Short abstract
Objective
Probiotics are beneficial in human health. In this study, we investigated the effect of probiotics on absorption of amlodipine, a dihydropyridine calcium antagonist used in the treatment of angina and hypertension, in a rabbit model.
Methods
Lactobacillus plantarum IS-10506 probiotic was administered for 14 days to male New Zealand rabbits. Blood samples were collected before and after probiotic supplementation. Amlodipine (10 mg) was then administered to all groups. Blood samples from a marginal vein were withdrawn at 5, 15, 30, 60, and 120 minutes to determine amlodipine concentrations in rabbit plasma.
Results
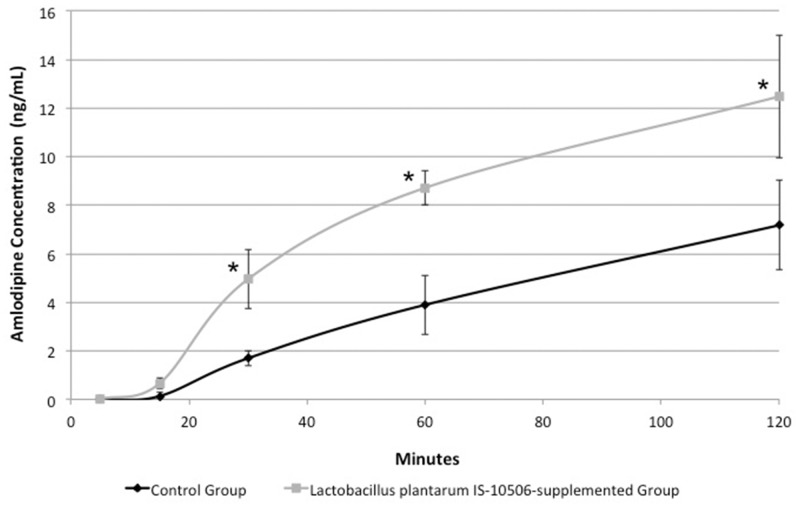
Amlodipine concentrations in the L. plantarum IS-10506 group were 4.95 ± 1.22, 8.71 ± 0.69, and 12.48 ± 2.53 ng/ml, and those in the control group were 1.69 ± 0.31, 3.89 ± 1.23, and 7.17 ± 1.85 ng/ml at 30, 60, and 120 minutes, respectively after administration of amlodipine. Amlodipine concentrations in the L. plantarum IS-10506 group were significantly higher than those in the control group at 30, 60, and 120 minutes after amlodipine administration.
Conclusion
Our results suggested that supplementation of L. plantarum IS-10506 significantly increases amlodipine plasma concentrations in rabbits.
Keywords: Probiotic, Lactobacillus plantarum, dadih, amlodipine, absorption, calcium channel blocker, hematology, gastrointestinal tract
Introduction
Oral administration is the easiest and most convenient way of drug administration.1 Factors that may affect the absorption rate are intestinal integrity, physiological status, gastrointestinal motility, site of drug absorption, membrane transporters, pre-systemic drug metabolism, and the effect of food or concomitant medication.2 The intestine has a substantial influence on drug biotransformation because of the presence of numerous enzymes, especially those produced by gut microbiota.3
In recent years, there has been some interest in the manipulation of the composition of intestinal microbiota by probiotics and prebiotics.4 The World Health Organization defined probiotics as “live organisms administered in adequate amounts to confer a health benefit for the host”.5 Probiotics have beneficial health effects via multiple mechanisms. These mechanisms include release of antibacterial substances (bacteriocins and bacteriocin-like inhibitory substances), secretion of non-specific antimicrobial substances, induction of production of antimicrobial compounds (defensins) by the host, direct enzymatic activities of probiotics within the gut lumen, reduction in luminal pH, inhibition of bacterial adherence, competition for nutrients, and immune responses.6–8 Probiotic bacteria may affect the expression and functionality of various proteins and membrane transporters of other bacteria in the gut via changing gut concentrations of certain polypeptides. These actions might be due to induction or suppression of membrane transporters or by the process of direct signaling.9
Previous studies have reported the potential of probiotics to enhance drug absorption.10,11 Supplementation of the probiotic Lactobacillus plantarum IS-10506 together with zinc increases the zinc status of Indonesian school children.10 Other reports have shown changes in gliclazide pharmacokinetics in diabetic rats that were pre-treated with a mixture of three probiotics (L. acidophilus, L. rhamnosus, and Bifidobacterium lactis) in a suspension prepared from freeze-dried probiotic powders. Probiotic treatment of diabetic rats increases gliclazide bioavailability and lowers blood glucose levels by insulin-independent mechanisms, suggesting that administration of probiotics may be a beneficial adjunct therapy in the treatment.11 Therefore, we hypothesized that probiotic supplementation increases drug absorption in the gastrointestinal tract.
