Short abstract
Objective
Early detection and prognosis prediction are critical to improve patient survival in pancreatic cancer. This study aimed to investigate whether interleukins could serve as indicators of prognosis in pancreatic cancer.
Methods
Sixty-eight patients with pancreatic cancer were enrolled in the study during the period between 2012 and 2014. The serum levels of a broad spectrum of interleukins in these patients were determined, including IL-1β, IL-2, IL-6, IL-8, IL-10, IL-13, IL-15, and IL-23.
Results
IL-6, IL-8, and IL-10 showed significant positive correlations with each other. Moreover, high levels of serum IL-6, IL-8, and IL-10 were independently strongly associated with poor survival of patients with pancreatic cancer.
Conclusions
Our results suggest that serum levels of IL-6, IL-8, and IL-10 could be useful markers for prediction of prognosis in patients with pancreatic cancer.
Keywords: Pancreatic cancer, interleukin, IL-6, IL-8, IL-10, prediction marker
Introduction
Pancreatic cancer is the fourth most common cause of cancer-related mortality in Europe and the United States;1 its fatality rate is nearly 100%, which is likely because of the difficulty of early diagnosis, as well as high rates of metastasis and therapeutic resistance.2,3 Moreover, its 5-year survival rate is approximately 5%, and the median survival period is less than 6 months for most patients.3 Pancreatic cancer is estimated to become the second leading cause of cancer death in the United States by 2020.4 Early detection and prediction of prognosis are critical for improving patient survival in pancreatic cancer.
The interleukins are an important group of cytokines, many of which play crucial roles in transmission of information, regulation of immune cell activation, and regulation of the inflammatory response. It has been reported that many interleukins are associated with the promotion of metastasis and angiogenesis, poor prognosis, and immune evasion; these include pro-inflammatory and anti-inflammatory cytokines, such as IL-1β, IL-6, IL-8, and IL-10.5–11 Notably, increasing evidence suggests that changes in interleukin expression are associated with poor prognosis in advanced pancreatic cancer.8,9
In this study, we examined serum levels of a broad spectrum of interleukins in 68 patients with pancreatic cancer—including IL-1β, IL-2, IL-6, IL-8, IL-10, IL-13, IL-15, and IL-23—in order to investigate the association between interleukins and poor prognosis in pancreatic cancer.
Materials and methods
Patients
Patients with pancreatic cancer who were treated in Fudan University Shanghai Cancer Center (FUSCC) were enrolled in our study. Patients with immunosuppressive diseases and immunosuppressive drugs were excluded from the present study. The Clinical Research Ethics Committee of FUSCC approved this study (050432-4-1212B) and all patients provided written informed consent. Relevant clinicopathological features, such as age, sex, tumor location, and TNM stage, were recorded for each patient. Clinicopathological features were classified by using the criteria of the Union for International Cancer Control (UICC) (seventh edition). Overall survival (OS) was defined as the time from the diagnosis to the date of death or last contact.
Interleukin assays
Five milliliters of peripheral venous blood were taken from the patient’s arm; serum was collected by centrifugation for 15 minutes at 1000 × g, then stored at −80°C until use. The serum levels of interleukins (IL-1β, IL-2, IL-6, IL-8, IL-10, IL-13, IL-15, and IL-23) were measured by using the Q-Plex multiplex array (Quansys Biosciences, Logan, UT, USA) on a Q-View Imager (Quansys Biosciences), in accordance with the manufacturer’s protocol. Briefly, a series of 1:2 dilutions were made of various interleukins with known concentrations, in order to generate a calibration curve. Fifty microliters of calibrators and samples per well were added into the Q-Plex™ Array 96-well plate and incubated for 1 hour at room temperature; the plate was then washed and incubated with detection mix for 1 hour at room temperature. Next, the plate was successively incubated with streptavidin-HRP and substrate mix for 15 minutes each at room temperature. Finally, the plate was imaged by using a 270-second exposure time on a Q-View™ Imager LS. Interleukin concentrations were analyzed by Q-View™ software.
