Short abstract
Objective
The consumption of opioid analgesics could be reduced by the use of analgesics with different mechanisms of action. We investigated whether additional treatment with dexmedetomidine or lidocaine could reduce opioid consumption.
Methods
We randomized 59 study participants into three groups and examined: (i) fentanyl consumption, (ii) consumption of piritramide, and (iii) cognitive function and neuropathic pain. The control group received continuous propofol infusion and fentanyl boluses. Continuous intravenous infusion of dexmedetomidine (0.5 µg/kg/h) was administered to the dexmedetomidine group and lidocaine (1.5 mg/kg/h) was administered to the lidocaine group.
Results
No reduction in fentanyl consumption was observed among the groups. However, we noted a significantly lower consumption of piritramide on the first and second postoperative day in the lidocaine group. Total consumption of piritramide was significantly lower in the lidocaine group compared with the control group.
Conclusions
Lidocaine and dexmedetomidine reduced intraoperative propofol consumption, while lidocaine reduced postoperative piritramide consumption.
Clinical trial registration: NCT02616523
Keywords: Analgesia, cognitive function, dexmedetomidine, laparoscopy, lidocaine, neuralgia
Background
Opioids are the mainstay of perioperative analgesia. However, the intraoperative use of large doses of opioids may be associated with several postoperative side effects such as postoperative hyperalgesia, increased analgesic consumption, prolonged sedation, ileus, urinary retention, postoperative nausea, and vomiting.1,2
Postoperative pain is complex in nature. Growing evidence suggests that its treatment should be multimodal and opioid-sparing.3 Efficient perioperative pain treatment is important to prevent the development of late neuropathic pain.
To improve pain therapy and reduce opioid demand, we combined standard fentanyl anaesthesia with the additional intravenous infusion of dexmedetomidine or lidocaine. Dexmedetomidine is a highly selective α2 adrenoceptor agonist and its perioperative intravenous administration is associated with a reduction in postoperative pain intensity, analgesic consumption, and nausea.4 It may preserve cognitive function in elderly patients.5 The administration of intravenous lidocaine is safe in the intraoperative period and has clear advantages, such as decreased intraoperative anaesthetic requirements, lower pain scores, reduced postoperative analgesic requirements, faster return of bowel function, and decreased length of hospital stay.6–8
In laparoscopic lower abdominal surgery, the reported incidence of clinically important neuropathic pain is low (approximately 5%) because it is associated with fewer nerve injuries compared with open surgery.9
We performed a prospective, randomized study combining opioids with dexmedetomidine or lidocaine, the intraoperative use of which can reduce opioid consumption during surgery and reduce pain intensity.10,11 These medications can also preserve cognitive function and reduce the incidence of neuropathic pain. The effects of intravenous lidocaine are consistent with previously published studies,6–8 but the effect of dexmedetomidine have been relatively unstudied in this patient population.
Our hypothesis was that participants receiving dexmedetomidine and lidocaine would require less fentanyl during surgery and less piritramide for postoperative pain compared with participants receiving only fentanyl intraoperatively. We assumed that cognitive function would be better preserved in participants receiving dexmedetomidine infusion. We observed the incidence of neuropathic pain in all participants.
Methods
This was a three-group randomized controlled trial following the CONSORT checklist, which evaluated the effect of supplementary analgesia with dexmedetomidine (DG), lidocaine (LG), and control group (CG). Inclusion criteria were adult participants (>18 years) scheduled for elective laparoscopic intestine resection, who had an American Society of Anesthesiologists (ASA) physical status I-III. Exclusion criteria were: allergy to α2 receptor agonists, uncontrolled arterial hypertension (cut point of 140/90 mmHg), second and third degree atrioventricular block, active alcohol and illegal drugs abuse, decompensated respiratory or cardiovascular disease, pregnancy, and chronic opioid therapy. Written informed consent was obtained from all participants before randomization. The Slovenian Ethics Committee approved the study (approval number no. 23/07/14).
A programme of enhanced recovery after surgery (ERAS) is not strictly followed in our surgical ward, primarily for resourcing reasons. Participants were mobilized immediately after surgery and could start to drink water as soon as they woke up from the anaesthesia. Participants received laxatives as required, and food was introduced on the third day after surgery, prior to which participants received parenteral nutrition.