In this study, we investigated the potential use of L. plantarum IS-10506 probiotic to enhance amlodipine concentrations in rabbit plasma. Amlodipine is a calcium channel blocking agent of a dihydropyridine derivative that is used for treating hypertension and angina.12 Because patients depend on amlodipine, this study examined the use of probiotics, as adjuvants, to increase the effectiveness of amlodipine therapy.
Material and methods
Materials
Microencapsulated L. plantarum IS-10506, which is a novel probiotic that is isolated from dadih (a traditional Indonesian fermented buffalo milk), was prepared as previously described.10 Amlodipine (amlodipine besylate), the internal standard nortriptyline hydrochloride (NOR), and 4-chloro-7-nitrobenzofurazan (NBD-Cl) were purchased from Sigma Aldrich (Singapore). Methanol, acetonitrile, ethanol, sulfuric acid, hydrochloric acid, boric acid, and sodium hydroxide were purchased from Merck (Darmstadt, Germany). All reagents that were used were analytical grade, except for methanol, which was high-performance liquid chromatography (HPLC) grade.
Stock solution of amlodipine was prepared by dissolving 10 mg of free base with 2 mL ethanol and diluting to 100 mL with water. A plasma standard with serial dilution of 2.5, 10, 40, 160, 640, and 1280 ng/mL was prepared to provide plasma calibration samples. NOR was prepared at a concentration of 100 µg/mL and diluted to obtain 50 ng/mL of working solution. NBD-Cl solution (0.08%, w/v) was freshly prepared in methanol. Teorell and Stenhagen buffer solution13 was composed of phosphoric acid, citric acid, 0.1 M sodium hydroxide, and 0.1 M hydrochloric acid, and adjusted to a pH of 8.6.
Rabbit experiments
Twelve male New Zealand White rabbits within the age range of 6 to 12 months old with a weight of 1.5 to 3 kg were used in this study. The rabbits were adapted to environmental conditions with individual stainless steel cages for 7 days and were clinically healthy before the study. Rabbits were allocated into two different groups: (1) pre-treated with L. plantarum IS-10506 at a dose of 1010 colony-forming units (CFU)/day for 14 days; and (2) a control group without pre-treatment of L. plantarum IS-10506. Both groups were then fed with a basal diet. A total of 1010 CFU/day for 14 days of supplementation provides good adhesion of L. plantarum IS-10506 to intestinal mucus.14 After 14 days, a suspension of amlodipine besylate (equivalent to 10 mg of free base) was administered to all rabbits. A total of 10 mg amlodipine was equivalent to 3 to 6 mg/kg body weight to reach a high distribution of amlodipine in the rabbits.15,16 Blood samples from the marginal vein were collected into EDTA tubes at 5, 15, 30, 60, and 120 minutes after amlodipine administration. The tubes were centrifuged, and the plasma samples were frozen at −80°C until HPLC analysis. The experimental protocol was approved by the Universitas Padjadjaran Ethical Committee for Health Research (Ethical Approval Number: 416/UN6.C1.3.2/KEPK/PN/2016).
Analysis of amlodipine
A total of 500 µL of plasma was transferred into a test tube, and then 100 µL IS, 100 µL NaOH 0.1 N, and 1 mL acetonitrile was added. After the solution was mixed for 1 minute on a vortex mixer and centrifuged for 15 minutes at 4500 g, 1 mL of aliquot was evaporated under a stream of nitrogen. The residue was dissolved in 100 µL methanol, followed by addition of 100 µL buffer solution (pH, 8.6) and 100 µL NBD-Cl solution. The solution was kept at 70°C for 20 minutes. After cooling, 100 µL 0.1 N H2SO4 was added.
