Short abstract
Hepatopulmonary fusion is a rare malformation that is often discovered during operative repair of right-sided congenital diaphragmatic defects. Based on a search using medical search engines, we only found 22 cases of hepatopulmonary fusion in the English literature worldwide to date. We describe herein a case of hepatopulmonary fusion with right-sided congenital diaphragmatic hernia in a female neonate who presented with respiratory distress. We discuss management of this case and review the relevant literature.
Keywords: Congenital diaphragmatic hernia, hepatopulmonary fusion, respiratory distress, neonate, liver, lungs, diaphragm
Introduction
Right-sided congenital diaphragmatic hernia (CDH) accounts for approximately 15% of CDH cases. Patients with right-sided CDH typically do not have symptoms at birth. Therefore, presentation and diagnosis of CDH are often delayed for such patients.1–3 The liver is the most commonly herniated organ for right-sided defects. On rare occasions, the herniated liver fuses with the hypoplastic right lung, causing hepatopulmonary fusion (HPF), which is often discovered during operative repair of the defect. We describe here a case of HPF with right-sided CDH and its management, and review the relevant literature.
Case Report
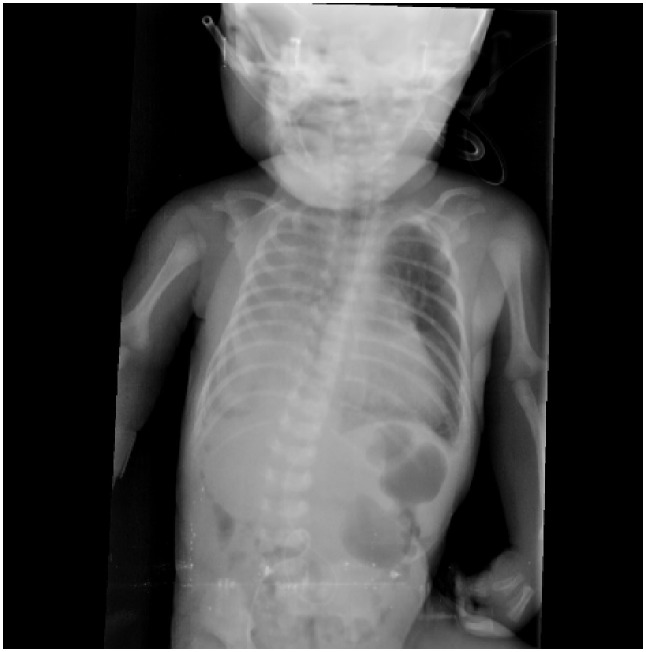
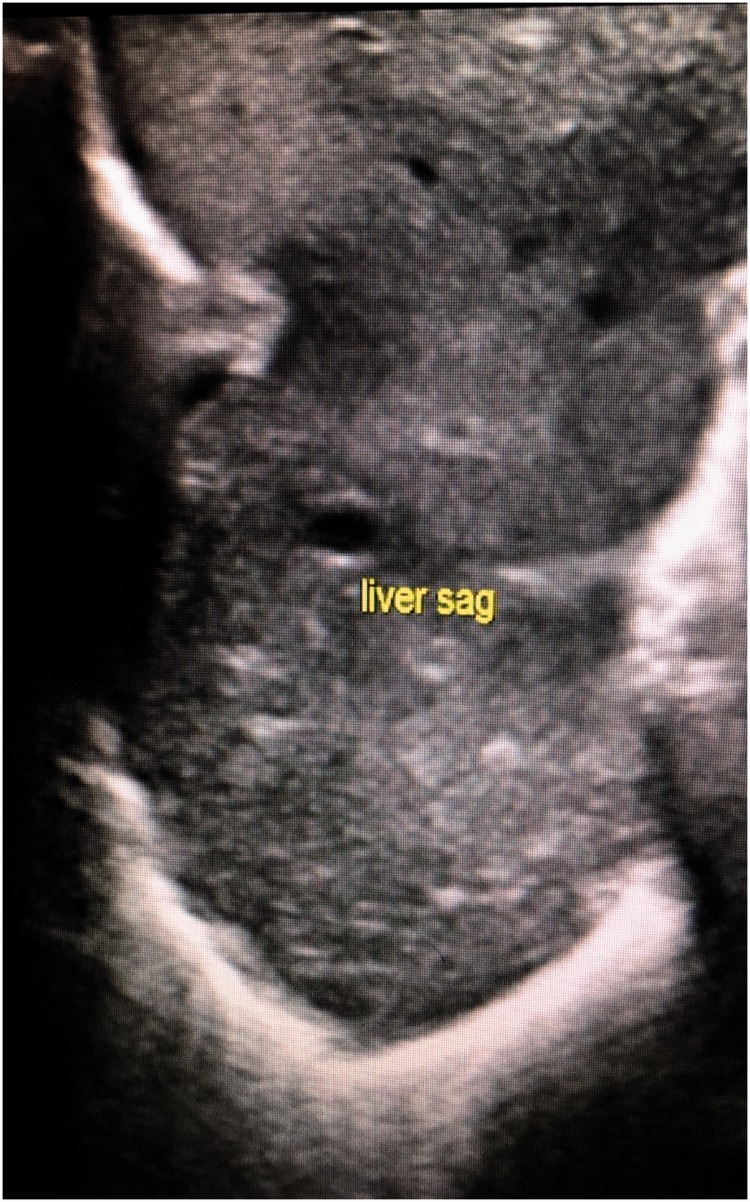
A full-term female neonate was admitted to neonatal intensive care immediately after birth with respiratory distress and referred to surgical service on the 2nd day. At that time, she was haemodynamically stable and maintained on 2 L of oxygen. Her breathing sounds were decreased on the right side. A chest radiograph showed opacification of the right hemi-thorax with a mediastinal shift (Figure 1). Ultrasound confirmed the presence of right-sided CDH and showed that the liver had herniated through the defect (Figure 2). An ultrasound of the abdomen and echocardiography were normal. These examinations were performed to check for other associated anomalies, and to assess cardiac function and pulmonary pressure. After 48 h of life, operative repair was planned for the patient. On exploration, a right postero-lateral diaphragmatic defect (Bochdalek hernia) was found, containing the right lobe of the liver, the right kidney with its suprarenal gland, and a loop of the colon. The liver, particularly its posterior aspect, was densely