Statistical analyses
Correlations of clinicopathological parameters and interleukin expression levels were analyzed by using Spearman’s χ2 test. Survival curves were calculated by the Kaplan-Meier method and compared by log-rank tests. The Cox multivariate model was used to assess the relationships between risk factors and survival. Data were analyzed with IBM SPSS Statistics for Windows, version 22.0 (IBM, Armonk, NY, USA). Statistical significance was set at p<0.05.
Results
Patient characteristics
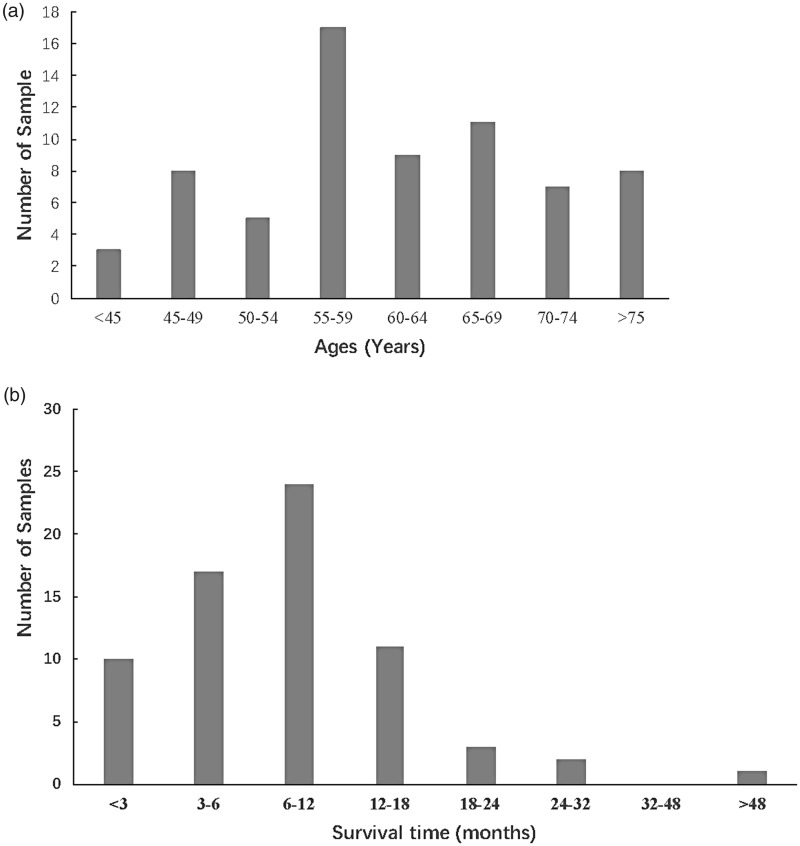
Sixty-eight patients with pancreatic cancer were enrolled in the study between 2012 and 2014. Among those patients, 23 were female, while 45 were male. The tumor stages comprised 23 cases of TNM III (33.8%) and 45 cases of TNM IV (66.2%); the tumor was located in the head and neck of the pancreas in 29 patients (42.6%), and in the body and tail of the pancreas in 39 patients (57.4%). The ages of the patients ranged from 28 to 82 years old; most were between 55–60 years old (Figure 1a). In addition, the survival periods of most patients (75%) were less than 1 year (Figure 1b).
Figure 1.
General characteristics of age and overall survival among all patients.
Interleukin expression levels
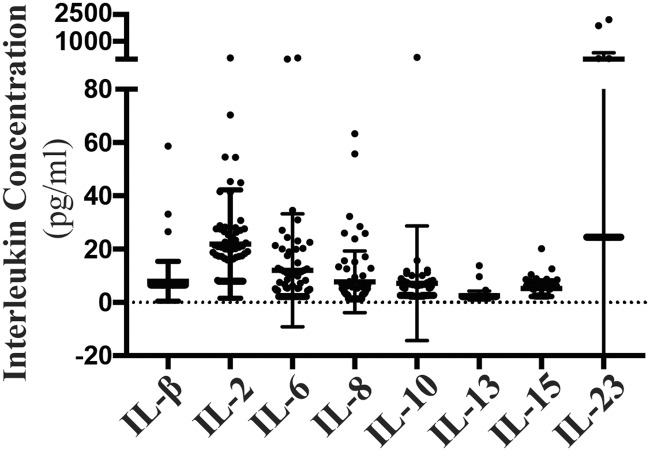
Levels of each interleukin were evaluated in all 68 patients; serum interleukin concentrations are shown in Figure 2. We explored the associations between survival time and interleukin expression levels; only levels of IL-6, IL-8, and IL-10 showed significant correlations with survival time (p<0.001 for all) (Table 1). Additionally, we examined the relationships between interleukin levels (IL-6, IL-8, and IL-10) and clinicopathological features, including sex, age, tumor location, and TNM stage; importantly, no significant correlations were found (Table 2). Intriguingly, IL-6, IL-8, and IL-10 showed significant correlations with each other (p<0.001 for all) (Table 3).
