Short abstract
Objective
This study was performed to identify a possible association of the clinical parameters of systemic sclerosis (SSc) and the socioeconomic status (SES) with oral health-related quality of life (OHrQoL) as measured by the Oral Health Impact Profile 49 (OHIP 49), taking into account the effect of educational level (as a proxy of SES) on oral health.
Methods
Subjects were recruited from the Croatian SSc Center of Excellence cohort. Detailed dental and clinical examinations were performed according to standardized protocols. The associations of OHrQoL with disease characteristics and socioeconomic status were examined.
Results
Thirty-one consecutive patients with SSc were enrolled (29 women; mean age, 56.45 ± 13.60 years). OHIP 49 scores were significantly correlated with disease activity and severity. Furthermore, OHrQoL was positively correlated with skin involvement as evaluated by the modified Rodnan skin score. Impaired OHrQoL was positively correlated with the severity of general, skin, gastrointestinal, and joint/tendon involvement. The OHIP 49 score differed between patients who were positive and negative for anti-topoisomerase I antibody. Higher OHIP 49 scores were detected in patients with lower SES (primary school educational level).
Conclusion
Collaboration between rheumatologists and dental professionals is required to improve dental care and oral health outcomes of SSc.
Keywords: Dental status, activity, severity, socioeconomic status, OHIP 49, systemic sclerosis
Introduction
Systemic sclerosis (SSc) is a multisystem connective tissue disease characterized by tissue thickening and fibrosis of the skin, often with involvement of internal organs including the gut, lungs, pulmonary arteries, heart, and kidneys.1 SSc can be divided into limited cutaneous SSc and diffuse cutaneous SSc.2 The etiology of SSc is still unknown.3 SSc is a rare disease. Several surveys have recently been performed in an attempt to define the prevalence of SSc, a disease carrying considerable morbidity and mortality. The prevalence of SSc appears to be higher in the United States (where it has been estimated at 24.2 per 100,000)4 than in Europe (where recent studies in Iceland, England, France, and Greece have estimated rates ranging from 7.1 to 15.8 per 100,000).5–8 The prevalence of SSc in Split-Dalmatia County, Croatia was estimated at 15.6 cases per 100,000 adults.9
SSc is characterized by microangiopathy, dysregulated immune function, and tissue remodeling, which commonly involves the oral cavity. The mouth, oral cavity, and teeth can be affected in patients with SSc, leading to difficulties with speech, feeding, and reduced quality of life (QoL). Patients with SSc have less saliva production, smaller interdental distances, more missing teeth, and more periodontal disease than healthy people.10 The etiology of excess periodontal disease is probably multifactorial and remains unclear. In patients with SSc, the diminished interincisal distance is associated with disease severity, and the decreased saliva production is associated with concomitant Sjögren’s syndrome–related antibodies.11 Furthermore, according to the results from a recent Canadian Oral Health study, radiologic findings of SSc that clearly differ from patients without SSc are erosions of the mandible and widening of the periodontal ligament.12
People with a lower socioeconomic status (SES) have worse health outcomes. Several measures of SES are used, such as vocation, education level, income, and employment status.13–15 SSc often begins in middle age; therefore, the disease usually appears many years after finishing education. Education level as a surrogate of SES has been used in clinical trials of patients with cancer. Herndon et al.16 evaluated more than 6000 patients with breast cancer and concluded that post-trial survivorship plans need to focus on women with a low social status as measured by education. Considering the course and prognosis of the disease, there was a presumption that a low education level (a surrogate of SES completed long before SSc onset) may be associated with worse outcomes in patients with SSc. In another previous study, however, education level was not associated with a poorer prognosis in patients with SSc after adjusting for confounding factors, and the correlation between disease severity and oral health-related QoL (OHrQoL) in patients with SSc was not confimed.11,17,18 The relationship between SES and general health has been extensively investigated. A close link between SES and health is well established; however, whether low SES as measured by the educational level contributes to impaired OHrQoL in patients with SSc is unknown. Transition countries, such as Croatia, have more marked socioeconomic differences than do well-developed countries. The aim of the present study was to identify a possible correlation of the clinical parameters of SSc and SES with OHrQoL as measured by the Oral Health Impact Profile 49 (OHIP 49) taking into account the effect of educational level (as a proxy of SES) on oral health in the Croatian SSc cohort.
