Abstract
The excess risk of cancer observed in patients with type 2 diabetes (T2DM) may have been influenced by detection bias. The aim of this study was to examine the real association by evaluating time-varying site-specific cancer risks in newly diagnosed T2DM patients. A total of 51,324 registered cancer-free individuals newly diagnosed with T2DM between 2004 and 2014 were linked with the Shanghai Cancer Registry and the Vital Statistics through September 2015. A total of 2920 primary, invasive cancer cases were identified during 325,354 person-years period. Within 1 year following diabetes onset, participants with T2DM had higher risks of total, lung and rectal cancer in men and total, liver, pancreas, thyroid, breast and uteri cancer in women. Thereafter the incidence for overall cancer decreased and then increased along with follow-up time, with the upward trend varying by cancer, suggesting potential detection bias. After the initial 1-year period, standardized incidence ratios (SIR) and 95% CIs for overall cancer were 0.80 (95% CI 0.76–0.85) in men and 0.93 (95% CI 0.88–0.99) in women, but a higher risk of breast and thyroid cancers were observed in women, with SIR and 95% CI being 1.13 (1.01, 1.28) and 1.37 (1.11, 1.63), respectively. Our results suggest that T2DM patients are at higher risk of certain cancers; this risk particularly increases shortly after diabetes diagnosis, which is likely to be due to detection bias caused by increased ascertainment. Prevention of female breast and thyroid cancers should be paid attention in Chinese individuals with T2DM.
Keywords: cancer incidence, detection bias, type 2 diabetes
Introduction
Type 2 diabetes (T2DM) has been associated with an elevated risk of liver, pancreas, endometrium, colorectal, breast and bladder cancers and a decreased risk of prostate cancer (1, 2). Several possible mechanisms have been proposed for these associations (3). For example, T2DM may play a positive role in carcinogenesis through hyperglycemia or hyperinsulinemia that stimulates the insulin and insulin-like growth factor (IGF) axis (4). In addition, the anti-diabetic agent metformin may decrease the risk of cancer possibly by attenuating insulin/IGF-1 signaling, inhibiting target of rapamycin and mitochondrial complex I in the electron transport chain in mammals, activating adenosine monophosphate (AMP)-activated kinase and reducing endogenous reactive oxygen species production and associated DNA damage and mutations (5).
Recently, the excess risk of cancer observed in individuals with T2DM has been considered to be inflated by reporting bias (6), detection bias (7) or confounding effects (1). Johnson et al. (8) and Geier (9) observed increased incidence rates of cancers among those diagnosed with cancers within 3 months or 1 year of T2DM diagnosis, followed by lower rates with increasing time since diagnosis, suggesting the possibility of detection bias.
China ranks first in the number of individuals with prevalent T2DM worldwide (10). Multiple studies have reported an increased risk of cancer in T2DM patients in China (11, 12, 13), implicating an important public health significance in cancer prevention in the population. These previous studies, however, did not take potential detection bias into consideration, which may have resulted in an overestimated risk of cancer in the population. In this retrospective cohort study based on a standardized T2DM management system in Shanghai, China, we estimated the risk of cancer, overall and by site, by the time since diagnoses in Chinese patients newly diagnosed with T2DM, to evaluate the influence of T2DM on cancer risk over time, and thus, exclude the potential detection bias.
Materials and methods
Study population and data sources
This retrospective cohort study was designed based on the database of the Standardized Management System of Diabetes in Minhang District, an administrative area with over one million residents of Shanghai, China. Details of the management system have been described elsewhere (13). In brief, this system was established in 2004 and included 65,500 residents living in the Minhang District newly diagnosed with T2DM according to the 1999 criteria of the World Health Organization (WHO) (14). After excluding those less than 20 years old and those previously diagnosed with cancer, 51,324 patients newly diagnosed with T2DM between January 2004 and December 2014 were included in this study. Demographic characteristics, diagnosis date of T2DM and other cancer risk factors such as BMI and drug use information were registered as an electronic health record in the system.
Identification of cancer cases
The outcome of this study was incidence of any primary cancer. Incident cancers and all deaths in the patients up to September 30, 2015, were identified through record linkage with the Shanghai Cancer Registry and the Shanghai Vital Statistics using a unique identification card number (15). We evaluated the top ten common specific cancers defined based on the International Classification Diseases codes (ICD-10), namely, Stomach (C16), Colon (C18), Rectal (C19–C20), Liver (C22), Pancreas (C25), Trachea/bronchus and lung (C33–C34), Prostate (C61), Breast (C50), Corpus uteri (C53) and Thyroid (C73). The record linkage was conducted in April 2017.
This study was approved by the Institutional Review Board (IRB) of the Center of Disease Prevention and Control of Minhang district, Shanghai, China (NO: EC-P-2012-002). Verbal informed consent was obtained from all enrolled patients.
Statistical analysis
Person-years (PYs) of observation were calculated from the date that T2DM was first diagnosed to the date of diagnosis of any primary cancer, or date of death, or end date of observation (September 30, 2015), whichever occurred first. The period of observation was further split into five intervals (within 1, 1–2, 2–4, 4–6 and >6 years) to evaluate cancer risk over time. Crude incidence rates (CIR) of cancer, with 95% CIs based on Poisson distribution, were calculated by dividing the number of patients with cancer by the person-time at risk. Age-standardized rates (ASR) were computed using the World Standard Population as the reference population. Standardized incidence ratios (SIRs) for all and site-specific cancers were calculated as the ratio between the observed and the expected number of cancer cases. The expected number of cases was calculated according to the age-, sex- and calendar-year-specific incidence rates reported by the Shanghai Cancer Registry. SIRs were standardized by sex and 5-year age group in the general population in the Minhang district. Restricted cubic splines functions (RCS) was used to describe the incidence trend of all cancers along with age and period of observation (16).
All data analyses were performed using SAS, version 9.2 for windows (SAS Institute). All tests were two-sided and P values less than 0.05 were considered as significant.