Randomization and blinding
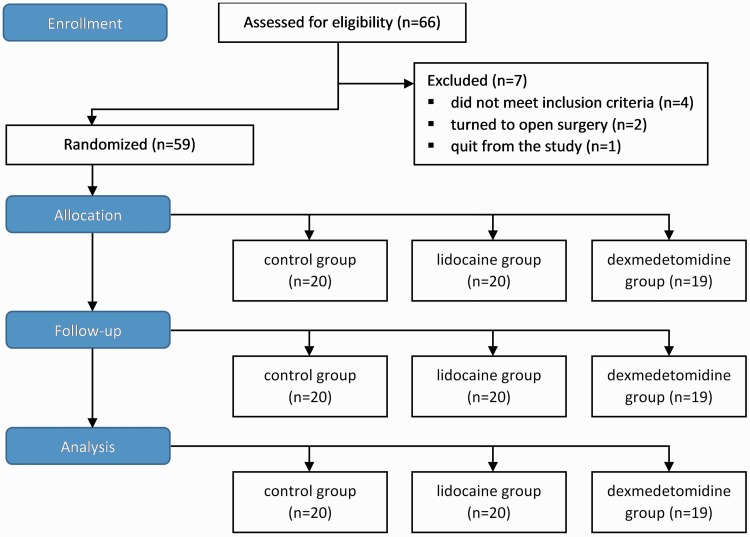
Simple randomization was performed using 66 sealed envelopes (22 for each group), indicating group assignment and describing the anaesthetic protocol (Figure 1). All envelopes were identical and were shuffled prior to distribution. Each participant randomly chose one envelope that assigned him/her to one of the three groups. Four participants did not meet the inclusion criteria and were therefore not included in the study, two laparoscopic procedures were converted to open surgery, and one participant in the DG withdrew from the study for personal reasons.
Figure 1.
Flow diagram of the study.
This was a single-blind trial, involving one anaesthesiologist who randomized and performed the study and was the only individual who knew which group each participant was assigned to. All other staff and the participants were blind to the study group allocation, participant management, and data collection.
Prior to surgery, the participants were randomly allocated into three groups. Participants in the LG received infusion of lidocaine (Xylocaine, AstraZeneca, London, UK) (1.5 mg/kg/h),12,13 participants in the DG received infusion of dexmedetomidine (Dexdor, Orion Pharma, Espoo, Finland) (0.5 µg/kg/h), and participants in the CG received infusion of normal saline.14 We stopped infusion of lidocaine and dexmedetomidine at the end of surgery.
Clinical management
Anaesthesia and perioperative care were managed according to standard local guidelines. Prior to surgery, we assessed cognitive function using a mini mental state examination (MMSE).15
Standard clinical monitoring included electrocardiogram, non-invasive blood pressure, pulse oximetry, and bispectral index (BIS, Vista, Covidien, Zaltbommel, Netherlands). Induction with analgesic fentanyl (Fentanyl Torrex, Chiesi, Austria) (2 µg/kg), sedative propofol (Propoven, Fresenius Kabi AG, Bad Homburg, Germany) (1.5–2.5 mg/kg), and the muscle relaxant rocuronium (Esmeron, MSD, NY, USA) (0.6 mg/kg) was performed. Participants were mechanically ventilated using tidal volumes of 6–8 mL/kg and an oxygen/air mixture (FiO2: 40%, respiratory rate 10–14/min). All of the participants received an intravenous bolus of fentanyl (2 µg/kg) at the time of incision16 and whenever the analgesia nociception index (ANI) dropped below 50 during the operation.17 Anaesthesia was maintained with intravenous infusion of propofol (4–6 mg/kg/h) according to the BIS value, which was maintained between 40 and 55.17
All participants were given paracetamol 1 g at the time of anastomosis construction.
ANI monitoring (Mdoloris Medical Systems) was used for pain measurement. ANI is an online heart rate variability analysis proposed for the assessment of the anti-nociception/nociception balance. The values range from 0 to 100, based on the degree of parasympathetic activation (100 represents high parasympathetic modulation and low stress level). We maintained ANI values between 50 and 70 with fentanyl boluses, thus providing adequate analgesia.18
Muscle relaxation was measured with TOF (train of four) and was maintained at the level of deep muscle relaxation (train of four, TOF 0 and post-tetanic count, PTC 1–2) with additional boluses of rocuronium (20–30 mg).
Surgeons experienced in laparoscopic intestine resections performed the procedures using standard three trocar techniques. Intra-abdominal pressure was maintained at 12 mmHg throughout the procedure. Propofol infusion was stopped at the end of the final suture. Residual neuromuscular blockade was antagonized with sugammadex. After surgery, piritramide was delivered using a patient-controlled analgesia (PCA) device (continuous infusion rate 0.5 mg/h, lockout 30 min, bolus 1.5 mg).
If participants complained of pain according to visual analogue scale (VAS) (0 corresponding to no pain and 10 to the worst imaginable pain) values higher than 3, rescue analgesics were delivered such as a bolus of piritramide 3 mg intravenously, then paracetamol 1 g intravenously over a period of 6 hours, and finally metamizole 30 mg/kg/12h intravenously. After 1 hour in the post-anaesthesia care unit (PACU), and VAS ≤3 was achieved, participants were transferred to intensive care in the clinical department of abdominal surgery. Vital signs were recorded continuously and pain scored every hour with VAS.
Medical staff blinded to treatment allocation and with no access to the intraoperative records performed all outcome assessments.