Materials and methods
Study design and population
This single-center, cross-sectional study was conducted from August 2015 to February 2017. To be eligible, patients had to fulfill the classification criteria of definite SSc proposed by the American College of Rheumatology (ACR).19 The distinction between limited cutaneous SSc and diffuse cutaneous SSc was made according to the criteria defined by LeRoy et al.2 The SSc disease duration was defined as the duration of time since onset of the first non-Raynaud’s phenomenon manifestation. Patients with other systemic diseases, including concomitant Sjögren’s syndrome, were excluded from this study. This study was conducted in compliance with the Helsinki Declaration and its revisions. The protocol was approved by the University Hospital Split Ethics Committee. All patients provided written informed consent prior to enrolling in the study.
The patients underwent a physical examination and comprehensive laboratory evaluation including a full blood count and measurement of the erythrocyte sedimentation rate, renal and liver function indices, C3 and C4 levels, and antinuclear and anti-extractable nuclear antigen antibodies. Anti-centromere antibodies were tested by indirect immunofluorescence on HEp-2 cells, and anti-Scl70 antibodies were determined by enzyme-linked immunosorbent assay.
Instrumental investigations included electrocardiography, esophagography, chest radiography, pulmonary function testing with the diffusing capacity for carbon monoxide (DLCO) adjusted for the hemoglobin concentration, and Doppler echocardiography to evaluate the left ventricular ejection fraction and estimate the pulmonary artery systolic pressure (sPAP). If no tricuspid regurgitation could be detected, the sPAP was presumed normal; the estimated sPAP was considered abnormal if it was >35 mmHg. The coefficient of variation of the pulmonary function test with DLCO was <5%.
Measures of disease severity and activity
Generally, joint/tendon, muscle, renal, skin, vascular, pulmonary, and cardiac involvement were evaluated based on a severity scale classification proposed by Medsger et al.20 The validated Medsger Severity Scale was used to determine organ-specific disease severity, scaled from 0 to 36.20 Disease activity was assessed according to Valentini et al.21 Skin involvement was assessed using the modified Rodnan skin score (mRSS), ranging from 0 to 51.22 One assessor (M.R.) evaluated all disease severity and activity measures.
Measures of OHrQoL
The self-administered Croatian version of the OHIP 49 was used to measure the dental outcome in terms of its influence on OHrQoL.23 The OHIP is a 49-item measure with statements divided into 7 theoretical domains: functional limitation, pain, psychological discomfort, physical disability, psychological disability, social disability, and handicap.24 Responses to each of the 49 OHIP statements were based on the Likert scale (i.e., 0 = never, 1 = hardly ever, 2 = occasionally, 3 = fairly often, and 4 = very often). Thus, lower scores indicate better OHrQoL. Patients were also offered an option of “don’t know” for each question; however, if more than nine responses were left blank or marked “don’t know,” the questionnaire was discarded and the patient was excluded from the study.
Measures of health assessment
The Scleroderma Health Assessment Questionnaire (SHAQ) consists of 20 items distributed among 8 domains and has 5 additional domains that assess dysfunctions caused by the symptoms of systemic scleroderma. A visual analog scale (VAS) is used for the five additional domains. The VAS is documented in paper form using a 100-mm-long horizontal line, and patients are instructed to place a cross on the line that most accurately represents their level of dysfunction. The VAS scores are transferred to a 100-value scale using a millimeter tape measure and converted to subscores ranging from 0 (no disability) to 3 (maximal disability). The overall score of the questionnaire is the sum of each of the five VAS subscores plus the scores for the eight domains, divided by 13.25 A second assessor (D.M.K.), different from the one evaluating the disease activity and severity, evaluated the SHAQ.