Results
Among the 51,324 cohort members, all of whom were newly diagnosed with T2DM by physicians between 2004 and 2014, 24,124 were men (47%) and 27,200 were women (53%) (Table 1). The average age at diagnosis was 60 years (interquartile range: 53–68 years). With an average BMI of 24.4 ± 3.1 in the population, the prevalence of overweight and obesity were 35.3 and 4.1%, respectively in men and 33.7 and 5.8%, respectively, in women. Patients were observed for an average of 6.65 years (interquartile range: 3.75–8.75 years), with 2.2% having less than 1 year of observation.
Table 1.
Demographic and clinical characteristics of individuals with type 2 diabetes, Shanghai, China.
| Demographic and clinical characteristics | Men (N = 24,124) | Women (N = 27,200) | Total (N = 51,324) |
|---|---|---|---|
| Age at diabetes diagnosis (years) | |||
| Median, IQRa | 60 (53, 68) | 60 (54, 69) | 60 (53, 68) |
| Age groups, N (%) | |||
| <40 | 805 (3.3) | 487 (1.8) | 1292 (2.5) |
| 40–49 | 3238 (13.4) | 2860 (10.5) | 6098 (11.9) |
| 50–59 | 7612 (31.6) | 9333 (34.3) | 16,945 (33.0) |
| 60–69 | 7399 (30.7) | 7946 (29.2) | 15,345 (29.9) |
| 70–79 | 4135 (17.1) | 5018 (18.5) | 9153 (17.8) |
| ≥80 | 935 (3.9) | 1556 (5.7) | 2491 (4.9) |
| BMI at diabetes diagnosis (kg/m2) | |||
| Median, IQRa | 24.2 (22.5, 26.2) | 24.1 (22.1, 26.6) | 24.2 (22.3, 26.4) |
| BMI categories, N (%) | |||
| <18.5 | 451 (1.9) | 664 (2.5) | 1115 (2.2) |
| 18.5–24.9 | 14,131 (58.7) | 15,723 (58.0) | 29,854 (58.4) |
| 25.0–29.9 | 8488 (35.3) | 9129 (33.7) | 17,617 (34.4) |
| ≥30.0 | 986 (4.1) | 1575 (5.8) | 2561 (5.1) |
| Year of diabetes diagnosis, N (%) | |||
| 2004 | 1676 (7.0) | 1960 (7.2) | 3636 (7.1) |
| 2005 | 2044 (8.5) | 2311 (8.5) | 4355 (8.5) |
| 2006 | 2483 (10.3) | 2925 (10.8) | 5408 (10.5) |
| 2007 | 2477 (10.3) | 2877 (10.6) | 5354 (10.4) |
| 2008 | 2969 (12.3) | 3475 (12.8) | 6444 (12.6) |
| 2009 | 2055 (8.5) | 2337 (8.6) | 4392 (8.6) |
| 2010 | 2220 (9.2) | 2392 (8.8) | 4612 (9.0) |
| 2011 | 1981 (8.2) | 2155 (7.9) | 4136 (8.1) |
| 2012 | 3022 (12.5) | 3326 (12.2) | 6348 (12.4) |
| 2013 | 1768 (7.3) | 1936 (7.1) | 3704 (7.2) |
| 2014 | 1429 (5.9) | 1506 (5.5) | 2935 (5.7) |
| Follow-up time (years) | |||
| Median, IQR | 6.3 (3.7,8.8) | 6.7 (3.8, 8.8) | 6.7 (3.8, 8.8) |
| Time groups, N (%) | |||
| <5 | 9455 (39.1) | 10,263 (37.7) | 19,718 (38.4) |
| 5–9 | 11,633 (48.2) | 13,368 (49.1) | 25,001 (48.7) |
| ≥10 | 3036 (12.5) | 3569 (13.1) | 5505 (12.9) |
aIQR, interquartile range.
After 324,116 person-years of observation (151,288 person-years in men and 174,066 in women), 2920 cancer cases were identified, resulting in an incidence of 900.9/100,000 person-years for all cancers. The incidence of overall cancer was higher in men (970.3/100,000 (95% CI: 921.9–1021.3/100,000)) than in women (834.2 per 100,000 person-years (95% CI: 792.3–878.2/100,000)) (Table 2). After adjusting for age according to the standard world population, the ASRs were 345.0 per 100,000 person-years in men and 285.80 per 100,000 in women. Lung cancer was the most common cancer in men, followed by cancers of stomach, colon, prostate, liver, rectum, pancreas and thyroid. In women, breast cancer was the most common cancer, followed by cancer of lung, colon, thyroid, stomach, pancreas, rectum, cervix uteri and liver. Compared with the general population, the T2DM patients had higher risk of colon cancer in both men and women, with SIRs being 1.21 (1.02–1.40) and 1.20 (1.01–1.38), respectively. The women patients had an increased risk of breast (SIR: 1.34, 95% CI: 1.18–1.50) and thyroid cancer (SIR: 1.55, 95% CI: 1.27–1.82).
Table 2.
Cancer incidence in patients with type 2 diabetes, Shanghai, China, 2002–2014.