adherent to the diaphragm and chest wall. The liver was abnormally lobulated and firmly fused with the hypoplastic ipsilateral lung. Throughout the dissection, no distinct plane between the lung and liver could be established, even after prolonged effort. During the dissection, a tear in the inferior vena cava occurred near its junction with the hepatic vein. This tear was immediately controlled and repaired. The liver, colon, and kidney with its suprarenal gland were placed back in the abdomen, and repair of the diaphragmatic defect was performed. A portion of the liver that we failed to separate from the lung remained, and the diaphragmatic defect was repaired around it without causing any tissue compression. The patient was moved to the neonatal intensive care unit and intubated and ventilated. Unfortunately, during the postoperative period, the patient persistently exhibited respiratory failure, pulmonary hypertension, and hemodynamic instability. She died on the third postoperative day.
Figure 1.
Chest X-ray shows left side opacity with mediastinal shift to the right side
Figure 2.
Ultrasound image showing a herniated liver inside the chest
Verbal consent for publication was obtained from the parents.
Discussion
HPF is a rare malformation in patients with right-sided CDH. The spectrum of HPF ranges from only fibrovascular communication to complete parenchymal fusion that can be difficult to divide.4 We describe one case of HPF with right-sided CDH that involved tissue fusion between the lower lobe of the lungs and the superior surface of the liver. A review of English literature using the search engines PubMed, Scopus, and Google scholar database was performed by searching with the following keywords: congenital diaphragmatic hernia, hepatopulmonary fusion, hepatic pulmonary fusion, and liver and lung fusion. We also searched references in the retrieved articles to identify other reported cases. Only 22 cases of HPF were identified (Table 1).4–18 The embryological basis for development of HPF is not yet understood. One theory for this development is that failure in formation of the diaphragm during early gestation allows hepatic tissue to herniate into the chest during the 10th week of gestation. Subsequently, the liver may fuse to the primitive lung. Another theory is that HPF is the primary anomaly that then interferes with complete development of the diaphragm.5
Table 1.
