Short abstract
Objective
To evaluate the association between serum copper levels and lung cancer risk.
Methods
We searched the electronic PubMed, WanFang, CNKI, and SinoMed databases to identify studies including information on serum copper levels and lung cancer. Standard mean differences and corresponding 95% confidence intervals were calculated using Stata 12.0 software. We performed a meta-analysis on the identified studies overall and according to geographic location. We also evaluated heterogeneity among the studies and the occurrence of publication bias.
Results
Thirty-three articles including 3026 cases and 9439 controls were included in our study. The combined results showed that serum copper levels were higher in patients with lung cancer compared with controls without lung cancer, though the results showed high heterogeneity. In a subgroup analysis according to geographic location, significant associations between copper levels and lung cancer were found for both Asian and European populations. No publication bias was detected in this meta-analysis.
Conclusions
High serum copper levels could increase the risk of lung cancer, suggesting that environmental copper exposure may be a risk factor for the development of lung cancer.
Keywords: Serum, copper level, lung cancer, meta-analysis, cancer risk, copper exposure
Introduction
Lung cancer results from the uncontrolled growth of lung tissue cells, which may also cause metastasis.1 Lung cancer is the leading cause of cancer-related death, both in China and worldwide, with 1- and 5-year survival rates of only 42% and 15%, respectively.2 Lung cancer is reportedly the most common cancer among men and women, representing huge social and economic burdens in both developed and developing countries.3 Although antioxidant vitamins and photochemicals have shown protective trends, the roles of trace metals in lung cancer risk remain poorly studied.4,5
Copper is an essential trace metal that plays a key role in maintaining DNA integrity through avoiding oxidative DNA damage or affecting gene mutations.6,7 However, although some studies have reported higher serum copper levels in patients with lung cancer compared with controls,8–10 others found no significant association11,12 or indeed a converse association.13 The effect of serum copper levels on lung cancer risk thus remains controversial. We conducted a meta-analysis to determine the relationship between serum copper levels and lung cancer, and evaluated potential heterogeneities among previous studies.
Methods
Study selection
We performed a comprehensive search of the literature for studies examining serum copper levels and lung cancer up to April 1st, 2018. The PubMed, WanFang, CNKI, and SinoMed databases were searched using the terms “copper concentration” or “copper levels” or “copper” or “Cu” or “trace element” in combination with “lung cancer” or “lung tumor”. Furthermore, references in the relevant articles were also searched to identify other eligible articles.
Inclusion and exclusion criteria
Two investigators (XPZ and QY) independently searched and reviewed articles for eligibility using the following inclusion criteria: 1) studies focusing on patients with lung cancer; 2) observational studies; 3) numbers, mean and standard deviation of serum copper levels for cases and controls available; 4) studies on humans; and 5) studies published in English or Chinese.
Data extraction
Two of the authors (XPZ and QY) independently extracted the following data from the included studies and recorded it in a spreadsheet: 1) first author’s name; 2) publication year; 3) study design; 4) country; 5) number of cases and controls; 6) sex of cases; 7) age; 8) mean and standard deviation of serum copper levels in cases and control; and 9) serum determination method. Other relevant data were also extracted from individual studies.
Statistical analysis
The meta-analysis was carried out using Stata 12.0 software (StataCorp, College Station, TX, USA). Continuous outcomes between serum copper levels and lung cancer were evaluated by calculating the standard mean deviation (SMD) and 95% confidence interval (CI).14 We performed meta-analyses on the identified studies overall and also carried out a subanalysis according to geographic location. The copper concentration in the serum was converted into µmol/L for all studies. Statistical heterogeneity was assessed based on Q and I2 tests.15 The results were combined using a random-effects model. The high between-study heterogeneity was explored by meta-regression analysis.16 Publication bias was evaluated by visual investigation of Begg’s filled funnel plots17 and Egger’s regression asymmetry test.18
Results
Search results and characteristics
The initial screening identified 87, 20, 87, and 79 articles from the PubMed, WanFang, CNKI, and SinoMed databases, respectively. Two additional records were identified through other sources. Figure 1 shows a flow diagram of the study. A total of 33 articles8–13,19–45 involving 3026 lung cancer patients and 9439 controls was finally considered suitable for this study. The characteristics of each study are shown in Table 1.
Figure 1.
