain resolved, and she resumed a normal menstrual cycle.
Immediate post-operative endocrine investigations (Table 1) showed a reduction in oestradiol and FSH and a slight rise in LH. Electrolytes, cortisol and free T4 remained normal.
Pelvic ultrasound showed a slightly bulky left ovary, but with two normal follicles 2–3 cm in size.
Post-operative MRI pituitary at 3 months showed removal of the majority of the pituitary adenoma, although a small amount of abnormal pituitary tissue remained in the right pituitary fossa.
Discussion
The majority of gonadotroph adenomas are described as clinically non-functioning and do not present with symptoms secondary to hormone hypersecretion. More commonly, gonadotroph adenomas present with neurological symptoms secondary to mass effects or are discovered as an incidental finding on imaging performed for another reason.
Although gonadotroph adenomas are considered non-functioning, most do produce intact gonadotrophins or their subunits. Approximately 35% of gonadotroph adenomas secrete sufficient LH or FSH to raise serum levels, but clinical syndromes are rare (4).
Clinical syndromes secondary to hormone hypersecretion have been described:
Premenopausal women: The most common presenting features are menstrual irregularity, spontaneous vaginal bleeding and infertility. Ovarian stimulation has also been reported but is usually mild; however, cases of multiple ovarian cysts resulting in ovarian torsion have been reported (5, 6). Persistently elevated FSH levels lead to recruitment of multiple dominant follicles and raised oestradiol levels, resulting in abdominal pain and distension. The clinical picture is similar to ovarian hyperstimulation syndrome secondary to exogenous FSH used in fertility treatment.
Males: FSH hypersecretion leading to testicular enlargement has been reported (7).
Children: Precocious puberty has been described in both girls and boys with functioning gonadotroph adenomas (7).
No clinical syndrome has been described in post-menopausal women. With increasing age, ovaries atrophy and become insensitive to FSH stimulation.
Cote et al. report a case series of seven patients (5 men and two women) with functioning gonadotroph adenomas over a 17-year period (8). Similar to our case, both female patients presented with clinical symptoms due to a functioning gonadotroph adenoma (8). The first presented with ovarian torsion due to bilateral enlarged polycystic ovaries. The second presented with oligomenorrhea and multiple follicular cysts requiring aspiration. None of the male patients presented with symptoms suggestive of hormone excess (8).
This lady presented with abdominal pain secondary to multiple large ovarian cysts. In addition she developed fibromatosis, which as far as we are aware has not previously been reported in association with functioning gonadotroph adenomas.
Fibromatosis, also called desmoid tumours, are locally aggressive tumours but without metastatic potential. Desmoid tumours are rare, with an estimated incidence in the general population of between two and four per million populations per year (9). Although the pathogenesis is poorly understood, they have been associated with high levels of oestrogen, for example, in women during or following pregnancy.
The expression of oestrogen receptors in fibromatosis has been evaluated: 53 out of 59 cases (89%) were positive for oestrogen receptor beta, but no cases were positive for oestrogen receptor alpha (10). In addition, patients treated with tamoxifen had a better outcome than those treated with surgery or radiation alone (10). Interestingly, tamoxifen acts on oestrogen receptor alpha and inhibits cell proliferation, and this study found that fibromatosis is oestrogen receptor alpha negative (10).
Therefore, the hormone sensitivity is controversial and the evidence suggesting an association between high oestrogen levels and desmoid tumours is limited to retrospective cases (11). But the development of fibromatosis in this case may be secondary to high oestrogen levels.
Investigations
The main biochemical finding is elevated oestradiol levels, which can range from mild to extremely elevated (7). Serum FSH levels can be normal or mildly elevated. Serum LH levels are usually suppressed.
Cote et al. found elevated FSH levels in six out of seven patients with a functioning gonadotroph adenoma (8). In the remaining patient, elevated gonadal steroids with inappropriately normal FSH and LH levels were found (8). Two female patients were included in the series; one patient had raised FSH with a normal oestradiol level. The other had a normal FSH with a raised oestradiol level. They both had suppressed LH levels. In patients with a pituitary adenoma, clinicians should consider a diagnosis of a functioning gonadotroph adenoma when there is an increase in FSH and or gonadal steroids.
Prolactin levels can be elevated, most likely due to compression of the pituitary stalk (7). A full pituitary hormone profile should be requested to ensure the rest of the pituitary axis is intact.
Imaging of the pelvis reveals bilateral ovarian enlargement, with multiple cysts often large in size (10).
Pituitary MRI reveals a macroadenoma in the majority of cases of functioning gonadotroph adenomas, frequently with suprasellar extension and cavernous sinus invasion (7).