Figure 2.
Serum interleukin concentrations in patients with pancreatic cancer. Standard deviation (SD) is shown as error bars.
Table 1.
Relationships between cytokine expression and survival time.
| IL-1β | IL-2 | IL-6 | IL-8 | IL-10 | IL-13 | IL-15 | IL-23 | |
|---|---|---|---|---|---|---|---|---|
| Survival time | ||||||||
| Correlation coefficient | 0.047 | −0.02 | −0.516** | −0.414** | −0.431** | 0.021 | −0.158 | 0.046 |
| Sig. | 0.706 | 0.87 | <0.001 | <0.001 | <0.001 | 0.867 | 0.199 | 0.707 |
Table 2.
Relationships between expression of IL-6, IL-8, and IL-10 and various clinicopathological features.
| IL-6 | IL-8 | IL-10 | |
|---|---|---|---|
| TNM stage | |||
| Correlation coefficient | 0.16 | 0.134 | 0.157 |
| Sig. | 0.195 | 0.277 | 0.201 |
| Sex | |||
| Correlation coefficient | 0.077 | 0.021 | −0.015 |
| Sig. | 0.536 | 0.862 | 0.905 |
| Age | |||
| Correlation coefficient | 0.141 | −0.044 | 0.136 |
| Sig. | 0.254 | 0.719 | 0.267 |
| Survival time | |||
| Correlation coefficient | −0.516** | −0.414** | −0.431** |
| Sig. | <0.001 | <0.001 | <0.001 |
| Location | |||
| Correlation coefficient | 0.08 | 0.181 | −0.02 |
| Sig. | 0.52 | 0.139 | 0.872 |
Table 3.
Correlations among expression levels of IL-6, IL-8, and IL-10.
| IL-6 | IL-8 | IL-10 | |
|---|---|---|---|
| IL-6 | |||
| Correlation coefficient | 1 | 0.541** | 0.579** |
| Sig. | <0.001 | <0.001 | |
| IL-8 | |||
| Correlation coefficient | 0.541** | 1 | 0.515** |
| Sig. | <0.001 | <0.001 | |
| IL-10 | |||
| Correlation coefficient | 0.579** | 0.515** | 1 |
| Sig. | <0.001 | <0.001 |
Relationships between patient prognosis and levels of IL-6, IL-8, and IL-10
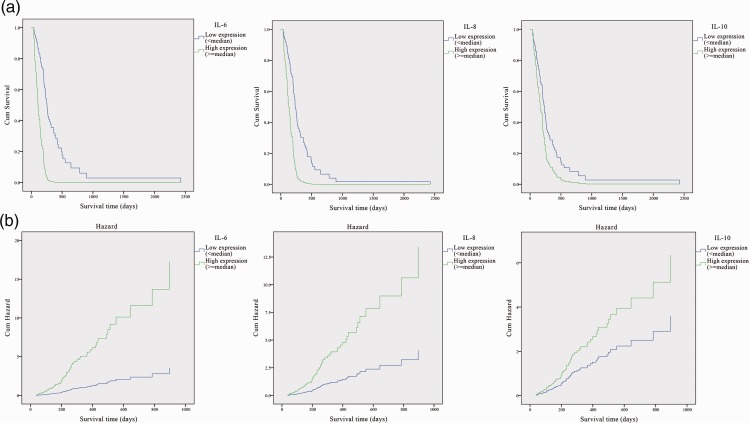
To further investigate the correlations between the levels of IL-6, IL-8, and IL-10 and the survival of pancreatic cancer patients, we performed univariate Cox analysis and Kaplan-Meier curves with log-rank analysis for OS. Patients with high IL-6, IL-8, or IL-10 expression (greater than median expression levels) exhibited a significantly shorter survival time, compared with patients with low expression (less than median expression levels) (p<0.001 for IL-6, p<0.01 for IL-8, and p<0.05 for IL-10) (Figure 3). Univariate Cox analysis revealed that a high expression level of either IL-6, IL-8, or IL-10 was associated with poor survival (IL-6: HR = 0.204, 95% CI: 0.107–0.410, p<0.001; IL-8: HR = 0.303, 95% CI: 0.135–0.683, p<0.01; IL-10: HR = 0.336, 95% CI: 0.125–0.900, p<0.05) (Table 4 and Figure 3).