| Men (N = 24,124) | Women (N = 27,200) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of cases | Person-years | CIRa (95% CI) (/100,000) | ASRb (/100,000) | SIRc (95% CI) | No of cases | Person-years | CIRa (95% CI) (/100,000) | ASRb (/100,000) | SIRc (95% CI) | |
| All cancers | 1468 | 151,288 | 970.3 (921.9, 1021.3) | 345.0 | 0.93 (0.88, 0.98) | 1452 | 174,066 | 834.2 (792.3, 878.2) | 476.3 | 1.07 (1.01, 1.12) |
| Site-specific cancersd | ||||||||||
| Stomach | 198 | 154,078 | 128.5 (111.8, 147.7) | 42.6 | 0.63 (0.56, 0.70) | 107 | 177,585 | 60.6 (49.9, 72.8) | 25.5 | 0.90 (0.73, 1.06) |
| Colon | 152 | 154,082 | 98.6 (84.1, 115.6) | 32.8 | 1.21 (1.02, 1.40) | 155 | 177,465 | 87.3 (74.6, 102.2) | 26 | 1.20 (1.01, 1.38) |
| Rectum | 92 | 154,220 | 59.7 (48.6, 73.2) | 20.6 | 0.97 (0.77, 1.17) | 74 | 177,601 | 41.7 (33.1, 52.3) | 12 | 0.97 (0.74, 1.19) |
| Liver | 126 | 154,311 | 81.7 (68.6, 97.2) | 28.0 | 0.98 (0.82, 1.16) | 53 | 177,761 | 28.8 (22.8, 39.0) | 8.7 | 0.77 (0.56, 0.97) |
| Pancreas | 74 | 154,432 | 47.9 (38.1, 60.2) | 15.2 | 1.09 (0.84, 1.33) | 75 | 177,742 | 42.2 (33.6, 52.9) | 40.9 | 1.18 (0.91, 1.45) |
| Lung | 264 | 154,127 | 171.3 (151.8, 193.3) | 53.1 | 0.67 (0.59, 0.76) | 166 | 177,604 | 93.5 (80.3, 108.8) | 27.8 | 0.82 (0.70, 0.94) |
| Breast | 272 | 176,778 | 153.9 (136.2, 172.9) | 101.6 | 1.34 (1.18, 1.50) | |||||
| Corpus uteri | 53 | 177,604 | 29.8 (22.8, 39.1) | 14.5 | 1.07 (0.73, 1.41) | |||||
| Prostate | 134 | 154,074 | 86.9 (73.4, 103.0) | 23.5 | 1.11 (0.92, 1.30) | |||||
| Thyroid | 22 | 154,431 | 14.2 (9.4, 21.6) | 8.6 | 1.07 (0.63, 1.52) | 119 | 177,458 | 67.1 (56.0, 80.0) | 73.7 | 1.55 (1.27, 1.82) |
aCIR, crude incidence rate; bASR, age-standardized rate based on the World Standard Population; cSIR, standard incidence ratios; dordered by ICD-O code of each cancer.
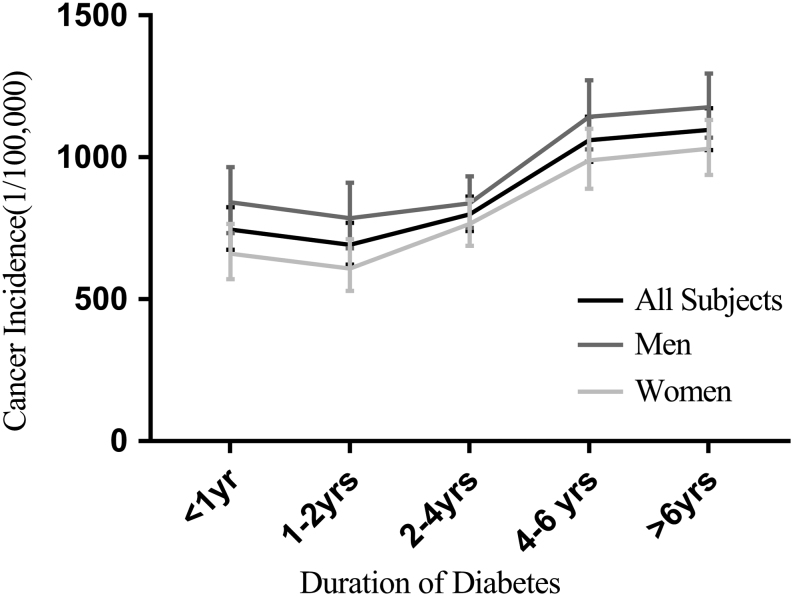
The incidence of overall, lung and rectal cancers was higher within the first year after diagnosis of T2DM in men, and then decreased in the next year and then increased afterward (Fig. 1, Table 3 and Supplementary Fig. 1, see section on supplementary data given at the end of this article). A similar pattern was observed in women for overall, liver, pancreas, thyroid, breast and corpus uteri cancers (Table 3). As shown in Supplementary Fig. 1, an evident peak in cancer incidence was observed within 1 year of T2DM diagnosis in almost all cancer types, except for colon, stomach, liver and prostate cancer.
Figure 1.
Incidence of overall cancer in Chinese diabetes patients along observation time.
Table 3.
Incidence and 95% CI of overall and site-specific cancers along with follow-up time in diabetes patients of Shanghai, China.
