Short abstract
Objective
Breast cancer has become the most common cancer in women in China, and the clinicopathological features differ from those in Western patients. This study was performed to investigate the distribution of programmed cell death protein 1 (PD-1)+/PD-1− tumor-infiltrating lymphocytes (TILs) and its association with clinicopathological features among Chinese patients with breast cancer.
Methods
In total, 133 consecutive patients with primary breast cancer were recruited into this cross-sectional study from 2012 to 2013. TILs were measured by cell counts under high-power fields (HPFs). Immunohistochemistry was used to detect PD-1 expression on tumor-infiltrating lymphocytes in the microenvironment.
Results
The median cell counts of the overall TILs, PD-1+ TILs, and PD-1− TILs were 80, 18, and 55/HPF, respectively. The number of PD-1− TILs was significantly lower in older than younger patients (50 vs. 60/HPF). Patients with positive E-cadherin expression had more PD-1− TILs than patients with negative E-cadherin expression (57 vs. 27/HPF). The Ki-67 index was positively correlated with the cell counts of PD-1+ TILs, and the correlation coefficient was 0.29.
Conclusions
PD-1 expression on TILs had different clinicopathological features in Chinese patients with breast cancer. E-Cadherin expression was associated with PD-1− TILs; however, Ki-67 expression was associated with PD-1+ TILs.
Keywords: Chinese breast cancer, E-cadherin, Ki-67, programmed cell death protein 1, tumor-infiltrating lymphocytes, immunohistochemistry
Introduction
Breast cancer (BC) is the most common cancer in women in China, and the estimated incidence rate in 2015 was 53.87/105 (185,585 new cases) and 40.14/105 (132,432 new cases) in urban and rural areas of China, respectively.1 Chinese women with BC have different clinicopathological features than Western women with BC: the expression rate of hormone receptors is lower and the expression rate of human epidermal growth factor receptor 2 is higher.2
Tumor-infiltrating lymphocytes (TILs) in the microenvironment reportedly affect cancer development, prognosis, and treatment efficacy. A high level of TILs promotes the efficacy of chemotherapy3 and is associated with a better prognosis of BC.4 Phenotypic TILs have particular biological functions and prognostic features. CD4+ and CD8+ T lymphocytes are effector T cells called helper and cytotoxic T cells. High numbers of CD4+ and CD8+ TILs lead to a better prognosis of BC.5 Programmed cell death protein 1 (PD-1) expressed on T cells and PD-1+ TILs confer inhibitory biological functions of effector T cells.6 PD-1 links to programmed cell death ligand 1 receptor (PD-L1) on the surface of cancer cells and initiates suppressive immune functions.7 The expression of PD-1 on T cells is considered “T cell exhaustion”8 and indicates the exhausted functions of lymphocytes. High levels of intratumoral PD-1+ TILs are correlated with worse survival in patients with BC.9 Therapies that block the PD-1/PD-L1 axis might reverse inhibitory properties and increase anti-cancer immunity. This study was performed to investigate the distribution of PD1+ and PD1− phenotypes of TILs and their relationship with clinicopathological characteristics to illustrate the particular microenvironment of BC in Chinese patients.
Patients and methods
Ethical approval
All procedures performed in this study involving human participants were approved by the ethics committee of Beijing Shijitan Hospital, Capital Medical University (Approval no. 2016062). The study was performed in accordance with the ethical standards of the 1964 Helsinki declaration and its later amendments.
Informed consent
Because of the retrospective nature of the study and the fact that some patients were lost to follow-up, the requirement for formal consent was waived.
Patients
Consecutive patients with a pathological diagnosis of primary invasive ductal BC at an operable stage were recruited into this cross-sectional study from 1 January 2012 to 31 December 2013. All patients underwent surgical treatment at the Department of Breast Surgery, Beijing Shijitan Hospital, Capital Medical University.
Tissue collection
Archival formalin-fixed, paraffin-embedded samples were collected from all patients. Slides of 4-μm thickness were used to determine histopathological features. Histological grading of BC was evaluated according to the Nottingham modification of the Bloom–Richardson system.