PCA was discontinued on the third postoperative day or later, when there was no further need for rescue analgesics (VAS score < 3) and when participants could receive oral doses of paracetamol 500 mg (4 × 1 per day) and diclofenac 75 mg (2 × 1 per day). Participants on oral analgesics were transferred to the surgical ward and discharged home when the wound was healing per primam, with no pain or complications.
Outcome measures and data collection
The primary outcome variable was fentanyl consumption during surgery. Secondary outcome measures were the consumption of piritramide (total piritramide administered through PCA and boluses in milligrams) in the PACU and in the surgical ward on the first and the second postoperative day, as well as the incidence of neuropathic pain 2 months after surgery. VAS and consumption of piritramide were re-evaluated 2 days after surgery. Cognitive function was evaluated again by MMSE on the second day.
Two months after the operation, neuropathic pain was evaluated with pain questionnaires (DN4 and Pain detect questionnaires). The following data were collected: demographic characteristics of participants, intraoperative consumption of propofol, fentanyl, consumption of piritramide in the PACU, MMSE score before surgery and on the second postoperative day, consumption of piritramide on the first and the second postoperative day on the surgical ward, and neuropathic pain 2 months after surgery. Fentanyl and propofol consumption were measured during surgery.
Statistical analysis
The sample size requirement was based on data from a previous pilot study that included nine participants (three in each group), whose fentanyl requirements (mean±standard deviation) were 0.322 ± 0.029 mg/hour in CG, 0.116 ± 0.027 mg/hour in LG, and 0.124 ± 0.015 mg/hour in DG. The CliniCalc Sample Size calculator was used to determine the minimum number of patients required for adequate study power by providing the anticipated distribution of the control group (that is, equal to fentanyl requirements in the pilot study of 0.322 ± 0.029 mg/hour) and the anticipated outcome of the study group, expressed as the 40% change in the mean value. At an alpha risk of 0.05, two final participants per group (after potential dropouts) would provide 80% power and detect 40% reduction in fentanyl consumption in LG and DG. The low number of required participants stems from the very low standard deviations in the participant groups in the pilot study.
Because the tests for normal distribution (Kolmogorov–Smirnov and Shapiro–Wilk for smaller samples) rejected the null hypothesis that the majority of variables (all variables except fentanyl consumption per time unit and consumption of piritramide on the first postoperative day) were normally distributed, we applied the non-parametric Kruskal–Wallis test for independent samples (for rejecting the null hypothesis that the distribution of variables is the same across patient groups) along with the median test for independent samples (for rejecting the null hypothesis that the medians are the same across patient groups). Both tests indicated that there are significant overall differences between the groups in (p-values of the Kruskal-–Wallis test are given): propofol consumption per time unit per body weight (p<0.001), consumption of piritramide on the first postoperative day (p=0.010), consumption of piritramide on the second postoperative day (p=0.001), total consumption of piritramide per body weight (p=0.020), and average VAS on the second postoperative day (p=0.035). To further analyse these differences in greater detail, we applied the Mann–Whitney test for each pair of patient groups (DG vs. CG, CG vs. LG, and DG vs. LG) for different distributions. The results of the statistical comparisons between the groups are shown in Table 2. The analyses were performed using IBM SPSS Statistics, version 25.0 (IBM Corp., Armonk, NY, USA). We considered a p-value of less than 0.05 as statistically significant.
