Short abstract
Vasopressin is a locally-injected vasoconstrictor used to reduce bleeding during gynaecological surgery. However, even in these cases, vasopressin can induce adverse effects, including bradycardia, myocardial infarction and cardiac arrest. Elevated blood concentrations of vasopressin may induce the sympathoinhibitory reflex by increasing blood pressure and augment the sympathoinhibitory reflex by activating the area postrema. In addition, pneumoperitoneum formation needed for laparoscopy as well as physiological changes caused by steep Trendelenburg positions used during robotic surgeries may cause bradycardia. Shoulder braces used to prevent slipping from a steep Trendelenburg position may also be hazardous. This case report describes a 31-year-old female patient who underwent a scheduled robotic-assisted laparoscopic myomectomy in a steep Trendelenburg position. The patient experienced a cardiac arrest 2 min after the vasopressin injection and was treated accordingly. There were no abnormal findings on the postoperative laboratory studies, chest X-ray and electrocardiogram. The patient also had clear consciousness with no other notable symptoms. The patient was discharged on postoperative day 2. The report discusses the potential adverse effects of local vasopressin injection during robotic-assisted laparoscopic myomectomy.
Keywords: Bradycardia, cardiac arrest, laparoscopy, myomectomy, robotics, vasopressin
Introduction
Vasopressin increases blood pressure by contracting the smooth muscles of blood vessels. It can be used in patients with hypotension unresponsive to catecholamine, as well as in cases of shock and cardiopulmonary resuscitation.1 In obstetrics and gynaecology, local vasopressin injections are used to reduce bleeding during myomectomy, excision of ovarian endometriomas and conization.2 However, a high concentration of vasopressin in the blood can induce gastrointestinal and myocardial ischaemia and, in severe cases, myocardial infarction or cardiac arrest.1,2 There have also been reports of bradycardia, anaphylaxis and bronchospasm.1,2 Bradycardia could also be induced by the Trendelenburg position during robotic surgery.3,4 This current report describes a case of cardiac arrest following severe bradycardia caused by local intramyometrial injection of vasopressin in a patient with uterine leiomyoma undergoing robotic-assisted laparoscopic myomectomy and reviews the relevant literature.
Case report
A 31-year-old female patient (height 163 cm, weight 60 kg) was scheduled for a robotic-assisted laparoscopic myomectomy on 11 August 2017 after being diagnosed with uterine leiomyoma. A myomectomy was scheduled because she had a history of uterine leiomyoma that was discovered 4 years earlier and had grown to 8 cm. She was unmarried and nulligravida. She had no notable medical history. Preoperative laboratory studies and chest X-ray did not reveal any abnormalities. An electrocardiogram (ECG) showed a normal sinus rhythm. Vital signs measured on the ward before surgery were blood pressure (BP) 110/70 mmHg, heart rate (HR) 76 beats per minute (bpm), body temperature 36.9°C and respiration rate 20 breaths/min.
The patient was transferred to the operating room and monitored with noninvasive blood pressure monitoring equipment, three-lead continuous electrocardiography, pulse oximetry and bispectral index. Before induction, the patient’s vital signs were BP 141/82 mmHg, HR 56 bpm, body temperature 36.5°C, respiration rate 13 breaths/min and oxygen saturation 100%. Anaesthesia was induced with 120 mg propofol and 40 mg rocuronium. A 7.0-mm endotracheal tube was inserted without difficulty. Anaesthesia was maintained with sevoflurane 2–3 vol%, air 2.0 l/min, O2 1.0 l/min and a continuous infusion of 0.05 µg/kg per min remifentanil. After skin incision, CO2 was insufflated into the abdomen while maintaining an intraabdominal pressure <12 mmHg. Mechanical ventilation was set to the pressure-controlled mode with a peak airway pressure of 20 cmH2O and a respiration rate of 15 breaths/min. The patient was placed into a 30° Trendelenburg position to install the robotic device. At the time, vital signs were stable, with BP 152/108 mmHg, HR 68 bpm and oxygen saturation 100%. Vasopressin was diluted in 0.9% normal saline to achieve a concentration of 0.5 U/ml in two 20-ml syringes. After the robotic device was installed, a 15-gauge injection cannula was used to confirm that no blood was aspirated from the leiomyoma. All of the diluted vasopressin (40 ml) was injected into the leiomyoma. Two minutes after injecting the vasopressin, the patient’s HR dropped to 29 bpm. Soon after, the heart stopped beating. The ECG showed a flat line and pulse oximetry also showed a flat plethysmographic waveform. The administration of sevoflurane and remifentanil was stopped immediately and the patient was ventilated with 100% oxygen. The patient was then injected with 0.5 mg atropine with a full drop of fluids. The surgery was also paused, the robotic device removed, and the patient was moved back into a supine position. At 40 s after the atropine injection, the patient’s heart began beating again, and HR returned to 28 bpm. The patient’s HR gradually recovered further, so no additional treatment was given. After 1 min, the HR recovered to 61 bpm, with a BP of 126/89 mmHg and oxygen saturation of 100%. There were no changes of ST segment on the ECG. BP at the time of the cardiac arrest