Immunohistochemistry
The expression status of PD-1, CK7, CK20, Ki-67, and E-cadherin (E-Cad) was evaluated by immunohistochemistry (IHC) on 4-μm-thick formalin-fixed, paraffin-embedded slides. Monoclonal antibodies to PD-1 (mouse anti-human, #UMAB199), CK7 (rabbit anti-human, #EP16), CK20 (rabbit anti-human, #EP23), Ki-67 (mouse anti-human, #MIB1), and E-Cad (mouse anti-human, #NCH-38) were purchased from Beijing Zhong Shan Golden Bridge Biotechnology Co., Ltd. (Beijing, China). Sections were baked at 60°C in a dehydration oven for 60 minutes, dewaxed for 20 minutes, and washed in graded alcohol of 100%, 100%, 95%, and 75% for 2 minutes, respectively. Sections were washed with phosphate-buffered saline (PBS) five times for 2 minutes each. Antigen retrieval was carried out using EnVision™ FLEX Target Retrieval Solution (Agilent, Santa Clara, CA, USA) for 2 minutes 30 s. The sections were then cooled to room temperature for 20 minutes, washed with PBS five times for 2 minutes each, incubated with 3% hydrogen peroxide for 15 minutes at room temperature, washed with PBS five times for 2 minutes each, and sealed with 5% serum at 37°C for 15 minutes; the liquid was then discarded. Finally, a moderate primary antibody was added, and the sections were left at 4°C overnight. They were then washed with PBS five times for 2 minutes each, DAB was added for 5 to 10 minutes, and AP/Red was added for 10 to 15 minutes. PD-1, CK7, CK20, Ki-67, and E-Cad detection was visualized with DAB. Slides were counterstained with hematoxylin.
A full assessment of the average number of TILs in the tumor area was conducted by pathologists. TILs were evaluated within the borders of the invasive tumor, excluding tumor zones with crush artifacts, necrosis, regressive hyalinization, and the previous core biopsy site. All mononuclear cells (including lymphocytes and plasma cells) were scored, but polymorphonuclear leukocytes were excluded. The average number of TILs was counted in 10 high-power fields (HPF, ×400) in IHC sections (4 µm).
Expression of PD-1 in the cytoplasm of lymphocytes, expression of CK7 and CK20 in the cytoplasm of BC cells, expression of Ki-67 in the nucleus of BC cells, and expression of E-Cad on the cytomembrane of BC cells are all shown by the color brown. We counted the number of PD-1-positive cells in 1000 TILs and calculated the expression rate. The Ki-67 index was estimated among 1000 BC cells. A Ki-67 index of >1% was defined as positive expression.
Statistical analysis
All analyses were conducted with SPSS Statistics for Windows, Version17.0 (SPSS Inc., Chicago, IL, USA). The median and interquartile range were used to describe the TIL counts. Age was transformed in a categorical scale by the median of 55 years. TIL phenotypes were compared by Wilcoxon tests among age, nerve invasion, vascular invasion, and axillary lymph node involvement groups. The association of TIL phenotypes with histological grade was estimated by Spearman correlation tests. Wilcoxon tests were used to estimate the difference in TILs phenotypes between positive and negative expression of CK7, CK20, and E-Cad. The Spearman correlation test was used to measure the relationship between the Ki-67 index and cell counts of TIL phenotypes. All analyses were two-sided, and the significance level was 0.05.
Results
In total, 133 patients were included in the present study. Their average age was 57.8 years (Table 1). A total of 11.3% of patients had a histological cancer diagnosis of grade I, 32.3% of patients had vascular invasion, 17.3% of patients had nerve invasion, and 54.2% of patients had axillary lymph node metastasis (Table 1). The average Ki-67 index was 30% (Table 1). The expression rate of E-Cad, CK20, and CK7 was 94.9%, 8.2%, and 86.8%, respectively (Table 1).
Table 1.
Patients’ characteristics
| Items | |
|---|---|
| Age, years | 57.8 ± 13.6 |
| Histological grade | |
| I | 14 (11.3) |
| II | 82 (66.1) |
| III | 28 (22.6) |
| Vascular invasion | |
| No | 86 (67.7) |
| Yes | 41 (32.3) |
| Nerve invasion | |
| No | 101 (82.1) |
| Yes | 22 (17.3) |
| Axillary lymph node metastasis | |
| No | 22 (45.8) |
| Yes | 26 (54.2) |
| E-Cadherin expression | |
| No | 6 (5.1) |
| Yes | 112 (94.9) |
| Ki-67 index | 30% ± 25% |
| CK20 expression | |
| No | 56 (91.8) |
| Yes | 5 (8.2) |
| CK7 expression | |
| No | 9 (13.2) |
| Yes | 59 (86.8) |
Data are presented as mean ± standard deviation or n (%)
Age was significantly associated with the TIL count. The median TIL count among patients of advanced age was 60/HPF, which was lower than that among young patients (80/HPF, p < 0.05) (Table 2). Age was not associated with the PD1+ TIL count; however, patients of advanced age tended to have lower PD-1− TIL counts than younger patients (50/HPF vs. 60/HPF, p < 0.05) (Table 2). Histological grade, vascular invasion, nerve invasion, and axillary lymph node metastasis were not significantly associated with phenotypes of TILs (Table 2).