Table 2.
Statistical analysis of differences between groups using the Mann–Whitney test. Significant differences are indicated in bold type in the last three columns.
| Group | N | Median | IQR | Min | Max | p-value for difference to DG | p-value for difference to CG | p-value for difference to LG | |
|---|---|---|---|---|---|---|---|---|---|
| Propofol consumption per time unit per body weight [mg/min/kg] | DG | 19 | 0.102 | 0.040 | 0.080 | 0.190 | / | 0.001 | 0.647 |
| CG | 20 | 0.146 | 0.060 | 0.080 | 0.270 | 0.001 | / | 0.001 | |
| LG | 20 | 0.099 | 0.030 | 0.070 | 0.150 | 0.647 | 0.001 | / | |
| Fentanyl consumption per time unit per body weight [mg/min/kg] | DG | 19 | 0.00005 | 0.000 | 0.0000 | 0.0001 | / | 0.084 | 0.749 |
| CG | 20 | 0.0001 | 0.000 | 0.0000 | 0.0001 | 0.084 | / | 0.102 | |
| LG | 20 | 0.00005 | 0.000 | 0.0000 | 0.0001 | 0.749 | 0.102 | / | |
| Recovery consumption: piritramide [mg] | DG | 19 | 3.000 | 9.000 | 0.000 | 15.000 | / | 0.708 | 0.942 |
| CG | 20 | 5.000 | 3.000 | 0.000 | 12.000 | 0.708 | / | 0.656 | |
| LG | 20 | 3.000 | 6.000 | 0.000 | 30.000 | 0.942 | 0.656 | / | |
| Recovery consumption: metamizole [mg] | DG | 19 | 0.000 | 0.000 | 0.000 | 2.500 | / | 0.946 | 0.946 |
| CG | 20 | 0.000 | 0.000 | 0.000 | 2.500 | 0.946 | / | 1.000 | |
| LG | 20 | 0.000 | 0.000 | 0.000 | 2.500 | 0.946 | 1.000 | / | |
| Consumption of piritramide per body weight – day of surgery [mg/kg] | DG | 19 | 0.295 | 0.240 | 0.157 | 0.638 | / | 0.361 | 0.673 |
| CG | 20 | 0.343 | 0.178 | 0.237 | 0.571 | 0.361 | / | 0.787 | |
| LG | 20 | 0.313 | 0.130 | 0.168 | 0.637 | 0.673 | 0.787 | / | |
| Consumption of piritramide per body weight – first post-op day [mg/kg] | DG | 19 | 0.475 | 0.255 | 0.286 | 0.900 | / | 0.152 | 0.144 |
| CG | 20 | 0.582 | 0.149 | 0.318 | 0.857 | 0.152 | / | 0.002 | |
| LG | 20 | 0.427 | 0.177 | 0.216 | 0.806 | 0.144 | 0.002 | / | |
| Consumption of piritramide per body weight – second post-op day [mg/kg] | DG | 19 | 0.463 | 0.432 | 0.267 | 1.109 | / | 0.768 | 0.003 |
| CG | 20 | 0.513 | 0.137 | 0.318 | 0.656 | 0.768 | / | 0.001 | |
| LG | 20 | 0.354 | 0.134 | 0.216 | 0.725 | 0.003 | 0.001 | / | |
| Total consumption of piritramide per body weight [mg/kg] | DG | 19 | 1.172 | 0.825 | 0.909 | 2.491 | / | 0.555 | 0.116 |
| CG | 20 | 1.433 | 0.420 | 0.906 | 2.040 | 0.555 | / | 0.003 | |
| LG | 20 | 1.133 | 0.324 | 0.600 | 2.168 | 0.116 | 0.003 | / | |
| Day of the first defecation | DG | 19 | 3.000 | 1.000 | 1.000 | 6.000 | / | 0.106 | 0.124 |
| CG | 20 | 3.000 | 1.000 | 1.000 | 5.000 | 0.106 | / | 0.578 | |
| LG | 20 | 2.500 | 2.750 | 1.000 | 6.000 | 0.124 | 0.578 | / | |
| Length of stay [days] | DG | 19 | 7.000 | 3.000 | 5.000 | 25.000 | / | 0.041 | 0.134 |
| CG | 20 | 6.000 | 2.000 | 4.000 | 15.000 | 0.041 | / | 0.460 | |
| LG | 20 | 6.000 | 1.000 | 5.000 | 14.000 | 0.134 | 0.460 | / | |
| Blood loss on time unit [mL] | DG | 19 | 0.000 | 0.556 | 0.000 | 2.632 | / | 0.647 | 0.502 |
| CG | 20 | 0.000 | 0.850 | 0.000 | 3.425 | 0.647 | / | 0.903 | |
| LG | 20 | 0.000 | 0.828 | 0.000 | 4.237 | 0.502 | 0.903 | / | |
| Mini mental test difference | DG | 19 | 0.000 | 0.000 | –4.000 | 3.000 | / | 0.203 | 0.271 |
| CG | 20 | 0.000 | 0.750 | –2.000 | 2.000 | 0.203 | / | 0.770 | |
| LG | 20 | 0.000 | 0.000 | –3.000 | 1.000 | 0.271 | 0.770 | / |
Results
We recruited 59 participants between June 2014 and April 2015 (Figure 1). Table 1 displays the descriptive statistics for each individual group (DG, CG, and LG). The type of surgery performed was laparoscopic intestine resection (hemicolectomy) in all participants (13 carcinomas in DG, 15 in CG, and 18 in LG; other patients had benign intestinal tumours).
Table 1.
Patient characteristics. Data are presented as ‘median (IQR) [min–max]’ for continuous variables and n (%) for binary variables.
