Abstract
Fluid challenge during right heart catheterization has been used for unmasking pulmonary hypertension (PH) related to left-sided heart disease. We evaluated the clinical and hemodynamic factors affecting the response to fluid challenge and investigated the role of fluid challenge in the classification and management of PH patients. We reviewed the charts of 67 patients who underwent fluid challenge with a baseline pulmonary arterial wedge pressure (PAWP) of ≤ 18 mmHg. A positive fluid challenge (PFC) was defined as an increase in PAWP to > 18 mmHg after 500 mL saline infusion. Clinical characteristics and echocardiographic and hemodynamic parameters were compared between PFC and negative fluid challenge (NFC). PFC was associated with female sex, increased BMI, and hypertension. A greater rise in PAWP was observed in PFC (6.8 ± 2.3 vs. 3.8 ± 2.7 mmHg, P = 0.001). A larger increase in PAWP correlated with a lower transpulmonary gradient (r = –0.42, P < 0.001), diastolic pulmonary gradient (r = –0.42, P < 0.001), and pulmonary vascular resistance (r = –0.38, P < 0.001). We found 100% of the patients with PFC were classified as WHO group 2 PH compared to 49% of the NFC patients (P < 0.001). Fewer patients with PFC were started on advanced PH therapies and more were discharged from PH clinic. A PFC and the magnitude of PAWP increase after saline loading are associated with parameters related to left heart disease. In our population, fluid challenge appeared to influence the classification of PH and whether patients are started on therapy or discharged from clinic.
Keywords: pulmonary hypertension, left heart disease, fluid challenge, right heart catheterization, hemodynamics
Introduction
Pulmonary hypertension (PH) can occur as a consequence of several conditions.1 The most common form of PH is pulmonary hypertension associated with left heart disease (PH-LHD).2–4 PH-LHD arises from increased pulmonary venous pressures.5–7 Often, the increase in pulmonary arterial pressures is simply related to passive transmission of high pulmonary venous pressure (isolated post-capillary PH).8,9 However, in some cases pulmonary arterial remodeling can develop from venous congestion, resulting in a pre-capillary component to the PH.9–13
Differentiating pre-capillary and post-capillary PH relies on pulmonary arterial wedge pressure (PAWP). A mean pulmonary arterial pressure (mPAP) of ≥ 25 mmHg with PAWP >15 mmHg is required for diagnosis of PH-LHD.2,14 However, a normal PAWP does not exclude PH-LHD as a PAWP <15 mmHg can be seen in resting or volume deplete states. The current medical therapies approved for pulmonary arterial hypertension (PAH) can be ineffective or potentially have adverse effects when administered to patients with left-sided heart disease or valvular heart disease.15–17 Therefore, there has been an interest in using fluid loading with saline to unmask occult post-capillary PH.18–21
There has been a lack of consensus on the role of fluid challenge in identifying PH-LHD.22 There is still uncertainty about the volume of infusate or the cut-off PAWP value for a pathological response.19,23 In addition, the diagnostic relevance and impact of fluid challenge on clinical decision-making remains unknown. In this retrospective study, hemodynamic responses to fluid challenge in a cohort of patients referred for right heart catheterization (RHC) were examined. Finally, we evaluated the influence of fluid challenge on the classification and management of a real-world PH population.