Clinically functioning gonadotroph adenomas are rare; therefore, investigations to rule out other causes should be considered. The differential diagnosis includes polycystic ovarian syndrome (PCOS), ovarian hyperstimulation syndrome secondary to exogenous FSH and ovarian neoplasms.
Management
Transphenoidal surgery is recommended as first-line treatment for clinically functioning gonadotroph adenomas. The aim is to achieve complete resection, whilst preserving normal pituitary tissue. This should lead to resolution of the clinical symptoms and normalisation of the biochemical changes.
The diagnosis should be considered to try and prevent women undergoing either cystectomy or oophorectomy for management of enlarging, painful or torted ovarian cysts.
Post-operative endocrine testing is essential to ensure resolution of the biochemical changes, and preservation of normal pituitary function.
Medical treatments including dopamine agonists, somatostatin analogues, GnRH agonists and GnRH antagonists have all been used in individual cases with inconsistent results. Tumour shrinkage has not been observed; therefore, they are not recommended as primary treatment (7).
Prognosis
There is limited data on long-term outcomes in patients with functioning gonadotroph adenomas. Remission in six out of seven patients was reported by Cole et al.; however, the follow-up period was short (median 10 months; range 4–213 months) (8). Cases of remission up to 3 years following surgery have been reported (6), but long-term follow-up is required due to the risk of recurrence. Repeat surgery, radiotherapy and medical treatment have all been used for treatment of recurrent tumours (3).
Conclusion
Clinically functioning gonadotroph adenomas are rare but can present with significant symptoms including large ovarian cysts and menstrual irregularities. More commonly, gonadotroph adenomas are clinically silent and present either with mass effects or as an incidental finding.
Functioning gonadotroph adenomas should be considered in women presenting with ovarian hyperstimulation, as surgical treatment of the pituitary tumour can result in the resolution of symptoms and avoiding the need for either cystectomy or oophorectomy. Women with ovarian hyperstimulation are likely to be referred to gynaecology; therefore, it is important that gynaecologists have an awareness of this association. If the hormonal profile shows elevated levels along the hypothalamic pituitary gonadal axis, this should prompt referral to endocrinology for further review.
Clinicians should also be aware of the possibility of an association between fibromatosis and high oestrogen states present in functioning gonadotroph adenomas.
Patient’s perspective
I consider myself a person with a very positive outlook and yet by week 6 following my cystectomy no amount of positivity was overshadowing the lower abdominal pain and nausea. I knew that the largest cysts had been taken out as I was shown images post surgery and yet I really did not feel well at all. My GP ensured that I had a large supply of codeine and naproxen which I relied on to get through normal days and be able to function for several months. I was devastated to learn of the ovarian torsion and resultant loss of the right ovary. I felt that I had learned to cope with the pain rather than react to it quickly enough to avoid the oophorectomy.
Following my surgery in June, I had 3-month recovery at home and returned to work at the beginning of September. It was at this time that I noticed a lump that hurt to touch on my right side under my ribs. I asked my GP a lot of questions to understand if it was related to prior surgeries or if I had strained myself due to driving and working. He thought it was highly unlikely and referred me for a scan. From that initial scan, further investigations were made and I was given a diagnosis of fibromatosis quite quickly. It was overwhelming that I was now facing another surgery whilst still experiencing lower abdominal pain due to an enlarged ovary. My gynaecology consultant was very supportive and considered medication to suppress ovulation to avoid loss of the left ovary whilst awaiting surgery for the removal of the desmoid tumour. I had discussions with an oncologist under Plastics to consider the impact of medication and ultimately did not go down the route of medication as the side effects and potential impact of oestrogen on the fibromatosis was a risk I did not want to take. I felt as though the different disciplines were doing well in their area of expertise but sometimes did not connect the fact that one treatment would impact on another part of my body and that the pain was all borne by the same patient. I asked the consultants whether the gynaecology surgeon could operate to remove the large ovarian cysts that were already approximately 10 cm and therefore at risk of torsion straight after the resection without the need for a separate surgery. This was not an option and in hindsight I am relieved that I did not have to deal with a wound in another area after the resection. It was incredibly painful and a very tough recovery.