was not measured because the noninvasive BP measurement interval was set to 5 min. After confirming that the vital signs had stabilized, anaesthesia was restarted and surgery was resumed. The total duration of anaesthesia was 5 h and 3 min; and the total length of surgery was 3 h and 54 min. Total blood loss was approximately 300 ml and urine output was 480 ml. To reverse the neuromuscular blockade, 0.4 mg glycopyrrolate and 15 mg pyridostigmine were administered intravenously. The endotracheal tube was removed after confirming that the patient had recovered spontaneous breathing and consciousness. The patient’s vital signs after arriving at the recovery room were BP 132/99 mmHg, HR 93 bpm, body temperature 36.2°C and respiration rate 17 breaths/min. The patient was transferred to the ward after being observed for 1 h in the recovery room.
There were no abnormal findings on the postoperative laboratory studies, chest X-ray and ECG. The patient also had clear consciousness with no other notable symptoms. The patient was discharged on postoperative day 2.
Discussion
Uterine leiomyomas are common benign uterine tumours in women. In most cases, they are asymptomatic, discovered by chance during routine health examinations and do not require treatment. Patients who show symptoms such as abnormal uterine bleeding, pain, miscarriage and infertility are first treated nonsurgically. Surgery may be considered if the leiomyoma is unresponsive to nonsurgical treatment or if it is large and causes discomfort. A myomectomy or hysterectomy can be performed in these cases, and the type of surgery is chosen on the basis of the patient age and preference.5 Uterine arteries and ovarian arteries supply blood to the uterus. Arcuate arteries, which are formed from the anastomosis of the two arteries, run along the uterine wall and supply blood to the myometrium. When a uterine leiomyoma develops, the normal vasculature is altered, and the arcuate arteries run in various directions. For this reason, myomectomy causes vascular damage regardless of the direction of the incision and results in heavy blood loss.2,5 To reduce bleeding, vasopressin is injected locally. This is also helpful for securing a good surgical field of view and decreasing the need for a blood transfusion. In addition, the low risk of ischaemic uterine injury or thromboembolism contributes to the wide use of vasopressin for gynaecological surgeries.2,6
Vasopressin is a peptide hormone consisting of nine amino acids that is produced in the hypothalamus and is stored and released by the posterior pituitary gland. The normal plasma concentration of vasopressin is <4 pg/ml. Vasopressin is secreted in response to stimuli such as hyperosmotic plasma, hypotension and hypovolaemia, and it has a half-life of 10–35 min.1 During anaesthesia, vasopressin is used to increase blood pressure by elevating vascular tension in cases wherein plasma vasopressin concentrations are relatively low, including in cases of hypotension unresponsive to catecholamine, septic shock, vasodilatory shock and haemorrhagic shock.1 Vasopressin receptors are classified into V1, V2, and V3.1 V1 and V2 receptors are distributed in the periphery, whereas V1 and V3 receptors are found in the central nervous system.1 Because the uterus has V1 receptors, local injection can induce not only vasoconstriction but also uterine contraction.1 In addition, because vasopressin has a similar affinity for oxytocin receptors as oxytocin, local injection of vasopressin is used during gynaecological surgeries for uterine contraction.1,7 However, a high concentration of vasopressin in the blood can cause adverse events, such as bradycardia, myocardial ischaemia, myocardial infarction and cardiac arrest.1,2 Cardiac arrest due to local injection of vasopressin can be described as follows. Local injection of vasopressin into the uterine leiomyoma results in an increased vasopressin concentration in the blood, which in turn causes vasoconstriction and an increase in blood pressure. A sudden increase in blood pressure activates the baroreceptors in the aortic arch and carotid sinus, which induce the sympathoinhibitory reflex in the nucleus tractus solitarius, thereby lowering cardiac contractibility and heart rate and, in severe cases, causing cardiac arrest.1,2,7–9 Neurons in the area postrema are connected to the nucleus tractus solitarius. Vasopressin in the blood binds to the V1 receptors in the area postrema and augments reflex sympathoinhibition in the nucleus tractus solitarius.7,10 Ideally, up to 50 ml of 0.1 U/ml vasopressin could be used for myomectomy, but vasopressin can be diluted to below 1 U/ml depending on the use. However, the total dose of vasopressin reported to have been used in bradycardia or cardiac arrest cases exceeded 5 U, which suggests that the vasopressin concentration should not exceed that limit.2 In this current case, vasopressin was prepared at a concentration of 0.5 U/ml and blood vessel injection via aspiration was avoided. However, it is possible that rapidly injecting 20 U of vasopressin using a 15-gauge injection cannula may have resulted in vasopressin being absorbed into the blood vessels, as the uterine leiomyoma had a rich supply of blood vessels. In addition, the cardiovascular incident occurred 2 min after injecting the vasopressin, further suggesting that the cardiac arrest occurred as a result of an elevated vasopressin concentration in the blood.
