Abstract
Development of a preventive strategy against tubular damage associated with proteinuria is of great importance. Recently, free fatty acid (FFA) toxicities accompanying proteinuria were found to be a main cause of tubular damage, which was aggravated by insufficiency of peroxisome proliferator-activated receptor alpha (PPARα), suggesting the benefit of PPARα activation. However, an earlier study using a murine acute tubular injury model, FFA-overload nephropathy, demonstrated that high-dose treatment of PPARα agonist (0.5% clofibrate diet) aggravated the tubular damage as a consequence of excess serum accumulation of clofibrate metabolites due to decreased kidney elimination. To induce the renoprotective effects of PPARα agonists without drug accumulation, we tried a pretreatment study using low-dose clofibrate (0.1% clofibrate diet) using the same murine model. Low-dose clofibrate pretreatment prevented acute tubular injuries without accumulation of its metabolites. The tubular protective effects appeared to be associated with the counteraction of PPARα deterioration, resulting in the decrease of FFAs influx to the kidney, maintenance of fatty acid oxidation, diminution of intracellular accumulation of undigested FFAs, and attenuation of disease developmental factors including oxidative stress, apoptosis, and NFκB activation. These effects are common to other fibrates and dependent on PPARα function. Interestingly, however, clofibrate pretreatment also exerted PPARα-independent tubular toxicities in PPARα-null mice with FFA-overload nephropathy. The favorable properties of fibrates are evident when PPARα-dependent tubular protective effects outweigh their PPARα-independent tubular toxicities. This delicate balance seems to be easily affected by the drug dose. It will be important to establish the appropriate dosage of fibrates for treatment against kidney disease and to develop a novel PPARα activator that has a steady serum concentration regardless of kidney dysfunction.
Keywords: Free fatty acid toxicity, Fibrates, Kidney disease, Proximal tubule, PPAR
Introduction
Tubulointerstitial damage is closely correlated with aggravation of kidney function in various kidney diseases, and existing tubular protective strategies are still insufficient (D’Amico, 1999). Therefore, it is of great importance to develop a novel beneficial therapy against tubulointerstitial damage. Recently, considerable attention has been paid to proteinuric toxicity as a causative factor associated with tubulointerstitial injury. Indeed, earlier studies have suggested that proteinuric toxicity is related to various macromolecules including free fatty acids (FFAs) (Kamijo et al., 2007a), albumin (Tang et al., 2003), transferrin (Chen et al., 1998), complement factors (Nangaku et al., 1999), and oxidized LDL (Ong and Moorhead, 1994). Among these molecules, we recently reported the importance of FFA toxicity accompanying proteinuria, using a murine experimental model called “protein-overload nephropathy” (Kamijo et al., 2007a). “Protein-overload nephropathy” is an established model frequently used for investigating the relationship between proteinuria and tubulointerstitial damage (Suzuki et al., 2001). In this model, massive proteinuria is artificially induced by excess increase of serum protein due to intraperitoneal administration of bovine serum albumin (BSA) without major glomerular injury; severe proximal tubular injuries develop as a consequence. In our earlier study, we used this model to compare FFA-binding BSA to FFA-free BSA, and demonstrated that FFAs were the main toxic molecules, as FFA-free BSA was less toxic. Overloading of FFAs is more important than that of protein itself for developing tubular injuries; therefore we defined the protein overload model using external BSA containing FFAs as “FFA-overload nephropathy (FAON)”. This earlier study also characterized the details of the FFA toxicities accompanying proteinuria. The excess amounts of FFAs binding to BSA are filtrated through the undamaged glomeruli, and reabsorbed via endocytosis into proximal tubular epithelial cells (PTECs). The absorbed FFAs are subjected to catabolism; however excess influx of FFAs caused accumulation of undigested FFAs in PTECs. Electron microscopic analyses demonstrated that excess loading of FFAs to PTECs induced mitochondrial swelling and rupture, nuclear shrinkage, disruption of the brush border, and the extravasation of cell contents. These organelle injuries resulted in vacuolation of PTECs, tubular dilation, tubular hyaline cast formation, detachment of PTECs from the tubular basement membrane, and an increase of tubular damage marker molecules including osteopontin and vimentin. Biochemical and pathological analyses also demonstrated that the increase of FFA influx to kidneys, intracellular accumulation of undigested FFAs, decreased fatty acid oxidation capacity, increased oxidative stress, promotion of apoptosis and activation of the NFκB signaling pathway in PTECs were closely related to tubular damage. These developments cause acute kidney dysfunction, resulting in urine volume reduction, insufficient excretion of urine protein induced by external BSA, systemic water retention, and accumulation of uremic toxin. With excess increase of body water, pulmonary congestion appeared and became fatal. By discontinuing FFA-overload treatment before reaching a lethal situation, these tubular changes were reversed with regenerative epithelial proliferation, and kidney function was recovered. The severity of the tubular damage could be controlled by the dosage of overloaded FFA.
Furthermore, we found that the acute tubular injury caused by FFA toxicity was enhanced in peroxisome proliferator-activated receptor alpha (PPARα)-null mice. PPARα, a member of the steroid/nuclear receptor superfamily, is a transcriptional regulator that regulates mitochondrial fatty acid oxidation, and its insufficiency is known to lead to a decrease in fatty acid oxidation (Kamijo et al., 2002; Aoyama et al., 1998; Watanabe et al., 2000). We demonstrated that the very low capacity for fatty acid oxidation in Ppara-null mice enhanced FFA toxicities in FAON. This result suggests that activation of PPARα might be a therapeutic option for kidney diseases with proteinuria.
Fibric acid derivatives (fibrates) are representative ligands of PPAR that are prescribed for the treatment of hyperlipidemia. Fibrates are relatively high-affinity ligands that bind to PPARα and increase the transcriptional activity of this receptor, resulting in enhanced fatty acid oxidation and a reduction of plasma triglyceride levels (Schoonjans et al., 1996).Therefore, fibrates might be beneficial agents for protecting PTECs from FFA toxicities accompanying proteinuria. To confirm this hypothesis, we previously tried an experiment using wild-type (WT) mice with FAON that were administered high-dose clofibrate (0.5% clofibrate-containing diet); however, this treatment could not exert significant tubular protective effects (Kamijo et al., 2007a). In this animal model, excess serum accumulation of fibrates occurred due to decreased drug elimination via kidney dysfunction. This drug accumulation was probably related to over-activation of PPARα and overproduction of reactive oxygen species (ROS), and disease progression of the treated mice. In order to prevent excess drug accumulation, we tried another experimental protocol using low-dose fibrates. Since the serum accumulation of fibrates had been reported to increase with kidney dysfunction, we employed a pretreatment protocol in which the fibrate treatment was started before an acute kidney injury episode. This study shows that stimulation of the PPARα function via pretreatment using low-dose fibrates may be useful for the prevention of acute tubulointerstitial injuries accompanying FFA toxicity of proteinuria.
Materials and methods
Animals and experimental design.
SV/129 female mice were used for this study (age, 26 weeks; bodyweight, 26 to 30 g). The mice were maintained in a facility free of specific pathogens, housed in a temperature- and light-controlled environment(25 °C; 12-h light/dark cycle) and given tap water ad libitum. All procedures were performed in accordance with the guidelines of Shinshu University, the National Institutes of Health, and the Association for Assessment and Accreditation of Laboratory Animal Care. The mice were divided into two groups, regular-diet and clofibrate-pretreatment groups (n = 14 for each group, respectively). A conventional rodent chow was crushed to powder, and 0.1% (drug weight/food weight) clofibrate was admixed. The clofibrate-pretreatment group was fed this 0.1% clofibrate-containing diet throughout the experimental period from two weeks before BSA injection. We measured body weight and daily food consumption every day using the Roden CAFE system (Oriental Yeast Co., Tokyo, Japan). Body weights did not change significantly throughout the experimental period, and did not differ between the regular-diet and clofibrate-pretreatment groups (day 0, 25.8±0.8 vs. 25.5±1.5 g; day 10, 25.3±0.8 vs. 25.0±1.6 g; day 17, 25.3±1.0 vs. 25.5±1.7 g for the regular-diet and clofibrate-diet group, respectively). Food consumption was also stable and similar in the regular-diet and clofibrate-pretreatment groups (day 0, 1.3±0.2 vs. 1.3±0.5; day 10, 1.4±0.6 vs. 1.2±0.7; day 17, 1.3±0.5 vs. 1.4±0.8 g/day/mouse for regular-diet and clofibrate-diet group, respectively). Using these data, the mean± SD of clofibrate dosage was calculated as 52.8±7.8 mg/kg body weight/ day. Clofibrate was obtained from Wako (Tokyo, Japan). Both groups of mice were given daily intraperitoneal bolus injections of 0.375 g FFAs-binding BSA in 1 ml sterile saline every day, for 17 consecutive days. BSA was obtained from Sigma Chemical (St. Louis, MO, catalog No. A4503, fraction V, albumin ≥96%). From this commercial data, calculated dosage of albumin was 0.360 to 0.375 g/day/mouse. FFAs content of the BSA (A4503) was calculated via the measurement of FFAs concentration in the BSA solution, and it was 2.56±0.37 μEq/g of A4503. Therefore, the dosage of FFAs was 0.96±0.14 μEq/day/ mouse. The concentration of endotoxins in the BSA solution was measured using a highly sensitive endotoxin-specific assay (ES-test; Wako, Osaka, Japan), and it was very low (18±3.4 pg/ml). No mice of either group died except for protocol sacrifice throughout the experimental period. The numbers of mice euthanized for analyses according to the protocol were as follows: n=3 for each group at day 0, n=5 for each group at day 10, and n=6 for each group at day 17.
Histopathological analyses.