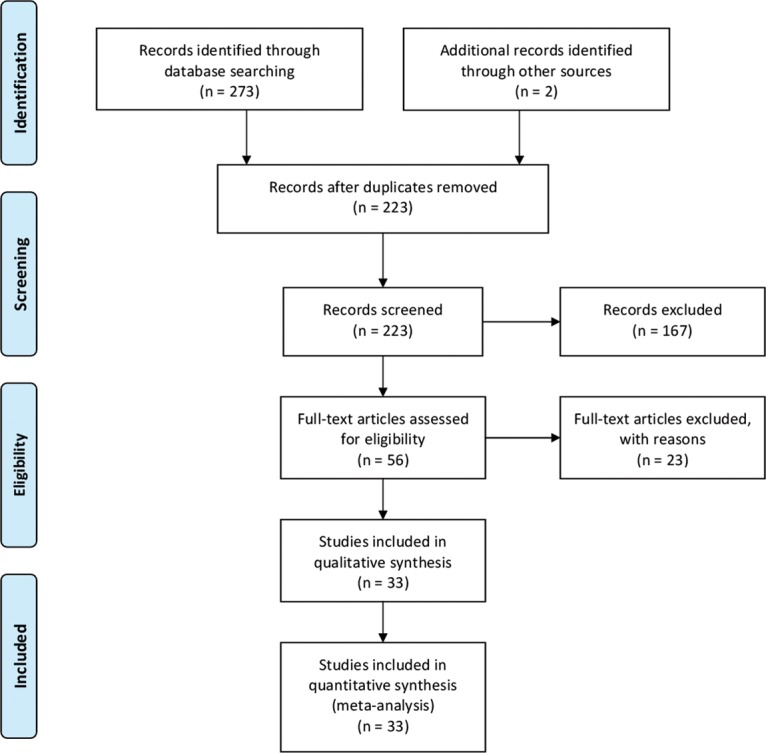
Study selection process for this meta-analysis
Table 1.
Characteristics of all included studies
| Study, year [ref] | Country | Age (year) (range or mean ± SD) | Study type |
Lung cancer cases |
Controls |
Method of copper measurement | |||
|---|---|---|---|---|---|---|---|---|---|
| n | Female (%) | Copper: mean ± SD | n | Copper: mean ± SD | |||||
| Sun et al. 1991 [8] | China | 30–75 | Case-control | 91 | 0.00 | 1.267 ± 0.278 (µg/mL) | 138 | 0.921 ± 0.198 (µg/mL) | AAS (IL-951, USA) |
| Sun et al. 1991 [8] | China | 30–75 | Case-control | 13 | 100.00 | 1.468 ± 0.416 (µg/mL) | 114 | 1.111 ± 0.324 (µg/mL) | AAS (IL-951, USA) |
| Cobanoglu et al. 2010 [13] | Turkey | 54 ± 8.29 | Case-control | 30 | 33.33 | 0.977 ± 0.316 (µg/dL) | 20 | 1.748 ± 0.198 (µg/dL) | UNICAM-929 spectrophotometer (Unicam Ltd., Cambridge, UK) |
| Diez et al. 1989 [19] | Spain | 60 ± 7 | Case-control | 64 | 7.81 | 1.4 ± 0.316 (µg/mL) | 100 | 1 ± 0.182 (µg/mL) | AAS (Perkin-Elmer 5.000) |
| Huhti et al. 1980 [20] | Finland | 37–80 | Case-control | 149 | 5.37 | 1.42 ± 0.3 (mg/L) | 19 | 1.03 ± 0.26 (mg/L) | AAS (Perkin-Elmer Model 303) |
| Jin et al. 2011 [9] | China | 34.9 ± 21.3 | Case-control | 154 | 10.39 | 1.624 ± 0.818 (µg/mL) | 154 | 1.285 ± 0.524 (µg/mL) | AAS (Wako Pure Chemical Industries, Osaka, Japan) |
| Oyama et al. 1994 [21] | Japan | 26–83 | Case-control | 109 | 34.86 | 122.9 ± 3.77 (µg/dL) | 53 | 109.5 ± 5.39 (µg/dL) | AAS (Wako Pure Chemical Industries, Osaka, Japan) |
| Zowczak et al. 2001 [22] | Poland | 42–87 | Case-control | 14 | 14.29 | 22.9 ± 6.2 (µmol/L) | 18 | 15 ± 1.5 (µmol/L) | Flame AAS (Perkin Elmer) |
| Feng et al. 2006 [23] | China | 18–82 | Observation trials | 13 | NA | 19 ± 2.36 (µmol/L) | 36 | 14.92 ± 2.71 (µmol/L) | Flame AAS |
| Zhang et al. 1997 [24] | China | 25–80 | Case-control | 64 | 40.63 | 1.512 ± 0.374 (mg/L) | 31 | 1.061 ± 0.157 (mg/L) | AAS |
| Jin et al. 2001 [25] | China | 45–70 | Case-control | 40 | 7.50 | 21.7 ± 6.55 (µmol/L) | 46 | 17.2 ± 2.48 (µmol/L) | AAS |