Table 2.
Relationship between PD-1 TILs and clinical characteristics
| TILs | p | PD1+TILs | p | PD1−TILs | p | |
|---|---|---|---|---|---|---|
| Age, years* | ||||||
| ≤55 | 80 (70) | 0.024 | 26 (33) | 0.066 | 60 (54) | 0.048 |
| >55 | 60 (60) | 16 (24) | 50 (51) | |||
| Histological grade† | ||||||
| I | 60 (50) | 0.203 | 19 (28) | 0.17 | 41 (37) | 0.217 |
| II | 80 (73) | 17 (31) | 60 (60) | |||
| III | 70 (110) | 23 (51) | 51 (65) | |||
| Vascular invasion† | ||||||
| No | 65 (83) | 0.141 | 18 (34) | 0.716 | 50 (53) | 0.070 |
| Yes | 80 (60) | 15 (30) | 68 (44) | |||
| Nerve invasion,* | ||||||
| No | 80 (70) | 0.836 | 18 (30) | 0.652 | 56 (51) | 0.763 |
| Yes | 60 (130) | 23 (48) | 40 (82) | |||
| Axillary lymph node metastasis* | ||||||
| No | 100 (140) | 0.425 | 28 (28) | 0.562 | 79 (92) | 0.306 |
| Yes | 90 (88) | 21 (43) | 65 (49) | |||
Data are presented as median (interquartile range). *Wilcoxon test, †Spearman correlation test
PD-1, programmed cell death protein 1; TILs, tumor-infiltrating lymphocytes
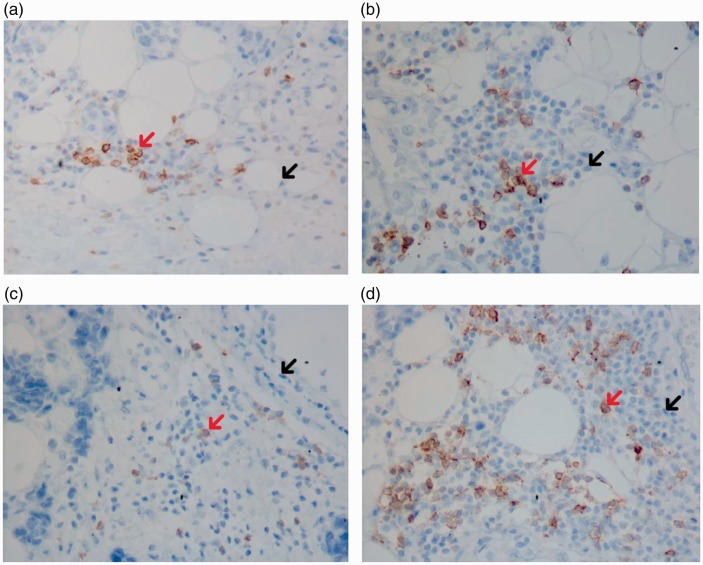
We also found no associations between the expression of CK7 and CK20 and the phenotypes of TILs (Table 3). E-Cad expression was positively correlated with PD-1− TILs: the median PD-1− TIL count was 27/HPF and 57/HPF among patients with negative and positive E-Cad expression, respectively (p < 0.05) (Table 2). On pathological slides, patients with negative E-Cad expression had fewer PD-1− TILs (Figure 1(a)) than patients with positive E-Cad expression (Figure 1(b)). The Ki-67 index was significantly associated with the TIL counts (correlation coefficient, 0.22; p < 0.05) (Table 3). The Ki-67 index was positively associated with the PD1+ TIL count but not the PD1− TIL counts (correlation coefficient, 0.29; p < 0.05) (Table 3). The PD-1+ TIL counts were lower among patients with negative Ki-67 expression (Figure 1(c)) than among patients with positive Ki-67 expression (Figure 1(d)).
Table 3.