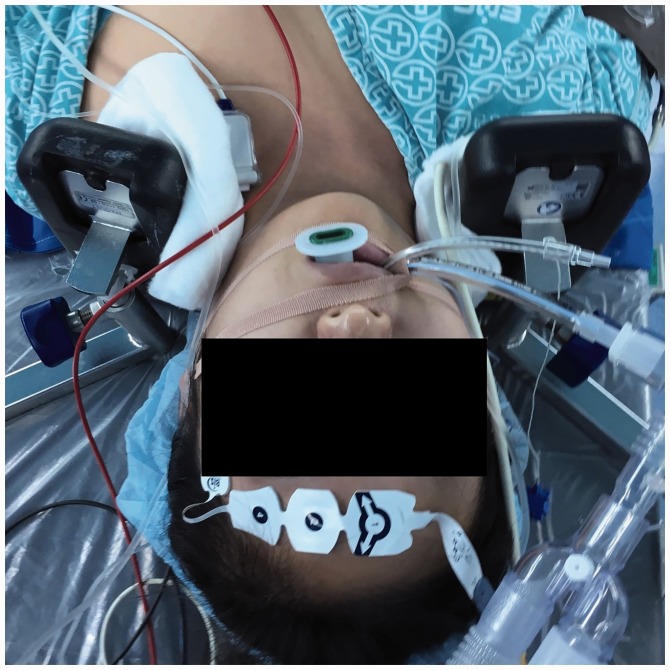
There are other risk factors for cardiac arrest. CO2 is insufflated when forming the pneumoperitoneum. When intraabdominal pressure rises because of CO2 insufflation, the peritoneum stretches, which induces the vagal-mediated cardiovascular reflex, resulting in bradycardia and cardiac arrest.11,12 CO2 should be insufflated while maintaining an intraabdominal pressure below 12–15 mmHg. However, even if the peak pressure of the CO2 insufflator is set to 12 mmHg, high flow insufflations may cause the peak pressure to exceed the setting and induce vagal responses.12 Owing to the nature of robotic-assisted laparoscopic surgery, the patient must be in a steep Trendelenburg position to install the device, and intrapelvic structures are exposed during surgery.13,14 However, venous return increases immediately after changing to the Trendelenburg position. This increases stroke volume, and cardiac output then activates baroreceptors, which induces the baroreflex and may lower the heart rate by diminishing sympathetic activity. The reduced HR is recovered to the baseline HR approximately 30 min after changing to the Trendelenburg position.3,4 Moreover, in a steep Trendelenburg position, pulmonary compliance and functional residual capacity are decreased while peak inspiratory pressure is increased, making ventilation difficult and causing hypercapnia.14 Further, CO2 absorbed from the pneumoperitoneum may also cause hypercapnia, which in turn increases the risk for arrhythmia.11,14 Because the trocar and other devices are fixed onto the patient during robotic-assisted surgeries, a change of patient position causes the incision site to be torn or makes it difficult to proceed with the surgery. Thus, the patient is braced on the shoulder or fixed with a string or antiskid foam material in the steep Trendelenburg position so that the patient does not slide down.13,14 In the current case, braces were placed on both shoulders to prevent sliding and pads were added to prevent pressure injury. However, the braces and pads may have pressed down on the patient’s neck (Figure 1). Although sufficient space between the braces and the neck was requested prior to surgery, if this had not been achieved, then the braces may have compressed the carotid sinus and lowered sympathetic activity, thereby causing a decrease in HR.8
Figure 1.