Tissues from kidneys and livers in each group were fixed in 4% paraformaldehyde. Deparaffinized sections were stained with hematoxylin and eosin, periodic acid Schiff, or periodic acid-methenamine-silver. For semiquantitative histologic analyses, more than 20 randomly selected glomeruli from each kidney section were examined. To evaluate glomerular size, the diameters of glomeruli were measured using an objective micrometer. Degrees of tubulointerstitial damage were estimated using a scale that ranged from 0 to 3 (0, normal; 1, mild; 2, moderate; 3, severe), which was categorized as tubular dilatation, tubular atrophy, or tubular hyaline cast formation. Indices were calculated using the following formula: Index = (no × 0) + (n1 × 1) + (n2 × 2) + (n3 × 3) / Σn (Σn>20). These histopathologic analyses were performed in a blinded manner by two observers who were unaware of the study protocol. The MEBSTAIN Apoptosis Kit II (Medical & Biologic Laboratories, Nagoya, Japan) and streptavidin-conjugated peroxidase (DakoCytomation, Glostrup, Denmark) were used for TUNEL staining. Ten randomly selected microscopic fields magnified ×200 were examined for each section, and the mean number of TUNEL-positive cell nuclei per 1000 PTECs was determined for each mouse.
Immunoblot analyses.
Renal cortex extracts were subjected to SDS-polyacrylamide gel electrophoresis and then transferred to nitrocellulose membranes. The membranes were incubated with a primary antibody followed by incubation with an alkaline phosphatase-conjugated secondary antibody. Immunoblotting was performed using antibodies against rat long-chain acyl-CoA synthetase (LACS) (Shindo and Hashimoto, 1978), very long-chain acyl-CoA dehydrogenase (VLCAD) (Aoyama et al., 1993, 1995, 1989), mitochondrial trifunctional protein α and β subunits (TPα and TPβ) (Uchida et al., 1992), short chain-specific 3-ketoacyl-CoA thiolase (T1) (Miyazawa et al., 1980), peroxisomal bifunctional protein (PH) (Osumi and Hashimoto, 1980), peroxisomal thiolase (PT) (Miyazawa et al., 1980), and catalase (Furuta et al., 1986). Primary antibodies to glutathione peroxidase-1 (GPx-1), Cu,Zn-superoxide dismutase (SOD), Mn-SOD, Bcl-2, Bcl-xL, Bax, and tBid were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Band intensity was quantified densitometrically, normalized to that of actin, and subsequently expressed as fold changes relative to that of control mice (regular-diet group of mice at day 0).
Analyses of mRNA.
Analyses of mRNA were performed using quantitative real-time PCR. One microgram of total RNA, extracted from the renal cortex of each mouse in each group, was reverse-transcribed using oligo(dT) primers and Superscript reverse transcriptase (Invitrogen, Carlsbad, CA). The cDNAs were quantified with an ABI PRISM 7700 sequence detection system (-Applied Biosystems, Foster City, CA) using specific primers and SYBR Green double-stranded DNA binding dye I. The specific primers were designed as shown in Table 1. Glyceraldehyde-3-phosphate dehydrogenase was used as the internal control for PCR amplification.
Table 1.
Primer sequences for quantitative real-time PCR assay.
| Gene name | Primers | GenBank access no. |
|---|---|---|
| PPARα | Forward: 5′-CCTCAGGGTACCACTACGGAGT-3′ | NM_011144 |
| Reverse: 5′-GCCGAATAGTTCGCCGAA-3′ | ||
| PPARγ | Forward: 5′-TTCCACTATGGAGTTCATGCTTGT-3′ | NM_011146 |
| Reverse: 5′-TCCGGCAGTTAAGATCACACCTA-3′ | ||
| IκBα | Forward: 5′-GCCCCGCACAGCCATGTTTCAG-3′ | U36277 |
| Reverse: 5′-CATGGAGTCCAGGCCGCTGTCGTG-3′ | ||
| COX-2 | Forward: 5′-TGACCCCCAAGGCTCAAATATG-3′ | NM_011198 |
| Reverse: 5′-ACCCAGGTCCTCGCTTATGAT-3′ | ||
| ICAM1 | Forward: 5′-TCCGGACTTTCGATCTTCCA-3′ | M31585 |
| Reverse: 5′-GAGCTTCAGAGGCAGGAAACA-3′ | ||
| TNFα | Forward: 5′-CAGCCGATGGGTTGTACCTT-3′ | NM_013693 |
| Reverse: 5′-GTGGGTGAGGAGCACGTAGTC-3′ |
PPARα, peroxisome proliferator-activated receptor α; PPARγ, peroxisome proliferator-activated receptor γ; COX-2, cyclooxygenase 2; ICAM1, intercellular adhesion molecule 1; TNFα, tumor necrosis factor α.
Miscellaneous methods.
Throughout the experimental period, urine collections were carried out daily. Urine protein concentrations were measured as described previously (Kamijo et al., 2002). Serum urea nitrogen was measured with a clinical analyzer (i-STAT 200; i-STAT Corporation, East Windsor, NJ). Serum concentration and tissue content of protein were measured using a BCA protein assay kit (-Pierce, Rockford, IL). Serum concentrations and tissue contents of FFAs and total phospholipids (PL), and serum concentration of aspartate amino transferase (AST), alanine transaminase (ALT), and γ-glutamyltranspeptidase (γ-GTP) were measured using an assay kit purchased from Wako (Osaka, Japan). Serum concentrations and tissue contents of triglycerides (TG) and total cholesterol (TC) were measured using an assay kit purchased from Sekisui Medical (Tokyo, Japan). Fatty acid composition in BSA solution and that in serum and total lipid extraction of kidney were quantified by gas chromatography at the SRL Co. (Tokyo, Japan). Palmitic acid β-oxidation activity was measured as described previously (Aoyama et al., 1998). Renal contents of malondialdehyde and 4-hydroxynonenal (MDA/HNE) were measured using a colorimetric assay kit (Bioxytech LPO-586; Oxis International, Inc., Portland, OR, USA). Plasma concentrations of clofibric acid (clofibrate metabolite) were measured using HPLC as described elsewhere (Barra et al., 1994).
Statistical analysis
Analysis of significant differences with respect to the interactive effects of the two factors (fibrate treatment and FFA-overload treatment) was performed using one-way ANOVA. Throughout the manuscript, significant differences from the respective day 0 group are indicated with asterisks (*P<0.05, **P<0.01, and ***P<0.001) while significant differences between regular-diet and clofibrate-diet groups are indicated with number signs (#P<0.05, ##P<0.01, and ###P<0.001).
Results
Pretreatment by low-dose clofibrate prevents tubular damage caused by FFA-overload
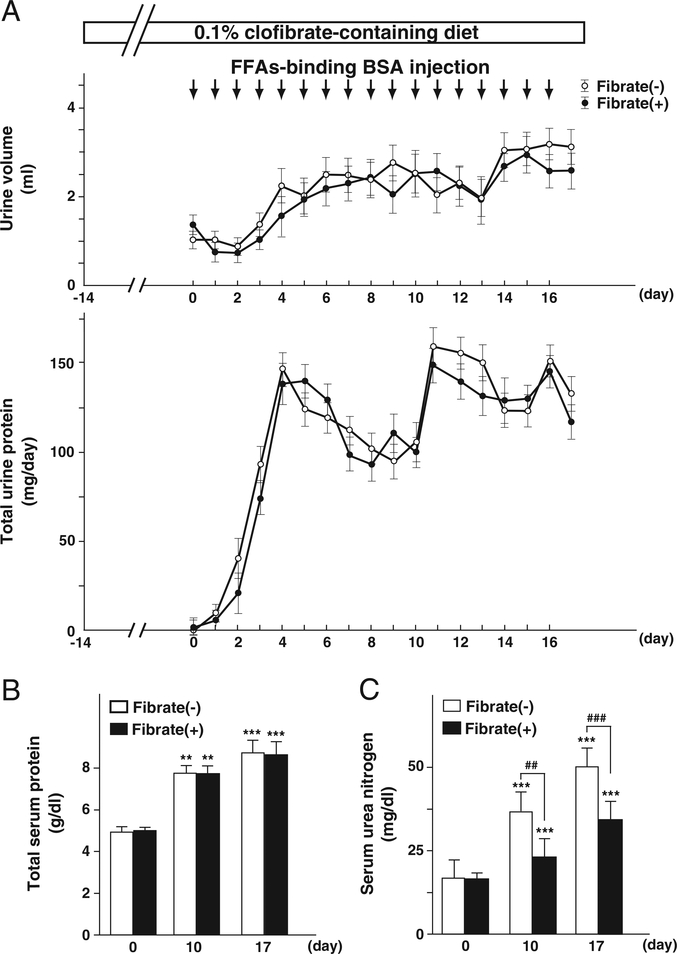
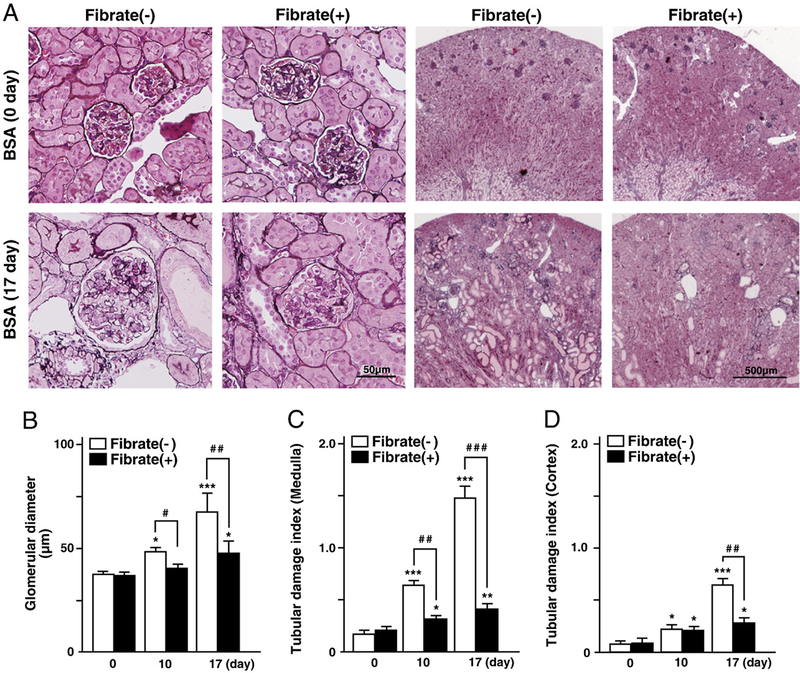
Daily intraperitoneal injections of FFAs-binding BSA were administered to regular-diet and clofibrate-pretreatment groups of mice for 17 consecutive days. Daily urine volume, daily urinary protein excretion, and total serum protein of both groups gradually increased with repeated injections, and were identical in the two diet groups (Figs. 1A and B). The protein contents in major organs in both groups, including kidney and liver, were not affected significantly by the BSA treatment (data not shown). Serum concentration of urea nitrogen was also increased in both diet groups; however, it was higher in the regular-diet group than the clofibrate-pretreatment group (Fig. 1C). To evaluate kidney damage, we carried out histopathological analyses. Light microscopy did not detect any glomerular pathological changes except for glomerular hypertrophy, suggesting secondary glomerular hyperfiltration. This pathological analysis revealed obvious tubular damage including tubular atrophy, tubular dilatation, and tubular hyaline cast formation in the regular-diet group at day 17, while these pathologic changes were not significantly observed in the clofibrate-pretreatment group (Fig. 2). These findings suggest that clofibrate pretreatment prevented the tubular damage caused by the FFAs-binding BSA injections. Throughout the experimental period, the plasma concentration of clofibric acid, a clofibrate metabolite, did not exceed toxic levels as evaluated in past studies (25±13 μg/ml for the pretreatment group before BSA injection, 63±26 μg/ml after BSA injection at day 10, 163±47 μg/ml at day 17). Serum hepatic damage markers including AST, ALT, and γ-GTP were not increased, and no pathological change of liver was detected in either group (data not shown), suggesting that hepatic damages were scarcely induced via the processes of FFAs-binding BSA injections and clofibrate treatment.