| Zhang et al. 1994 [26] | China | 59 ± 9 | Case-control | 40 | 10.00 | 29.67 ± 5.34 (µmol/L) | 24 | 18.84 ± 2.98 (µmol/L) | AAS |
| Xu et al. 1993 [11] | China | 56 ± 7.5 | Case-control | 42 | 9.52 | 19.14 ± 4.29 (µmol/L) | 40 | 19.61 ± 1.88 (µmol/L) | AAS |
| Zhou et al. 1995 [27] | China | 39–69 | Case-control | 186 | 31.18 | 1.481 ± 0.163 (µg/mL) | 150 | 1.035 ± 0.094 (µg/mL) | AAS |
| Chen et al. 1994 [28] | China | 37–72 | Case-control | 58 | 25.86 | 20.1 ± 5.6 (mol/L) | 100 | 18.5 ± 5.1 (mol/L) | AAS (MFX-ID) |
| Luo et al. 1996 [29] | China | 40–70 | Case-control | 35 | NA | 17.94 ± 4.09 (µmol/L) | 22 | 9.76 ± 1.89 (µmol/L) | AAS |
| Mo et al. 1995 [30] | China | 58.5 | Case-control | 57 | 21.05 | 153.44 ± 33.38 (µg/dL) | 46 | 93.77 ± 12.86 (µg/dL) | AAS |
| He et al. 1995 [31] | China | 34–72 | Case-control | 143 | 39.16 | 24.194 ± 9.135 (µmol/L) | 50 | 17.402 ± 5.264 (µmol/L) | AAS |
| Wei et al. 2002 [32] | China | 22–76 | Case-control | 79 | 41.77 | 1.093 ± 0.073 (µg/mL) | 32 | 0.867 ± 0.039 (µg/mL) | AAS ( p-100, PE Co., USA) |
| Huang et al. 1999 [33] | China | 40–72 | Case-control | 27 | 14.81 | 1.341 ± 0.304 (µg/mL) | 45 | 1.084 ± 0.182 (µg/mL) | AAS |
| Zhao et al. 1993 [34] | China | 43–62 | Case-control | 46 | 13.04 | 21.36 ± 4.6 (µmol/L) | 50 | 15.76 ± 4.2 (µmol/L) | AAS (BJKP-36, Beijing, China) |
| He et al. 2011 [10] | China | 38–69 | Case-control | 104 | 29.81 | 23.15 ± 3.16 (µmol/L) | 122 | 14.52 ± 1.75 (µmol/L) | AAS |
| Chen et al. 1998 [35] | China | 47–72 | Case-control | 43 | 32.56 | 19.08 ± 3.41 (µmol/L) | 180 | 13.85 ± 2.36 (µmol/L) | AAS (A670, Shimadzu, Japan) |
| Liang et al. 1992 [36] | China | 61 | Case-control | 57 | 21.05 | 28.75 ± 9.7 (µmol/L) | 80 | 19.76 ± 3.56 (µmol/L) | AAS (WFX-ID, China) |
| Huang et al. 1998 [12] | China | 25–65 | Case-control | 136 | 19.12 | 21.453 ± 5.783 (µmol/L) | 7101 | 20.713 ± 5.508 (µmol/L) | AAS (AA670/C2H2, Shimadzu) |
| Wang et al. 2003 [37] | China | 28–69 | Case-control | 50 | 40.00 | 1.04 ± 0.2 (µg/L) | 60 | 0.77 ± 0.22 (µg/L) | AAS |
| Cheng et al. 2011 [38] | China | 37–68 | Case-control | 197 | 32.99 | 1.19 ± 0.13 (µmol/L) | 93 | 0.87 ± 0.35 (µmol/L) | AAS |