My gynaecology consultant was very determined to find answers as to why my pain was recurring. She asked fellow colleagues for other opinions and requested a brain MRI. It was the MRI that led to my diagnosis and ongoing care under Endocrinology and Neurosurgery. When the endocrine consultant broke the news to me, I was devastated to know that I needed more surgery but a small part of me was relieved to have answers as to why I had been living with constant lower abdominal pain and nausea. I also understood now, why earlier surgeries had treated symptoms but did not get to the bottom of my condition. I still questioned whether there was a link to the fibromatosis and did not get an affirmative response except to say that it was responsive to oestrogen. The treatment for the pituitary macro adenoma was presented to me and it was a terrifying thought that I would have anyone go into my head especially as all the pain I had was in my stomach and affecting my periods. I am almost 6 months into recovery from the transphenoidal surgery and have not been experiencing the lower abdominal pain and nausea. I feel fatigued and fall asleep easily. I limit what I take on at the moment to the essentials and rest more. I am very grateful to all the Southmead Hospital employees who have been very caring, compassionate and thorough in treating me. It has felt like a lengthy illness in respect of normal day to day life and family impact but I am extremely grateful to be pain free.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Patient consent
Written informed consent has been obtained from the patient for publication of this article and the accompanying image.
Author contribution statement
The case report was written by Dr Chloe Broughton. The report has been reviewed by Dr Kathryn Lonnen who is responsible for making the patients’ diagnosis, management and ongoing care.
References
- 1.Ezzat S, Asa S, Couldwell W, Barr C, Dodge W, Vance M, McCutcheon I. The prevalence of pituitary adenomas. Cancer 2004. 101 613–619. ( 10.1002/cncr.20412) [DOI] [PubMed] [Google Scholar]
- 2.Yamada S, Ohyama K, Taguchi M, Takeshita A, Morita K, Takano K, Sano T. A study of the correlation between morphological findings and biological activities in clinically non-functioning pituitary adenomas. Neurosurgery 2007. 61 580–584. ( 10.1227/01.NEU.0000290906.53685.79) [DOI] [PubMed] [Google Scholar]
- 3.Karapanou O, Tzanela M, Tamouridis N, Tsagarakis S. Gonadotroph pituitary macroadenoma inducing ovarian hyperstimulation syndrome: successful response to octreotide therapy. Hormones 2012. 11 199–202. ( 10.14310/horm.2002.1347) [DOI] [PubMed] [Google Scholar]
- 4.Ho DM, Hsu CY, Ting LT, Chiang H. The clinicopathological characteristics of gonadotroph cell adenoma: a study of 118 cases. Human Pathology 1997. 28 905 ( 10.1016/S0046-8177(97)90005-8) [DOI] [PubMed] [Google Scholar]
- 5.Kanaya M, Baba T, Kitajima Y, Ikeda K, Shimuza A, Morishita M, Honnma H, Endo T, Saito T. Continuous follicle-stimulating hormone exposure from pituitary adenoma causes periodic follicle recruitment and atresia, which mimics ovarian hyperstimulation syndrome. International Journal of Women's Health 2012. 4 427–431. ( 10.2147/IJWH.S33386) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sicilia V, Earle J, Mezitis SG. Multiple ovarian cysts and oligomenorrhoea as the initial manifestation of a gonadotropin secreting pituitary macroadenoma. Endocrine Practice 2006. 12 417–442. ( 10.4158/EP.12.4.417) [DOI] [PubMed] [Google Scholar]
- 7.Ntali G, Capatina C, Grossman A, Karavitaki N. Functioning gondaotroph adenomas. Journal of Clinical Endocrinology and Metabolism 2014. 99 4423–4433. ( 10.1210/jc.2014-2362) [DOI] [PubMed] [Google Scholar]
- 8.Cote DJ, Smith TR, Sandler CN, Gupta T, Bale TA, Bi WL, Dunn IF, De Girolami U, Woodmansee WW, Kaiser UB, Laws ER., Jr Functional gonadotroph adenomas: case series and report of literature. Neurosurgery 2016. 79 823–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reitamo JJ, Häyry P, Nykyri E, Saxén E. The desmoid tumor. I. Incidence, sex-, age- and anatomical distribution in the Finnish population. American Journal of Clinical Pathology 1982. 77 665 ( 10.1093/ajcp/77.6.665) [DOI] [PubMed] [Google Scholar]
- 10.Santos GA, Cunha IW, Rocha RM, Mello CA, Guimarães GC, Fregnani JH, Lopes A. Evaluation of estrogen receptor alpha, estrogen receptor beta, progesterone receptor, and cKIT expression in desmoids tumors and their role in determining treatment options. BioScience Trends 2010. 4 25–30. [PubMed] [Google Scholar]
- 11.Lewis JJ, Boland PJ, Leung DH, Woodruff JM, Brennan MF. The enigma of desmoid tumors. Annals of Surgery 1999. 229 866–872. ( 10.1097/00000658-199906000-00014) [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a