| Group DG (n=19) | Group CG (n=20) | Group LG (n=20) | |
|---|---|---|---|
| Age [years] | 68 (25) [42–83] | 58 (20) [36–85] | 63.5 (16) [36–79] |
| Body weight [kg] | 70 (25) [47–101] | 74 (20) [49–102] | 80 (16) [54–125] |
| BMI [kg/m2] | 25.7 (6) [18.1–34.9] | 24.4 (5.5) [18.2–29.4] | 28.2 (6.5) [19.8–38.6] |
| Duration of surgery [min] | 115 (65) [55–190] | 115 (71) [70–235] | 123 (34) [60–220] |
| Male sex (%) | 9 (47.4%) | 10 (50%) | 12 (60%) |
| ASA classification (I/II/III) | 0 (0%) /11 (58%) /8 (42%) | 4 (20%) /14 (70%) /2 (10%) | 1 (5%) /13 (65%) /6 (30%) |
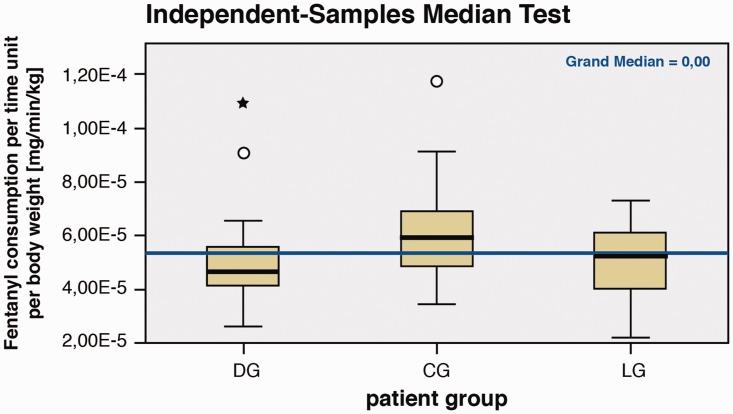
The experimental results shown in Table 2 indicate that no significant differences in intraoperative fentanyl consumption were observed between the groups (Figure 2). In the PACU, there was no difference found in piritramide consumption between the groups.
Figure 2.
Fentanyl consumption per patient group.
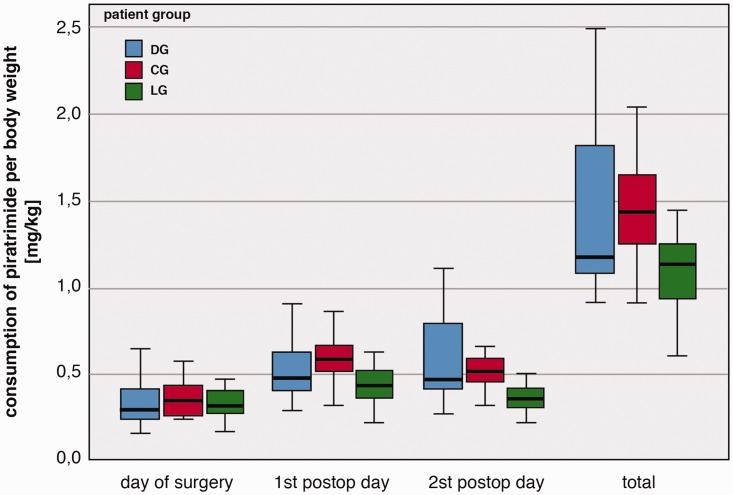
The comparisons of piritramide consumption are also illustrated in Figure 3. From these results, we can see that the consumption of piritramide was significantly lower in LG compared with CG on the first (p=0.002) and second postoperative day (p=0.001). On the second postoperative day, significantly lower piritramide consumption was noted in LG compared with DG (p=0.003). Total consumption of piritramide was also significantly lower in LG compared with CG (p=0.003).
Figure 3.
Piritramide consumption per body weight among the patient groups.
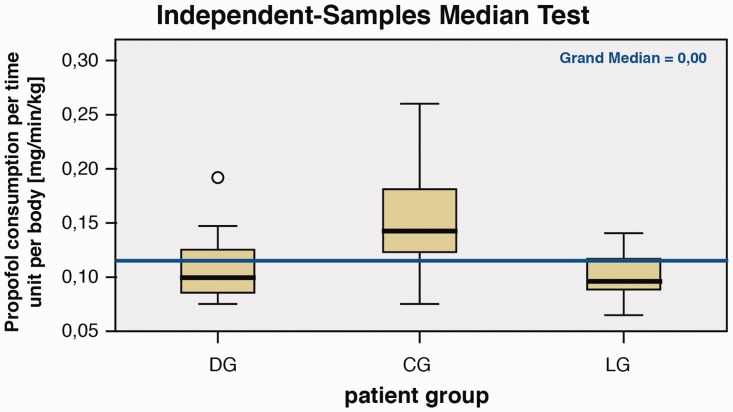
As shown in Table 2 and in Figure 4, there was a significantly lower consumption of propofol (per time unit and per body weight) in DG compared with CG (p=0.001) and in LG compared with CG (p=0.001) during surgery.
Figure 4.
Propofol consumption per patient group.