Methods
Study population
This was a retrospective, single-center study approved by clinical research ethics board at the University of British Columbia. Clinic charts of patients referred to our PH clinic from 1 July 2013 to 30 April 2017 were reviewed. Vancouver General Hospital Pulmonary Hypertension clinic is the sole PH referral center for the province of British Columbia in Canada with a large referral base of 4 million. All the RHCs for our patients were performed by a single operator (NB). All the patients who underwent fluid challenge during RHC between the study period and had a PAWP ≤15 mmHg at baseline were included in this study. The diagnosis of PH required a mPAP ≥25 mmHg. There were 92 patients referred for evaluation of PH who underwent fluid challenge of whom 67 patients met our inclusion criteria. The decision to perform fluid challenge was left at the discretion of operator (NB) or the referring physicians and was mostly performed in patients with risk factors for left heart disease but normal resting hemodynamics. All patients received 500 mL of normal saline over 5 min. A positive fluid challenge (PFC) was defined as an increase in PAWP to >18 mmHg with saline infusion while in negative fluid challenge (NFC) PAWP remained ≤18 mmHg. Those patients who underwent fluid challenge but had a PAWP >15 mmHg at baseline were excluded. In cases in which left heart catheterization was concurrently performed with RHC, we also excluded the patients with discordant PAWP and left ventricular end-diastolic pressure (i.e. PAWP of ≤15 mmHg and LVEDP of >15 mmHg).
Patient characteristics, echocardiography, and hemodynamics
Co-morbidities were assessed through a structured chart review. Obesity was defined as a body mass index (BMI) of ≥30 kg/m2 based on the National Institute of Health guidelines.24 Arrhythmia was described as any history of sustained arrhythmia. In the vast majority of our patients, this was atrial fibrillation or flutter with similar distribution between the PFC and NFC cohorts (Table S1 Supplementary Appendix).
All patients had a two-dimensional transthoracic echocardiogram within a year of RHC. Right ventricular (RV) function was qualitatively assessed as either normal, mildly, moderately, or severely dysfunctional by the interpreting echocardiographer. All the other parameters were measured according to the American Society of Echocardiography guidelines.25,26
During RHC, the zero line was set at the mid-thoracic height. All measurements were obtained from hemodynamic tracing at end-diastole and at end-expiration. Only four of the patients (three in the NFC and one in the PFC) were in an atrial fibrillation rhythm at the time of RHC. In patients with atrial fibrillation, all pressures were carefully measured as averages over 3–5 RR intervals depending on the heart rate, to ensure accuracy of the recordings. Cardiac output (CO) was measured via the thermodilution method. There were no patients with significant unrepaired intracardiac shunts in our analysis. The transpulmonary gradient (TPG), diastolic pulmonary gradient (DPG), and pulmonary vascular resistance (PVR) were calculated in the standard manner, as previously described.27 The left ventricular end-diastolic transmural pressure (LVTMP) was calculated as PAWP – RAP.28
We also examined the relationship between change in PAWP for patients with lower baseline DPG, TPG, and PVR compared to higher values. Low DPG, TPG, and PVR were defined as a DPG < 7 mmHg, TPG < 12 mmHg, and PVR ≤3 Wood units, respectively. These thresholds were chosen based on values commonly chosen in the literature.1,9,29 Patients were seen in follow-up after fluid challenge by one of the three experienced PH specialists (NB, JS, RL). The physicians’ World Health Organization (WHO) PH classification, assessment, and management plan was determined from the chart notes from the visit immediately following the RHC with fluid challenge.
Statistical analysis
All data were analyzed with IBM SPSS statistical software package. Continuous variables were measured as means ± standard deviations and categorical variables were measured as percentages. The PFC and NFC groups were compared with the independent two-sample t-test or non-parametric Mann–Whitney–Wilcoxon test when normal distribution was not achieved. Using linear regression, we investigated the association between the change in PAWP with fluid and DPG, TPG, and PVR. The Pearson correlation coefficient (r) was calculated to assess the strength of association. For all comparisons, statistical significance was determined as a P value of < 0.05.
Results
Sixty-seven patients met the inclusion criteria. Of these, 12 patients had PFC (18%) and 55 had NFC (82%). Baseline demographics and echocardiographic characteristics of patients with PFC and NFC are compared in Table 1. Those patients with PFC were more likely to be women, have an increased BMI, and higher prevalence of hypertension. Other cardiovascular risk factors such as age, diabetes, or renal insufficiency were not statistically different between the two groups. On echocardiogram, those patients with a PFC had larger left atrial volume index but less evidence of RV dysfunction compared to NFC.