The chromatographic system consisted of liquid chromatography (Waters e2695; Waters Associates, Milford, MA, USA) with a fluorescence detector (Waters 2475 FLR, Waters Associates), LiChrospher RP 18 column (125 mm × 4 mm, inner diameter; Merck, Darmstadt, Germany) and LiChrospher RP 18 guard column (4 mm × 4 mm, inner diameter, Merck). The mobile phase used was methanol-phosphoric acid 0.01% (v/v) (65:35) with a flow rate of 1 mL/minute. Detection was at an excitation wavelength of 480 nm and emission wavelength of 535 nm. Calibration curves were constructed by analyzing a series of plasma calibration samples that were spiked with concentrations ranging from 2.5 to 1280 ng/mL. Linear regression showed that the value of the coefficient of determination was r2 = 0.9989. The mean recovery was 61.68% ± 5.96%. The lower limit of quantification (LLOQ) was 2.5 ng/mL. The coefficient variation was less than 15% for quality control samples and the LLOQ, while the percentage difference for accuracy was less than 15% for quality control samples and less than 20% for the LLOQ.
Hematological analysis
Blood samples were analyzed by using the Samsung LABGEOHC10 Hematology Analyzer (Samsung Electronics Co., Suwon, Korea) for analysis of white blood cells, lymphocytes, monocytes, granulocytes, red blood cells (RBC), hemoglobin, hematocrit (HCT), mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, and red cell distribution width. All of the procedures followed the manufacturer’s instructions. The quality control material from the manufacturer was run on a daily basis throughout the entire study period.
Statistical analysis
Differences between groups were analyzed using independent t-tests. P values of less than 0.05 were considered significant. SPSS (IBM Corp., Armonk, NY, USA) was used for statistical analysis.
Results
The HPLC chromatogram of amlodipine and NOR in rabbit plasma is shown in Figure 1. Amlodipine concentrations are shown in Figure 2. There was no significance difference in amlodipine concentrations between the L. plantarum IS-10506 supplementation group and the control group at 5 and 15 minutes after amlodipine administration. However, amlodipine concentrations in the L. plantarum IS-10506 group were significantly higher than those in the control group at 30, 60, and 120 minutes after amlodipine administration (P = 0.001).
Figure 1.
Chromatogram of amlodipine and nortriptyline as an internal standard in rabbit plasma
AML: amlodipine; NOR: nortriptyline hydrochloride.
Figure 2.
Amlodipine concentrations in rabbit plasma with and without Lactobacillus plantarum IS-10506 supplementation.
L. plantarum IS-10506 supplementation affected some blood hematological parameters (Table 1). L. plantarum IS-10506 supplementation led to a significantly higher RBC count and HCT compared with the control group (both P < 0.05). These two parameters may play roles in drug absorption.
Table 1.
Effect of Lactobacillus plantarum IS-10506 supplementation on hematological parameters
| Hematological parameter | Control group | Lactobacillus plantarum IS-10506 | P value |
|---|---|---|---|
| (mean ± SD) | (mean ± SD) | ||
| White blood cell count (103/µL) | 9.773 ± 3.401 | 9.029 ± 2.178 | 0.820 |
| Lymphocytes (103/µL) | 3.143 ± 1.363 | 2.633 ± 1.139 | 0.673 |
| Monocytes (103/µL) | 1.852 ± 1.036 | 1.501 ± 0.818 | 0.768 |
| Granulocytes (103/µL) | 4.782± 2.713 | 4.894 ± 1.899 | 0.995 |
| Lymphocytes (%) | 0.328 ± 0.127 | 0.307 ± 0.140 | 0.939 |
| Monocytes (%) | 0.188 ± 0.092 | 0.161 ± 0.071 | 0.823 |
| Granulocytes (%) | 0.484 ± 0.207 | 0.533± 0.117 | 0.830 |
| Red blood cell count (106/µL) | 4.657 ± 1.192 | 5.677 ± 0.358 | 0.040 |
| Hemoglobin (g/dL) | 11.167 ± 1.965 | 12.678 ± 0.531 | 0.088 |
| Hematocrit (%) | 0.277 ± 0.056 | 0.329 ± 0.016 | 0.021 |
| Mean corpuscular volume (fL) | 60.167 ± 4.622 | 57.889 ± 3.140 | 0.389 |
| Mean corpuscular hemoglobin (pg) | 24.4 ± 2.519 | 22.356 ± 0.930 | 0.045 |
| Mean corpuscular hemoglobin concentration (g/dL) | 40.533± 1.727 | 38.611 ± 1.643 | 0.056 |
| Red cell distribution width (%) | 0.174 ± 0.018 | 0.171 ± 0.013 | 0.879 |
A P value of less than 0.05 was considered as significant.