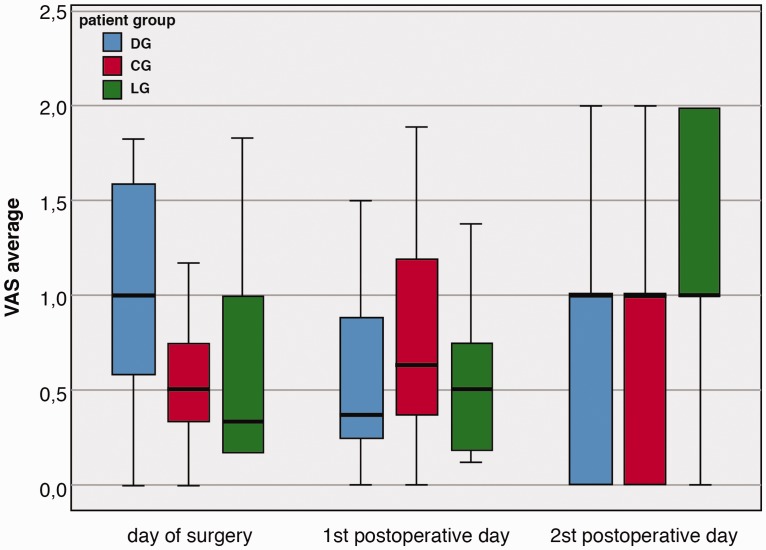
Table 2 and Figure 5 also show that the average VAS on the day of surgery was lower in CG than in DG (p=0.039). Additionally, although the medians of the average VAS in all three groups on the second postoperative day were the same, the distribution of the average VAS differed significantly between DG and LG (p=0.028) and between CG and LG (p=0.023).
Figure 5.
Average VAS score per patient group. VAS = visual analogue scale
There were no differences in cognitive function observed before or after surgery between the groups.
There was a significant difference observed between CG and DG in the length of stay (p=0.041).
There were no reports of neuropathic pain 2 months after surgery (all participants answered the questionnaire).
In addition to the statistical testing, we noted that five participants experienced adverse events during hospitalization. Two patients developed delirium (according to the delirium observation scale), 1 from CG and 1 from LG; both patients were recovered alcoholics. One participant from DG presented with an incarcerated hernia after the first operation and was discharged from hospital on the fourth day after a hernioplasty. Two participants from LG presented with lower urinary tract infections which were treated prior to discharge from hospital. No nausea or vomiting were recorded for any of the participants.
Discussion
The purpose of fast-track colorectal surgery is to enhance patient recovery through the optimization of perioperative care.19 To reduce peri- and postoperative opioid consumption in our study, medications with known analgesic effects (lidocaine and dexmedetomidine) were used intraoperatively.
Intraoperative anaesthetic drug consumption
For pain assessment during anaesthesia, we used ANI monitoring. According to its mechanism of action, certain drugs (atropine, ephedrine), participants with pacemakers, and an apnoea period (intubation time) interact with ANI values.20,21 In such cases, ANI values do not reflect real values because of interference by these events with parasympathetic tone.22 Despite the pneumoperitoneum formation, we did not observe severe bradycardia or hypotension among the patients and there was no requirement to use atropine or ephedrine. None of the groups included participants with a pacemaker, and it was deemed unimportant for the study to interpret ANI values during the intubation time.
A reduction in propofol consumption was observed in both LG and DG compared with CG, which is in accordance with the findings of previous studies.23
Influence of anaesthesia type on postoperative recovery
It has previously been reported that dexmedetomidine reduced opioid consumption intraoperatively and lowered postoperative pain for up to 24 hours in classic colorectal surgery.24 However, this effect has not been studied in laparoscopic surgery,25–27 where lower piritramide consumption has not been observed. In previous studies, both dexmedetomidine and lidocaine used intraoperatively had an opioid-sparing effect, while in several studies, lidocaine reduced pain in open28 and laparoscopic colorectal surgery.29 In the present study, intraoperative infusion of lidocaine exerted an analgesic effect on the first and second postoperative day in comparison with dexmedetomidine, where the duration of its analgesic effect was shorter.
In our study, lidocaine and dexmedetomidine failed to improve bowel function. This lack of effect may be attributable to the absence of an early mobilization programme on the surgical ward.
According to the MMSE results, no cognitive decline was found in any observed group. To eliminate the influence of drugs on cognitive function, we avoided those with a known effect of worsening cognitive function, such as midazolam. Propofol anaesthesia (closely monitored with BIS) is known to preserve cognitive performance postoperatively in comparison with inhalation-based anaesthesia.30 It has been reported that dexmedetomidine preserves cognitive function.31–32 We might have identified some differences between the groups with a larger number of participants or by using more-sensitive psychological tests. One participant with Crohn disease in LG developed delirium in the late postoperative period, likely because of long-term immunomodulation therapy, but his cognitive function was not worse immediately after surgery according to MMSE.
Influence of anaesthesia type on the incidence of postoperative neuropathic pain
In our study, no neuropathic pain was recorded for any of the participants 2 months after surgery when all, without exception, responded to a questionnaire.
The literature estimates there is up to a 5% risk that neuropathic pain will appear because of injury to the iliohypogastric-ilioinguinal nerve associated with the fascial closure of laparoscopic incisions in the lower abdomen.9 However, no study to date has demonstrated skin nerve injury and the resulting development of neuropathic pain in minimally invasive abdominal surgery.