| Xie et al. 2000 [39] | China | 35–68 | Case-control | 64 | 45.31 | 25.3 ± 6.3 (µmol/L) | 100 | 22.1 ± 1.7 (µmol/L) | AAS |
| Du et al. 1996 [40] | China | 22–73 | Case-control | 73 | 31.51 | 21.3 ± 4.3 (µmol/L) | 63 | 15.3 ± 3.4 (µmol/L) | AAS |
| Zhu et al. 1997 [41] | China | NA | Case-control | 56 | NA | 21.05 ± 3.56 (µmol/L) | 118 | 16.01 ± 2.13 (µmol/L) | AAS (3030, Perkin Elmer Zeeman, USA) |
| Zhang et al. 2000 [42] | China | 25–77 | Case-control | 310 | 17.74 | 1.151 ± 0.264 (µg/mL) | 48 | 1.068 ± 0.233 (µg/mL) | AAS (180-80, Shimadzu, Japan) |
| Hu et al. 2000 [43] | China | 36–77 | Case-control | 56 | 17.86 | 1.508 ± 0.379 (µg/mL) | 60 | 1.403 ± 0.148 (µg/mL) | AAS |
| Guo et al. 1994 [44] | China | 55.1 | Case-control | 26 | 26.92 | 2.81 ± 1.54 (µg/mL) | 26 | 0.82 ± 0.21 (µg/mL) | AAS (AA-40p, Varian, USA) |
| Han et al. 1999 [45] | China | NA | Case-control | 400 | NA | 1.12 ± 0.43 (µg/mL) | 100 | 0.87 ± 0.26 (µg/mL) | AAS (3030, PE Co., USA) |
AAS, atomic absorption spectrophotometry; SD, standard deviation; NA, not available
Serum copper levels and risk of lung cancer
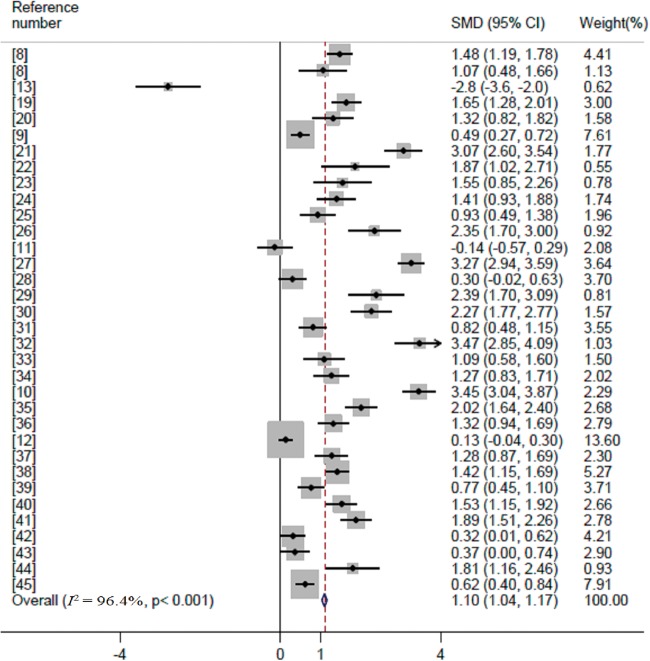
In the overall analysis, lung cancer patients had significantly higher serum copper levels than controls (summary SMD=1.103, 95%CI=1.040–1.165, Z=34.55, P for Z test <0.001), with significant between-study heterogeneity (I2=96.4%, P<0.001) (Figure 2).
Figure 2.
Forest plot of the association between serum copper levels and lung cancer risk. SMD, standard mean error; CI, confidence interval
Thirty-two of the included 33 articles were case-control studies, and the result for these was consistent with the overall result (summary SMD=1.099, 95%CI=1.036–1.162, Z=34.30, P for Z test <0.001). In a stratified analysis according to geographic location, the associations between serum copper levels and lung cancer were significant for both Asian (summary SMD=1.078, 95%CI=1.013–1.142, Z=32.88, P for Z test <0.001] and European populations (summary SMD=1.568, 95%CI=1.292–1.845, Z=11.13, P for Z test <0.001). Detailed results are shown in Table 2.
Table 2.