Measures of SES
SES was measured by education level (“did not complete high school” and “completed high school or more”). Education is only a part of the total SES but was used as a surrogate because the exposure to “highest education level completed” would have occurred long before SSc onset, and we wanted to determine whether education level is predictive of SSc outcomes.
Statistical methods
All analyses were performed with the SPSS 22.0 statistical package (IBM Corp., Armonk, NY, USA). A p value of <0.05 was considered statistically significant for all data analyses. The distribution of continuous variables in the groups is expressed as mean ± standard deviation. Comparison between two groups was performed using the Mann–Whitney U test. Associations between qualitative variables were analyzed with the chi-square (χ2) test, χ2 test for linear trend, or Fisher’s exact test when indicated. Linear correlations between continuous variables were evaluated using Spearman’s rank coefficient.
Results
Patient demographics
Thirty-one consecutive patients with SSc were enrolled in this study (Table 1). All patients fulfilled the ACR criteria for a diagnosis of SSc.19 Most patients were women (94%). The patients’ mean age was 56 ± 14 years, and their mean disease duration from onset of the first non-Raynaud’s phenomenon manifestation was 7 years (range, 1–28 years). Sjögren’s syndrome was ruled out in all patients by negative anti-Ro and anti-La antibodies. With respect to the disease subset, 90% of patients had the diffuse cutaneous disease form. All patients were Caucasian, and 35% had a high school education or more. The median physician-assessed disease severity and activity were 11.5 (range, 4–22) and 4 (range, 2–7), respectively. The mean OHIP 49 score was 44 ± 21, and the mean SHAQ score was 0.93 ± 0.73.
Table 1.
Patient demographics in SSc cohort.
| Characteristic | ||
|---|---|---|
| Age (years) | 56.45 ± 13.60 | |
| Sex | Female | 29 (93.55) |
| SSc duration (years) | 7 (1–28) | |
| SSc subset | lcSScdcSSc | 3 (9.67)28 (90.33) |
| OHIP 49 | 43.68 (21.07) | |
| SHAQ | 0.92 (0.73) | |
| Autoantibody pattern | ANA positivity (negative for ACA and anti-Scl70) | 2 (6.45) |
| ACA | 10 (32.26) | |
| Anti-Scl 70 | 18 (58.06) | |
| Educational level | Primary school | 20 (64.52) |
| High school or more | 11 (35.48) | |
| mRSS | Mild/moderate | 22 (70.97) |
| Severe/end-stage | 9 (29.03) | |
| GI tract involvement | Present | 21 (67.74) |
| Joint/tendon involvement | Present | 27 (87.10) |
| Muscular involvement | Present | 21 (67.74) |
| Digital ulcers | Present | 6 (19.35) |
| Peripheral vascular involvement | Present | 27 (87.10) |
| Lung involvement | Present | 27 (87.10) |
| Chest radiography | Normal | 22 (70.97) |
| Intestinal lung disease | 9 (29.03) | |
| FVC | 91.23 ± 21.77 | |
| DLCO | 68.33 ± 20.16 | |
| Estimated sPAP | High | 4 (12.90) |
| Heart involvement | Present | 24 (77.42) |
Data are expressed as mean ± standard deviation, absolute number (%), or median (range).
SSc, systemic sclerosis; lcSSc, limited cutaneous systemic sclerosis; dcSSc, diffuse cutaneous systemic sclerosis; OHIP 49, Oral Health Impact Profile 49; SHAQ, Scleroderma Health Assessment Questionnaire; ANA, antinuclear antibodies; ACA, anti-centromere antibodies; Anti-Scl70, anti-Scl70 antibodies; mRSS, modified Rodnan skin score; GI, gastrointestinal; FVC, forced vital capacity, % predicted; DLCO, diffusing capacity for carbon monoxide, % predicted; sPAP, pulmonary artery systolic pressure.