Table 1.
Baseline demographic, echocardiographic, and hemodynamic data.
| NFC (n = 55) | PFC (n = 12) | P value | |
|---|---|---|---|
| Demographics and co-morbidities | |||
| Age (years) | 67.8 ± 13.5 | 69.7 ± 8.5 | 0.54 |
| Sex (% female) | 51 | 92 | 0.01 |
| Obesity (%) | 16 | 50 | 0.01 |
| Hypertension (%) | 67 | 92 | 0.03 |
| Diabetes (%) | 22 | 42 | 0.16 |
| Arrhythmia (%) | 40 | 50 | 0.53 |
| OSA (%) | 27 | 25 | 0.88 |
| Renal insufficiency (%) | 24 | 17 | 0.59 |
| Scleroderma disorders (%) | 20 | 33 | 0.40 |
| Other connective tissue diseases (%) | 18 | 17 | 0.12 |
| Echocardiographic parameters | |||
| LV mass index (g.m–2) | 84.9 ± 30.2 | 89.9 ± 29.2 | 0.49 |
| LA volume index (mL/m2) | 35.6 ± 14.4 | 46.0 ± 14.1 | 0.03 |
| RV diameter (mm) | 39.3 ± 9.1 | 39.1 ± 10.2 | 0.94 |
| RA diameter (mm) | 39.2 ± 8.3 | 38.5 ± 8.5 | 0.81 |
| PASP (mmHg) | 59.2 ± 18.7 | 55.5 ± 13.9 | 0.45 |
| RV dysfunction (%) | 49 | 17 | 0.02 |
| RHC hemodynamic data | |||
| RA pressure (mmHg) | 6.7 ± 3.4 | 7.3 ± 3.3 | 0.64 |
| Cardiac output (L.min–1) | 4.6 ± 1.3 | 4.21 ± 0.65 | 0.12 |
| PVR (Wood unit) | 5.6 ± 4.0 | 3.0 ± 1.4 | <0.001 |
| TPG (mmHg) | 21.5 ± 10.2 | 14.6 ± 5.6 | 0.003 |
| DPG (mmHg) | 10.6 ± 8.1 | 3.3 ± 5.7 | 0.001 |
| Systolic PAP (mmHg) | 53.6 ± 17.2 | 44.9 ± 8.2 | 0.01 |
| Diastolic PAP (mmHg) | 21.7 ± 7.9 | 16.6 ± 5.8 | 0.02 |
| Mean PAP (mmHg) | 32.9 ± 10.0 | 26.8 ± 5.8 | 0.009 |
| LVTMP pre-fluid (mmHg) | 4.4 ± 3.1 | 6.1 ± 2.5 | 0.08 |
DPG, diastolic pulmonary gradient; LA, left atrium; LV, left ventricle; OSA, obstructive sleep apnea; PAP, pulmonary arterial pressure; NFC, negative fluid challenge; PASP, pulmonary arterial systolic pressure; PFC, positive fluid challenge; PVR, pulmonary vascular resistance; TPG, transpulmonary gradient; RA, right atrium; RHC, right heart catheterization; RV, right ventricle; LVTMP, left ventricular transmural pressure.