Overall and subgroup analyses of relationship between serum copper levels and lung cancer risk
| Study | No. of studies | SMD (95% CI) |
Z test |
Heterogeneity test |
||
|---|---|---|---|---|---|---|
| Z | P-value | I2 (%) | P-value | |||
| All | 33 | 1.103 (1.040–1.165) | 34.55 | <0.001 | 96.4 | <0.001 |
| Geographic location | ||||||
| Europe | 3 | 1.568 (1.292–1.845) | 11.13 | <0.001 | 0.0 | 0.444 |
| Asia | 30 | 1.078 (1.013–1.142) | 32.88 | <0.001 | 96.6 | <0.001 |
| Study type | ||||||
| Case-control | 32 | 1.099 (1.036–1.162) | 34.30 | <0.001 | 96.5 | <0.001 |
| Observation trials | 1 | – | – | – | – | – |
SMD, standard mean deviation; CI, confidence interval
Between-study heterogeneity
Significant evidence of between-study heterogeneity was detected when we pooled the overall results. We therefore performed univariate meta-regression analysis to explore the source of the high heterogeneity. No specific covariate (publication year, geographic location, case number) accounted for this high heterogeneity.
Publication bias and sensitivity analysis
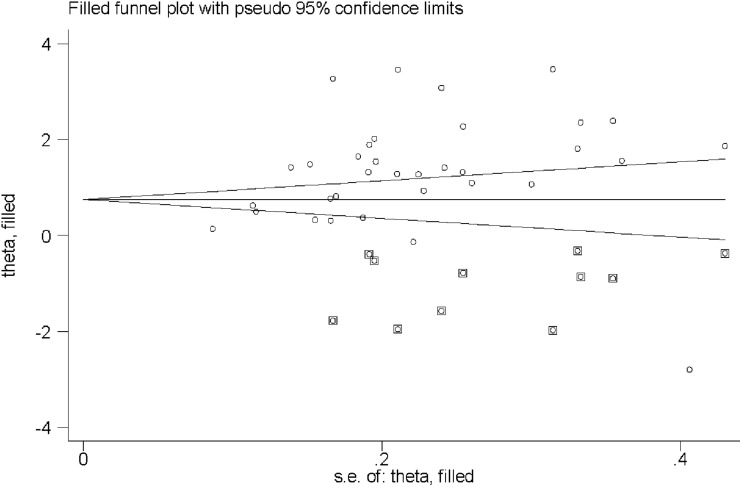
Egger’s regression asymmetry test (P=0.103) and Begg’s filled funnel plots (Figure 3) detected no publication bias.
Figure 3.
Filled funnel plots of the association between serum copper levels and lung cancer risk. Open circles represent studies included in this meta-analysis, circles in squares represent missing studies. s.e., standard error
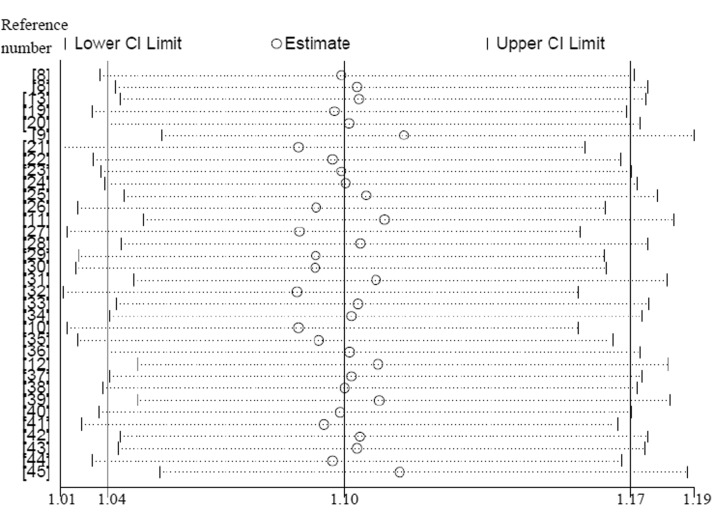
Sensitivity analysis showed no apparent effect on the overall merged SMD after deleting any individual study, indicating that no single study influenced the overall effect (Figure 4).
Figure 4.
Sensitivity analysis of the association between serum copper levels and lung cancer risk. CI, confidence interval. Meta-analysis results with the indicated study omitted
Discussion
Previous analyses have shown inconsistent results regarding the relationship between serum copper levels and lung cancer, probably due to limited sample sizes. We therefore conducted a meta-analysis of pooled data to obtain a comprehensive result and showed that elevated serum copper levels may increase the risk of lung cancer. Furthermore, serum copper levels were higher in lung cancer patients than in controls in both European and Asian populations.