OHrQoL
The OHIP 49 score was positively correlated with disease activity (p = 0.005, r = 0.4872, Spearman’s rank coefficient) and severity (p = 0.016, r = 0.4303). Furthermore, OHrQoL as measured by the OHIP 49 was positively correlated with skin involvement as evaluated by the mRSS (p = 0.003, r = 0.5207). Impaired quality of oral health was positively correlated with the severity of general, skin, gastrointestinal, and joint/tendon involvement (p = 0.003, r = 0.506; p = 0.003, r = 0.511; p < 0.001, r = 0.591; and p = 0.02, r = 0.391, respectively). The OHIP 49 score differed between patients who were positive and negative for anti-topoisomerase I antibody (p < 0.001, Fisher’s exact test). OHrQoL as measured by the OHIP 49 was positively correlated with the health assessment of patients with SSc as evaluated by the SHAQ (p = 0.01, r = 0.413).
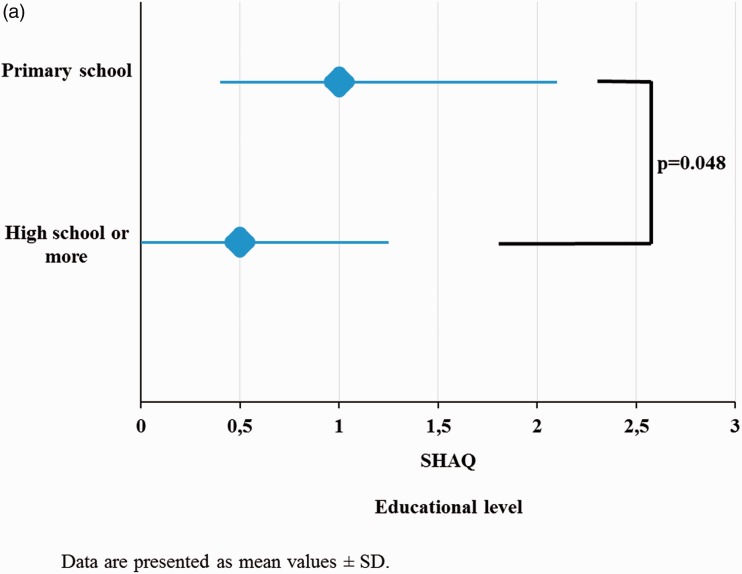
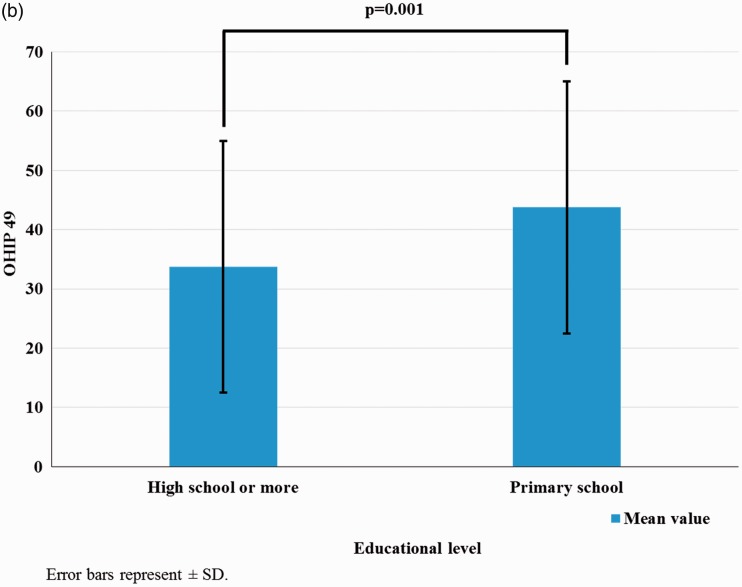
Health assessment and SES
Health assessment in patients with SSc as measured by the SHAQ on the basis of the educational level (as a proxy of SES) was significantly impaired in patients with lower SES (Figure 1(a)). Higher OHIP 49 scores were detected in patients with lower SES (primary school educational level, p = 0.001) (Figure 1(b)). SES as measured by the educational level was not correlated with physician-assessed disease severity or activity.