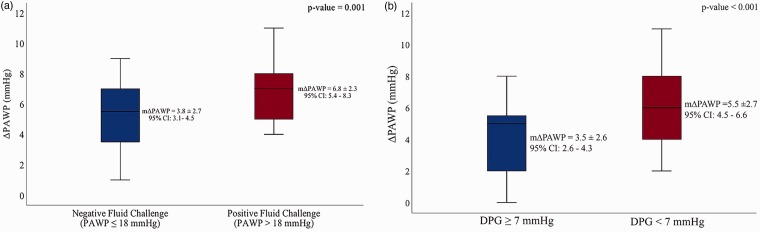
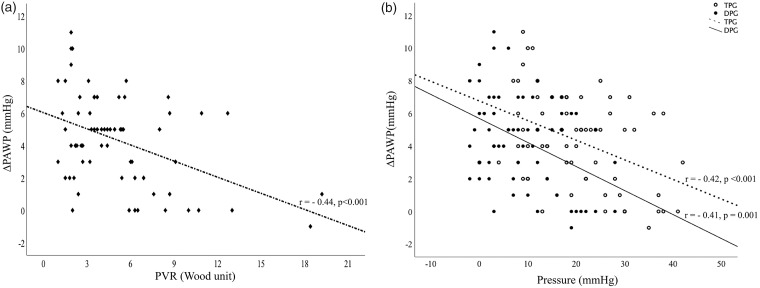
Hemodynamic data obtained during RHC are also summarized in Table 1. Although there was no difference in pulmonary artery systolic pressure (PASP) on echocardiography, pulmonary arterial pressures measured during RHC were significantly lower in the PFC patients compared to NFC. PAWP was higher in PFC both at baseline (13.3 ± 1.8 vs. 11.1 ± 2.5, P < 0.001) and after fluid challenge (20.2 ± 0.7 vs. 14.9 ± 2.8, P < 0.001). As expected, PFC was associated with a significantly greater increase in PAWP after fluid challenge compared to NFC (6.8 ± 2.3 vs. 3.8 ± 2.7, P value = 0.001) (Fig. 1a). This was independent of the starting PAWP value. As shown in Fig. 2, there was a moderate but statistically significant inverse correlation between change in PAWP with saline infusion and baseline DPG, TPG, and PVR. This indicates that parameters related to pre-capillary PH are associated with lesser increases in PAWP with fluid challenge. Similarly, larger increases in PAWP were related to parameters associated with isolated post-capillary PH (IpcPH), such as DPG < 7 mmHg (Fig. 1b). Of 41 patients with apparent PAH at baseline (i.e. mPAP ≥25 mmHg, PAWP < 15 mmHg, and PVR >3 Wood units), 18 (44%) patients met hemodynamic criteria for combined pre- and post-capillary PH (CpcPH) after fluid challenge (Figure S2 Supplementary Appendix).
Fig. 1.
Change (Δ) in PAWP after 500 mL of saline infusion (a) in patients with PFC and NFC and (b) in patients with DPG ≥ 7 mmHg and DPG < 7 mmHg. There was a greater increase in PAWP after fluid challenge in PFC compared to NFC and in patients with normal DPG (i.e. < 7 mmHg) compared to DPG ≥ 7 mmHg. mΔPAWP = mean (PAWPpost-fluid – PAWPpre-fluid). CI, confidence interval.
Fig. 2.
Relationship between (a) ΔPAWP* and PVR and (b) ΔPAWP and TPG (empty circles) and DPG (bold circles). A line of best fit was drawn as a linear correlation between ΔPAWP and DPG (solid line), TPG (dashed line), and PVR. Pearson correlation coefficient (r) revealed a moderate inverse relationship between ΔPAWP and both TPG/DPG and PVR. *ΔPAWP = (PAWPpost-fluid – PAWPpre-fluid).
Finally, a retrospective analysis of PH specialists’ classification and management of patients who underwent fluid challenge was performed and the results are recorded in Table 2. In several cases, the specialists felt that PH was multifactorial; hence, more than one WHO PH group was chosen as the underlying etiology. Patients with PFC were more likely to be classified as WHO group 2 PH and were less likely to be started on PAH-targeted therapy and followed in our specialized PH clinic.
Table 2.