Previously reported cases of hepatopulmonary fusion
| Study | Patients (n) |
Sex/age | Associated findings | Outcome at report time |
|---|---|---|---|---|
| Katz et al. (1998)9 | 1 | Sex not mentioned/ full-term neonate |
Right congenital diaphragmatic hernia | Died |
| Slovis et al. (2000)4 | 6 | Two males and four females/neonates |
Five patients had systemic arterial and venous circulation to a fused lung, two had left-sided congenital heart disease, and two had sequestrations | Two patients died Four patients survived |
| Keller et al. (2003)5 | 1 | Male/full term neonate | Right diaphragmatic hernia | Survived |
| Robertson et al. (2006)8 | 1 | Male/neonate | Right diaphragmatic hernia | Died |
| Taide et al. (2010)10 | 1 | Sex not mentioned/ 7 months |
Right diaphragmatic hernia | Survived |
| Gander et al. (2010)6 | 1 | Male/3 months | Right diaphragmatic hernia | Survived |
| Chandrashekhara et al. (2011)11 |
1 | Male/11 years | Pulmonary sequestration receiving arterial supply from the descending aorta and celiac trunk | Survived |
| Castle et al. 201112 | 1 | Male/neonate | Right-sided congenital diaphragmatic hernia, duodenal atresia, and an imperforate anus | Survived |
| Hamilton et al. (2014)13 | 1 | Male/3 months | Anomalous right pulmonary venous return, and azygos continuation of the inferior vena cava | Died |
| Lin et al. (2012)14 | 3 | Male/8 months Female/6 years Female/neonate |
Two patients with diaphragmatic hernia and one with pentalogy of Cantrell | Survived Survived Died before the operation |
| Saurabh et al. (2013)15 | 1 | Male/neonate | Right-sided diaphragmatic hernia, thumb and index finger syndactyly, and multiple clefts in the vertebrae | Died |
| Olenik et al. (2014)7 | 1 | Male/neonate | Right diaphragmatic hernia | Survived |
| Laamiri, et al. (2016)16 | 1 | Male/neonate | Right diaphragmatic hernia | Died |
| Jain et al. (2017)17 | 1 | Female/2 months | Right diaphragmatic hernia | Died |
| Takezo et al. (2017)18 | 1 | Female/diagnosed at 33 weeks’ gestation. Operated on at 1 day old |
Right diaphragmatic hernia | Not mentioned |
The clinical presentation of HPF is that of Congenital diaphragmatic hernia, which is commonly discovered during the early neonatal period, when respiratory distress is the dominant feature. However, asymptomatic cases of HPF at birth present late during infancy and are discovered incidentally in a chest X-ray performed for other reasons.6 However, HPF is typically not diagnosed preoperatively and is most often discovered during surgical exploration.7 HPF should be suspected preoperatively when there is opacification of the right hemi-thorax without a mediastinal shift to the contralateral side. This lack of shifting of the mediastinum is believed to be related to the presence of a hypoplastic lung on the affected side.4 Exceptions to the above-mentioned description of HPF can occur. Mediastinal shift may be detected in HPF cases if the defect is large and the bowel and other organs are herniated into the chest, as observed in our case and described in other reports.4,6 With routine use of ultrasound in antenatal care, CDH can be diagnosed antenatally. Ultrasonographic features suggesting right CDH include the absence of bowel loops in the abdominal cavity, bowel loops in the chest, and presence of the liver inside the chest. Moreover, when a colour Doppler study is added, it may show the umbilical segment of the portal vein bowing towards the left and portal branches to the lateral segment of the left hepatic lobe in the direction of or above the diaphragm. Additionally, the gallbladder is observed above the diaphragm, and an echogenic space representing the left hepatic lobe is found between the left border of the heart and the stomach.19–21 Although CDH can be diagnosed by ultrasound, the diagnosis of HPF is difficult prenatally. However, with increased use of foetal magnetic resonance imaging (MRI) as a diagnostic tool for CDH once suspected on prenatal ultrasound, HPF can be identified before delivery and thus before postnatal surgical exploration. Postnatally, MRI is still the diagnostic modality of choice for HPF. Demonstration of venous drainage from the lung parenchyma into the intrahepatic part of the inferior vena cava could be a diagnostic indicator of HPF.5 Echocardiography is usually performed to assess cardiac function, associated anomalies, and features of pulmonary hypertension, but demonstration of hepatic veins draining directly into the right atrium also indicates diagnosis of HPF. In our case, neither computed tomography nor MRI was performed because we did not consider HPF, and only ultrasound was performed.