SD: standard deviation.
Discussion
We performed an in vivo experimental study in rabbits to investigate the effect of L. plantarum IS-10506 supplementation on plasma amlodipine concentrations. The chromatogram of amlodipine and NOR showed that there was good separation of amlodipine and NOR (Figure 1). Our finding of an increase in amlodipine concentrations in the L. plantarum IS-10506 group (Figure 2) is in agreement with previous studies. Al Salami and co-workers showed an increase in gliclazide bioavailability in diabetic rats that were pre-treated with a mixture of three probiotics (L. acidophilus, L. rhamnosus, and Bifidobacterium lactis).11 Furthermore, Matuskova and co-workers showed that concomitant supplementation with the probiotic Escherichia coli strain Nissle 1917 was responsible for better amiodarone absorption from the gastrointestinal tract, which led to increased bioavailability of amiodarone.17
We performed hematological analysis in the control group and L. plantarum IS-10506-supplemented group. We found that that there was significant increase in RBC and HCT in the L. plantarum IS-10506-supplemented group (Table 1). RBC play roles in tissue oxygen delivery. RBC may sense tissue O2 requirements via their degree of deoxygenation when they travel through the microcirculation and release vasodilatory compounds that enhance blood flow in hypoxic tissues;18 this vasodilatory effect indicates decreased blood flow. Furthermore, HCT is a global hematological marker of the amount of hemoglobin in blood.19
Enhancement of blood flow caused by L. plantarum IS-10506 supplementation may potentially increase absorption of amlodipine. This possibility is consistent with a study by Crouthamel et al.20 who showed that a reduction in blood flow resulted in progressive impairment of sulfaethidole absorption. Moreover, higher levels of RBC and HCT indicate a lower sedimentation rate. Lowering the sedimentation rate leads to an increase of plasma protein. Amlodipine is a drug bound with plasma protein. Therefore, increasing plasma protein can increase the absorption of amlodipine.
Additionally, L. plantarum is a prokaryote that has ATP-binding cassette transporters. Prokaryotic cells are bacteria that can either be exporters or importers.21 Amlodipine is a calcium channel blocker that is transported via binding with ATP-binding cassette transporters.22 The presence of L. plantarum provides more ATP-binding cassette transporters to transport amlodipine via the intestinal tract and causes enhancement of amlodipine absorption.
Our study has some limitations. In the current preliminary study, we did not use a hypertensive rabbit model group and non-surviving L. plantarum IS-10506 pre-treatment group. This is because of a lack of data on detection of L. plantarum IS-10506 at the end of treatment. We also did not investigate the dose response of amlodipine absorption to amlodipine administration and pre-treatment of L. plantarum IS-10506 or a normal reference lactic acid bacterium. These limitations are currently under investigation in a follow-up study in our laboratory. Despite these limitations, our study suggests that supplementation of L. plantarum IS-10506 significantly increases amlodipine plasma concentrations in rabbits. These results may be due to the ability of L. plantarum IS-10506 to enhance blood hematological parameters that play roles in drug absorption.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This work was financially supported by The Indonesian Ministry of Research, Technology, and Higher Education (Grant-in-aid for International Research Collaboration and Publication) for RA.
References
- 1.Sastry SV, Nyshadham JR, Fix JA. Recent technological advances in oral drug delivery - a review. Pharm Sci Technolo Today 2000; 3: 138–145. [DOI] [PubMed] [Google Scholar]
- 2.Martinez MN, Amidon GL. A mechanistic approach to understanding the factors affecting drug absorption: a review of fundamentals. J Clin Pharmacol 2002; 42: 620–643. [DOI] [PubMed] [Google Scholar]
- 3.Laube B, Winkler S, Ladstetter B, et al. Establishment of a novel in vitro system for studying the interaction of xenobiotic metabolism of liver and intestinal microflora. Arch Toxicol 2000; 74: 379–387. [DOI] [PubMed] [Google Scholar]
- 4.Gareau MG, Sherman PM, Walker WA. Probiotics and the gut microbiota in intestinal health and disease. Nat Rev Gastroenterol Hepatol 2010; 7: 503–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joint FAO/WHO Working Group. Guidelines for the Evaluation of Probiotics in Food. http://www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf (2002, accessed September 22nd, 2017).