Physicians’ classification and management of patients after fluid challenge.
| NFC (n = 55) | PFC (n = 12) | P value | |
|---|---|---|---|
| WHO PH classification* | |||
| Group 1, PAH | 23 (42) | 1 (8) | 0.03 |
| Group 2, PH-LHD | 27 (49) | 12 (100) | <0.001 |
| Group 3, PH-Lung disease | 24 (44) | 4 (33) | 0.52 |
| Group 4, CTEPH | 3 (5) | 2 (17) | 0.19 |
| Group 5, Miscellaneous | 3 (5) | 1 (8) | 0.75 |
| Initiation of PAH therapy | 28 (51) | 0 (0) | <0.001 |
| Discharged from PH clinic | 15 (27) | 8 (67) | 0.02 |
Values are presented as n (%).
Some patients were classified as multifactorial PH with contributions from more than one WHO group
CTEPH, chronic thromboembolic PH; LHD, left heart disease; PAH, pulmonary arterial hypertension.
In a sensitivity analysis, we used a threshold of 15 mmHg for PFC, as this was the threshold used in some previous studies. The results did not change appreciably and are presented in the Supplementary Appendix.
Discussion
In our population of patients being evaluated for PH, patients with a PFC had a higher prevalence of several demographic, echocardiographic, and hemodynamic features associated with left heart disease. This is similar to the findings of previous studies that investigated the clinical characteristics of patients with PH-LHD.30–32 However, certain key variables related to diastolic dysfunction, such as diabetes and age, did not correlate with PFC. Such discrepancies have also been observed in other studies and are thought to be due to the small number of patients who underwent fluid challenge.18,20 The observed distinct sex distribution in the hemodynamic response to saline loading could be due to the sex-related differences in left ventricular diastolic indices.33 The higher prevalence of heart failure with preserved ejection fraction (HFpEF) and more pronounced impairment in left ventricular relaxation in women suggest that fluid challenge might be higher yield in women.33–35 As expected, PFC was associated with structural changes in the left heart including left atrial dilatation. We did not note any difference in RA and RV size; this may have been on the basis of low sample size or mixed pre-capillary and post-capillary phenotype in the PFC group. However, we also found that RV dysfunction was less common in PFC. It is not entirely clear whether the presence of RV dysfunction attenuates the increase in preload to the left heart associated with a fluid bolus or whether patients in the NFC group had an enriched population of advanced pre-capillary PH with RV dysfunction. The latter hypothesis would be supported by the higher pulmonary pressures noted during RHC in the NFC group. Based on our findings, however, systolic pulmonary artery pressure (sPAP) measurement by echocardiography might be less useful in predicting response to saline loading as there might be possible sources of pressure underestimation particularly in patients with RV dysfunction.36
Currently, post-capillary PH is further classified into IpcPH with a normal DPG and PVR, and CpcPH with a DPG ≥7 mmHg or PVR >3 Wood units.1,2,37 In the past, the TPG was also used as a discriminatory variable.38 The association we found between low DPG, TPG, PVR, and response to saline loading demonstrates that a PFC is more likely when hemodynamic measures of pre-capillary PH are less elevated. Additionally, we observed that the magnitude of the increase in PAWP had an inverse correlation with baseline DPG, TPG, and PVR. This finding suggests that the change in PAWP may provide added discriminatory power over and above the final PAWP achieved post fluid challenge. Further research is required to determine whether the magnitude of the change in PAWP with saline loading should be incorporated into the definition of a PFC.
On the basis of hemodynamics alone, for a threshold of 15 mmHg and 18 mmHg for PFC, approximately 1/2 and 1/6 of our study patients meeting the hemodynamic criteria for PAH at baseline would be reclassified as CpcPH after fluid challenge, respectively. This suggests substantial implications to diagnosis and therapy. We found evidence for this in the specialists’ classification and approaches to therapy and follow-up. Patients with PFC were more commonly diagnosed with PH-LHD. We found that fewer patients with PFC were started on PAH therapies and were more likely to be discharged from clinic. These observations should however be taken with caution as this was a retrospective analysis and there were likely additional factors influencing the physicians’ clinical diagnosis and management. It should also be noted that the presence of elevated PA pressures, low wedge pressure, and elevated PVR does not necessarily secure a diagnosis of PAH.39 While hemodynamics are an essential component of PAH diagnosis, they are not absolute. Many of the patients in our study who underwent saline loading had phenotypic risk factors for LHD despite normal PAWP at baseline, and a NFC does not necessarily imply a classification of PAH or initiation of pulmonary vasodilator therapy. Furthermore, some of the patients in our study where classified as having WHO group 3 or group 4 PH. The role of fluid challenge in these populations is not well established.