Figure 1.
(a) Health assessment measured by the SHAQ based on the educational level. (b) OHIP 49 scores based on the educational level
SHAQ, Scleroderma Health Assessment Questionnaire; OHIP 49, Oral Health Impact Profile 49; SD, standard deviation
Comparison with control group
To understand the impact of SSc on OHrQoL, our SSc cohort was compared with a control group of similar age and sex (Table 2). There was no statistically relevant difference in educational level or smoking status between the two groups. The use of medications associated with dry mouth was significantly higher in the SSc cohort (p < 0.01). Significantly higher OHIP 49 scores were found in the SSc cohort than in the control group (p < 0.0001).
Table 2.
Comparison of SSc cohort with control group
| Characteristic | SSc cohort(n = 31) | Control group(n = 31) | p value |
|---|---|---|---|
| Female | 29 (93.55) | 30 (96.77) | 0.81 |
| Age (years) | 56.45 ± 13.60 | 53.70 ± 12.03 | 0.32 |
| Completed high school or more | 11 (35.48) | 16 (51.61) | 0.04 |
| Current smoker | 2 (6.45) | 3 (9.67) | 0.22 |
| Use of drugs associated with dry mouth | 21 (67.74) | 10 (32.25) | <0.01 |
| OHIP 49 | 43.67 ± 21.06 | 16.00 ± 19.60 | <0.0001 |
Data expressed as absolute number (%) or mean ± standard deviation
SSc, systemic sclerosis; OHIP 49, Oral Health Impact Profile 49
Discussion
Patients with SSc can have a high risk of developing caries, periodontal conditions, and infections due to a number of factors including hyposalivation, impaired oral hygiene practices, and immunosuppressive drug use.26 Previous studies have shown that OHrQoL is not captured by the physician’s assessment of disease activity and severity in patients with SSc.11,18 Contrary to previous studies, we found that disease severity and activity were related to the OHIP 49 score.18 Creating questionnaires with greater specificity for orofacial symptoms might improve early detection of these changes, which is a particularly important aspect that is regularly overlooked and undertreated. Dry mouth is a frequent yet underreported symptom and may be an early manifestation of autonomic involvement in patients with Parkinson’s disease. Cersosimo et al.27 used a specifically designed questionnaire for assessment of dry mouth in patients with Parkinson’s disease. Our data suggest that OHrQoL is correlated with the severity of general, skin, gastrointestinal, and joint/tendon involvement in patients with SSc. The disease subset and autoantibody profile could play a role in the oral manifestation of SSc. Compared with previous studies, our SSc cohort had different characteristics in regards to the disease subset, autoantibody profile, and educational level. Our mean OHIP 49 score was comparable with that in a previous study (44 vs 41, respectively), but the rate of patients with the education level of “completed high school or more” was lower (35% vs 50%, respectively).18 Considering that previous studies were conducted in well-developed countries with excellent medical care, the differences in these results could be due to the level of medical care provided to patients. According to published data from the World Health Organization in 2014, living in Croatia compared with Canada means that an individual spends an average of 64.35% less on health care.28
The present study has several limitations: the OHIP 49 has not been validated specifically for SSc, the sample size was small, and the disease duration differed among patients. The level of education was an independent factor impacting the dental status based on the adjusted analysis, but a multivariate analysis could not be performed because of the small sample size. Furthermore, we did not examine oral health and self-reported oral health attitudes and behaviors. There is a complex interaction among OHrQoL, oral health-related attitudes and behaviors, and oral health status as recently reported in a cross-sectional study by Vigu et al.29 We assume that patients with SSc with higher educational levels take better care of their oral health. Education beyond primary school was associated with better oral QoL and better health assessment in patients with SSc. Croatia is a country in transition and shows obvious differences in SES compared with Canada. Hence, we postulate that better medical care provided to patients and a higher educational level are possible reasons for the differences between the studies. Lower SES was associated with impaired OHrQoL. Therefore, collaboration between rheumatologists and dental professionals is required to improve dental care and oral health outcomes for patients with SSc. Providing comprehensive information about oral health at the very beginning of SSc could possibly decrease inequalities in dental health by educational level. Larger studies are necessary to confirm our results.
