The recent article by Bach et al. (2015), raises the issue of “parity-conditioning bias” when assessing the effect of environmental contaminants on human fecundity, an issue previously addressed in our manuscript on Perfluoroalkyl acids (PFAAs) and Time to Pregnancy (TTP) (Vélez et al., 2015). Following the publication by Fei et al. (2009) that first suggested a negative impact of selected PFAAs on human fecundity, concerns have been raised about the possibility of reverse causation in parous women (i.e., parous women with longer TTP have higher PFAAs levels because they have long interpregnancy intervals allowing re-accumulation of PFAAs) (Olsen et al., 2009; Whitworth et al., 2012). Based on this, Bach et al. restricted their sample to nulliparous women and concluded that this approach should be adopted in future studies. We will argue that conditioning (i.e., adjusting, stratifying, or restricting) on parity is redundant and would cause over-adjustment, as parity is the result, among other factors, of proven fecundability.
In their supplemental material, Bach et al. (2015), present Directed Acyclic Graphs (DAgs) to support their analytical model. Using supplementary Figure 1A in an unrestricted setting (i.e., nulliparous and multiparous women), and assuming that the question of interest is whether the exposure PFAA1 is associated with current TTP, the authors claim that the association is not subject to parity conditioning bias because nulliparity must precede PFAA levels (temporality must be maintained). Bach et al. argue that “considering the timely order, nulliparity per se must precede the concentrations of PFAA at the relevant time of the exposure, and hence nulliparity cannot be considered an intermediate factor between the exposure and the outcome” (Bach et al., 2015). However, we would argue that this setting is not likely to be free of parity-conditioning bias as 1) PFAAs are persistent in the environment and may be a relevant marker of exposure despite temporality concerns, and 2) that restricting by nulliparity may in fact be imperfectly adjusting for an intermediate as nulliparity could be considered a proxy for fecundability even when evaluating current TTP (Schisterman et al., 2009).
First, since PFAAs are persistent in the environment, in nulliparous women their levels are probably as high, if not higher, at the time of conception than during the first trimester of pregnancy. In fact, compared to lipophilic compounds, the magnitude of changes for PFAAs during pregnancy and lactation appear minimal, as indicated by the relatively small changes in maternal serum concentrations during pregnancy or through 6 months postpartum reported in a pregnancy cohort study (Fromme et al., 2010). In addition, the correlation between repeated measurements of PFAAs in two consutive pregnancies is moderate to high, and seems not to be affected by adjustment for reproductive factors as indicated in a recent study reporting Pearson correlation coefficients of 0.80 for perfluorooctane sulfonate (PFOS); 0.50 for perfluorooctanoate (PFOA); and 0.74 for perfluorohexane sulfonate (PFHxS) (Papadopoulou et al., 2015). Moreover, PFAAs have the capacity to bind to serum albumin (Han et al., 2003), which may account for breast milk concentrations being ~1000 times lower than blood concentrations (Fromme et al., 2010; Kato et al., 2011). Furthermore, independently of parity, women are continuously exposed to PFAAs, not only due to the long half-lives of these chemicals, but also through an estimated daily uptake of 2–3 ng/kg of PFOS and PFOA, with 90% coming from dietary sources (Fromme et al., 2009).
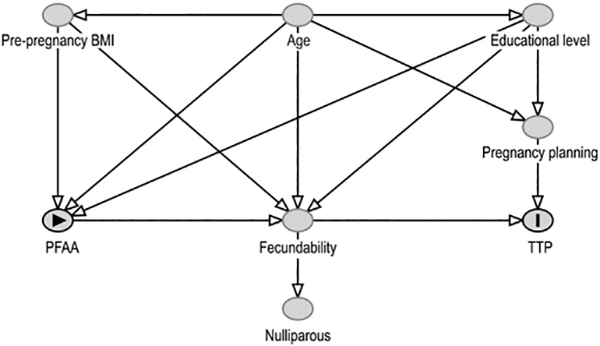
Secondly, Bach et al. consider that it is necessary “to only study nulliparous women in order to eliminate the risk of confounding by factors related to previous pregnancies and childbirths, in particular when the setting is a birth cohort”. However, in fecundity studies restriction by parity does not eliminate this potential risk even in nulliparous women because TTP is an endpoint of several conditional processes underlying human conception, implantation, and the viability of the conceptus (Weinberg and Wilcox, 2008). Hence, restriction by parity, which is a marker of proven fecundity even in the first pregnancy, could be considered as over-adjusting for all the factors that intervene in the achievement of that pregnancy since the preconception period until birth. This concept is partially described in their supplementary Figure 2, where fecundability is situated in the middle of PFAA and TTP, acting as a mediator. We argue that parity could thus be considered as a marker (though perhaps imperfect) of fecundability and could be represented as a consequence of fecundability. By adding nulliparous women to their DAG we reinforce the concept of potential over-adjustment bias, as this implies selection by a marker of fecundability (Fig. 1).