Figure 3.
IL-6, IL-8, and IL-10 levels were associated with overall survival (OS) in patients with pancreatic cancer. Kaplan-Meier curves (a) and Hazard ratios (b) for OS for all cases. The median level for each group was selected as the cut-off.
Table 4.
Univariate analyses for overall survival according to interleukin levels in patients with pancreatic cancer.
| p value | Exp (B) | 95.0% CI for Exp (B) |
||
|---|---|---|---|---|
| Lower | Upper | |||
| High level of IL-6 | 0 | 0.211 | 0.097 | 0.462 |
| High level of IL-8 | 0.036 | 0.35 | 0.131 | 0.933 |
| High level of IL-10 | 0.03 | 0.336 | 0.125 | 0.9 |
Discussion
Pancreatic cancer is a highly malignant digestive system tumor with rapid progression and poor prognosis.4 Therefore, the identification of predictive markers for patient prognosis is critical for therapeutic approach in pancreatic cancer. In this study, we found that serum expression levels of IL-6, IL-8, and IL-10 were associated with OS in patients with pancreatic cancer. High levels of IL-6, IL-8 or IL-10 were positively correlated with poor prognosis in pancreatic cancer.
IL-6 is an important interleukin secreted by macrophages, T cells, and B cells, as well as many other cells; notably, it can act as either a pro-inflammatory cytokine or an anti-inflammatory myokine.12 It can regulate immune response, acute phase response, and hematopoietic function; furthermore, it can play an important role in the body’s anti-infection and immune response. Dysregulation of IL-6 expression can cause many diseases, and its clinical manifestations primarily include elevated IL-6 levels at the onset of disease.13,14 Elevated IL-6 levels are associated with tumor development, degree of rejection, and treatment outcome.15–18 In addition, previous studies have revealed that IL-6 level is an potential prognostic factor in pancreatic cancer,19 renal cell cancer,20 and ovarian cancer;21,22 moreover, serum levels of IL-6 and IL-1β can predict the efficacy of gemcitabine in patients with advanced pancreatic cancer.23 In this study, we found that a high level of serum IL-6 was correlated with shorter survival and worse prognosis in pancreatic cancer. However, serum levels of IL-1β did not show a significant correlation with poor patient survival in our study.
IL-8 is an important mediator of inflammatory diseases, which plays an important role in anti-infection, immune response, and tumor suppression. IL-8 also has strong chemotactic effects on specific and nonspecific immune cells; these primarily comprise chemotactic and activating effects on neutrophils, as well as chemotactic effects on lymphocytes and basophils. As a major inflammatory factor, significantly elevated levels of IL-8 are found at sites of inflammation and serum in the presence of infection and certain autoimmune diseases.24 IL-8 also functions in the proliferation of normal cells and tumor cells. Recent studies have shown that IL-8 can promote the development of various cancers, such as pancreatic cancer, nasopharyngeal tumor, colorectal cancer, and lung cancer;25–28 notably, it serves as a prognostic marker in several cancers.29–31 Consistent with previous studies, the present study showed that a high level of serum IL-8 was predictive of shorter survival times and poor prognosis in pancreatic cancer.