| Specific cancers | Incidence (95% CI) (/100,000) | ||||
|---|---|---|---|---|---|
| Within 1 year | 1–2 years | 2–4 years | 4–6 years | >6 years | |
| All cancers | |||||
| All subjects | 746.0 (674.7, 824.8) | 691.7 (621.6, 769.8) | 799.3 (741.1, 862.1) | 1060.6 (983.8, 1143.4) | 1097.4 (1026.1, 1173.7) |
| Men | 841.6 (733.2, 966.1) | 786.0 (678.9, 910.0) | 837.9 (752.1, 933.6) | 1143.2 (1028.0, 1271.5) | 1176.9 (1069.3, 1295.3) |
| Women | 661.2 (571.1, 765.5) | 608.5 (529.4, 711.6) | 765.4 (688.6, 850.8) | 989.1 (889.3, 1100.0) | 1030.4 (937.7, 1132.1) |
| Lung | |||||
| All subjects | 97.8 (65.6, 117.7) | 91.9 (68.6, 123.1) | 101.9 (82.7, 125.8) | 163.9 (134.8, 196.9) | 181.2 (154.1, 213.1) |
| Men | 132.9 (94.0, 187.9) | 113.2 (77.0, 166.2) | 135.6 (103.8, 176.9) | 233.3 (184.9, 294.3) | 220.1 (177.0, 273.6) |
| Women | 47.9 (27.8, 82.5) | 73.1 (45.6, 114.6) | 72.6 (51.6, 102.1) | 102.1 (73.7, 141.6) | 148.5 (116.5, 189.4) |
| Colon | |||||
| All subjects | 62.5 (44.2, 88.3) | 81.7 (59.9, 111.3) | 69.2 (53.6, 89.3) | 106.6 (84.4, 134.8) | 131.8 (108.9, 159.4) |
| Men | 78.9 (50.3, 123.7) | 91.4 (59.6, 140.2) | 77.8 (59.6, 140.2) | 118.3 (54.7, 110.7) | 122.4 (91.4, 163.9) |
| Women | 47.9 (27.8, 82.5) | 73.19 (46.6, 114.6) | 61.6 (42.5, 89.3) | 96.5 (68.9, 135.1) | 139.7 (108.7, 179.5) |
| Rectum | |||||
| All subjects | 48.8 (32.9, 72.2) | 32.7 (20.0, 53.3) | 50.4 (37.4, 67.9) | 51.7 (36.9, 72.4) | 59.6 (44.9, 79.0) |
| Men | 74.7 (47.1, 118.6) | 34.8 (17.4, 69.5) | 70.3 (48.5, 101.8) | 36.1 (20.0, 65.2) | 73.3 (50.3, 106.9) |
| Women | 25.8 (12.3, 54.1) | 30.8 (15.4, 61.5) | 32.9 (19.9, 54.7) | 65.3 (43.3, 98.2) | 48.0 (31.3, 73.6) |
| Stomach | |||||
| All subjects | 60.5 (42.6, 86.0) | 73.5 (53.0, 101.9) | 90.3 (72.2, 112.9) | 123.4 (99.2, 153.3) | 99.4 (79.8, 123.7) |
| Men | 95.5 (63.5, 143.8) | 113.1 (77.0, 166.1) | 107.9 (80.1, 145.6) | 174.2 (133.1, 228.0) | 144.2 (110.1, 188.7) |
| Women | 29.5 (14.7, 58.9) | 38.5 (20.7, 71.5) | 74.8 (53.4, 104.7) | 79.5 (54.9, 115.1) | 61.7 (42.3, 90.0) |
| Liver | |||||
| All subjects | 35.1 (22.1, 55.8) | 26.5 (15.4, 45.7) | 48.0 (35.4, 65.2) | 82.1 (62.9, 107.2) | 65.7 (50.1, 85.9) |
| Men | 41.5 (22.3, 77.1) | 52.2 (29.6, 91.9) | 67.7 (46.4, 98.7) | 124.7 (90.7, 171.3) | 105.8 (77.3, 144.7) |
| Women | 29.5 (14.7, 58.9) | 3.8 (0.5, 27.2) | 30.8 (18.2, 51.9) | 45.3 (27.8, 74.0) | 31.9 (18.9, 53.9) |
| Pancreas | |||||
| All subjects | 52.7 (36.1, 76.8) | 53.1 (36.1, 77.9) | 39.8 (28.4, 55.7) | 51.7 (36.9, 72.3) | 34.7 (23.9, 50.2) |
| Men | 53.9 (31.3, 92.9) | 69.6 (42.6, 113.6) | 37.6 (22.7, 62.4) | 55.7 (34.6, 89.6) | 35.2 (20.4, 60.6) |
| Women | 51.6 (30.5, 87.1) | 38.4 (20.7, 71.5) | 41.8 (26.6, 65.5) | 48.2 (29.9, 77.5) | 34.2 (20.6, 56.8) |
| Thyroid | |||||
| All subjects | 29.3 (17.6, 48.6) | 22.4 (12.4, 40.5) | 36.3 (25.5, 51.6) | 56.3 (40.8, 77.7) | 58.3 (43.8, 77.6) |
| Men | 4.2 (0.6, 299.5) | 4.3 (0.6, 30.8) | 10.0 (3.8, 26.7) | 22.9 (10.9, 48.1) | 24.4 (12.7, 46.8) |
| Women | 51.6 (30.5, 87.1) | 38.5 (20.7, 71.5) | 59.4 (40.7, 86.6) | 85.2 (59.6, 121.9) | 87.0 (63.3, 119.6) |
| Prostatea | 58.1 (34.4, 98.1) | 87.0 (56.1, 134.9) | 77.8 (54.7, 110.7) | 124.9 (90.9, 171.7) | 84.3 (59.3, 119.9) |
| Breastb | 154.8 (114.4, 209.5) | 134.8 (96.8, 187.8) | 161.1(128.1, 202.7) | 165.6 (128.0, 214.2) | 147.6 (115.5, 188.6) |
| Corpus uterib | 51.6 (30.5, 87.1) | 26.9 (12.8, 56.5) | 26.4 (15.1, 46.7) | 22.8 (11.4, 45.6) | 27.5 (15.6, 48.5) |
aAmong men only; bamong women only.
To reduce the potential influence of detection bias in the ascertainment of cancer outcomes, we excluded the events and person-years during the first year of observation, and calculated SIRs of cancer, overall and by site, standardized to the general population of Minhang district (Table 4). In men, the risks of rectal (SIR: 0.79, 95% CI: 0.61–0.97), lung (SIR: 0.60, 95% CI: 0.52–0.67) and stomach cancer (SIR: 0.82, 95% CI: 0.69–0.94) were significantly lower in those with T2DM than in the general population. Among women, the risks of thyroid (SIR: 1.37, 95% CI: 1.11, 1.63) and breast (SIR: 1.13, 95% CI: 1.01, 1.28) cancer were significantly higher in those with T2DM, whereas the risk of lung cancer (SIR: 0.76, 95% CI: 0.64, 0.88) and liver cancer (SIR: 0.65, 95% CI: 0.46, 0.84) was lower. Non-significant associations were observed for other cancers, although there was a suggestion of a lower incidence of stomach cancer among women with T2DM.
Table 4.