It should be noted that at present there is a lack of consensus on how a fluid challenge is best performed and interpreted. In this study, we chose 18 mmHg as our PAWP threshold for defining a hemodynamically significant fluid challenge. A lower threshold of 15 mmHg was used in earlier studies with fluid challenge.18,20 However, increases in filling pressures to >15 mmHg have been shown in healthy individuals with 1–2 L of saline infusion,19 and similarly might be seen after 500 mL of volume loading in some individuals without left heart disease.23 Consequently, an upper limit of 18 mmHg has recently been proposed.21 When we used the traditional cut-off value of 15 mmHg for PAWP, 57% of patients were classified as having PFC, but the baseline clinical associations and hemodynamic responses to saline loading remained largely unchanged. Additionally, the use of a weight-based volume of infusion rather than a fixed 500-mL load has been suggested.19,22 There has been recent evidence emerging on the potential prognostic relevance of fluid challenge which further calls for additional standardization in fluid-loading protocols.40
Limitations
We acknowledge that our study has several limitations. First, this was a retrospective, single-center review with a limited sample size. The small sample size could explain the fact that we did not find significantly higher prevalence of some of the established risk factors for left-sided heart disease. Second, the decision to perform a fluid challenge was left to the discretion of the referring PH specialist or the PH specialist performing the RHC. Thus, there may have been a selection bias and fluid challenge might have been performed more commonly in patients with risk factors for LHD. Third, a PAWP cut-off for a pathologic response to saline loading is not yet well standardized. To account for this, we analyzed our data at PAWP threshold of 18 mmHg and 15 mmHg and found similar results. Finally, our specialists’ classification and management of PH patients undergoing fluid challenge are unlikely to be purely ascribable to the hemodynamic responses to saline loading. Nonetheless, the specialists in our center were all unanimous in the diagnosis of PH-LHD and decision against starting PAH therapy when PAWP rose above 18 mmHg with fluid challenge, highlighting a consistent diagnostic approach. However, further prospective analysis on the utility of fluid challenge and the effect it has on physicians’ clinical decision making is required.
Conclusion
A PFC was associated with many clinical, echocardiographic, and hemodynamic factors related to left-sided heart disease. However, not all risk factors correlated. Extent of change in PAWP may be useful in the interpretation of the fluid challenge results. In our population, fluid challenge results were associated with measurable changes in classification of PH and whether patients are started on therapy or discharged from clinic. However, further work is needed before implementing updated guidelines around the role of saline loading in routine hemodynamic assessment of PH.
