Abstract
Aims:
The aryl hydrocarbon receptor (AHR) is a ligand-activated transcription factor that mediates the toxicity of environmental pollutants. It is also implicated in the regulation of the immune system. Ahr-null macrophages overproduce several proinflammatory cytokines following LPS-mediated stimulation, suggesting that AHR affects the balance between the inflammatory M1 and anti-inflammatory M2 phenotypes. Therefore, the present study aimed to examine whether the loss of AHR modifies macrophage polarization.
Materials and methods:
Peritoneal macrophages from wild-type and Ahr-null mice were differentiated into M1 or M2 phenotype by treatment with LPS/IFNγ or IL-4, and several M1 and M2 markers were determined by qPCR and ELISA assays. Macrophage phagocytic capacity was determined through phagocytosis of yeast and Leishmania major infection assays. Nitric oxide (NO) and urea production, and arginase activity were also determined.
Key findings:
When macrophages were polarized to the M1 phenotype, Ahr-null cells presented a mixed response; higher levels of IL-1β, IL-6, IL-12, and TNFα were observed after IFNγ- and LPS-mediated activation. However, Ahr-null cells also exhibited decreased NO production and phagocytic capacity. When macrophage was polarized to the M2 phenotype, Ahr-null cells exhibited lower levels of Fizz1, Ym1, and IL-10. In contrast, arginase activity was increased when compared to wild-type macrophages. In addition, macrophages from Ahr-null mice were more susceptible to L. major infection.
Significance:
Disruption of the Ahr gene alters macrophage polarization when compared to WT macrophage. These changes may affect the development and resolution of several diseases such as bacterial or parasitic infections.
Keywords: Arginase, Aryl hydrocarbon receptor, Leishmaniasis, Macrophage, Nitric oxide
1. Introduction
Macrophages are essential components of innate immunity and are involved in regulation of adaptive immunity. These cells respond to a wide variety of environmental signals by producing molecules that modulate several processes, such as host defense against infections and wound healing. To accomplish these functions, macrophage acquires two major phenotypes: the classic inflammatory M1 phenotype and the alternative anti-inflammatory M2 phenotype (reviewed in: [20]). Activation with pro-inflammatory cytokines, such as interferon gamma (IFNγ), or Toll-like receptor ligands, such as lipopolysaccharide (LPS), polarizes cells to the M1 phenotype. In contrast, activation of macrophage with Th2 cytokines, such as interleukin 4 or 13 (IL-4 or IL-13, respectively), polarizes cells to the M2 phenotype. M1 macrophage exhibits microbicidal activity, secrete pro-inflammatory cytokines, such as IL-1β, IL-6, IL-12, and tumor necrosis factor alpha (TNFα), and produce reactive oxygen species (ROS). This phenotype promotes and amplifies the Th1 response. Conversely, M2 macrophages secrete anti-inflammatory cytokines, such as IL-10, increase levels of arginase activity, and upregulate the production of mannose receptors, inflammatory zone one (Fizz1), and the eosinophilic chemotactic factor, Ym1 ([16], reviewed in: [29]). M2 macrophage protects the host from excessive injury and allows wound healing. Imbalances of M1/M2 macrophage polarization result in inflammatory alterations associated with several diseases such as infections, obesity, asthma, sepsis and cancer (reviewed in: [15]). Therefore, identifying the molecular components involved in macrophage polarization is central to finding targets for the treatment of inflammatory diseases.
The aryl hydrocarbon receptor (AHR), a member of the basic helix-loop-helix, Per-Arnt-Sim transcription factor family, is a ligand-activated receptor that mediates the toxicity of environmental pollutants, such as 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) ([22], reviewed in: [31]). Upon binding to TCDD, AHR translocates to the nucleus, dimerizes with the AHR nuclear-translocator protein (ARNT), binds to xenobiotic-responsive elements, and upregulates the expression of a battery of genes that encode xenobiotic-metabolizing enzymes, such as the cytochrome P450s (CYP1A1, CYP1A2, CYP1B1), NAD(P)H quinone oxidoreductase, and UDP-glucuronosyltransferase-6 (reviewed in: [7]).
Although AHR functions as part of an adaptive chemical response, several studies demonstrated that this transcription factor is also implicated in regulation of the immune system through different mechanisms. Targeting AHR disrupts the immune system by decreasing lymphocyte accumulation in the spleen and lymph nodes and overrides the thymic atrophy induced by TCDD [5,6]. AHR also plays an important role in the differentiation of IL-17-producing helper T cells as well as Treg cells [24]. In contrast, several studies identify the AHR as a negative regulator of the immune system; lymphocytes from Ahr-null mice produce more IFNγ and IL-12 compared to wild-type (WT) cells [26], Ahr-null mice are more susceptible to endotoxic shock induced by LPS, and macrophages from Ahr-null mice produce higher levels of proinflammatory cytokines compared to WT cells [11]. The Ahr-null mouse also exhibits an exacerbated immune response against Leishmania major or Toxoplasma gondii infections accompanied by an increase in TNFα serum levels [4,27]. To further determine the role of AHR in the immune response, the present study examined whether AHR modifies macrophage polarization by evaluating M1/M2 markers.