IL-10 is a negative regulatory factor, primarily secreted by Th2 cells, activated B cells, monocytes, and macrophages; it is involved in immune response, tumor development, and other biological regulation.32 Importantly, IL-10 also plays a role in autoimmune diseases, serious infectious diseases, and transplantation immunity; IL-10 acts as a growth factor in certain tumor cells (e.g., myeloma cells), and maintains cell growth and proliferation.33,34 IL-10 has been reported to serve at the crossroads of immune stimulation and immune suppression in cancer, as well as development of tumor metastasis.35–37 A high level of serum interleukin-10 was associated with poor prognosis in multiple myeloma38 and thyroid cancer.39 Similarly, we found that a high level of serum IL-10 was associated with poor prognosis in pancreatic cancer.
IL-6, IL-8, and IL-10 are all involved in the inflammatory response; we found that, in patients with pancreatic cancer, the expression levels of these three interleukins were significantly positively correlated with each other, implying a role for inflammation in the development of pancreatic cancer. Chronic pancreatitis is closely correlated with pancreatic cancer in clinical analyses. A prior study analyzed 1663 patients with pancreatic cancer and found that the risk of pancreatic cancer increased by 7.2-fold among patients with pancreatitis; this risk increased by up to 10-fold in patients older than 55 years of age.40 Similarly, our results showed a significant positive correlation between high levels of IL-6, IL-8, or IL-10 and poor prognosis in patients with pancreatic cancer. Thus, attenuation of the inflammatory response through inhibition of the expression of important inflammatory factors, such as IL-6, IL-8, or IL-10, may improve patient prognosis.
IL-13, IL-15, and IL-23 have been suggested as potential independent prognostic factors for OS in several cancers.41–43 However, none of these interleukins showed significant correlations with the OS of patients with pancreatic cancer in the present study. Nevertheless, certain limitations must be considered in future studies. The main limitation of the present study was the limited number of patients, which may influence the statistical evaluation and strength of correlations between serum levels of interleukins and OS. A larger sample size is necessary to confirm the reliability of the present findings.
Taken together, our results suggested that high levels of serum IL-6, IL-8, or IL-10 were strongly associated with poor survival, and that these cytokines can be regarded as useful markers to predict prognosis in patients with pancreatic cancer, as well as potential therapeutic targets.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This work was supported by the National Natural Science Foundation of China (81102004, 81100934) and Shanghai Municipal Health Bureau (2010QJ032A).
References
- 1.Roshani R, McCarthy F, Hagemann T. Inflammatory cytokines in human pancreatic cancer. Cancer Lett 2014; 345: 157–163. DOI: 10.1016/j.canlet.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 2.Hariharan D, Saied A, Kocher HM. Analysis of mortality rates for pancreatic cancer across the world. HPB (Oxford) 2008; 10: 58–62. DOI: 10.1080/13651820701883148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee ES, Lee JM. Imaging diagnosis of pancreatic cancer: a state-of-the-art review. World J Gastroenterol 2014; 20: 7864–7877. DOI: 10.3748/wjg.v20.i24.7864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chari ST, Kelly K, Hollingsworth MA, et al. Early detection of sporadic pancreatic cancer: summative review. Pancreas 2015; 44: 693–712. DOI: 10.1097/MPA.0000000000000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang RF, Wang SX, Zhang FR, et al. Interleukin-1alpha, 6 regulate the secretion of vascular endothelial growth factor A, C in pancreatic cancer . Hepatobiliary Pancreat Dis Int 2005; 4: 460–463. [PubMed] [Google Scholar]