A previous meta-analysis suggested that patients with thyroid cancer had higher copper levels than healthy controls.46 Another meta-analysis showed that serum copper levels were markedly higher in patients with bladder cancer compared with individuals without bladder cancer.47 Furthermore, a recent study found higher serum copper levels in patients with cervical cancer than in controls.48 The current results are consistent with the above studies. The reason why serum copper levels may be elevated in patients with lung cancer may be related to copper metabolism. Serum copper levels in healthy people are associated with ceruloplasmin,49 which is normally catabolized in the liver following cleavage of its terminal sialic acid chains by neuraminidase.50 It has been suggested51 that ceruloplasmin may be resialylated at the tumor cell surface or in the peripheral blood in patients with neoplasms, thus inhibiting its catabolism and potentially explaining the increase in serum copper levels in patients with malignant tumors.
This meta-analysis had several important strengths. First, the study included a large numbers of cases and participants, yielding a comprehensive result. Second, removing each individual study from the analysis had no apparent effect on the overall merged SMD, indicating that the results were stable. Third, no small study effect was detected by Egger’s regression asymmetry test or Begg’s filled funnel plots.
However, several limitations also need to be considered when interpreting the results. First, most of the included studies involved Asian populations and only three studies were from Europe. Although subgroup analysis identified significant associations between serum copper and lung cancer in both these subgroups, future studies in European and other populations are warranted to clarify the relationship between serum copper levels and lung cancer risk. Second, lung cancer is a complex disease with a variety of etiologic factors, including environmental and genetic factors. It is therefore possible that other factors may have influenced the results. Third, although most of the included studies measured copper levels using atomic absorption spectrophotometry, the use of instruments produced by different companies could have led to inconsistent measurements. Finally, significant heterogeneity between studies was observed in this meta-analysis. However, the heterogeneity was mainly related to the strength of the association rather than the direction of the risk estimate, suggesting that the findings in relation to the investigated outcome were promising. Furthermore, an investigation of potential covariates by meta-regression analysis found no significant contribution of publication year, geographic location, sex, or case number to the high between-study heterogeneity. No single study accounted for the significant between-study heterogeneity or influenced the overall result according to sensitivity analysis.
Conclusions
This meta-analysis concluded that serum copper levels tend to be higher in patients with lung cancer than in controls without lung cancer. Environmental copper exposure may thus increase the risk of lung cancer.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This work was supported by the “Psychological therapy and related research on chronic pain in cancer patients” (C2015-86).
References
- 1.Sato M, Shames DS, Gazdar AF, et al. A translational view of the molecular pathogenesis of lung cancer. J Thorac Oncol 2007; 2: 327–343. [DOI] [PubMed] [Google Scholar]
- 2.Walser T, Cui X, Yanagawa J, et al. Smoking and lung cancer: the role of inflammation. Proc Am Thorac Soc 2008; 5: 811–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Minguet J, Smith KH, Bramlage P. Targeted therapies for treatment of non-small cell lung cancer–Recent advances and future perspectives. Int J Cancer 2016; 138: 2549–2561. [DOI] [PubMed] [Google Scholar]
- 4.Ruano-Ravina A, Figueiras A, Freire-Garabal M, et al. Antioxidant vitamins and risk of lung cancer. Curr Pharm Des 2006; 12: 599–613. [DOI] [PubMed] [Google Scholar]