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Galiè N, Humbert M, Vachiery J-L, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67–119. [DOI] [PubMed] [Google Scholar]
- 2.Vachiéry J-L, Adir Y, Barberà JA, et al. Pulmonary hypertension due to left heart diseases. J Am Coll Cardiol 2013; 62: D100–D108. [DOI] [PubMed] [Google Scholar]
- 3.Oudiz RJ. Pulmonary hypertension associated with left-sided heart disease. Clin Chest Med 2007; 28: 233–241. [DOI] [PubMed] [Google Scholar]
- 4.Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013; 62: D34–D41. [DOI] [PubMed] [Google Scholar]
- 5.Fang JC, DeMarco T, Givertz MM, et al. World Health Organization Pulmonary Hypertension Group 2: Pulmonary hypertension due to left heart disease in the adult—a summary statement from the Pulmonary Hypertension Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2012; 31: 913–933. [DOI] [PubMed] [Google Scholar]
- 6.Gerges M, Gerges C, Pistritto A-M, et al. Pulmonary hypertension in heart failure. Epidemiology, right ventricular function, and survival. Am J Respir Crit Care Med 2015; 192: 1234–1246. [DOI] [PubMed] [Google Scholar]
- 7.Lam CSP, Roger VL, Rodeheffer RJ, et al. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol 2009; 53: 1119–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guazzi M, Borlaug BA. Pulmonary hypertension due to left heart disease. Circulation 2012; 126: 975–990. [DOI] [PubMed] [Google Scholar]
- 9.Rosenkranz S, Gibbs JSR, Wachter R, et al. Left ventricular heart failure and pulmonary hypertension. Eur Heart J 2016; 37: 942–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ooi H, Colucci WS, Givertz MM. Endothelin mediates increased pulmonary vascular tone in patients with heart failure: demonstration by direct intrapulmonary infusion of sitaxsentan. Circulation 2002; 106: 1618–1621. [DOI] [PubMed] [Google Scholar]
- 11.Dupuis J, Guazzi M. Pathophysiology and clinical relevance of pulmonary remodelling in pulmonary hypertension due to left heart diseases. Can J Cardiol 2015; 31: 416–429. [DOI] [PubMed] [Google Scholar]
- 12.Assad TR, Brittain EL, Wells QS, et al. Hemodynamic evidence of vascular remodeling in combined post- and precapillary pulmonary hypertension. Pulm Circ 2016; 6: 313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aronson D, Eitan A, Dragu R, et al. Relationship between reactive pulmonary hypertension and mortality in patients with acute decompensated heart failure. Circ Heart Fail 2011; 4: 644–650. [DOI] [PubMed] [Google Scholar]
- 14.McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 Expert Consensus Document on Pulmonary Hypertension: A Report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: Developed in Collaboration With the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation 2009; 119: 2250–2294. [DOI] [PubMed] [Google Scholar]
- 15.Kaluski E, Cotter G, Leitman M, et al. Clinical and hemodynamic effects of bosentan dose optimization in symptomatic heart failure patients with severe systolic dysfunction, associated with secondary pulmonary hypertension–a multi-center randomized study. Cardiology 2008; 109: 273–280. [DOI] [PubMed] [Google Scholar]
- 16.Califf RM, Adams KF, McKenna WJ, et al. A randomized controlled trial of epoprostenol therapy for severe congestive heart failure: The Flolan International Randomized Survival Trial (FIRST). Am Heart J 1997; 134: 44–54. [DOI] [PubMed] [Google Scholar]
- 17.Bermejo J, Yotti R, García-Orta R, et al. Sildenafil for Improving Outcomes after VAlvular Correction (SIOVAC) investigators. Sildenafil for improving outcomes in patients with corrected valvular heart disease and persistent pulmonary hypertension: a multicenter, double-blind, randomized clinical trial. Eur Heart J 2018; 39: 1255–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fox BD, Shimony A, Langleben D, et al. High prevalence of occult left heart disease in scleroderma-pulmonary hypertension. Eur Respir J 2013; 42: 1083–1091. [DOI] [PubMed] [Google Scholar]