- 6.Fooks LJ, Gibson GR. Probiotics as modulators of the gut flora. Br J Nutr 2002; 88(Suppl 1): S39–S49. [DOI] [PubMed] [Google Scholar]
- 7.Gillor O, Etzion A, Riley MA. The dual role of bacteriocins as anti- and probiotics. Appl Microbiol Biotechnol 2008; 81: 591–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vineeth S, Saireddy S, Keerthi T, et al. Efficacy of Bacillus clausii and Saccharomyces boulardii in treatment of acute rotaviral diarrhea in pediatric patients. Indones J Clin Pharm 2017; 6: 91–98. [Google Scholar]
- 9.de Oliveira GLV, Leite AZ, Higuchi BS, et al. Intestinal dysbiosis and probiotic applications in autoimmune diseases. Immunology 2017; 152: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Surono IS, Martono PD, Kameo S, et al. Effect of probiotic L. plantarum IS-10506 and zinc supplementation on humoral immune response and zinc status of Indonesian pre-school children. J Trace Elem Med Biol 2014; 28: 465–469. [DOI] [PubMed] [Google Scholar]
- 11.Al-Salami H, Butt G, Fawcett JP, et al. Probiotic treatment reduces blood glucose levels and increases systemic absorption of gliclazide in diabetic rats. Eur J Drug Metab Pharmacokinet 2008; 33: 101–106. [DOI] [PubMed] [Google Scholar]
- 12.Haria M, Wagstaff AJ. Amlodipine. A reappraisal of its pharmacological properties and therapeutic use in cardiovascular disease. Drugs 1995; 50: 560–586. [DOI] [PubMed] [Google Scholar]
- 13.Ostling S, Virtama P. A modified preparation of the universal buffer described by Teorell and Stenhagen. Acta Physiologica 1946; 11: 289–293. [Google Scholar]
- 14.Collado MC, Surono IS, Meriluoto J, et al. Potential probiotic characteristics of Lactobacillus and Enterococcus strains isolated from traditional dadih fermented milk against pathogen intestinal colonization. J Food Prot 2007; 70: 700–705. [DOI] [PubMed] [Google Scholar]
- 15.Stopher DA, Beresford AP, Macrae PV, et al. The metabolism and pharmacokinetics of amlodipine in humans and animals. J Cardiovasc Pharmacol 1988; 12(Suppl 7): S55–S59. [DOI] [PubMed] [Google Scholar]
- 16.Yeung PK, Mosher SJ, Pollak PT. Liquid chromatography assay for amlodipine: chemical stability and pharmacokinetics in rabbits. J Pharm Biomed Anal 1991; 9: 565–571. [DOI] [PubMed] [Google Scholar]
- 17.Matuskova Z, Anzenbacherova E, Vecera R, et al. Administration of a probiotic can change drug pharmacokinetics: effect of E. coli Nissle 1917 on amidarone absorption in rats. PloS One 2014; 9: e87150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jensen FB. The dual roles of red blood cells in tissue oxygen delivery: oxygen carriers and regulators of local blood flow. J Exp Biol 2009; 212: 3387–3393. [DOI] [PubMed] [Google Scholar]
- 19.Yang Z, Craddock RC, Milham MP. Impact of hematocrit on measurements of the intrinsic brain. Front Neurosci 2014; 8: 452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crouthamel WG, Diamond L, Dittert LW, et al. Drug absorption VII: influence of mesenteric blood flow on intestinal drug absorption in dogs. J Pharm Sci 1975; 64: 664–671. [DOI] [PubMed] [Google Scholar]
- 21.Sharom FJ. ABC multidrug transporters: structure, function and role in chemoresistance. Pharmacogenomics 2008; 9: 105–127. [DOI] [PubMed] [Google Scholar]
- 22.Krishna R, Yu L. Biopharmaceutics application in drug development. New York: Springer, 2008. [Google Scholar]