- 6.Lesina M, Kurkowski MU, Ludes K, et al. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell 2011; 19: 456–469. DOI: 10.1016/j.ccr.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Li M, Zhang Y, Feurino LW, et al. Interleukin-8 increases vascular endothelial growth factor and neuropilin expression and stimulates ERK activation in human pancreatic cancer. Cancer Sci 2008; 99: 733–737. DOI: 10.1111/j.1349-7006.2008.00740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsuo Y, Ochi N, Sawai H, et al. CXCL8/IL-8 and CXCL12/SDF-1alpha co-operatively promote invasiveness and angiogenesis in pancreatic cancer. Int J Cancer 2009; 124: 853–861. DOI: 10.1002/ijc.24040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y, Shi M, Yu GZ, et al. Interleukin-8, a promising predictor for prognosis of pancreatic cancer. World J Gastroenterol 2012; 18: 1123–1129. DOI: 10.3748/wjg.v18.i10.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verma G, Bhatia H, Datta M. Gene expression profiling and pathway analysis identify the integrin signaling pathway to be altered by IL-1 beta in human pancreatic cancer cells: role of JNK. Cancer Lett 2012; 320: 86–95. DOI: 10.1016/j.canlet.2012.01.036. [DOI] [PubMed] [Google Scholar]
- 11.Bellone G, Smirne C, Mauri FA, et al. Cytokine expression profile in human pancreatic carcinoma cells and in surgical specimens: implications for survival. Cancer Immunol Immun 2006; 55: 684–698. DOI: 10.1007/s00262-005-0047-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kishimoto T. Interleukin-6: from basic science to medicine–40 years in immunology. Annu Rev Immunol 2005; 23: 1–21. DOI: 10.1146/annurev.immunol.23.021704.115806. [DOI] [PubMed] [Google Scholar]
- 13.Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol 2015; 16: 448–457. DOI: 10.1038/ni.3153. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol 2014; 6: a016295. DOI: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lippitz BE, Harris RA. Cytokine patterns in cancer patients: a review of the correlation between interleukin 6 and prognosis. Oncoimmunology 2016; 5: e1093722. DOI: 10.1080/2162402X.2015.1093722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tchirkov A, Khalil T, Chautard E, et al. Interleukin-6 gene amplification and shortened survival in glioblastoma patients. Br J Cancer 2007; 96: 474–476. DOI: 10.1038/sj.bjc.6603586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sansone P, Storci G, Tavolari S, et al. IL-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. J Clin Invest 2007; 117: 3988–4002. DOI: 10.1172/JCI32533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sapochnik M, Haedo MR, Fuertes M, et al. Autocrine IL-6 mediates pituitary tumor senescence. Oncotarget 2017; 8: 4690–4702. DOI: 10.18632/oncotarget.13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ebrahimi B, Tucker SL, Li D, et al. Cytokines in pancreatic carcinoma: correlation with phenotypic characteristics and prognosis. Cancer 2004; 101: 2727–2736. DOI: 10.1002/cncr.20672. [DOI] [PubMed] [Google Scholar]
- 20.Kallio J, Hamalainen M, Luukkaala T, et al. Resistin and interleukin 6 as predictive factors for recurrence and long-term prognosis in renal cell cancer. Urol Oncol 2017; 35: 544. e525–544. e531. DOI: 10.1016/j.urolonc.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 21.Kawabata A, Yanaihara N, Nagata C, et al. Prognostic impact of interleukin-6 expression in stage I ovarian clear cell carcinoma. Gynecol Oncol 2017; 146: 609–614. DOI: 10.1016/j.ygyno.2017.06.027. [DOI] [PubMed] [Google Scholar]
- 22.Dalal V, Kumar R, Kumar S, et al. Biomarker potential of IL-6 and VEGF-A in ascitic fluid of epithelial ovarian cancer patients. Clin Chim Acta 2018; 482: 27–32. DOI: 10.1016/j.cca.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 23.Mitsunaga S, Ikeda M, Shimizu S, et al. Serum levels of IL-6 and IL-1beta can predict the efficacy of gemcitabine in patients with advanced pancreatic cancer. Br J Cancer 2013; 108: 2063–2069. DOI: 10.1038/bjc.2013.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russo RC, Garcia CC, Teixeira MM, et al. The CXCL8/IL-8 chemokine family and its receptors in inflammatory diseases. Expert Rev Clin Immunol 2014; 10: 593–619. DOI: 10.1586/1744666X.2014.894886. [DOI] [PubMed] [Google Scholar]