Standard incidence ratios of cancers in diabetes patients of Shanghai, China.
| Sites | Men (N = 24,124) | Women (N = 27,200) | ||||
|---|---|---|---|---|---|---|
| No. of cases | SIRa (95% CI) | No. of cases | SIRa (95% CI) | |||
| Observed | Expected | Observed | Expected | |||
| All cancers | 1266 | 1580 | 0.80 (0.76, 0.85) | 1273 | 1360 | 0.93 (0.88, 0.99) |
| Sub-site cancers | ||||||
| Colon | 133 | 125 | 1.07 (0.88, 1.25) | 142 | 129 | 1.10 (0.92, 1.28) |
| Rectum | 74 | 94 | 0.79 (0.61, 0.97) | 67 | 76 | 0.88 (0.67, 1.10) |
| Lung | 232 | 389 | 0.60 (0.52, 0.67) | 153 | 200 | 0.76 (0.64, 0.88) |
| Stomach | 175 | 215 | 0.82 (0.69, 0.94) | 99 | 119 | 0.93 (0.67, 1.00) |
| Liver | 116 | 127 | 0.92 (0.75, 1.08) | 45 | 69 | 0.65 (0.46, 0.84) |
| Pancreas | 61 | 68 | 0.90 (0.68, 1.13) | 61 | 63 | 0.96 (0.72, 1.21) |
| Thyroid | 21 | 20 | 1.03 (0.60, 1.47) | 105 | 76 | 1.37 (1.11, 1.63) |
| Prostate | 120 | 119 | 1.01 (0.82, 1.18) | |||
| Breast | 230 | 202 | 1.13 (1.01, 1.28) | |||
| Corpus uteri | 39 | 40 | 0.98 (0.67, 1.20) | |||
Cancer cases diagnosed within one year of diabetes diagnosis excluded from the analysis.
aSIR, standard incidence ratios.
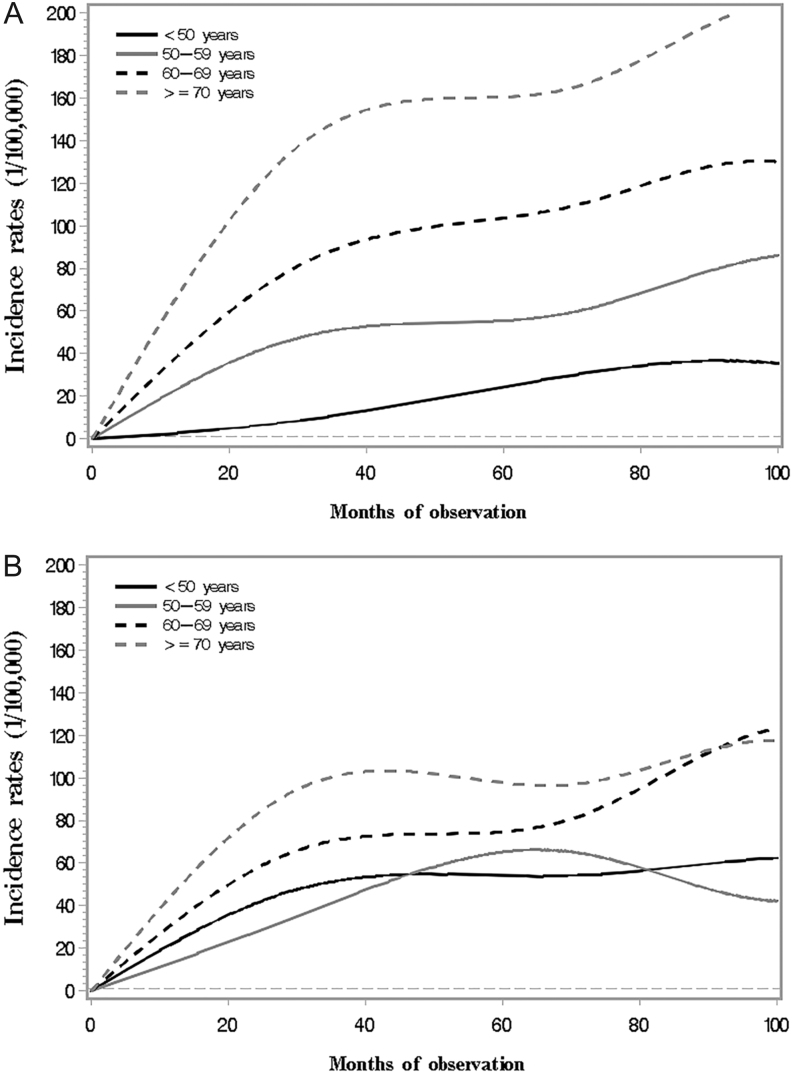
We further curved the cancer incidence along follow-up time in T2DM patients by age group after excluding the first year of follow-up. As shown in Fig. 2, the cancer incidence rates were much higher in older groups than in the younger groups along follow-up time in men, but the incidence seemed to be much closer in women at different age over time, particularly for the younger age groups.
Figure 2.
Incidence of overall cancer in Chinese diabetes patients along observation time by age groups (A. Men; B. Women).