Fig. 1.
Systemic effects of low-dose clofibrate pretreatment in FFAs-binding BSA-injected mice. (A) Daily urine volume and daily urinary protein excretion. (B and C) Serum concentrations of total protein and urea nitrogen, respectively. The clofibrate-pretreatment group was fed a 0.1% clofibrate-containing diet from two weeks before daily FFAs-binding BSA injection. The start time point of FFAs-binding BSA injection was designated as day 0. Values represent means±SD (n = 3, 5, and 6 for each group at day 0, 10, and 17, respectively).
Fig. 2.
Light microscopic analyses of kidney injuries. (A) Representative micrographs of the kidney in FFAs-binding BSA-injected mice. Kidney sections were stained with periodic acid methenamine-silver (PAM). (B–D) Quantification of glomerular diameters and semiquantification of tubular damage indices (medulla and cortex). Values are means±SD (n=3 for each group at day 0; n=5 for each group at day 10; n=6 for each group at day 17).
Analyses of the tubular protective effects of clofibrate pretreatment
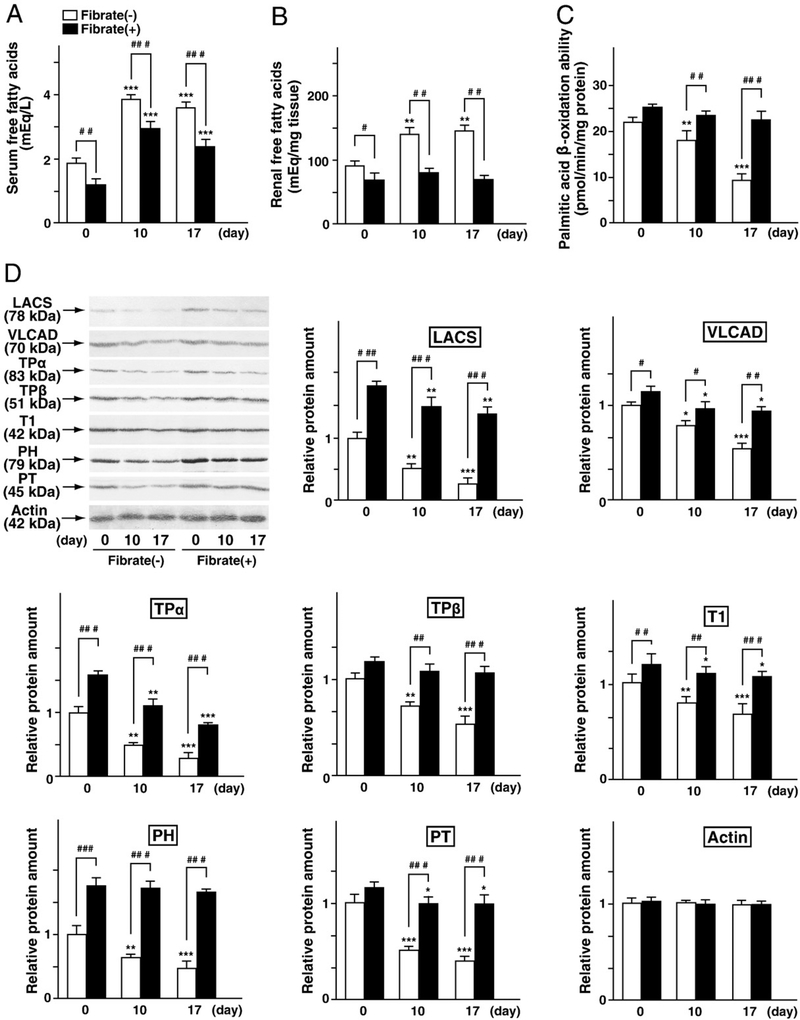
Earlier studies have suggested that tubular injuries caused by FFA-overload were enhanced via PPARα deficiency followed by an increase of FFA influx to the kidney, decrease of fatty acid oxidation, intracellular accumulation of undigested FFAs, increased oxidative stress, increased apoptosis, and activation of NFκB signaling (Kamijo et al., 2007a). Therefore, these factors were examined. Initially, alterations of lipids in serum and kidney were compared in the regular-diet and the clofibrate-pretreatment groups. The concentrations of FFAs and TG in the serum and kidney at day 0 (at the start point of BSA injection) were lower in the clofibrate-pretreatment group than in the regular-diet group (Figs. 3A and B and Table 2). The concentrations of PL and TC at day 0 did not differ significantly between the two diet groups (Table 2). FFAs-binding BSA injections caused a marked increase in the serum and renal FFA concentrations in the regular-diet group at days 10 and 17; however, these changes were suppressed in the clofibrate-pretreatment group (Figs. 3A and B). FFAs-binding BSA injections did not affect the concentrations of TG, PL and TC, indicating that the FFAs-binding BSA injections certainly introduced overloading of FFAs (Figs. 3A and B and Table 2). The FFAs binding to BSA included palmitic acid (16:0), stearic acid (18:0), oleic acid (18:1), and linoleic acid (18:2), and their composition was approximately similar to the composition of fatty acids in serum and lipid extraction of kidney tissue (Table 3). Clofibrate pretreatment and the FFA-overload treatment scarcely affected the fatty acid composition of the serum and kidney. These results suggest that clofibrate pretreatment and FFA-overload treatment caused marked quantitative changes of FFAs, but not qualitative changes, in the serum and kidney. Fatty acid β-oxidation ability at day 0 was identical in the two diet groups (Fig. 3C). The renal contents of some of the fatty acid catabolic enzymes (representative PPARα target genes) at day 0 were increased in the clofibrate pretreatment diet groups (Fig. 3D). FFA-overload treatment decreased fatty acid oxidation and protein levels of these enzymes in the regular-diet group in a time-dependent manner, while these reductions were attenuated in the clofibrate-pretreatment group (Figs. 3C and D). These findings suggest that low-dose clofibrate pretreatment allowed for the maintenance of renal fatty acid catabolism.
Fig. 3.
Analysis of renal fatty acid metabolism. (A and B) Serum concentration and renal content of FFAs in FFAs-binding BSA-injected mice, respectively. (C) Palmitic acid β-oxidation capacity in the renal cortex. Values are means±SD (n=3 for each group at day 0; n=5 for each group at day 10; n=6 for each group at day 17). (D) Immunoblot analyses of fatty acid-metabolizing enzymes, including LACS, VLCAD, TPα, TPβ, T1, PH, and PT. Renal cortical lysates (20 μg protein) from all kidneys of each group were used. Immunoblots were performed in triplicate. Band intensity was quantified densitometrically, normalized to that of actin, and subsequently expressed as fold changes relative to that of control mice (regular-diet group at day 0).
Table 2.
Serum concentration and kidney tissue contents of triglyceride, total cholesterol, and total phospholipids in regular-diet and fibrate-diet groups of mice.
| Regular-diet |
Fibrate-diet |
|||
|---|---|---|---|---|
| Day 0 | Day 17 | Day 0 | Day 17 | |
| Serum | ||||
| Triglyceride (mg/dl) | 88.6±8.5 | 76.4±7.9 | 59.3±6.7## | 56.9±9.2## |
| Total cholesterol (mg/dl) | 128.6±12.7 | 133.8±16.6 | 131.6±13.8 | 132.4±18.5 |
| Phospholipid (mg/dl) | 129.4±13.7 | 132.3±15.2 | 124.8±14.5 | 131.7±11.9 |
| Kidney | ||||
| Triglyceride (mg/g tissue) | 5.6±1.4 | 5.2±1.4 | 4.1±1.9# | 3.9±1.2# |
| Total cholesterol (mg/g tissue) |
3.9±1.9 | 4.2±1.7 | 4.3±1.9 | 3.9±1.8 |
| Phospholipid (mg/g tissue) | 14.5±2.5 | 13.8±3.4 | 13.9±4.6 | 14.3±3.5 |
Values represent means±SD.