Fig. 1.
Directed Acyclic Graph adapted from Bach et al. (2015).
Thus, our conclusion differs to that of Bach et al. in that we consider that neither adjustment nor stratification for parity should be conducted when studying the reproductive adverse effects of PFAAs, as this will introduce over-adjustment bias. Furthermore, restriction to nulliparous women in future studies as proposed by Bach et al. will compromise the internal validity of the study at the expense of costly laboratory analysis such as PFAAs.
Contributor Information
M.P. Vélez, Department of Obstetrics and Gynecology, Kingston General Hospital, Queen’s University, Kingston, Canada.
T.E. Arbuckle, Population Studies Division, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, Canada
W.D. Fraser, Sainte-Justine University Hospital Research Centre, University of Montreal, Montreal, Canada Department of Obstetrics and Gynecology, University of Sherbrooke, Sherbrooke, Quebec, Canada.
S.L. Mumford, Epidemiology Branch, Division of Intramural Population Health Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Rockville, MD, United States
References
- Bach CC, Bech BH, Nohr EA, Olsen J, Matthiesen NB, Bossi R, et al. , 2015. Serum perfluoroalkyl acids and time to pregnancy in nulliparous women. Environ. Res 142, 535–541. [DOI] [PubMed] [Google Scholar]
- Vélez MP, Arbuckle TE, Fraser WD, 2015. Maternal exposure to perfluorinated chemicals and reduced fecundity: the MIREC study. Hum. Reprod 30 (3), 701–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei C, McLaughlin JK, Lipworth L, Olsen J, 2009. Maternal levels of per-fluorinated chemicals and subfecundity. Hum. Reprod 24 (5), 1200–1205. [DOI] [PubMed] [Google Scholar]
- Olsen GW, Butenhoff JL, Zobel LR, 2009. Perfluoroalkyl chemicals and human fetal development: an epidemiologic review with clinical and toxicological perspectives. Reprod. Toxicol 27 (3–4), 212–230. [DOI] [PubMed] [Google Scholar]
- Whitworth KW, Haug LS, Baird DD, Becher G, Hoppin JA, Skjaerven R, et al. , 2012. Perfluorinated compounds and subfecundity in pregnant women. Epidemiology 23 (2), 257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schisterman EF, Cole SR, Platt RW, 2009. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology 20 (4), 488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromme H, Mosch C, Morovitz M, Alba-Alejandre I, Boehmer S, Kiranoglu M, et al. , 2010. Pre- and postnatal exposure to perfluorinated compounds (PFCs). Environ. Sci. Technol 44 (18), 7123–7129. [DOI] [PubMed] [Google Scholar]
- Papadopoulou E, Haug LS, Sabaredzovic A, Eggesbo M, Longnecker MP, 2015. Reliability of perfluoroalkyl substances in plasma of 100 women in two consecutive pregnancies. Environ. Res 140, 421–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Snow TA, Kemper RA, Jepson GW, 2003. Binding of perfluorooctanoic acid to rat and human plasma proteins. Chem. Res. Toxicol 16 (6), 775–781. [DOI] [PubMed] [Google Scholar]
- Kato K, Wong LY, Jia LT, Kuklenyik Z, Calafat AM, 2011. Trends in exposure to polyfluoroalkyl chemicals in the U.S. Population: 1999–2008. Environ. Sci. Technol 45 (19), 8037–8045. [DOI] [PubMed] [Google Scholar]
- Fromme H, Tittlemier SA, Volkel W, Wilhelm M, Twardella D, 2009. Per-fluorinated compounds – exposure assessment for the general population in Western countries. Int. J. Hyg. Environ. Health 212 (3), 239–270. [DOI] [PubMed] [Google Scholar]
- Weinberg CR, Wilcox AJ, 2008. Methodological issues in reproductive epidemiology In: Rothman KJ, Greenland S, Lash TL (Eds.), Modern Epidemiology. Lippincott Williams & Wilkins, Philadelphia, pp. 620–640. [Google Scholar]