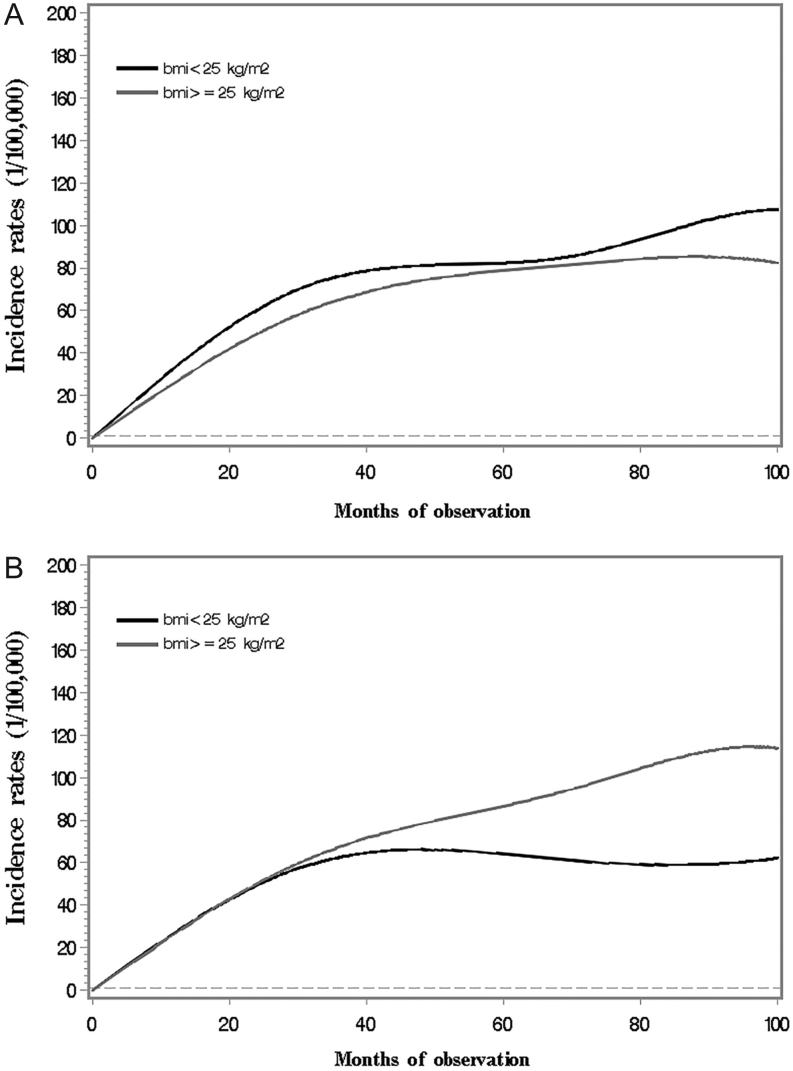
Figure 3A shows the increasing pattern of cancer incidence over time by BMI in men. The cancer incidence in overweight men appeared slightly lower than that in normal weight men. The incidence, on the other hand, was observed to increase at a similar rate in overweight and normal weight women during the initial 2-year observation, and then showed a flattening along further observations in normal weight women and a continuous upward trend in overweight women.
Figure 3.
Incidence of overall cancer in Chinese diabetes patients along observation time by BMI, adjusted for age (A. Men; B. Women).
Discussion
In this population-based retrospective study based on a standardized T2DM management data, we examined the cancer incidence by time since T2DM diagnosis in patients from the Minhang District of Shanghai, China. We observed a lower risk of overall cancer and several specific cancers, but an increased risk of female breast and thyroid cancers over a 10-year period. The cancer risk, either increased or decreased, was not influenced by detection bias because cancers that occurred within 1 year of T2DM diagnosis were excluded from the analysis. Our findings have implications for understanding the T2DM–cancer association and highlight the need to emphasize cancer prevention in Chinese T2DM patients.
Numerous studies have reported a positive association between T2DM and cancer risk in western populations (2, 17, 18, 19), with the strongest associations being observed for liver, pancreatic and endometrial cancers and more moderate associations observed for breast, colorectal and bladder cancer. An inverse association has been consistently observed for prostate cancer, while the evidence has been inconsistent for other cancers including lung and stomach cancers (2, 17, 18, 19). Hyperglycemia or hyperinsulinemia may partially explain some of the observed positive associations. It has been suggested that hyperglycemia in T2DM patients may increase cell oxidation, cause DNA damage and increase hyperinsulinemia (1). Increased concentrations of insulin and insulin-like growth factors (IGF) have been demonstrated to stimulate cell proliferation in many organs (20).
The lower incidence of overall cancer in our subjects was somewhat inconsistent with these previous results. One explanation for the inconsistency may be the different levels of exposure to common risk factors such as obesity, unhealthy diet, physical inactivity, hyperinsulinemia and hyperglycemia in Chinese populations (3, 4). Moreover, use of hypoglycemic drugs may also play a role (21). Of the most commonly used hypoglycemic drugs, metformin has been associated with a decreased risk of cancer (5), while use of insulin and sulfonylureas has been related to increased risk of cancer. Insulin injection is not used to treat T2DM patients as commonly in China as in other countries, with only 11% in China (22) vs 27% in the US (23), 33% in Colombia (24) and 18% in Korea (25). On the contrary, metformin was recommended as the first-line treatment of T2DM in the Chinese Guidelines in 2007 (26, 27). Since then, metformin has been the most widely used oral hypoglycemic agent in China. It is reported that in outpatients with T2DM who had received oral anti-diabetic drugs, 53.7% used metformin and 42.7% used sulfonylureas (28). The more common use of metformin may have contributed to lower risk of overall cancer in the population. Detection bias may also contribute to the differences observed between our study and prior studies (7, 29). Newly diagnosed T2DM patients may visit medical institutions more frequently and thus may be more likely to be detected for cancer. Johnson et al. (8) observed an increased risk for colorectal, lung, liver, cervical, endometrial, ovarian, pancreatic and prostate cancers evaluated during the first 3 months after T2DM onset in Canadian citizens with T2DM. In our previous report on Chinese individuals with T2DM, we also observed an elevated incidence for almost every cancer examined, but we did not account for any potential detection bias in that study (13). Finally, carcinogenesis is a multistage process lasting for years to decades, depending on the specific cancer (30). Longer follow-up and detailed information on use of medications are needed to confirm our results.
It is interesting that women with T2DM had a higher risk of thyroid and breast cancer than their counterparts in the general population. The dis-regulation of sex hormones caused by insulin resistance in T2DM patients may help to explain the results. Insulin resistance has been suggested to lead to decreased production of circulating sex hormone-binding globulin and thus enhanced bioactivity of estradiol (31) which would increase the risk of breast cancer. Moreover, T2DM may affect the mitogenic pathway of the follicular cells through the role of elevated insulin levels, long-term elevation of thyroid-stimulating hormone (TSH), vitamin D deficiency and exposure to high levels of glucose and triglycerides, all of which may increase the risk of thyroid cancer (32), particularly in women whose high level of estrogen is a potent growth factor both for benign and malignant thyroid cells (33).
Strengths and limitations
The strengths of this study include the use of validated data sources for both identification of the study population and of cancer outcomes. We used a population-based administrative system that covers 90% of the whole population in the Minhang District of Shanghai to identify individuals newly diagnosed with T2DM. The information recorded in the system enabled us to design a large-scale population-based retrospective cohort study and minimize selection bias. Moreover, the Shanghai Cancer Registry and the Shanghai Vital Statistics, as a part of the National Program of Cancer Registries with high quality of data (34) that has been used in previous epidemiologic studies (13, 15, 35), provide us reliable sources to identify incident cancer cases and all-cause deaths in the population.