Table 3.
Fatty acid composition in BSA solution, and that in serum and total lipid extraction of kidney of regular-diet and fibrate-diet group of mice.
| BSA in saline | Serum |
Kidney |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Regular-diet |
Fibrate-diet |
Regular-diet |
Fibrate-diet |
|||||||
| Name | Day 0 | Day 17 | Day 0 | Day 17 | Day 0 | Day 17 | Day 0 | Day 17 | ||
| Lauric acid | C12:0 | >0.01 | 1.11±0.29 | 0.89±0.59 | 0.99±0.33 | 1.07±0.44 | 1.04±0.61 | 1.16±0.74 | 0.99±0.76 | 0.96±0.44 |
| Myristic acid | C14:0 | 0.16 | 1.28±0.83 | 1.32±0.42 | 0.89±0.44 | 1.18±0.37 | 1.08±0.59 | 0.89±0.30 | 0.73±0.34 | 0.79±0.21 |
| Palmitic acid | C16:0 | 18.2 | 14.23±0.76 | 16.98±0.45 | 15.28±0.66 | 12.59±0.26 | 23.75±3.89 | 24.03±2.35 | 23.83±4.81 | 24.38±2.39 |
| Palmitoleic acid | C16:1 | 2.3 | 2.34±0.25 | 2.26±0.64 | 2.66±0.36 | 2.42±0.24 | 6.35±1.37 | 4.11±2.64 | 4.36±2.49 | 4.74±1.22 |
| Stearic acid | C18:0 | 24.4 | 18.64±0.19 | 19.22±0.78 | 17.98±0.19 | 22.23±0.48 | 6.84±1.58 | 7.74±1.85 | 6.96±2.47 | 7.67±2.37 |
| Oleic acid | C18:1 | 22.6 | 16.43±0.37 | 17.99±0.86 | 16.68±0.27 | 18.81±0.74 | 25.78±4.62 | 26.03±6.33 | 25.63±5.80 | 26.16±8.47 |
| Linoleic acid | C18:2 | 19.2 | 14.82±0.61 | 11.38±0.53 | 13.47±0.16 | 13.49±0.31 | 20.05±3.59 | 21.27±2.18 | 20.49±6.22 | 21.04±4.67 |
| r-Linolenic acid | C18:3 | >0.01 | 0.23±0.68 | 0.56±0.14 | 0.42±0.09 | 0.28±0.28 | 1.98±0.80 | 1.53±0.69 | 2.15±1.37 | 1.31±0.81 |
| Linolenic acid | C18:3 | >0.01 | 0.32±0.32 | 0.16±0.19 | 0.44±0.22 | 0.28±0.15 | 0.31±0.31 | 0.42±0.28 | 0.72±0.13 | 0.37±0.32 |
| Arachidic acid | C20:0 | >0.01 | 0.23±0.16 | 0.34±0.25 | 0.28±0.14 | 0.38±0.28 | 1.91±0.22 | 1.37±0.18 | 1.83±0.26 | 1.37±0.17 |
| Eicosenoic acid | C20:1 | >0.01 | 0.48±0.38 | 0.55±0.24 | 0.51±0.27 | 0.32±0.19 | 0.89±0.28 | 0.43±0.11 | 0.79±0.26 | 0.70±0.33 |
| Eicosadienoic acid | C20:2 | >0.01 | 0.19±0.11 | 0.12±0.13 | 0.22±0.09 | 0.37±0.51 | 0.68±0.36 | 0.36±0.34 | 0.61±0.29 | 0.33±0.10 |
| Dihomo-r-linolenic acid | C20:3 | 1.12 | 1.53±0.64 | 1.43±0.52 | 1.39±0.48 | 1.69±0.44 | 0.82±0.27 | 0.67±0.36 | 0.76±0.51 | 0.64±0.47 |
| Arachidonic acid | C20:4 | 8.1 | 16.88±0.87 | 15.82±0.48 | 17.39±0.85 | 14.73±0.54 | 3.31±1.36 | 4.36±1.61 | 3.18±2.36 | 3.80±1.67 |
| Eicosapentaenoic acid | C20:5 | >0.01 | 0.75±0.27 | 0.68±0.16 | 0.79±0.46 | 0.81±0.34 | 0.74±0.41 | 0.95±0.55 | 0.82±0.48 | 0.62±0.61 |
| Behenic acid | C22:0 | >0.01 | 0.21±0.64 | 0.18±0.17 | 0.37±0.38 | 0.26±0.27 | 0.16±0.13 | 0.32±0.34 | 0.57±0.18 | 0.62±0.23 |
| Erucic acid | C22:1 | 1.31 | 0.15±0.14 | 0.26±0.23 | 0.13±0.17 | 0.37±0.20 | 0.21±0.15 | 0.18±0.26 | 0.34±0.18 | 0.23±0.24 |
| Docosatetranoic acid | C22:4 | >0.01 | 0.25±0.78 | 0.12±0.66 | 0.17±0.53 | 0.21±0.48 | 0.15±0.37 | 0.28±0.41 | 0.46±0.32 | 0.27±0.28 |
| Docosapentanoic aid | C22:5 | >0.01 | 0.35±0.36 | 0.23±0.32 | 0.27±0.18 | 0.41±0.26 | 0.19±0.16 | 0.26±0.37 | 0.36±0.25 | 0.34±0.18 |
| Lignoceric acid | C24:0 | >0.01 | 0.16±0.84 | 0.37±0.36 | 0.21±0.28 | 0.18±0.14 | 0.27±0.23 | 0.12±0.27 | 0.17±0.34 | 0.26±0.43 |
| Docosahexaenoic acid | C22:6 | >0.01 | 5.18±0.31 | 3.87±0.74 | 4.89±0.36 | 5.29±0.58 | 0.57±0.83 | 0.37±0.37 | 0.75±0.81 | 0.90±0.59 |
| Nervonic acid | C24:1 | >0.01 | 0.79±0.58 | 0.68±0.12 | 0.57±0.08 | 0.74±0.25 | 0.66±0.06 | 0.53±0.14 | 0.89±0.11 | 0.74±0.03 |
Fatty acid composition was indicated as a percentage of total fatty acids, which was calculated using area counts of the chromatogram.
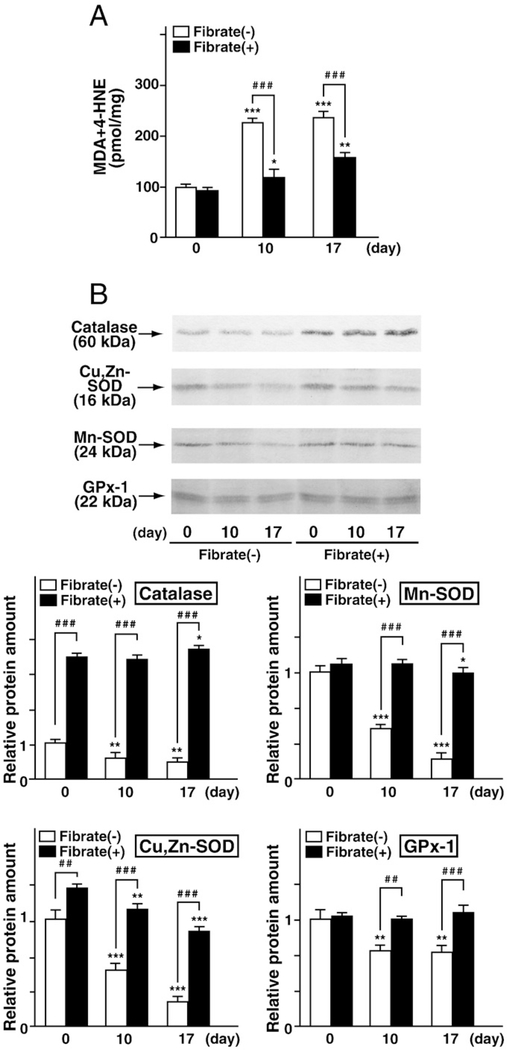
Next, renal oxidative stress was examined in both diet groups. Clofibrate pretreatment did not increase the renal contents of MDA/ HNE, a representative lipid peroxidation marker, at day 0. FFA-overload treatment increased this oxidative stress marker in both diet groups; however, the increase was moderated in the clofibrate-pretreatment group (Fig. 4A). Immunoblot analyses demonstrated that FFA-overload treatment decreased the expression of antioxidant enzyme proteins (i.e., catalase, Cu,Zn-SOD, Mn-SOD, and GPx-1) in the regular-diet group in a time-dependent manner. In the clofibrate-pretreatment group, the renal contents of several antioxidant enzymes, such as catalase and Cu,Zn-SOD, were increased at day 0, while that of Mn-SOD and GPx-1 was not affected by clofibrate pretreatment. This enhanced antioxidant status was maintained despite FFA-overload (Fig. 4B). These findings suggest that clofibrate pretreatment might exert antioxidative effects in the kidney.
Fig. 4.