Author contributions
Katica Parat: data collection, data analysis/interpretation, approval of article. Mislav Radić: concept/design, data collection, data analysis/interpretation, drafting of article, critical revision of article, approval of article. Katarina Boric: data collection, approval of article, statistical analysis. Dijana Perkovic: data collection, approval of article. Dolores Biočina Lukenda: data collection, approval of article. Dušanka Martinović Kaliterna: data collection, approval of article.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
No specific funding was received from any funding bodies in the public, commercial or not-for-profit sectors.
References
- 1.Bernatsky S, Joseph L, Pineau CA, et al. Scleroderma prevalence: demographic variations in a population-based sample. Arthritis Rheum 2009; 61: 400–404. [DOI] [PubMed] [Google Scholar]
- 2.LeRoy EC, Black C, Fleischmajer R, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol 1988; 15: 202–205. [PubMed] [Google Scholar]
- 3.Hunzelmann N. Developments into understanding the pathogenesis of systemic sclerosis. Expert Rev Dermatol 2013; 8: 267–276. [Google Scholar]
- 4.Mayes MD, Lacey JV, Jr, Beebe-Dimmer J, et al. Prevalence, incidence, survival, and disease characteristics of systemic sclerosis in a large US population. Arthritis Rheum 2003; 48: 2246–2255. [DOI] [PubMed] [Google Scholar]
- 5.Geirsson AJ, Steinsson K, Guthmundsson S, et al. Systemic sclerosis in Iceland. A nationwide epidemiological study. Ann Rheum Dis 1994; 53: 502–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allcock RJ, Forrest I, Corris PA, et al. A study of the prevalence of systemic sclerosis in northeast England. Rheumatology (Oxford) 2004; 43: 596–602. [DOI] [PubMed] [Google Scholar]
- 7.Le Guern V, Mahr A, Mouthon L, et al. Prevalence of systemic sclerosis in a French multi-ethnic county. Rheumatology (Oxford) 2004; 43: 1129–1137. [DOI] [PubMed] [Google Scholar]
- 8.Alamanos Y, Tsifetaki N, Voulgari PV, et al. Epidemiology of systemic sclerosis in northwest Greece 1981 to 2002. Semin Arthritis Rheum 2005; 34: 714–720. [DOI] [PubMed] [Google Scholar]
- 9.Radić M, Martinović Kaliterna D, Fabijanić D, et al. Prevalence of systemic sclerosis in split-Dalmatia county in Southern Croatia. Clin Rheumatol 2010; 29: 419–412. [DOI] [PubMed] [Google Scholar]
- 10.Baron M, Hudson M, Tatibouet S, et al. The Canadian systemic sclerosis oral health study: orofacial manifestations and oral health related quality of life in systemic sclerosis compared to the general population. Rheumatology (Oxford) 2014; 53: 1386–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baron M, Hudson M, Tatibouet S, et al. Relationship between disease characteristics and orofacial manifestations in systemic sclerosis: Canadian systemic sclerosis oral health study III. Arthritis Care Res (Hoboken) 2015; 67: 681–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dagenais M, MacDonald D, Baron M, et al. The Canadian systemic sclerosis oral health study IV: oral radiographic manifestations in systemic sclerosis compared with the general population. Oral Surg Oral Med Oral Pathol Oral Radiol 2015; 120: 104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winkleby MA, Jatulis DE, Frank E, et al. Socioeconomic status and health: how education, income, and occupation contribute to risk factors for cardiovascular disease. Am J Public Health 1992; 82: 816–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braveman PA, Cubbin C, Egerter S, et al. Socioeconomic status in health research: one size does not fit all. JAMA 2005; 294: 2879–2888. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez E. Keeping the unemployed healthy: The effect of means-tested and entitlement benefits in Britain, Germany, and the United States. Am J Public Health 2001; 91: 1403–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herndon JE, 2nd, Kornblith AB, Holland JC, et al . Effect of socioeconomic status as measured by education level on survival in breast cancer clinical trials. Psychooncology 2013; 22: 315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mansour S, Bonner A, Muangchan C, et al. Low socioeconomic status (measured by education) and outcomes in systemic sclerosis: data from the Canadian scleroderma research group. J Rheumatol 2013; 40: 447–454. [DOI] [PubMed] [Google Scholar]
- 18.Baron M, Hudson M, Tatibouet S, et al. The Canadian systemic sclerosis oral health study II: the relationship between oral and global health-related quality of life in systemic sclerosis. Rheumatology (Oxford) 2015; 54: 692–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masi AT, Rodnan GP, Medsger TA, et al.; Subcommittee for Scleroderma Criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Preliminary criteria for the classification of systemic sclerosis (scleroderma). Arthritis Rheum 1980; 23: 581–590. [DOI] [PubMed] [Google Scholar]
- 20.Medsger TA, Jr, Bombardieri S, Czirjak L, et al. Assessment of disease severity and prognosis. Clin Exp Rheumatol 2003; 21(3 Suppl 29): S42–S46. [PubMed] [Google Scholar]
- 21.Valentini G, Valentini G, Della Rossa A, et al. European multicentre study to define disease activity for systemic sclerosis. Identification of disease activity variables and development of preliminary activity indexes. Ann Rheum Dis 2001; 60: 592–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clements P, Lachenbruch P, Siebold J, et al. Inter and intraobserver variability of total skin thickness score (modified Rodnan TSS) in systemic sclerosis. J Rheumatol 1995; 22: 1281–1285. [PubMed] [Google Scholar]
- 23.Petricević N, Celebić A, Papić M, et al. The Croatian version of the oral health impact profile questionnaire. Coll Antropol 2009; 33: 841–847. [PubMed] [Google Scholar]
- 24.Slade GD. The oral health impact profile In: Slade GD. (ed) Measuring oral health and quality of life. Chapel Hill, NC, USA: Department of Dental Ecology, School of Dentistry, University of North Carolina, 1997, pp.93–104. [Google Scholar]
- 25.Rannou F, Poiraudeau S, Berezné A, et al. Assessing disability and quality of life in systemic sclerosis: construct validities of the cochin hand function scale, health assessment questionnaire (HAQ), systemic sclerosis HAQ, and medical outcomes study 36-item short form health survey. Arthritis Rheum 2007; 57: 94–102. [DOI] [PubMed] [Google Scholar]
- 26.Veale BJ, Jablonski RY, Frech TM, et al. Orofacial manifestations of systemic sclerosis. Br Dent J 2016; 221: 305–310. [DOI] [PubMed] [Google Scholar]
- 27.Cersosimo MG, Raina GB, Calandra CR, et al. Dry mouth: an overlooked autonomic symptom of Parkinson's disease. J Parkinsons Dis 2011; 1: 169–173. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization, European Office, Copenhagen, Denmark. European Health for All Database (HFA-DB) [Online]. 2005. Jan [cited 2018 Feb 22]. Available from: http://www.thehealthwell.info/node/5569
- 29.Vigu AL, Stanciu D, Lotrean LM, et al. Complex interrelations between self-reported oral health attitudes and behaviors, the oral health status, and oral health-related quality of life. Patient Prefer Adherence 2018; 12: 539–549. doi: 10.2147/PPA.S159621. eCollection 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]