There were several limitations in this study. First, as we used the data from an administrative system, we did not have access to data on lifestyle factors such as cigarette smoking, alcohol drinking, diet, physical activity and medication use. It is possible that excess risk of some cancers may be due to more prevalent of these risk factors in T2DM patients than in general population. However, stratified analysis by BMI at diabetes diagnosis did not observe a large difference in overall cancer incidence between overweight and normal weight patients. On the contrary, the patients may have adopted healthy lifestyles after diagnosis with diabetes, which may have biased our results toward null. Second, we calculated SIR using data from the general population in the Minhang District, not from a population without T2DM. Thus, given the high prevalence of T2DM in Chinese adults (12%) (36), we anticipate that the general population would include a large number of T2DM patients, diagnosed or undiagnosed, which, in turn, may have led to underestimated SIRs. Moreover, the T2DM patients who were enrolled in the management system may have higher motivation to visit clinics than those did not, which may also help to explain the increased cancer detection rates shortly after the enrollment in this population. Finally, the average 6-year observation for 51,324 patients provided only 325,354 person-years, which was not enough to produce sufficient number of cases for less common cancers such as pancreatic cancer in both men and women, thyroid cancer in men, and corpus uteri, liver, and rectal cancer in women.
In summary, this large-scale population-based study of Chinese individuals with T2DM observed a lower risk of overall cancer and several common cancers but an elevated risk of breast and thyroid cancers in women after excluding potential detection bias. However, the huge number of T2DM patients and the increasing prevalence of T2DM in China suggest that even a small excess risk of cancer can be significant in public health. Prevention of cancers, especially female breast and thyroid cancers, should be emphasized in Chinese T2DM patients.
Supplementary Material
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work was supported by the Three-year Action Plan on Public Health, Phase IV, Shanghai, China (15GWZK0801).
Author contribution statement
Y F drafted the paper. X Z and S S W contributed to statistical analysis and revision of the paper, X H and X D contributed to data collection and quality control. H F and W H X contributed to study design, statistical analysis and revision of the paper. All authors approved the final version.
Acknowledgements
The authors would like to thank the study participants and the staff members of the Communities in Minhang District of Shanghai, China, for their contribution to the study.
References
- 1.Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, Pollak M, Regensteiner JG, Yee D. Diabetes and cancer: a consensus report. Diabetes Care 2010. 33 1674–1685. ( 10.2337/dc10-0666) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jian Gang P, Mo L, Lu Y, Runqi L, Xing Z. Diabetes mellitus and the risk of prostate cancer: an update and cumulative meta-analysis. Endocrine Research 2015. 40 54–61. ( 10.3109/07435800.2014.934961) [DOI] [PubMed] [Google Scholar]
- 3.Hua F, Yu JJ, Hu ZW. Diabetes and cancer, common threads and missing links. Cancer Letters 2016. 374 54–61. ( 10.1016/j.canlet.2016.02.006) [DOI] [PubMed] [Google Scholar]
- 4.Cohen DH, LeRoith D. Obesity, type 2 diabetes, and cancer: the insulin and IGF connection. Endocrine-Related Cancer 2012. 19 F27–F45. ( 10.1530/ERC-11-0374) [DOI] [PubMed] [Google Scholar]
- 5.Gandini S, Puntoni M, Heckman-Stoddard BM, Dunn BK, Ford L, DeCensi A, Szabo E. Metformin and cancer risk and mortality: a systematic review and meta-analysis taking into account biases and confounders. Cancer Prevention Research 2014. 7 867–885. ( 10.1158/1940-6207.CAPR-13-0424) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JP. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ 2015. 350 g7607 ( 10.1136/bmj.g7607) [DOI] [PubMed] [Google Scholar]
- 7.De Bruijn KM, Ruiter R, de Keyser CE, Hofman A, Stricker BH, van Eijck CH. Detection bias may be the main cause of increased cancer incidence among diabetics: results from the Rotterdam Study. European Journal of Cancer 2014. 50 2449–2455. ( 10.1016/j.ejca.2014.06.019) [DOI] [PubMed] [Google Scholar]
- 8.Johnson JA, Bowker SL, Richardson K, Marra CA. Time-varying incidence of cancer after the onset of type 2 diabetes: evidence of potential detection bias. Diabetologia 2011. 54 2263–2271. ( 10.1007/s00125-011-2242-1) [DOI] [PubMed] [Google Scholar]
- 9.Geier AS, Wellmann J, Wellmann I, Kajuter H, Heidinger O, Hempel G, Hense HW. Cancer detection rates following enrolment in a disease management programme for type 2 diabetes. Diabetologia 2013. 56 1944–1948. ( 10.1007/s00125-013-2947-4) [DOI] [PubMed] [Google Scholar]
- 10.Yang SH, Dou KF, Song WJ. Prevalence of diabetes among men and women in China. New England Journal of Medicine 2010. 362 2425–6; author reply 2426. ( 10.1056/NEJMc1004671) [DOI] [PubMed] [Google Scholar]
- 11.Hu R, Gong W, Wang M, Pan J, Wu H, Fei F, He Q, Yu M. Association between type 2 diabetes mellitus and risk of cancers: a cohort study. Zhonghua Liu Xing Bing Xue Za Zhi 2015. 36 1384–1386. ( 10.3760/cma.j.issn.0254-6450.2015.02.002) [DOI] [PubMed] [Google Scholar]