Analysis of oxidative stress. (A) Quantification using a colorimetric assay of the lipid peroxidation marker malondialdehyde (MDA) with 4-hydroxynonenal (HNE). (B) Relative quantification using immunoblot analyses of antioxidant enzymes, including catalase, Cu, Zn-SOD, Mn-SOD, and GPx-1. Five micrograms of renal cortical lysate protein was used for catalase determinations; 20 μg of lysate protein was used for determination of other antioxidant enzymes. Immunoblotting and densitometric analyses were carried out in triplicate. Each protein amount was normalized to that of actin (see Fig. 3), and protein amounts relative to that of control mice are indicated. Values represent means±SD.
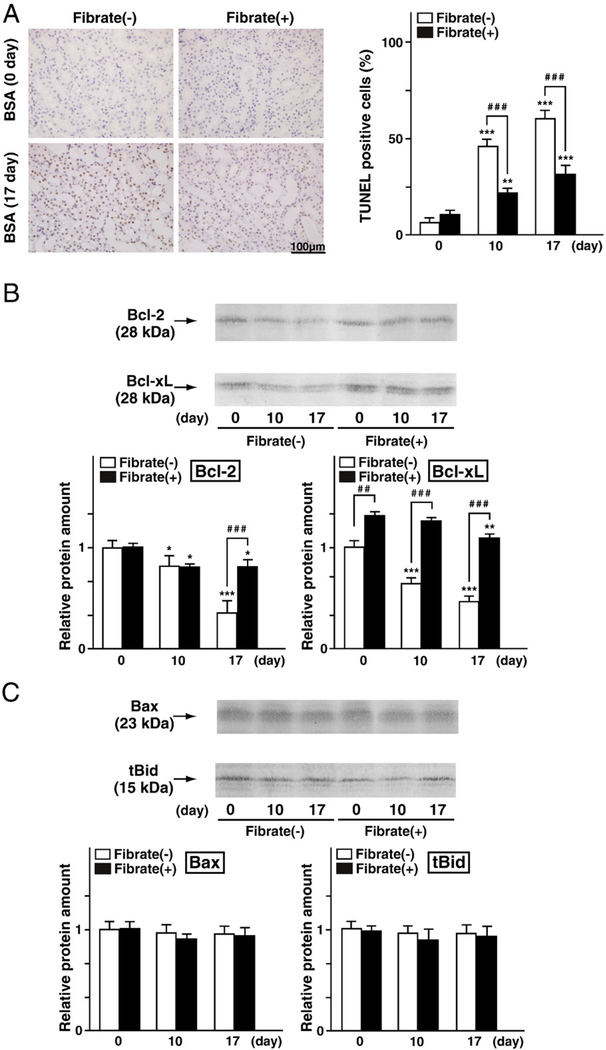
To examine apoptosis, TUNEL staining was carried out. Clofibrate pretreatment did not increase the number of TUNEL-positive PTECs at day 0. FFA-overload markedly increased the number of positive cells in the regular-diet group at days 10 and 17; this effect was moderated in the clofibrate-pretreatment group (Fig. 5A). To obtain biochemical confirmation of the apoptosis, the renal contents of Bcl-2 related proteins (Bcl-2 and Bcl-xL, antiapoptotic proteins; Bax and tBid, apoptosis-stimulatory proteins) were examined. Clofibrate pretreatment increased the renal contents of Bcl-xL, and did not affect those of the other enzymes at day 0. FFA-overload reduced the renal contents of Bcl-2 and Bcl-xL in the regular-diet group at days 10 and 17, while these reductions were moderated in the clofibrate-pretreatment group (Fig. 5B). FFA-overload induced no changes in the levels of Bax or tBid in either diet group (Fig. 5C). These findings suggest that clofibrate pretreatment might exert an antiapoptotic function in the kidney.
Fig. 5.
Analysis of apoptosis. (A) Representative light micrographs of sections stained by the TUNEL method. Percentages of TUNEL-positive cells in each group are indicated. (B) Relative quantification using immunoblot analyses of antiapoptotic proteins, including Bcl-2 and Bcl-xL. (C) Relative quantification using immunoblot analyses of apoptosis-stimulating proteins, including Bax and tBid. For immunoblotting, renal corticallysates (40 μg protein) from all kidneys from each group were used. Immunoblots and densitometric analyses were carried outin triplicate. Each protein amount was normalized to that of actin (see Fig. 3), and protein amounts relative to that of control mice are indicated. Values represent means±SD (n = 3 for each group at day 0; n=5 for each group at day 10; n=6 for each group at day 17).
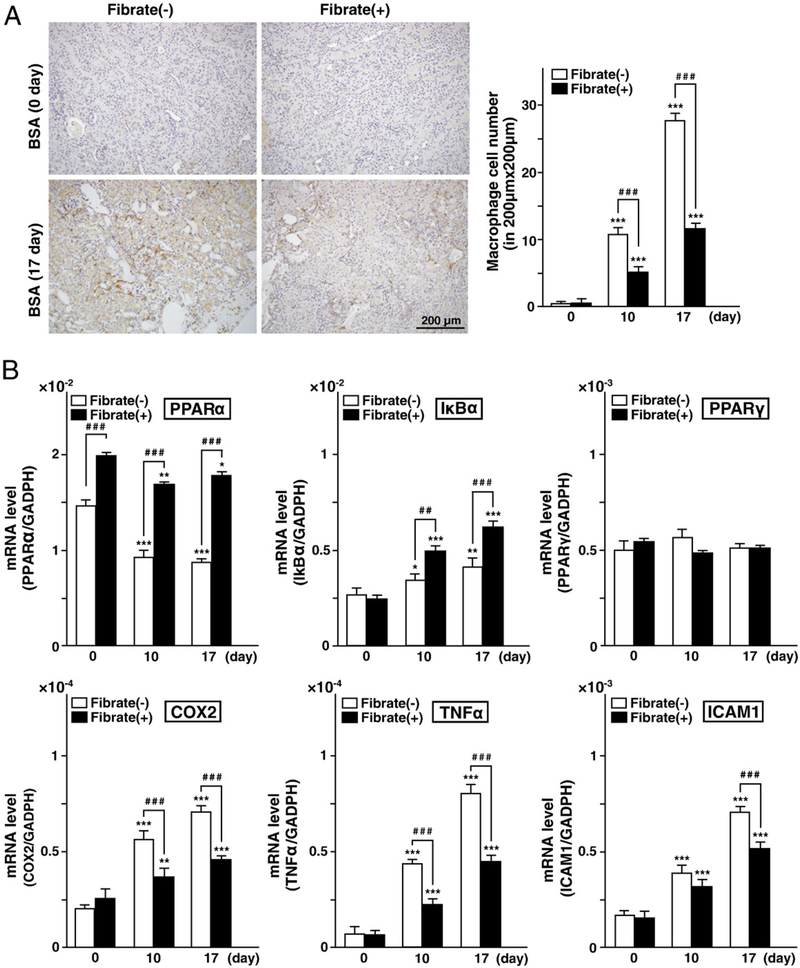
Finally, to evaluate the renal inflammatory cell invasion, an immunohistochemical analysis was conducted. Clofibrate pretreatment did not increase the number of macrophages in the interstitial area at day 0. FFA-overload markedly increased the number of macrophages in the regular-diet group at days 10 and 17, but this effect was moderated in the clofibrate-pretreatment group (Fig. 6A). Since renal expression of PPARα has been reported to inhibit the NFκB signaling pathway via induction of IκBα expression (Kamijo et al., 2007a, 2007b; Kono et al., 2009), PPARα and IκBα expressions were examined. Renal expression of PPARα in the regular-diet group was decreased in a time-dependent manner, indicating PPARα deterioration caused by renal injuries (Fig. 6B). On the other hand, clofibrate pretreatment increased PPARα expression at day 0, and maintained this elevated expression in spite of repeated FFA-overload. IκBα mRNA was increased in both diet groups at days 10 and 17, with a higher increase in the clofibrate-pretreatment group. Renal expression of PPARγ, which was also reported to have an effect of suppressing the NFκB signaling pathway, was not changed, while levels of mRNAs encoding target molecules of NFκB signaling (i.e., cyclooxygenase2 [COX2], tumor necrosis factor-α [TNFα], and intercellular adhesion molecule 1 [ICAM1]) showed increases in both diet groups at days 10 and 17 (Fig. 6B). However, these changes were moderated in the clofibrate-pretreatment group. These findings suggest that clofibrate pretreatment could maintain renal expression of PPARα, which in turn moderated the renal NFκB signaling pathway by upregulation of IκBα.
Fig. 6.
Analysis of inflammation. (A) Immunohistochemical analyses of renal tissues. Renal sections were stained for the macrophage marker F4/80. The numbers of macrophages of each group of mice are indicated. (B) mRNAs were obtained from all kidneys in each group of mice. Expression of mRNAs for factors related to the NFκB signaling pathway, including PPARα, PPARγ, IκBα, COX2, ICAM1, and TNFα, were measured with real-time PCR. GAPDH mRNA was used as an internal control. Amounts of mRNA are indicated as target gene copy number/GAPDH copy number. PCR reactions were carried out in triplicate. Values represent means±SD.