- 25.Ning Y, Lenz HJ. Targeting IL-8 in colorectal cancer. Expert Opin Ther Targets 2012; 16: 491–497. DOI: 10.1517/14728222.2012.677440. [DOI] [PubMed] [Google Scholar]
- 26.Lo MC, Yip TC, Ngan KC, et al. Role of MIF/CXCL8/CXCR2 signaling in the growth of nasopharyngeal carcinoma tumor spheres. Cancer Letters 2013; 335: 81–92. DOI: 10.1016/j.canlet.2013.01.052. [DOI] [PubMed] [Google Scholar]
- 27.Chen LY, Fan J, Chen H, et al. The IL-8/CXCR1 axis is associated with cancer stem cell-like properties and correlates with clinical prognosis in human pancreatic cancer cases. Sci Rep 2014; 4: 5911. DOI: 10.1038/srep05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bar-Eli M. Role of interleukin-8 in tumor growth and metastasis of human melanoma. Pathobiology 1999; 67: 12–18. DOI: 10.1159/000028045. [DOI] [PubMed] [Google Scholar]
- 29.Sanmamed MF, Carranza-Rua O, Alfaro C, et al. Serum Interleukin-8 reflects tumor burden and treatment response across malignancies of multiple tissue origins. Clin Cancer Res 2014; 20: 5697–5707. [DOI] [PubMed] [Google Scholar]
- 30.Ren Y, Poon RTP, Tsui HT, et al. Interleukin-8 serum levels in patients with hepatocellular carcinoma: correlations with clinicopathological features and prognosis. Clin Cancer Res 2003; 9: 5996–6001. [PubMed] [Google Scholar]
- 31.Benoy IH, Salgado R, Van Dam P, et al. Increased serum interleukin-8 in patients with early and metastatic breast cancer correlates with early dissemination and survival. Clin Cancer Res 2004; 10: 7157–7162. DOI: 10.1158/1078-0432.CCR-04-0812. [DOI] [PubMed] [Google Scholar]
- 32.Sabat R, Grutz G, Warszawska K, et al. Biology of interleukin-10. Cytokine Growth Factor Rev 2010; 21: 331–344. DOI: 10.1016/j.cytogfr.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 33.Saraiva M, O'Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol 2010; 10: 170–181. DOI: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 34.Ouyang WJ, Rutz S, Crellin NK, et al. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol 2011; 29: 71–109. DOI: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- 35.Dennis KL, Saadalla A, Blatner NR, et al. T-cell expression of IL10 is essential for tumor immune surveillance in the small intestine. Cancer Immunol Res 2015; 3: 806–814. DOI: 10.1158/2326-6066.Cir-14-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dennis KL, Blatner NR, Gounari F, et al. Current status of interleukin-10 and regulatory T-cells in cancer. Curr Opin Oncol 2013; 25: 637–645. DOI: 10.1097/Cco.0000000000000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sato T, Terai M, Tamura Y, et al. Interleukin 10 in the tumor microenvironment: a target for anticancer immunotherapy. Immunol Res 2011; 51: 170–182. DOI: 10.1007/s12026-011-8262-6. [DOI] [PubMed] [Google Scholar]
- 38.Wang H, Wang L, Chi PD, et al. High level of interleukin-10 in serum predicts poor prognosis in multiple myeloma. Br J Cancer 2016; 114: 463–468. DOI: 10.1038/bjc.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cunha LL, Morari EC, Nonogaki S, et al. Interleukin 10 expression is related to aggressiveness and poor prognosis of patients with thyroid cancer. Cancer Immunol Immun 2017; 66: 141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bracci PM, Wang F, Hassan MM, et al. Pancreatitis and pancreatic cancer in two large pooled case-control studies. Cancer Causes Control 2009; 20: 1723–1731. DOI: 10.1007/s10552-009-9424-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Formentini A, Braun P, Fricke H, et al. Expression of interleukin-4 and interleukin-13 and their receptors in colorectal cancer. Int J Colorectal Dis 2012; 27: 1369–1376. [DOI] [PubMed] [Google Scholar]
- 42.Gangemi S, Minciullo P, Adamo B, et al. Clinical significance of circulating interleukin-23 as a prognostic factor in breast cancer patients. J Cell Biochem 2012; 113: 2122–2125. [DOI] [PubMed] [Google Scholar]
- 43.Zhou HJ, Huang H, Shi JO, et al. Prognostic value of interleukin 2 and interleukin 15 in peritumoral hepatic tissues for patients with hepatitis B-related hepatocellular carcinoma after curative resection. Gut 2010; 59: 1699–1708. [DOI] [PubMed] [Google Scholar]