This is the first study to evaluate pain relief during laparoscopic intestine resection in combination with intraoperative dexmedetomidine infusion. An advantage of our study was the use of ANI monitoring for pain assessment, which has not yet been used for adjuvant anaesthesia with lidocaine or dexmedetomidine.
The limitation of our study was the lack of a starting bolus of lidocaine or dexmedetomidine, so that plasma concentrations increased slowly during surgery and may have not reached a sufficient level to reduce intraoperative pain demands, but were nevertheless sufficient to deepen the anaesthesia level and reduce propofol demand. A bolus of dexmedetomidine was not given because it may have had an effect on haemodynamic stability (bradycardia, hypotension), thus requiring treatment with vasoactive drugs or atropine, both of which can affect ANI measurement so that the objectivization of pain would not have been properly evaluated. For the purpose of comparison, we did not use a loading dose in LG. Another important limitation was that we used basic pain management in our study, meaning that we did not manage pain according to the WHO pain relief ladder.33 Non-opioid analgesics were used in low doses (paracetamol 4 × 500 mg, metamizole 30 mg/kg/12 h) as the participants were educated to ask for boluses of opioids when their VAS score was <3. Furthermore, the ERAS programme was not strictly followed in our surgical ward due to resourcing limitations.
Conclusions
Lidocaine infusion reduced opioid demand on the first and second postoperative day as well as total opioid demand. Both dexmedetomidine and lidocaine infusion reduced propofol consumption during surgery.
Author's contributions and authorship
L.A.: study design, patient recruitment, data collection, and writing the first draft of the paper
V.N.J.: study design, revision of the manuscript
N.P.L.: study design, patient recruitment, revision of the manuscript
Z.B.: study design, data analysis, revision of the manuscript
A.S.V.: study design, patient recruitment, revision of the manuscript
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology 2006; 104: 570–587. [DOI] [PubMed] [Google Scholar]
- 2.Bakan M, Umutoglu T, Topuz U, et al. Opioid-free total intravenous anaesthesia with propofol, dexmedetomidine and lidocaine infusions for laparoscopic cholecystectomy: a prospective, randomized, double-blinded study. Rev Bras Anestesiol 2015; 65: 191–199[in Portuguese, English Abstract]. [DOI] [PubMed] [Google Scholar]
- 3.Leslie JB, Viscusi ER, Pergolizzi JV, Jr, et al. Anesthetic routines: the anesthesiologist's role in GI recovery and postoperative ileus. Adv Prev Med 2010; 2011: 976904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramsay MA. Bariatric surgery: the role of dexmedetomidine. Semin Anesth Perioperat Med Pain 2006; 25: 51–56. [Google Scholar]
- 5.Chen J, Yan J, Han X. Dexmedetomidine may benefit cognitive function after laparoscopic cholecystectomy in elderly patients. Exp Ther Med 2013; 5: 489–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahn E, Kang H, Choi GJ, et al. Intravenous lidocaine for effective pain relief after a laparoscopic colectomy: a prospective, randomized, double-blind, placebo-controlled study. Int Surg 2015; 100: 394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim TH, Kang H, Choi YS, et al. Pre- and intraoperative lidocaine injection for preemptive analgesics in laparoscopic gastrectomy: a prospective, randomized, double-blind, placebo-controlled study. J Laparoendosc Adv Surg Tech A 2013; 23: 663–668. [DOI] [PubMed] [Google Scholar]
- 8.Kaba A, Laurent SR, Detroz BJ, et al. Intravenous lidocaine infusion facilitates acute rehabilitation after laparoscopic colectomy. Anesthesiology. 2007; 106: 11–18. [DOI] [PubMed] [Google Scholar]
- 9.Shin JH, Howard FM. Abdominal wall nerve injury during laparoscopic gynecologic surgery: incidence, risk factors, and treatment outcomes. J Minim Invasive Gynecol 2012; 19: 448–453. [DOI] [PubMed] [Google Scholar]
- 10.Gurbet A, Basagan-Mogol E, Turker G, et al. Intraoperative infusion of dexmedetomidine reduces perioperative analgesic requirements. Can J Anaesth 2006; 53: 646–652. [DOI] [PubMed] [Google Scholar]
- 11.Vigneault L, Turgeon AF, Côté D, et al. Perioperative intravenous lidocaine infusion for postoperative pain control: a meta-analysis of randomized controlled trials. Can J Anaesth 2011; 58: 22–37. [DOI] [PubMed] [Google Scholar]
- 12.McCarthy GC, Megalla SA. Impact of intravenous lidocaine infusion on postoperative analgesia and recovery from surgery. Drugs 2010; 70: 1149–1163. [DOI] [PubMed] [Google Scholar]