Tubular protective effects of other fibrates
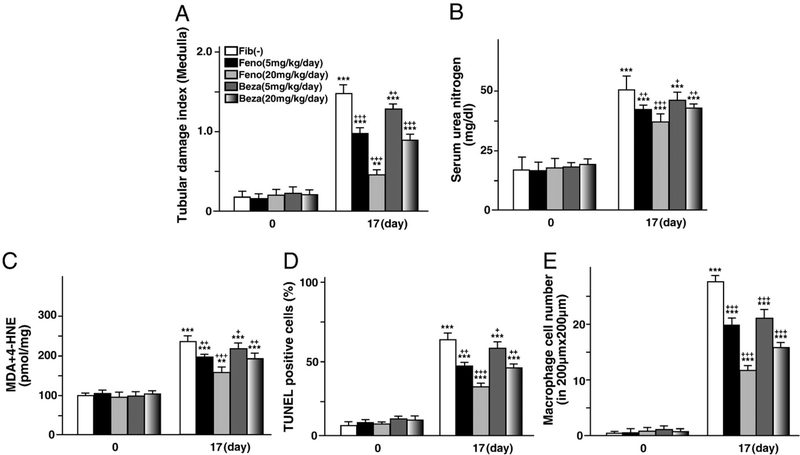
We next examined whether pretreatment using other types of fibrates would exert similar effects. We tested fenofibrate (PPARα-specific ligand, from Wako, Osaka, Japan), and bezafibrate, (PPARα and PPARβ ligand, from Kissei, Matsumoto, Japan). These fibrates (low dose, 5 mg/kg body weight; medium dose, 20 mg/kg body weight) were administered once daily by gavage from two weeks before FFA-overload treatment. The procedure of FFA-overload was the same as in the clofibrate experiment. Pretreatments of neither fibrate induced tubular damage, oxidative stress, apoptosis, or activation of NFκB signaling pathway at day 0. At day 17, both fibrate pretreatments exerted tubular protective effects in a dose-dependent manner, with the effects of fenofibrate tending to be stronger than those of bezafibrate (Fig. 7). These findings suggested that tubular protective effects are common to various types of fibrates, and that these effects might be dependent on PPARα activation.
Fig. 7.
Tubular protective effects of other fibrates including fenofibrate and bezafibrate. (A–E) Alterations of tubular damage, kidney function, oxidative stress, apoptosis and inflammation via pretreatment of fenofibrate or bezafibrate, respectively. Each fibrate was administered once daily by gavage (low dose, 5 mg/kg body weight; medium dose, 20 mg/kg body weight) from two weeks before FFAs-binding BSA injections. The start time point of FFAs-binding BSA injection is designated as day 0. The procedures of FFA-overload treatment and assessment of disease developmental factors were the same as in the clofibrate experiment. Values represent means±SD (n=5 for each group at day 0; n=5 for each group at day 17).
Tubular protective effects of clofibrate treatment were based on a PPARα-dependent mechanism
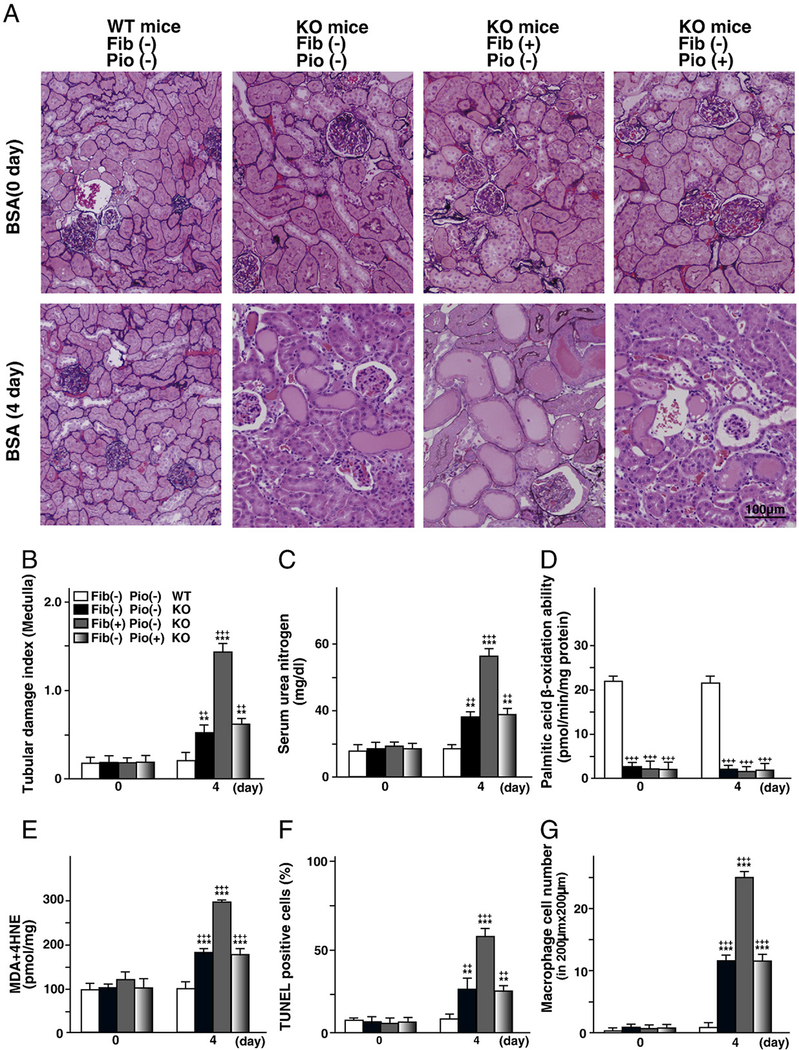
To determine whether these favorable properties of fibrates were dependent on PPARα, the effects of the clofibrate pretreatment on Ppara-null mice (SV129 genetic background, Lee et al., 1995) with FAON were examined. Since clofibrate is known to be a pan-agonist of PPARα and PPARγ (Willson et al., 2000), the effects of pretreatment by the PPARγ agonist pioglitazone were also examined in these mice. Ppara-null mice were fed a 0.1% clofibrate-containing or 0.05% pioglitazone-containing diet from two weeks before FFA-overload treatment. It was reported that Ppara-null mice were susceptible to FFA toxicity accompanying proteinuria, and that FFA-overload markedly decreased the survival rate in Ppara-null mice until day 4 (Kamijo et al., 2007a). Therefore, a reduced amount of FFAs-binding BSA (0.325 g/day) was administered to each group of mice for 4 days. Pretreatment by either agonist did not exert significant effects at day 0, and did not suppress the tubular damages caused by FFA-overload treatment at day 4. Surprisingly, the clofibrate-pretreatment group of Ppara-null mice exhibited more obvious tubular damage than the control group (Figs. 8A to C). The fatty acid oxidation capacity of the clofibrate-pretreatment group of Ppara-null mice was identical to that of other groups of Ppara-null mice (Fig. 8 D), while the oxidative stress, apoptosis and inflammation of this group were obviously increased (Figs. 8 E to G). These findings suggest that the beneficial effects of clofibrate pretreatment are dependent on PPARα function, and that the treatment might induce PPARα-independent tubular toxicity in the PPARα-deficient animals. Clofibrate might exert double action, PPARα-dependent tubular protective effects and PPARα-independent tubular toxic effects.
Fig. 8.
The absence of tubular protective effects of the pretreatment using clofibrate or pioglitazone in Ppara-null mice with FAON. (A–G) Alterations of pathologic findings, tubular damage, kidney function, fatty acid metabolism ability, oxidative stress, apoptosis and inflammation via pretreatment of clofibrate or pioglitazone in Ppara-null mice with FAON, respectively. Ppara-null mice were fed a 0.1% clofibrate-containing or 0.05% pioglitazone-containing diet from two weeks before FFAs-binding BSA injections. The start time point of FFAs-binding BSA injection is designated as day 0. A reduced amount of FFAs-binding BSA (0.325 g/day) was administered to each group for 4 days. The assessment procedure for disease developmental factors was the same as in the clofibrate experiment using wild-type (WT) mice. Data for the regular-diet WT mice, regular-diet Ppara-null mice, clofibrate-diet Ppara-null mice, and pioglitazone-diet Ppara-null mice at days 0 and 4 are indicated. Values represent means±SD (n = 3 for each group at day 0; n = 5 for each group at day 4).
Discussion
This study demonstrated that pretreatment using low-dose clofibrate reduced acute tubular injury caused by FFA-overload through a PPARα-dependent mechanism. Prevention of tubular injury appeared to be associated with the reduction of FFA influx, maintenance of fatty acid catabolism, diminution of intracellular accumulation of FFAs and suppression of oxidative stress, apoptosis, and NFκB activation.
FFAs-binding BSA injections led to increased levels of serum FFAs and the decrease of fatty acid oxidation in the regular-diet group of WT mice, resulting in excessive intracellular accumulation of FFAs. On the other hand, these metabolic changes were attenuated in the clofibrate-pretreatment group of WT mice. Since FFAs-binding BSA introduces many external FFAs, its injection causes a higher serum level of FFAs. Fibrate treatment is known to decrease serum FFA levels due to stimulation of intracellular catabolism of fatty acids in various organs including the liver, skeletal muscle and kidney (Kersten et al., 2000). Many studies have demonstrated that these metabolic effects of fibrates are mediated by transcriptional activation of PPARα, which enhances the expression of the fatty acid transport protein (FATP) (Martin et al., 1997), LACS (acyl-CoA conversion protein) (Schoonjans et al., 1995), and several mitochondrial and peroxisomal enzymes involved in fatty acid β-oxidation (Aoyama et al., 1998). Therefore, the decrease of serum FFA levels in the clofibrate-pretreatment group of WT mice would be derived from systemic PPARα activation. The reduction of serum FFAs might reduce the oversupply of FFAs to the kidney. An earlier study indicated that FFA-overload treatment also led to a reduction in levels and activities of renal fatty acid metabolic enzymes including LACS and β-oxidation enzymes, a consequence of tubular mitochondrial damage (Kamijo et al., 2007a). The genes encoding these enzymes are transcriptional targets of PPARα; therefore, PPARα activation via clofibrate pretreatment appeared to maintain the amounts of these enzymes. These results suggested that PPARα activation via clofibrate pretreatment would suppress the influx of FFAs to kidney, maintain fatty acid catabolism in PTECs, and diminish the intracellular accumulation of FFAs in PTECs.