There is no consensus about the optimal time for repairing CDH and no strong evidence to favour an early (within the first 24 h) or delayed approach (after period of stabilization).22 However, an early operation in the presence of compromised respiratory and unstable haemodynamic parameters is unsafe, and delayed repair may allow stabilization of borderline patients and thus improve prognosis.23 Nevertheless, there are no agreed criteria to define physiological stabilization. Some authors have defined these criteria as stable mean blood pressure for gestational age, preductal oxygen saturation in the range of 85%–95% on ≤50% oxygen, urine output >2 cc/kg/h, and serum lactate levels <3 mmol/l.24 Pulmonary artery pressure also determines stability. Some surgeons operate only if normal measures are maintained for at least 24 to 48 h based on echocardiography because they believe that pulmonary hypertension is a major risk of mortality. However, Rozmiarek and colleagues related mortality of CDH to associated complications (cardiac defects and renal failure) and initial blood gases rather than the timing of repair.25 In our case, we preferred to perform immediate repair because the neonate was haemodynamically stable and pulmonary pressure did not cause a severe shunt.
The aim of surgical repair in HPF cases is to reduce the herniated abdominal content and suture the diaphragmatic defect, which are difficult in cases involving inseparable lung and hepatic tissues. Many procedures have been described, including separation of the lungs and liver with repair of the defect, repair of the defect around the fused organs without separation, and partial hepatectomy and/or pneumonectomy.4,6,26 To achieve successful separation of the lung and liver, Robertson et al.8 used a Liga-Sure device because blunt dissection causes major air leakage.4,8 The prognosis of patients with HPF is poor, and thus patients often die during the perioperative period. High mortality among patients with HP is related to complications of lung hypoplasia, CDH and/or surgical procedures for HPF. These complications include respiratory failure, persistent pulmonary hypertension, right heart failure, congenital heart diseases, and thrombosis of the inferior vena cava.6,7 Table 1 shows that 12 of the reported patients survived, while nine children died.
We recommend that the possibility of HPF should be considered in any case of right-sided diaphragmatic hernia. Surgical management of such cases should not be rushed. When feasible, these cases should be assessed via a thorough investigation that invariably includes ultrasonography, a contrast computed tomography scan and/or MRI. This is because the majority of reported cases were discovered intra-operatively.17 Additionally, the only surrogate indicator mentioned in these previous reports was the absence of mediastinal shift, which was believed to be related to pulmonary hypoplasia. Furthermore, some cases of HPF were associated with mediastinal shift when the defect was large and there were intrathoracic herniated bowels. Before surgery for HPF in right-sided CDH, careful preparation and optimal stabilization are required. Additionally, all logistical preparations for a major surgical undertaking, potentially including hepatic or pulmonary lobectomy or pneumonectomy, should be performed.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Fisher JC, Jefferson RA, Arkovitz MS, et al. Redefining outcomes in right congenital diaphragmatic hernia. J Pediatr Surg 2008; 43: 373–379. [DOI] [PubMed] [Google Scholar]
- 2.Benya EC, Nussbaum-Blask AR, Selby DM. Colonic diverticulitis causing partial bowel obstruction in a child with cystic fibrosis. Pediatr Radiol 1997; 27: 918–919. [DOI] [PubMed] [Google Scholar]
- 3.Weber TR, Tracy T, jr, Bailey PV, et al. Congenital diaphragmatic hernia beyond infancy. Am J Surg 1991; 162: 643–646. [DOI] [PubMed] [Google Scholar]
- 4.Slovis TL, Farmer DL, Berdon WE, et al. Hepatic pulmonary fusion in neonates. AJR Am J Roentgenol 2000; 174: 229–233. [DOI] [PubMed] [Google Scholar]
- 5.Keller RL, Aaroz PA, Hawgood S, et al. MR imaging of hepatic pulmonary fusion in neonates. AJR Am J Roentgenol 2003; 180: 438–440. [DOI] [PubMed] [Google Scholar]