- 13.Koppert W, Weigand M, Neumann F, et al. Perioperative intravenous lidocaine has preventive effect on postoperative pain and morphine consumption after major abdominal surgery. Anesth Analg 2004; 98: 1050–1055. [DOI] [PubMed] [Google Scholar]
- 14.Tufanogullari B, White PF, Peixoto MP, et al. Dexmedetomidine infusion during laparoscopic bariatric surgery: the effect on recovery outcome variables. Anesth Analg 2008; 106: 1741–1748. [DOI] [PubMed] [Google Scholar]
- 15.Deiner S, Silverstein JH. Postoperative delirium and cognitive dysfunction. Br J Anaesth 2009; 103(Suppl 1): i41–i46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Penney R. Use of dexmedetomidine and ketamine infusions during scoliosis repair surgery with somatosensory and motor-evoked potential monitoring: a case report. AANA J 2010; 78: 446–450. [PubMed] [Google Scholar]
- 17.Jeanne M, Logier R, De Jonckheere J, et al. Heart rate variability during total intravenous anesthesia: effects of nociception and analgesia. Auton Neurosci 2009; 147: 91–96. [DOI] [PubMed] [Google Scholar]
- 18.Ledowski T, Tiong WS, Lee C, et al. Analgesia nociception index: evaluation as a new parameter for acute postoperative pain. Br J Anaesth 2013; 111: 627–629. [DOI] [PubMed] [Google Scholar]
- 19.Kehlet H, Wilmore DW. Fast‐track surgery. Br J Surg 2005; 92: 3–4. [DOI] [PubMed] [Google Scholar]
- 20.Yardeni IZ, Beilin B, Mayburd E, et al. The effect of perioperative intravenous lidocaine on postoperative pain and immune function. Anesth Analg 2009; 109: 1464–1469. [DOI] [PubMed] [Google Scholar]
- 21.Logier R, Jeanne M, Dassonneville A, et al. PhysioDoloris: a monitoring device for analgesia/nociception balance evaluation using heart rate variability analysis. Proceedings of 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology 2010. IEEE; 1194–1197. [DOI] [PubMed]
- 22.Boselli E, Daniela-Ionescu M, Bégou G, et al. Prospective observational study of the non-invasive assessment of immediate postoperative pain using the analgesia/nociception index (ANI). Br J Anaesth 2013; 111: 453–459. [DOI] [PubMed] [Google Scholar]
- 23.Duta S, Karol MD, Cohen T, et al. Effect of dexmedetomidine on propofol requirements in healthy subjects. J Pham Sci 2001; 90: 172–181. [DOI] [PubMed] [Google Scholar]
- 24.Cheung CW, Qiu Q, Ying AC, et al. The effects of intra‐operative dexmedetomidine on postoperative pain, side‐effects and recovery in colorectal surgery. Anaesthesia 2014; 69: 1214–1221. [DOI] [PubMed] [Google Scholar]
- 25.Hofer RE, Sprung J, Sarr MG, et al. Anesthesia for a patient with morbid obesity using dexmedetomidine without narcotics. Can J Anesth 2005; 52: 176–180. [DOI] [PubMed] [Google Scholar]
- 26.Sadhasivam S, Boat A, Mahmoud M. Comparison of patient-controlled analgesia with and without dexmedetomidine following spine surgery in children. J Clin Anesth 2009; 21: 493–501. [DOI] [PubMed] [Google Scholar]
- 27.Feld JM, Hoffman WE, Stechert MM, et al. Fentanyl or dexmedetomidine combined with desflurane for bariatric surgery. J Clin Anesth 2006; 18: 24–28. [DOI] [PubMed] [Google Scholar]
- 28.Herroeder S, Pecher S, Schönherr ME, et al. Systemic lidocaine shortens length of hospital stay after colorectal surgery: a double-blinded, randomized, placebo-controlled trial. Ann Surg 2007; 246: 192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tikuišis R, Miliauskas P, Samalavičius NE, et al. Intravenous lidocaine for post-operative pain relief after hand-assisted laparoscopic colon surgery: a randomized, placebo-controlled clinical trial. Tech Coloproctol 2014; 18: 373–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hadžimešić M, Imamović S, Uljić V, et al. Cognitive function recovery rate in early postoperative period: comparison of propofol, sevoflurane and isoflurane anesthesia. J Health Sci 2013; 3: 48–54. [Google Scholar]
- 31.Takahiko T, Tomoaki Y, Mayuko H, et al. Dexmedetomidine maintains cognitive function in volunteers: 12AP3‐3. Eur J Anaesthesiol 2013; 30: 188. [Google Scholar]
- 32.Goodwin HE, Gill RS, Murakami PN, et al. Dexmedetomidine preserves attention/calculation when used for cooperative and short-term intensive care unit sedation. J Crit Care 2013; 28: 1113.e7–1113.e10. [DOI] [PubMed] [Google Scholar]
- 33.Carlson CL. Effectiveness of the World Health Organization cancer pain relief guidelines: an integrative review. J Pain Res 2016; 9: 515–534. doi: 10.2147/JPR.S97759. [DOI] [PMC free article] [PubMed] [Google Scholar]