A previous study demonstrated that severe fatty acid catabolic insufficiency in Ppara-null mice with FAON caused intracellular accumulation of undigested FFAs (Kamijo et al., 2007a). These accumulated FFAs are prone to react with ROS generated through mitochondrial energy production, resulting in obvious lipid peroxide toxicity (Schrauwen and Hesselink, 2004). Furthermore, Ppara-null mice have a lower anti-oxidative capacity than WT mice, and therefore are exposed to more oxidative stress and tubular injury (Kamijo et al., 2007a). These results indicated that PPARα exerts antioxidative effects, as well as maintaining FFA influx and catabolism in PTECs. The present study demonstrated that clofibrate pretreatment increased or maintained the amount of antioxidant enzyme proteins in the renal cortex. For example, an increase of catalase protein was prominent in the clofibrate-pretreatment group of WT mice. Indeed, catalase is known to be expressed at higher levels in the kidney compared to other organs (Ishikawa et al., 1986), and this enzyme lowers hydrogen peroxide levels and thereby reduces the cellular injury caused by oxidative stress. Two recent studies reported that catalase deficiency enhanced oxidative stress in PTECs in a murine unilateral ureteral obstruction model and 5/6 nephrectomy model, followed by remarkable tubulointerstitial injury, fibrosis, and tubular cell apoptosis (Sunami et al., 2004; Kobayashi et al., 2005). Another study demonstrated that increased catalase activity inhibited cisplatin-induced nephrotoxicity (Ma et al., 2007). These studies indicated that catalase is one of the most important antioxidant enzymes for protecting PTECs from oxidative stress. It was reported that catalase promoters possess PPAR response elements and that PPARα is a main regulator of catalase gene expression (Girnun et al., 2002). Therefore, the induction of catalase through PPARα activation via clofibrate pretreatment might be involved in the inhibition of tubulointerstitial injury caused by FFA toxicity observed in this study. On the other hand, high-dose clofibrate pretreatment (0.5% clofibrate-containing diet) is reported to cause aggravation of tubular injuries in WT mice with FAON (Kamijo et al., 2007a). Some earlier studies reported that high-dose fibrate treatment could exert unfavorable effects through an overproduction of ROS due to a PPARα-dependent mechanism, such as induction of PPARα-regulated ROS-generating enzymes (acyl-CoA oxidase, cytochrome P450 4A, and NADPH oxidase) and enhancement of mitochondrial fatty acid β-oxidation (Kamijo et al., 2007a; Nakajima et al., 2010). Furthermore, the present study revealed the PPARα-independent toxicity of clofibrate-enhancing oxidative stress. These unfavorable actions of clofibrate might have outweighed its beneficial effects in the case of a high-dose treatment study. At this time, the mechanism of PPARα-independent toxicity via clofibrate treatment is unknown. Further analysis will be needed in the future.
Many earlier studies reported that FFA-overload led to the induction of tubular cell apoptosis (Arici et al., 2003). Our previous study demonstrated that this type of cell death was remarkably induced in Ppara-null mice under FAON, as these mice had lower expression of antiapoptotic proteins such as Bcl-2 and Bcl-xL (Kamijo et al., 2007a). The present study demonstrated that clofibrate pretreatment maintained the amount of antiapoptotic proteins in the renal cortex of WT mice on FAON. Several earlier studies also demonstrated antiapoptotic effects of PPARα ligands in the liver and kidney (Roberts et al., 2002). A study using cisplatin-treated mice further demonstrated that fibrate treatment could attenuate acute renal injury through the maintenance of the expressions of these antiapoptotic proteins (Nagothu et al., 2005). These results all point to the antiapoptotic effects of fibrates in the kidney.
It is well known that PPARα exerts suppressive effects on the NFκB signaling pathways in many organs via induction of IκBα expression (Kamijo et al., 2007b; Kono et al., 2009; Delerive et al., 2001; Li et al., 2005). A previous study reported that Ppara-null mice with FAON exhibited greater activation of the NFκB signaling pathway via the absence of IκBα induction, resulting in enhanced tubular inflammation (Kamijo et al., 2007a). The present work revealed that clofibrate treatment increased IκBα mRNA expression and inhibited the increased expression of mRNAs encoding NFκB target genes (i.e., COX2, TNFα, and ICAM1) in WT mice under FAON. These results suggest that fibrates can exert PPARα-dependent suppression of the NFκB signaling pathway in the kidney.
Taken together, the current results suggest that low-dose clofibrate pretreatment exerts a protective function against acute FFA toxicities via a PPARα-dependent mechanism. A very recent study using transgenic mice also demonstrated that over expression of tubular PPARα attenuates acute kidney injury caused by ischemia– reperfusion injury or treatment with cisplatin (Li et al., 2009). These favorable properties of PPARα activation are evident when the PPARα-dependent tubular protective effects outweigh the tubular toxicities of fibrates. This delicate balance seems to be easily affected by the drug dose. Most fibrates are excreted through the kidney, and kidney dysfunction causes the accumulation of the drugs, which can result in tubular damage (Lipscombe et al., 2001). Furthermore, there are known to be species differences in PPARα activation via fibrate treatment between rodents and humans (Gonzalez et al., 1998). Therefore, it will be important to establish the appropriate dosage of fibrates for treatment against human kidney disease. In the future, the development of a novel PPARα activator that has a steady serum concentration regardless of kidney dysfunction will be needed.
Acknowledgments
We would like to thank Matsuko Watanabe (Shinshu University School of Medicine, Japan) for assistance with pathologic analyses.
Abbreviations:
- AST
aspartate amino transferase
- ALT
alanine transaminase
- BSA
bovine serum albumin
- COX2
cyclooxygenase 2; FAON, free fatty acid overload nephropathy
- FATP
fatty acid transport protein
- FFA
free fatty acid
- Fibrates
fibric acid derivatives
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GPx-1
glutathione peroxidase-1
- γ-GTP
γ-glutamyltranspeptidase
- HNE
4-hydroxynonenal
- ICAM1
intercellular adhesion molecule 1
- LACS
long-chain acyl-CoA synthetase
- LDL
lowdensity lipoprotein
- MDA
malondialdehyde
- NFκB
nuclear factor-kappa B
- PAM
periodic acid-methenamine-silver
- PCR
polymerase chain reaction
- PH
peroxisomal bifunctional protein
- PL
total phospholipids
- PPAR
peroxisome proliferator-activated receptor
- PT
peroxisomal thiolase
- PTECs
proximal tubular epithelial cells
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- TC
total cholesterol
- TG
triglycerides
- TNFα
tumor necrosis factor α
- T1
short chain-specific 3-ketoacyl-CoA thiolase
- TPα and TPβ
mitochondrial trifunctional protein α and β subunits
- VLCAD
very long-chain acyl-CoA dehydrogenase
Footnotes
Conflict of interest statement
The authors declare that there are no conflicts of interest.
References
- Aoyama T, Yamano S, Waxman DJ, Lapenson DP, Meyer UA, Fischer V, Tyndale R, Inaba T, Kalow W, Gelboin HV, Gonzalez FJ, 1989. Cytochrome P-450 hPCN3, a novel cytochrome P-450 IIIA gene product that is differentially expressed in adult human liver. cDNA and deduced amino acid sequence and distinct specificities of cDNA-expressed hPCN1 and hPCN3 for the metabolism of steroid hormones and cyclosporine. J. Biol. Chem 264, 10388–10395. [PubMed] [Google Scholar]
- Aoyama T, Uchida Y, Kelley RI, Marble M, Hofman K, Tonsgard JH, Rhead WJ, Hashimoto T, 1993. A novel disease with deficiency of mitochondrial very-long-chain acyl-CoA dehydrogenase. Biochem. Biophys. Res. Commun 191, 1369–1372. [DOI] [PubMed] [Google Scholar]
- Aoyama T, Souri M, Ushikubo S, Kamijo T, Yamaguchi S, Kelley RI, Rhead WJ, Uetake K, Tanaka K, Hashimoto T, 1995. Purification of human very-long-chain acyl-coenzyme A dehydrogenase and characterization of its deficiency in seven patients. J. Clin. Invest 95, 2465–2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama T, Peters JM, Iritani N, Nakajima T, Furihata K, Hashimoto T, Gonzalez FJ, 1998. Altered constitutive expression of fatty acid-metabolizing enzymes in mice lacking the peroxisome proliferator-activated receptor alpha (PPARalpha). J. Biol. Chem 273, 5678–5684. [DOI] [PubMed] [Google Scholar]
- Arici M, Chana R, Lewington A, Brown J, Brunskill NJ, 2003. Stimulation of proximal tubular cell apoptosis by albumin-bound fatty acids mediated by peroxisome proliferator activated receptor-γ. J. Am. Soc. Nephrol 14, 17–27. [DOI] [PubMed] [Google Scholar]