Correlation between PD-1 TILs and other molecules in breast cancer
| TILs | p | PD1+TIL s | p | PD1−TILs | p | |
|---|---|---|---|---|---|---|
| CK7* | ||||||
| No | 90 (105) | 0.928 | 16 (56) | 0.562 | 70 (58) | 0.711 |
| Yes | 80 (110) | 25 (40) | 59 (71) | |||
| CK20* | ||||||
| No | 80 (113) | 0.130 | 24 (42) | 0.371 | 59 (72) | 0.087 |
| Yes | 130 (260) | 33 (71) | 98 (195) | |||
| E-Cad* | ||||||
| No | 45 (68) | 0.086 | 9 (34) | 0.265 | 27 (39) | 0.049 |
| Yes | 80 (80) | 19 (36) | 57 (63) | |||
| Ki67, correlation coefficient† | 0.22 | 0.016 | 0.29 | 0.001 | 0.16 | 0.076 |
Data are presented as mean (interquartile range)
PD-1, programmed cell death protein 1; TILs, tumor-infiltrating lymphocytes; E-Cad, E-cadherin. *Wilcoxon test, †Spearman correlation test
Figure 1.
Expression of E-Cad and Ki-67 and PD-1 TIL counts in different phenotypes. (a) Patients with negative E-Cad expression (IHC, ×400). (b) Patients with positive E-Cad expression (IHC, ×400). (c) Patients with negative Ki-67 expression (IHC, ×400). (d) Patients with positive Ki-67 expression (IHC, ×400). →PD-1+ TILs (red); →PD-1− TILs (black). PD-1+ TILs showed brown cytoplasm. Patients with negative E-Cad had fewer PD-1− TILs than those with positive E-Cad; patients with negative Ki-67 had fewer PD-1+ TILs than those with positive Ki-67. E-Cad, E-cadherin; PD-1, programmed cell death protein 1; TILs, tumor-infiltrating lymphocytes; IHC, immunohistochemistry
Discussion
TILs reflect autoimmunity and are composed of variable proportions of T cells, B cells, NK cells, and macrophages. Increased TIL counts are associated with improved overall survival in patients with epithelial ovarian carcinoma,10 colorectal cancer,11 endometrial cancer,12 and BC.13 TILs reportedly affect BC tumor biology and susceptibility to immunotherapy.14 A high amount of stromal and intratumoral TILs improve the chemotherapy sensitivity and provide a better prognosis.15,16
PD-1, a member of the CD28/CTLA-4 family of co-stimulatory receptors, transmits an inhibitory signal to T cells, suppresses immune responses, and contributes to immune tolerance and T-cell exhaustion.8,17 PD-1 is expressed on helper T cells, cytotoxic T cells, regulatory T cells, follicular T and B cells, and antigen-presenting cells including activated dendritic cells and monocytes.18 PD-1 expression is significantly higher on T cells infiltrating tumors than in normal tissue, but PD-1+ TILs display impaired effector function.19 The PD-1/PD-L1 signaling pathway maintains an immunosuppressive tumor microenvironment and attenuates antitumor immunity. Higher levels of PD-1+ TILs are associated with a poorer prognosis in patients with BC.9 Besides the prognosis, PD-1 polymorphism also affects the risk of BC in the Han Chinese population.20 Young patients tend to have higher levels of PD-1− TILs, reflecting the better immune system of young patients.
Ki-67 is a proliferative cell nuclear antigen, and the Ki-67 index is correlated with the cellular mitotic cycle. Ki-67 is expressed in all phases except G0 and early G1 and peaks in the M phase.21 The Ki-67 index is an indicator of malignant proliferation activity and an independent prognosticator for BC recurrence and survival.22,23 PD-1− TILs produce higher amounts of interleukin-2 and interferon-γ than PD-1+ TILs.19 PD-1− TILs seem to have higher immune activity than PD-1+ TILs. PD-1 expression on effector T cells exhausts antitumor immunity and compromises the control of tumor growth.24 PD-1 signaling affects tumor chemoresistance and metastasis.25 A high Ki-67 index indicates greater proliferative activity of cancer cells and might be related to the exhausted microenvironment as indicated by the high PD-1+ TIL counts.