In this case of a 31-year-old female patient who experienced a cardiac arrest during a scheduled robotic-assisted laparoscopic myomectomy in a steep Trendelenburg position, the space between the braces and the neck might have been insufficient to prevent compression of the carotid sinus and lowered sympathetic activity.
In conclusion, this current case report describes a case of cardiac arrest after a local injection of vasopressin while maintaining pneumoperitoneum in a steep Trendelenburg position. Bradycardia may have resulted from a compressed carotid sinus. Fortunately, the patient’s heart rate recovered while disassembling the robotic device and returning the patient to the supine position, and cardiopulmonary resuscitation was not performed. Surgeons should note that even when vasopressin is locally injected, some absorption into the blood may cause adverse events. They should ensure that vasopressin solutions are diluted well and slowly injected using a small gauge needle. Furthermore, interdepartmental communication is important, as the use of vasopressin may vary across medical departments. Anaesthesiologists should be aware of the physiological changes that occur in a steep Trendelenburg position and prepare for potential problems that may occur, noting that approach may be difficult in such situations. In addition, carefully observing the patient’s state before surgery is important.
Declaration of conflicting interests
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Park KS, Yoo KY. Role of vasopressin in current anesthetic practice. Korean J Anesthesiol 2017; 70: 245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeqja E, Qirko R. Vasopressine in laparoscopic myomectomy, a review over the effectiveness, dosage and possible complications. IOSR J Pharm 2016; 6: 23–27. [Google Scholar]
- 3.Oksar M, Akbulut Z, Ocal H, et al. Anesthetic considerations for robotic cystectomy: a prospective study. Braz J Anesthesiol 2014; 64: 109–115. [DOI] [PubMed] [Google Scholar]
- 4.Lowenstein L, Mustafa M, Burke YZ, et al. Steep Trendelenburg position during robotic sacrocolpopexy and heart rate variability. Eur J Obstet Gynecol Reprod Biol 2014; 178: 66–69. [DOI] [PubMed] [Google Scholar]
- 5.Hillard PJA. Benign diseases of the female reproductive tract In: Berek JS. (ed) Berek & Novak’s gynecology. 14th ed Philadelphia: Lippincott Williams & Wilkins, 2007, pp.479–481. [Google Scholar]
- 6.Saha MM, Khushboo, Biswas SC, et al. Assessment of blood loss in abdominal myomectomy by intramyometrial vasopressin administration versus conventional tourniquet application. J Clin Diagn Res 2016; 10: QC10–QC13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koshimizu TA, Nakamura K, Egashira N, et al. Vasopressin V1a and V1b receptors: from molecules to physiological systems. Physiol Rev 2012; 92: 1813–1864. [DOI] [PubMed] [Google Scholar]
- 8.Sun LS, Schwarzenberger J, Dinavahi R. Cardiac physiology In: Miller RD. (ed) Miller’s anesthesia. 8th ed Philadelphia: Elsevier, 2015, p.489. [Google Scholar]
- 9.Shinn HK, Song JH, Han JW, et al. Cardiac arrest caused by intramyometrial infiltration of vasopressin during dilation and curettage under general anesthesia: a case report. Korean J Anesthesiol 2007; 53: 664–667. [Google Scholar]
- 10.Hasser EM, Cunningham JT, Sullivan MJ, et al. Area postrema and sympathetic nervous system effects of vasopressin and angiotensin II. Clin Exp Pharmacol Physiol 2000; 27: 432–436. [DOI] [PubMed] [Google Scholar]
- 11.Aghamohammadi H, Mehrabi S, Mohammad Ali Beigi F. Prevention of bradycardia by atropine sulfate during urological laparoscopic surgery: a randomized controlled trial. Urol J 2009; 6: 92–95. [PubMed] [Google Scholar]
- 12.Jung KT, Kim SH, Kim JW, et al. Bradycardia during laparoscopic surgery due to high flow rate of CO2 insufflation. Korean J Anesthesiol 2013; 65: 276–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klauschie J, Wechter ME, Jacob K, et al. Use of anti-skid material and patient-positioning to prevent patient shifting during robotic-assisted gynecologic procedures. J Minim Invasive Gynecol 2010; 17: 504–507. [DOI] [PubMed] [Google Scholar]
- 14.Kaye AD, Vadivelu N, Ahuja N, et al. Anesthetic considerations in robotic-assisted gynecologic surgery. Ochsner J 2013; 13: 517–524. [PMC free article] [PubMed] [Google Scholar]