- 12.Ren X, Zhang X, Zhang X, Gu W, Chen K, Le Y, Lai M, Zhu Y. Type 2 diabetes mellitus associated with increased risk for colorectal cancer: evidence from an international ecological study and population-based risk analysis in China. Public Health 2009. 123 540–544. ( 10.1016/j.puhe.2009.06.019) [DOI] [PubMed] [Google Scholar]
- 13.Xu HL, Fang H, Xu WH, Qin GY, Yan YJ, Yao BD, Zhao NQ, Liu YN, Zhang F, Li WX, et al Cancer incidence in patients with type 2 diabetes mellitus: a population-based cohort study in Shanghai. BMC Cancer 2015. 15 852 ( 10.1186/s12885-015-1887-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabetic Medicine 1998. 15 539–553. () [DOI] [PubMed] [Google Scholar]
- 15.Huang Z, Wen W, Zheng Y, Gao YT, Wu C, Bao P, Wang C, Gu K, Peng P, Gong Y, et al Breast cancer incidence and mortality: trends over 40 years among women in Shanghai, China. Annals of Oncology 2016. 27 1129–1134. ( 10.1093/annonc/mdw069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Statistics in Medicine 2010. 29 1037–1057. ( 10.1002/sim.3841) [DOI] [PubMed] [Google Scholar]
- 17.El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clinical Gastroenterology and Hepatology 2006. 4 369–380. ( 10.1016/j.cgh.2005.12.007) [DOI] [PubMed] [Google Scholar]
- 18.Huxley R, Ansary-Moghaddam A, Berrington de Gonzalez A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. British Journal of Cancer 2005. 92 2076–2083. ( 10.1038/sj.bjc.6602619) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szablewski L. Diabetes mellitus: influences on cancer risk. Diabetes/Metabolism Research and Reviews 2014. 30 543–553. ( 10.1002/dmrr.2573) [DOI] [PubMed] [Google Scholar]
- 20.Furstenberger G, Senn HJ. Insulin-like growth factors and cancer. Lancet Oncology 2002. 3 298–302. ( 10.1016/S1470-2045(02)00731-3) [DOI] [PubMed] [Google Scholar]
- 21.Shlomai G, Neel B, LeRoith D, Gallagher EJ. Type 2 diabetes mellitus and cancer: the role of pharmacotherapy. Journal of Clinical Oncology 2016. 34 4261–4269. ( 10.1200/JCO.2016.67.4044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X, Zhan Y, Wang Q, Xu Y, Liu Y, Wang X, Ren Y. A sneak peak of the 2015 report on the market of type 2 diabetes mellitus diagnosis and treatment in China. Annals of Translational Medicine 2015. 3 120 ( 10.3978/j.issn.2305-5839.2015.06.09) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hampp C, Borders-Hemphill V, Moeny DG, Wysowski DK. Use of antidiabetic drugs in the U.S., 2003–2012. Diabetes Care 2014. 37 1367–1374. ( 10.2337/dc13-2289) [DOI] [PubMed] [Google Scholar]
- 24.Gaviria-Mendoza A, Sanchez-Duque JA, Medina-Morales DA, Machado-Alba JE. Prescription patterns and costs of antidiabetic medications in a large group of patients. Primary Care Diabetes 2018. 12 184–191. ( 10.1016/j.pcd.2017.11.002) [DOI] [PubMed] [Google Scholar]
- 25.Ko SH, Kim DJ, Park JH, Park CY, Jung CH, Kwon HS, Park JY, Song KH, Han K, Lee KU, et al Trends of antidiabetic drug use in adult type 2 diabetes in Korea in 2002–2013: nationwide population-based cohort study. Medicine 2016. 95 e4018 ( 10.1097/MD.0000000000004018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weng JP, Ji L, Jia W, Lu J, Zhou Z, Zou D, Zhu D, Chen L, Chen L, Guo L, et al Standards of care for type 2 diabetes in China. Diabetes/Metabolism Research and Reviews 2016. 32 442–458. ( 10.1002/dmrr.2827) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang W, Weng J. Early therapy for type 2 diabetes in China. Lancet Diabetes and Endocrinology 2014. 2 992–1002. ( 10.1016/S2213-8587(14)70136-6) [DOI] [PubMed] [Google Scholar]
- 28.Ji L, Lu J, Weng J, Jia W, Tian H, Zhu D, Xing X, Guo L. China type 2 diabetes treatment status survey of treatment pattern of oral drugs users. Journal of Diabetes 2015. 7 166–173. ( 10.1111/1753-0407.12165) [DOI] [PubMed] [Google Scholar]
- 29.Carstensen B, Witte DR, Friis S. Cancer occurrence in Danish diabetic patients: duration and insulin effects. Diabetologia 2012. 55 948–958. ( 10.1007/s00125-011-2381-4) [DOI] [PubMed] [Google Scholar]
- 30.Luebeck EG, Curtius K, Jeon J, Hazelton WD. Impact of tumor progression on cancer incidence curves. Cancer Research 2013. 73 1086–1096. ( 10.1158/0008-5472.CAN-12-2198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kazer RR. Insulin resistance, insulin-like growth factor I and breast cancer: a hypothesis. International Journal of Cancer 1995. 62 403–406. ( 10.1002/ijc.2910620408) [DOI] [PubMed] [Google Scholar]
- 32.Shih SR, Chiu WY, Chang TC, Tseng CH. Diabetes and thyroid cancer risk: literature review. Experimental Diabetes Research 2012. 2012 578285 ( 10.1155/2012/578285) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Derwahl M, Nicula D. Estrogen and its role in thyroid cancer. Endocrine-Related Cancer 2014. 21 T273–T283. ( 10.1530/ERC-14-0053) [DOI] [PubMed] [Google Scholar]
- 34.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA: A Cancer Journal for Clinicians 2016. 66 115–132. ( 10.3322/caac.21338) [DOI] [PubMed] [Google Scholar]
- 35.He D, Fang Y, Gunter MJ, Xu D, Zhao Y, Zhou J, Fang H, Xu WH. Incidence of breast cancer in Chinese women exposed to the 1959–1961 great Chinese famine. BMC Cancer 2017. 17 824 ( 10.1186/s12885-017-3794-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu Y, Wang L, He J, Bi Y, Li M, Wang T, Wang L, Jiang Y, Dai M, Lu J, et al Prevalence and control of diabetes in Chinese adults. JAMA 2013. 310 948–959. ( 10.1001/jama.2013.168118) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a