- Barra J, Taburet AM, Jardel A, Fessi H, Puisieux F, 1994. High-performance liquid chromatographic assay for the simultaneous determination of ethyl clofibrate and clofibric acid in plasma. Evaluation of plasma stability of ethyl clofibrate polylactic nanocapsules in human and rat plasmas. J. Chromatogr. B 661, 178–182. [DOI] [PubMed] [Google Scholar]
- Chen L, Boadle RA, Harris DC, 1998. Toxicity of holotransferrin but not albumin in proximal tubule cells in primary culture. J. Am. Soc. Nephrol 9, 77–84. [DOI] [PubMed] [Google Scholar]
- D’Amico G, 1999. Tubulointerstitium as predictor of progression of glomerular diseases. Nephron 83, 289–295. [DOI] [PubMed] [Google Scholar]
- Delerive P, Fruchart JC, Staels B, 2001. Peroxisome proliferator-activated receptors in inflammation control. J. Endocrinol 169, 453–459. [DOI] [PubMed] [Google Scholar]
- Furuta S, Hayashi H, Hijikata M, Miyazawa S, Osumi T, Hashimoto T, 1986. Complete nucleotide sequence of cDNA and deduced amino acid sequence of rat liver catalase. Proc. Natl Acad. Sci. USA 83, 313–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girnun GD, Domann FE, Moore SA, Robbins ME, 2002. Identification of a functional peroxisome proliferator-activated receptor response element in the rat catalase promoter. Mol. Endocrinol 16, 2793–2801. [DOI] [PubMed] [Google Scholar]
- Gonzalez FJ, Peters JM, Cattley RC, 1998. Mechanism of action of the nongenotoxic peroxisome proliferators: role of the peroxisome proliferator-activated receptor alpha. J. Natl Cancer Inst 90, 1702–1709. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Akerboom TPM, Sies H, 1986. Target Organ Toxicity. CRC Press, Boca Raton, FL, pp. 129–143. [Google Scholar]
- Kamijo Y, Hora K, Tanaka N, Usuda N, Kiyosawa K, Nakajima T, Gonzalez FJ, Aoyama T, 2002. Identification of functions of peroxisome proliferator-activated receptor alpha in proximal tubules. J. Am. Soc. Nephrol 13, 1691–1702. [DOI] [PubMed] [Google Scholar]
- Kamijo Y, Hora K, Kono K, Takahashi K, Higuchi M, Ehara T, Kiyosawa K, Shigematsu H, Gonzalez FJ, Aoyama T, 2007a. PPARα protects proximal tubular cells from acute fatty acid toxicity. J. Am. Soc. Nephrol 18, 3089–3100. [DOI] [PubMed] [Google Scholar]
- Kamijo Y, Hora K, Nakajima T, Kono K, Takahashi K, Ito Y, Higuchi M, Kiyosawa K, Shigematsu H, Gonzalez FJ, Aoyama T, 2007b. Peroxisome proliferator-activated receptor alpha protects against glomerulonephritis induced by long-term exposure to the plasticizer di-(2-ethylhexyl)phthalate. J. Am. Soc. Nephrol 18, 176–188. [DOI] [PubMed] [Google Scholar]
- Kersten S, Desvergne B, Wahli W, 2000. Roles of PPARs in health and disease. Nature 405, 421–424. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Sugiyama H, Wang DH, Toda N, Maeshima Y, Yamasaki Y, Masuoka N, Yamada M, Kira S, Makino H, 2005. Catalase deficiency renders remnant kidneys more susceptible to oxidant tissue injury and renal fibrosis in mice. Kidney Int. 68, 1018–1031. [DOI] [PubMed] [Google Scholar]
- Kono K, Kamijo Y, Hora K, Takahashi K, Higuchi M, Kiyosawa K, Shigematsu H, Gonzalez FJ, Aoyama T, 2009. PPARα attenuates the proinflammatory response in activated mesangial cells. Am. J. Physiol. Renal. Physiol 296, F328–F336. [DOI] [PubMed] [Google Scholar]
- Lee SS, Pineau T, Drago J, Lee EJ, Owens JW, Kroetz DL, Fernandez-Salguero PM, Westphal H, Gonzalez FJ, 1995. Targeted disruption of the alpha isoform of the peroxisome proliferator-activated receptor gene in mice results in abolishment of the pleiotropic effects of peroxisome proliferators. Mol. Cell. Biol. 15, 3012–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Gokden N, Okusa MD, Bhatt R, Portilla D, 2005. Anti-inflammatory effect of fibrate protects from cisplatin-induced ARF. Am. J. Physiol. Renal. Physiol 289, F469–F480. [DOI] [PubMed] [Google Scholar]
- Li S, Nagothu KK, Desai V, Lee T, Branham W, Moland C, Megyesi JK, Crew MD, Portilla D, 2009. Transgenic expression of proximal tubule peroxisome proliferator-activated receptor-alpha in mice confers protection during acute kidney injury. Kidney Int. 76, 1049–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipscombe J, Lewis GF, Cattran D, Bargman JM, 2001. Deterioration in renal function associated with fibrate therapy. Clin. Nephrol 55, 39–44. [PubMed] [Google Scholar]
- Ma SF,Nishikawa M,Hyoudou K, Takahashi R, Ikemura M, Kobayashi Y,Yamashita F, Hashida M, 2007. Combining cisplatin with cationized catalase decreases nephrotoxicity while improving antitumor activity. Kidney Int. 72, 1474–1482. [DOI] [PubMed] [Google Scholar]
- Martin G, Schoonjans K, Lefebvre AM, Staels B, Auwerx J, 1997. Coordinate regulation of the expression of the fatty acid transport protein and acyl-CoA synthetase genes by PPARα and PPARγ activators. J. Biol. Chem 272, 28210–28217. [DOI] [PubMed] [Google Scholar]
- Miyazawa S, Osumi T, Hashimoto T, 1980. The presence of a new 3-oxoacyl-CoA thiolase in rat liver peroxisomes. Eur. J. Biochem 103, 589–596. [DOI] [PubMed] [Google Scholar]
- Nagothu KK, Bhatt R, Kaushal GP, Portilla D, 2005. Fibrate prevents cisplatin-induced proximal tubule cell death. Kidney Int. 68, 2680–2693. [DOI] [PubMed] [Google Scholar]
- Nakajima T, Tanaka N, Li G, Hu R, Kamijo Y, Hara A, Aoyama T, 2010. Effect of bezafibrate on hepatic oxidative stress: comparison between conventional experimental doses and clinically-relevant doses in mice. Redox Rep. 15, 123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nangaku M, Pippin J, Couser WG, 1999. Complement membrane attack complex (C5b-9) mediates interstitial disease in experimental nephrotic syndrome. J. Am. Soc. Nephrol 10, 2323–2331. [DOI] [PubMed] [Google Scholar]
- Ong AC, Moorhead JF, 1994. Tubular lipidosis: epiphenomenon or pathogenetic lesion in human renal disease? Kidney Int. 45, 753–762. [DOI] [PubMed] [Google Scholar]
- Osumi T, Hashimoto T, 1980. Purification and properties of mitochondrial and peroxisomal 3-hydroxyacyl-CoA dehydrogenase from rat liver. Arch. Biochem. Biophys. 203, 372–383. [DOI] [PubMed] [Google Scholar]
- Roberts RA, Chevalier S, Hasmall SC, James NH, Cosulich SC, Macdonald N, 2002. PPARα and the regulation of cell division and apoptosis. Toxicology 181–182, 167–170. [DOI] [PubMed] [Google Scholar]
- Schoonjans K, Watanabe M, Suzuki H, Mahfoudi A, Krey G, Wahli W, Grimaldi P, Staels B, Yamamoto T, Auwerx J, 1995. Induction of the acyl-coenzyme Asynthetase gene by fibrates and fatty acids is mediated by a peroxisome proliferator response element in the C promoter. J. Biol. Chem 270, 19269–19276. [DOI] [PubMed] [Google Scholar]
- Schoonjans K, Staels B, Auwerx J, 1996. Role of the peroxisome proliferator-activated receptor (PPAR) in mediating the effects of fibrates and fatty acids on gene expression. J. Lipid Res 37, 907–925. [PubMed] [Google Scholar]
- Schrauwen P, Hesselink MK, 2004. Oxidative capacity, lipotoxicity, and mitochondrial damage in type 2 diabetes. Diabetes 53, 1412–1417. [DOI] [PubMed] [Google Scholar]
- Shindo Y, Hashimoto T, 1978. Acyl-coenzyme A synthetase and fatty acid oxidation in rat liver peroxisomes. J. Biochem 84, 1177–1181. [DOI] [PubMed] [Google Scholar]
- Sunami R, Sugiyama H, Wang DH, Kobayashi M, Maeshima Y, Yamasaki Y, Masuoka N, Ogawa N, Kira S, Makino H, 2004. Acatalasemia sensitizes renal tubular epithelial cells to apoptosis and exacerbates renal fibrosis after unilateral ureteral obstruction. Am. J. Physiol. Renal. Physiol 286, F1030–F1038. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Lopez-Franco O, Gomez-Garre D, Tejera N, Gomez-Guerrero C, Sugaya T, Bernal R, Blanco J, Ortega L, Egido J, 2001. Renal tubulointerstitial damage caused by persistent proteinuria is attenuated in AT1-deficient mice: role of endothelin-1. Am. J. Pathol 159, 1895–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S, Leung JC, Abe K, Chan KW, Chan LY, Chan TM, Lai KN, 2003. Albumin stimulates interleukin-8 expression in proximal tubular epithelial cells in vitro and in vivo. J. Clin. Invest 111, 515–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida Y, Izai K, Orii T, Hashimoto T, 1992. Novel fatty acid beta-oxidation enzymes in rat liver mitochondria. II. Purification and properties of enoyl-coenzyme A (CoA) hydratase/3-hydroxyacyl-CoA dehydrogenase/3-ketoacyl-CoA thiolasetrifunctional protein. J. Biol. Chem 267, 1034–1041. [PubMed] [Google Scholar]
- Watanabe K, Fujii H, Takahashi T, Kodama M, Aizawa Y, Ohta Y, Ono T, Hasegawa G, Naito M, Nakajima T, Kamijo Y, Gonzalez FJ, Aoyama T, 2000. Constitutive regulation of cardiac fatty acid metabolism through peroxisome proliferator-activated receptor alpha associated with age-dependent cardiac toxicity. J. Biol. Chem 275, 22293–22299. [DOI] [PubMed] [Google Scholar]
- Willson TM, Brown PJ, Sternbach DD, Henke BR, 2000. The PPARs: from orphan receptors to drug discovery. J. Med. Chem 43, 527–550. [DOI] [PubMed] [Google Scholar]