- 5.Mahabir S, Forman MR, Barerra SL, et al. Joint effects of dietary trace metals and DNA repair capacity in lung cancer risk. Cancer Epidemiol Biomarkers Prev 2007; 16: 2756–2762. [DOI] [PubMed] [Google Scholar]
- 6.Patrick L. Selenium biochemistry and cancer: a review of the literature. Altern Med Rev 2004; 9: 239–258. [PubMed] [Google Scholar]
- 7.Ho E. Zinc deficiency, DNA damage and cancer risk. J Nutr Biochem 2004; 15: 572–578. [DOI] [PubMed] [Google Scholar]
- 8.Sun ZP, Meng SX, Xu PY, et al. [Study on serum copper and zinc levels and copper/zinc ratio in 102 lung cancer patients]. Trace Elements and Health Research 1991; 1: 67–69. [Google Scholar]
- 9.Jin Y, Zhang C, Xu H, et al. Combined effects of serum trace metals and polymorphisms of CYP1A1 or GSTM1 on non-small cell lung cancer: a hospital based case-control study in China. Cancer Epidemiol 2011; 35: 182–187. [DOI] [PubMed] [Google Scholar]
- 10.He ZJ. [Detection of serum trace elements in lung cancer patients and its significance]. Med Innovation of China 2011; 8: 118–119. [Google Scholar]
- 11.Xu ZF, Sun YC, Zhang CW, et al. [Clinical significance of changes of serum copper, zinc and magnesium contents in patients with lung cancer]. J Second Military Med Univ 1993; 14: 195–196. [Google Scholar]
- 12.Huang ZY, Hu FD. [Comparative study of serum trace elements in patients with lung cancer]. Shanxi Clin Med J 1998; 17: 114–116. [Google Scholar]
- 13.Cobanoglu U, Demir H, Sayir F, et al. Some mineral, trace element and heavy metal concentrations in lung cancer. Asian Pac J Cancer Prev 2010; 11: 1383–1388. [PubMed] [Google Scholar]
- 14.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clin Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JP, Thompson SG. Controlling the risk of spurious findings from meta-regression. Stat Med 2004; 23: 1663–1682. [DOI] [PubMed] [Google Scholar]
- 17.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50: 1088–1101. [PubMed] [Google Scholar]
- 18.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diez M, Arroyo M, Cerdan FJ, et al. Serum and tissue trace metal levels in lung cancer. Oncology 1989; 46: 230–234. [DOI] [PubMed] [Google Scholar]
- 20.Huhti E, Poukkula A, Uksila E. Serum copper levels in patients with lung cancer. Respiration 1980; 40: 112–116. [DOI] [PubMed] [Google Scholar]
- 21.Oyama T, Matsuno K, Kawamoto T, et al. Efficiency of serum copper/zinc ratio for differential diagnosis of patients with and without lung cancer. Biol Trace Elem Res 1994; 42: 115–127. [DOI] [PubMed] [Google Scholar]
- 22.Zowczak M, Iskra M, Paszkowski J, et al. Oxidase activity of ceruloplasmin and concentrations of copper and zinc in serum of cancer patients. J Trace Elem Med Biol 2001; 15: 193–196. [DOI] [PubMed] [Google Scholar]
- 23.Feng JF, Li SL. . Correlation between oxidative stress and trace elements in blood of patients with cancer. Chin J Clinic Reh 2006; 10: 187–190. [Google Scholar]
- 24.Zhang Y. [Clinical significance of determination of serum lead, zinc and copper to zinc ratio in patients with lung cancer]. Trace Element Science in Guangdong 1997; 4: 32–34. [Google Scholar]
- 25.Jin ZJ, Qian LQ, Dong GQ, et al. [Measurement and analysis of serum copper, zinc and magnesium in patients with lung cancer and gastric cancer]. Shaanxi Med J 2001; 30: 165–166. [Google Scholar]
- 26.Zhang TQ, Yao RL, Song MC. [Copper, zinc and copper-zinc ratio in plasma of patients with lung cancer]. Shaanxi Med J 1994; 25: 349–351. [Google Scholar]
- 27.Zhou QH, Luo YY, Li CF, et al. A study on serum copper and zinc levels in patients with lung cancer . Chinese J Clin Thorac Cardiovasc Surg 1995; 2: 45–47. [Google Scholar]
- 28.Chen ZH, Chen NJ, Chen LJ, et al. [Detection of serum copper, zinc and iron in patients with lung cancer and lung infection and its clinical significance]. Journal of Guangzhou Medical College 1994; 12: 118–119. [Google Scholar]
- 29.Luo XR, Mao WG. Changes of serum copper and zinc in patients with lung cancer and its clinical significance. Hunan Med 1996; 13: 136–137. [Google Scholar]