E-Cad is responsible for calcium-mediated cell-to-cell adhesion.26 E-Cad promotes intercellular adhesion between homogeneous cells and inhibits the infiltration and metastasis of tumor cells.27 Down-regulation of E-Cad escalates exacerbation of the tumor grade and stage and the transition from adenoma to carcinoma.28 E-Cad is a marker of the epithelial phenotype, and down-regulation of E-Cad is a marker of epithelial–mesenchymal transition (EMT).29 In esophageal cancer cell lines, PD-L1 expression and its association with PD-1 are responsible for a more pronounced EMT phenotype.30 EMT phenotypes of lung adenocarcinoma have a higher number of PD-1+ TILs than do EMT negative phenotypes.31 We found that patients with BC with E-Cad expression had more PD-1− TILs and an apparently more active immune microenvironment than the patients with negative E-Cad expression. The BRAF-MAPK pathway reportedly activates the EMT process, triggers immunosuppressive cascades, and produces immunosuppressive molecules.32 EMT activation is associated with decreased E-Cad expression.32 Patients with BC have a detectable level of E-Cad in the peripheral blood.33 Down-regulation of E-Cad is positively correlated with the immunosuppressive status.32 High expression of E-Cad might be related to active immune function and increased PD-1− TIL counts.
The small sample size was a limitation of this study. The fact that stromal and intratumoral TILs were analyzed together is another limitation. We calculated the TIL counts by IHC, not flow cytometry.
An increased number of PD-1+ TILs is associated with higher proliferative activity of BC, and an increased number of PD-1- TILs is associated with an epithelial phenotype of BC. PD-1/PD-L1 blockade is a potential strategy for BC treatment, and further studies are warranted.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This study was financially supported by Beijing Municipal Administration of Hospitals (Q.K.S. grant number PX2018029), Beijing Shijitan Hospital, Capital Medical University (Q.K.S. grant number 2017-QB03), Beijing Key Laboratory of Cancer Therapeutic Vaccine (F.S. grant number 2017-KF01), Beijing Municipal Commission of Health and Family Planning (Q.K.S. grant number 2015-3-057), and Ministry of Railway (Q.K.S. grant number J2017Z604). The supporting organizations had no role in the study design or data collection, analysis, and interpretation.
References
- 1.Wang Q, Jiao J. Health disparity and cancer health disparity in China. Asia Pac J Oncol Nurs 2016; 3: 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng S, Bai JQ, Li J, et al. The pathologic characteristics of breast cancer in China and its shift during 1999-2008: a national-wide multicenter cross-sectional image over 10 years. Int J Cancer 2012; 131: 2622–2631. [DOI] [PubMed] [Google Scholar]
- 3.García-Martínez E, Gil GL, Benito AC, et al. Tumor-infiltrating immune cell profiles and their change after neoadjuvant chemotherapy predict response and prognosis of breast cancer. Breast Cancer Res 2014; 16: 488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gajewski TF Schreiber H, andFu YX.. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol 2013; 14: 1014–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seo AN, Lee HJ, Kim EJ, et al. Tumour-infiltrating CD8+ lymphocytes as an independent predictive factor for pathological complete response to primary systemic therapy in breast cancer. Br J Cancer 2013; 109: 2705–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chamoto K, Al-Habsi M, Honjo T. Role of PD-1 in immunity and diseases. Curr Top Microbiol Immunol 2017; 410: 75–97. [DOI] [PubMed] [Google Scholar]
- 7.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002; 8: 793–800. [DOI] [PubMed] [Google Scholar]
- 8.Flies DB, Sandler BJ, Sznol M, et al. Blockade of the B7-H1/PD-1 pathway for cancer immunotherapy. Yale J Biol Med 2011; 84: 409–421. [PMC free article] [PubMed] [Google Scholar]
- 9.Muenst S, Soysal SD, Gao F, et al. The presence of programmed death 1 (PD-1)-positive tumor-infiltrating lymphocytes is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat 2013; 139: 667–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomsová M, Melichar B, Sedláková I, et al. Prognostic significance of CD3+ tumor-infiltrating lymphocytes in ovarian carcinoma. Gynecol Oncol 2008; 108: 415–420. [DOI] [PubMed] [Google Scholar]
- 11.Mei Z, Liu Y, Liu C, et al. Tumour-infiltrating inflammation and prognosis in colorectal cancer: systematic review and meta-analysis. Br J Cancer 2014; 110: 1595–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bear HD, Anderson S, Brown A, et al. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from national surgical adjuvant breast and bowel project protocol B-27. J Clin Oncol 2003; 21: 4165–4174. [DOI] [PubMed] [Google Scholar]