- 6.Gander JW, Kadenhe-Chiweshe A, Fisher JC, et al. Hepatic pulmonary fusion in an infant with a right-sided congenital diaphragmatic hernia and contralateral mediastinal shift. J Pediatr Surg 2010; 45: 265–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olenik D, Codrich D, Gobbo F, et al. Hepatopulmonary fusion in a newborn. An uncommon intraoperatory finding during right congenital diaphragmatic hernia surgery: case description and review of literature. Hernia 2014; 18: 417–421. [DOI] [PubMed] [Google Scholar]
- 8.Robertson DJ, Harmon CM, Goldberg S. Right congenital diaphragmatic hernia associated with fusion of the liver and the lung. Journal of pediatric surgery 2006; 41: e9–e10. [DOI] [PubMed] [Google Scholar]
- 9.Katz S, Kidron D, Litmanovitz I, et al. Fibrous fusion between the liver and the lung: an unusual complication of right congenital diaphragmatic hernia. J Pediatr Surg 1998; 33: 766–767. [DOI] [PubMed] [Google Scholar]
- 10.Taide DV, Bendre PS, Kirtane JM, et al. Hepatic pulmonary fusion: a rare case. Afr J Paediatr Surg 2010; 7: 28. [DOI] [PubMed] [Google Scholar]
- 11.Chandrashekhara SH, Bhalla As, Gupta AK, et al. Hepatic pulmonary fusion: case report with review of literature. J Pediatr Surg 2011; 46: e23–e7. [DOI] [PubMed] [Google Scholar]
- 12.Castle SL, Naik-Mathuria BJ, Torres MB. Right-sided congenital diaphragmatic hernia, hepatic pulmonary fusion, duodenal atresia, and imperforate anus in an infant. J Pediatr Surg 2011; 46: 1432–1434. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton J, Jaroszewski D, Notrica D. Fatal complication after repair of a congenital diaphragmatic hernia associated with hepatopulmonary fusion, anomalous right pulmonary venous return, and azygos continuation of the inferior vena cava. Eur J Pediatr Surg 2014; 24: 350–352. [DOI] [PubMed] [Google Scholar]
- 14.Lin J, Durham MM, Ricketts R, et al. Hepatic pulmonary fusion: two cases with diaphragmatic hernia and one case with pentalogy of Cantrell. Fetal Pediatr Pathol 2012; 31: 401–409. [DOI] [PubMed] [Google Scholar]
- 15.Saurabh K, Kumar S, Chellani H, Aarya S. Hepatic pulmonary fusion: a rare association of right-sided congenital diaphragmatic hernia. Annals of Gastroenterology: Quarterly Publication of the Hellenic Society of Gastroenterology 2013; 26(1): 95–96. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3959522/ [PMC free article] [PubMed]
- 16.Laamiri R, Belhassen S, Ksia A, et al. Right Congenital Diaphragmatic Hernia Associated With Hepatic Pulmonary Fusion: a Case Report. J Neonatal Surg 2016; 5: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jain V, Yadav DK, Kandasamy D, et al. Hepatopulmonary fusion: a rare and potentially lethal association with right congenital diaphragmatic hernia. BMJ Case Rep 2017; 2017: bcr2016218227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takezoe T, Nomura M, Ogawa K, et al. Prenatally diagnosed, right-sided congenital diaphragmatic hernia complicated by hepatic pulmonary fusion and intrathoracic kidney.
- 19.Chinn DH, Filly RA, Callen PW, et al. Congenital diaphragmatic hernia diagnosed prenatally by ultrasound. Radiology 1983; 148: 119–123. [DOI] [PubMed] [Google Scholar]
- 20.Taylor GA, Atalabi OM, Estroff JA. Imaging of congenital diaphragmatic hernias. Pediatric radiology 2009; 39: 1. [DOI] [PubMed] [Google Scholar]
- 21.Vettraino IM, Lee W, Comstock CH. The evolving appearance of a congenital diaphragmatic hernia. J Ultrasound Med. 2002; 21: 85–89. [DOI] [PubMed] [Google Scholar]
- 22.Moyer VA, Moya FR, Tibboel D, et al. Late versus early surgical correction for congenital diaphragmatic hernia in newborn infants. The Cochrane Library. 2000. [DOI] [PubMed]
- 23.Langer JC, Filler RM, Bohn DJ, et al. Timing of surgery for congenital diaphragmatic hernia: is emergency operation necessary? J Pediatr Surg 1988; 23: 731–734. [DOI] [PubMed] [Google Scholar]
- 24.Kumar VH. Current Concepts in the Management of Congenital Diaphragmatic Hernia in Infants. Indian J Surg 2015; 77: 313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rozmiarek AJ, Qureshi FG, Cassidy L, et al. Factors influencing survival in newborns with congenital diaphragmatic hernia: the relative role of timing of surgery. J Pediatr Surg 2004; 39: 821–824. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka S, Kubota M, Yagi M, et al. Treatment of a case with right-sided diaphragmatic hernia associated with an abnormal vessel communication between a herniated liver and the right lung. J Pediatr Surg 2006; 41: e25–e8. [DOI] [PubMed] [Google Scholar]