- 30.Mo LN, Du KX, Liu GC. [Analysis of serum CEA and trace elements Zn and Cu in patients with lung cancer]. Radioimmunology 1995; 8: 354–355. [Google Scholar]
- 31.He WD. [Detection and analysis of some trace elements in serum of patients with lung cancer]. Jiujiang Med J 1995; 10: 69–71. [Google Scholar]
- 32.Wei L, Ji QM, Xue DY, et al. [The role of copper and zinc determination in patients with lung cancer]. Chin Tumor 2002; 11: 182–183. [Google Scholar]
- 33.Huang Y, Xu YF. [Analysis of serum copper and ferritin in patients with lung cancer]. Cancer Res 1999; 26: 275–277. [Google Scholar]
- 34.Zhao YM, Zhang XP. [The value of serum copper and zinc levels and their ratio in diagnosis of lung cancer]. Shanghai Med Inspection J 1993; 8: 120–121. [Google Scholar]
- 35.Chen WY. [Changes of serum trace elements copper and zinc in patients with lung cancer]. Zhejiang Med 1998; 20: 330–331. [Google Scholar]
- 36.Liang GM, Zhu XH, Wang X. Serum zinc and copper levels and copper/zinc ratios in patients with lung cancer . Cancer Res 1992; 19: 193–194. [Google Scholar]
- 37.Wang ZL, Zhang W, Zhang HY, et al. [Determination of serum trace elements and their clinical value in patients with lung cancer]. Clinical Focus 2003; 18: 183–185. [Google Scholar]
- 38.Cheng Z, Dai LL, Kang Y, et al. [Detection of trace elements of patients with lung cancers and pulmonary infections and its clinical significance]. Chin J Nosocomiol 2011; 21: 2006–2008. [Google Scholar]
- 39.Xie SY, Chen YX. [Analysis of 64 cases of lung cancer with trace elements copper and zinc]. Shanxi Med J 2000; 29: 83–84. [Google Scholar]
- 40.Du FL, Li ZM, Cao MJ, et al. [Determination of serum copper, zinc, magnesium and iron in patients with pulmonary tuberculosis, chronic bronchitis, pulmonary heart disease and lung cancer]. J Xi'an Med University 1996; 17: 348–350. [Google Scholar]
- 41.Zhu JJ, Duan XY, Liu JS. [The diagnostic value of serum copper and zinc concentrations in lung cancer, pulmonary tuberculosis and pulmonary infection]. China J Modern Med 1997; 7: 11–13. [Google Scholar]
- 42.Zhang Y, Li X. [Relationship of serum trace elements to lung cancer]. Trace Elements and Health Research 2000; 17: 15–17. [Google Scholar]
- 43.Hu ZH, Ren Q, Guo J, et al. [Significance of determination of serum zinc, copper and copper/zinc ratio on evaluating diagnosis therapeutic effect and prognosis of lung cancer]. Trace Element Science in Guangdong 2000; 7: 19–22. [Google Scholar]
- 44.Guo XH, Li PF, Peng FK, et al. [Relationship between serum zinc, copper, manganese and lung cancer]. Chin Pub Heal 1994; 10: 156–157. [Google Scholar]
- 45.Han CZ, Zhao XW, Jing JX, et al. [Evaluation of the serum copper zinc levels and copper/zinc ratio in the diagnosis of malignant tumor]. Chin Tumor 1999; 8: 572–573. [Google Scholar]
- 46.Shen F, Cai WS, Li JL, et al. The association between serum levels of selenium, copper, and magnesium with thyroid cancer: a meta-analysis. Biol Trace Elem Res 2015; 167: 225–235. [DOI] [PubMed] [Google Scholar]
- 47.Mao S, Huang S. Zinc and copper levels in bladder cancer: a systematic review and meta-analysis. Biol Trace Elem Res 2013; 153: 5–10. [DOI] [PubMed] [Google Scholar]
- 48.Zhang M, Shi M, Zhao Y. Association between serum copper levels and cervical cancer risk: A meta-analysis. Biosci Rep 2018; 38 https://www.ncbi.nlm.nih.gov/pubmed/29519960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gubler CJ, Lahey ME, Cartwright GE, et al. Studies on copper metabolism. IX. The transportation of copper in blood. J Clin Invest 1953; 32: 405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Den Hamer CJ, Morell AG, Scheinberg IH, et al. Physical and chemical studies on ceruloplasmin. IX. The role of galactosyl residues in the clearance of ceruloplasmin from the circulation. J Biol Chem 1970; 245: 4397–4402. [PubMed] [Google Scholar]
- 51.Fisher GL, Shifrine M. Hypothesis for the mechanism of elevated serum copper in cancer patients. Oncology 1978; 35: 22–25. [DOI] [PubMed] [Google Scholar]