- 13.Ismael G, Hegg R, Muehlbauer S, et al. Subcutaneous versus intravenous administration of (neo)adjuvant trastuzumab in patients with HER2-positive, clinical stage I-III breast cancer (HannaH study): a phase 3, open-label, multicentre, randomised trial. Lancet Oncol 2012; 13: 869–878. [DOI] [PubMed] [Google Scholar]
- 14.Salgado R, Denkert C, Demaria S, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs working group 2014. Ann Oncol 2015; 26: 259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma Y, Mattarollo SR, Adjemian S, et al. CCL2/CCR2-dependent recruitment of functional antigen-presenting cells into tumors upon chemotherapy. Cancer Res 2014; 74: 436–445. [DOI] [PubMed] [Google Scholar]
- 16.Alizadeh D, Larmonier N. Chemotherapeutic targeting of cancer-induced immunosuppressive cells. Cancer Res 2014; 74: 2663–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bour-Jordan H, Esensten JH, Martinez-Llordella M, et al. Intrinsic and extrinsic control of peripheral T-cell tolerance by costimulatory molecules of the CD28/ B7 family. Immunol Rev 2011; 241: 180–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yao S, Chen L. PD-1 as an immune modulatory receptor. Cancer J 2014; 20: 262–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmadzadeh M, Johnson LA, Heemskerk B, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood 2009; 114: 1537–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hua Z, Li D, Xiang G, et al. PD-1 polymorphisms are associated with sporadic breast cancer in Chinese Han population of Northeast China. Breast Cancer Res Treat 2011; 129: 195–201. [DOI] [PubMed] [Google Scholar]
- 21.MacCallum DE, Hall PA. The location of pKi67 in the outer dense fibrillary compartment of the nucleolus points to a role in ribosome biogenesis during the cell division cycle. J Pathol 2000; 190: 537–544. [DOI] [PubMed] [Google Scholar]
- 22.Yerushalmi R, Woods R, Ravdin PM, et al. Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol 2010; 11: 174–183. [DOI] [PubMed] [Google Scholar]
- 23.Inwald EC, Klinkhammer-Schalke M, Hofstädter F, et al. Ki-67 is a prognostic parameter in breast cancer patients: results of a large population-based cohort of a cancer registry. Breast Cancer Res Treat 2013; 139: 539–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu X, Gibbons RM, Harrington SM, et al. Endogenous tumor-reactive CD8+T cells are differentiated effector cells expressing high levels of CD11a and PD-1 but are unable to control tumor growth. Oncoimmunology 2013; 2: e23972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Black M, Barsoum IB, Truesdell P, et al. Activation of the PD-1/PD-L1 immune checkpoint confers tumor cell chemoresistance associated with increased metastasis. Oncotarget 2016; 7: 10557–10567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, Zhao J, Zhang PY, et al. MicroRNA-10b targets E-cadherin and modulates breast cancer metastasis. Med Sci Monit 2012; 18: BR299–BR308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adhikary A, Chakraborty S, Mazumdar M, et al. Inhibition of epithelial to mesenchymal transition by E-cadherin up-regulation via repression of slug transcription and inhibition of E-cadherin degradation: dual role of scaffold/matrix attachment region-binding protein 1 (SMAR1) in breast cancer cells. J Biol Chem 2014; 289: 25431–25444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ye X, Weinberg RA. Epithelial-mesenchymal plasticity: a central regulator of cancer progression. Trends Cell Biol 2015; 25: 675–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng G, Lyons JG, Tan TK, et al. Disruption of E-cadherin by matrix metalloproteinase directly mediates epithelial-mesenchymal transition downstream of transforming growth factor-beta1 in renal tubular epithelial cells. Am J Pathol 2009; 175: 580–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen L, Xiong Y, Li J, et al. PD-L1 expression promotes epithelial to mesenchymal transition in human esophageal cancer. Cell Physiol Biochem 2017; 42: 2267–2280. [DOI] [PubMed] [Google Scholar]
- 31.Kim S, Koh J, Kim MY, et al. PD-L1 expression is associated with epithelial-to-mesenchymal transition in adenocarcinoma of the lung. Hum Pathol 2016; 58: 7–14. [DOI] [PubMed] [Google Scholar]
- 32.Vergara D, Simeone P, Franck J, et al. Translating epithelial mesenchymal transition markers into the clinic: novel insights from proteomics. EuPA Open Proteom 2016; 10: 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vergara D, Bianco M, Pagano R, et al. An SPR based immunoassay for the sensitive detection of the soluble epithelial marker E-cadherin. Nanomedicine. 2018; 14: 1963–1971. [DOI] [PubMed] [Google Scholar]