- 19.Fujimoto N, Borlaug BA, Lewis GD, et al. Hemodynamic responses to rapid saline loading clinical perspective: the impact of age, sex, and heart failure. Circulation 2013; 127: 55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robbins IM, Hemnes AR, Pugh ME, et al. High prevalence of occult pulmonary venous hypertension revealed by fluid challenge in pulmonary hypertension. Circ Heart Fail 2014; 7: 116–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D’Alto M, Romeo E, Argiento P, et al. Clinical relevance of fluid challenge in patients evaluated for pulmonary hypertension. Chest 2017; 151: 119–126. [DOI] [PubMed] [Google Scholar]
- 22.Andersen MJ, Olson TP, Melenovsky V, et al. Differential hemodynamic effects of exercise and volume expansion in people with and without heart failure. Circ Heart Fail 2015; 8: 41–48. [DOI] [PubMed] [Google Scholar]
- 23.Borlaug BA. Invasive assessment of pulmonary hypertension: time for a more fluid approach? Circ Heart Fail 2014; 7: 2–4. [DOI] [PubMed] [Google Scholar]
- 24.Clinical Guidelines on the Identification, Evaluation and Treatment of Overweight and Obesity in Adults–The Evidence Report. National Institutes of Health. Obes Res 1998; 6(Suppl. 2): 51S–209S. [PubMed] [Google Scholar]
- 25.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010; 23: 685–713. [DOI] [PubMed] [Google Scholar]
- 26.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015; 28: 1–39.e14. [DOI] [PubMed] [Google Scholar]
- 27.Gerges C, Gerges M, Lang MB, et al. Diastolic pulmonary vascular pressure gradient: a predictor of prognosis in “out-of-proportion” pulmonary hypertension. Chest 2013; 143: 758–766. [DOI] [PubMed] [Google Scholar]
- 28.Obokata M, Reddy YNV, Pislaru SV, et al. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation 2017; 136(1): 6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naeije R, D’Alto M. The diagnostic challenge of group 2 pulmonary hypertension. Prog Cardiovasc Dis 2016; 59: 22–29. [DOI] [PubMed] [Google Scholar]
- 30.Thenappan T, Shah SJ, Gomberg-Maitland M, et al. Clinical characteristics of pulmonary hypertension in patients with heart failure and preserved ejection fraction. Circ Heart Fail 2011; 4: 257–265. [DOI] [PubMed] [Google Scholar]
- 31.Farr G, Shah K, Markley R, et al. Development of pulmonary hypertension in heart failure with preserved ejection fraction. Prog Cardiovasc Dis 2016; 59: 52–58. [DOI] [PubMed] [Google Scholar]
- 32.Robbins IM, Newman JH, Johnson RF, et al. Association of the metabolic syndrome with pulmonary venous hypertension. Chest 2009; 136: 31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okura H, Takada Y, Yamabe A, et al. Age- and gender-specific changes in the left ventricular relaxation: a Doppler echocardiographic study in healthy individuals. Circ Cardiovasc Imaging 2009; 2: 41–46. [DOI] [PubMed] [Google Scholar]
- 34.Scantlebury DC, Borlaug BA. Why are women more likely than men to develop heart failure with preserved ejection fraction? Curr Opin Cardiol 2011; 26: 562–568. [DOI] [PubMed] [Google Scholar]
- 35.Duca F, Zotter-Tufaro C, Kammerlander AA, et al. Gender-related differences in heart failure with preserved ejection fraction. Sci Rep 2018; 8: 1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.D’Alto M, Di Marco GM, D’Andrea A, et al. Invasive and noninvasive evaluation for the diagnosis of pulmonary hypertension: how to use and how to combine them. Heart Fail Clin 2018; 14: 353–360. [DOI] [PubMed] [Google Scholar]
- 37.Guazzi M, Naeije R. Pulmonary hypertension in heart failure: pathophysiology, pathobiology, and emerging clinical perspectives. J Am Coll Cardiol 2017; 69: 1718–1734. [DOI] [PubMed] [Google Scholar]
- 38.Naeije R, Vachiery J-L, Yerly P, et al. The transpulmonary pressure gradient for the diagnosis of pulmonary vascular disease. Eur Respir J 2013; 41: 217–223. [DOI] [PubMed] [Google Scholar]
- 39.Borlaug BA, Nishimura RA, Sorajja P, et al. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail 2010; 3(5): 588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.D’Alto M, Motoji Y, Romeo E, et al. Fluid challenge predicts clinical worsening in pulmonary arterial hypertension. Int J Cardiol 2018; 261: 167–171. [DOI] [PubMed] [Google Scholar]