2. Materials and methods
2.1. Materials
Fetal bovine serum (FBS) was purchased from Hyclone (Logan, UT, USA). RPMI-1640 media, L-glutamine, non-essential amino acids, antibiotics/antimycotics, and sodium pyruvate were purchased from Invitrogen Life Technologies (Carlsbad, CA, USA). Recombinant mouse IFNγ and IL-4 were obtained from BD Pharmingen (San Jose, CA, USA). LPS (Escherichia coli, O55:B5) was purchased from Sigma (St. Louis, MO, USA).
2.2. Animals
The Ahr-null mice described previously [5] were genotyped by polymerase chain reaction (PCR) as reported elsewhere [26]. WT littermates were used as control mice. C57BL/6 mice were housed in a pathogen-free facility, fed with autoclaved Purina rodent chow (St. Louis, MO, USA), and provided with water ad libitum. All animal studies were performed according to the Guide for the Care and Use of Laboratory Animals, as adopted and promulgated by the U.S. National Institutes of Health and the Mexican Regulation of Animal Care and Maintenance (NOM-062-ZOO-1999, 2001).
2.3. Cell culture and stimulation
Resident macrophages were harvested from the peritoneal cavity of 8–10 week-old male mice. Briefly, 5 ml of ice-cold, sterile RPMI-1640 media was injected into the peritoneal cavity, and the area massaged for 5 min. The medium was aspirated from five to six mice, pooled, and collected by centrifugation at 2000 rpm for 10 min at 4 °C, and then washed twice. Cell viability was assessed by Trypan blue exclusion. Macrophage were adjusted to 1 × 106 cell/ml in RPMI-1640 media supplemented with 10% FBS, 2 mM L-glutamine, 0.5% non-essential amino acids, 0.5% sodium pyruvate, 100 U/ml penicillin, and 100 μg/ml streptomycin. One milliliter of the cell suspension was transferred into a 12-well polystyrene tissue culture plate and incubated 2 h at 37 °C in humidified air containing 5% CO2. Suspended cells were washed twice with RPMI-1640 media, and the remaining adherent macrophages were replenished with supplemented RPMI-1640 media. Macrophages were differentiated to the M1 or M2 phenotype by treatment with LPS/IFNγ (20 ng/ml, 1 μg/ml, respectively) or IL-4 (20 ng/ml).
2.4. Real-time quantitative PCR (qPCR) analysis
Total RNA was prepared from cell extracts using TRIzol reagent according to the manufacturer’s instructions (Invitrogen, Camarillo, CA, USA). RNA was quantified spectrophotometrically at OD260. RNA integrity was evaluated by electrophoresis of RNA samples on 1% agarose gels. cDNA for the qPCR assay was prepared from 2 μg total RNA using oligo dT and the SuperScript First-Strand Synthesis kit (Invitrogen, Carlsbad, CA, USA). PCR reactions were conducted in a StepOne Real-Time PCR System (Applied Biosystems, Branchburg, NJ, USA) and analyzed using the comparative threshold cycle method. The mRNAs encoding IL-1β, IL-6, IL-12, TNFα, or 18S ribosomal RNA (rRNA, endogenous) were amplified in a single PCR reaction to allow for normalization of the mRNA data. The PCR reaction mixture contained 2 μl of cDNA, 1× TaqMan Universal PCR Master Mix (Applied Biosystems, Branchburg, NJ, USA), 0.9 μM primers, and 0.25 μM probes. The sequences for the primers and probes used for Il1b, Il6, Il12, Tnfa, mRNAs and 18S were obtained from Applied Biosystems (Branchburg, NJ, USA) with identification numbers Mm00434228_m1, Mm00446190_m1, Mm00434165_m1, Mm00443258_m1, and Mm00507222_s1, respectively.
2.5. Cytokine quantification
After macrophage stimulation, the supernatants were collected and mouse IL-6 and TNFα (OPtEIA ELISA KIT BD; San Jose, CA, USA) as well as mouse 12/IL-23 (p40), IL-1β, and IL-10 (ELISA MAX Deluxe sets; Biolegend, San Diego, CA, USA) were determined by enzyme-linked immunosorbent assays (ELISAs) according to the manufacturer’s instructions.
2.6. Nitric oxide and urea determination
Nitric oxide (NO) production was assessed by nitrite accumulation in the culture media using the Griess reagent. Briefly, 100 μl of culture supernatant was mixed with 100 μl of 5.8% phosphoric acid, 1% sulphanilamide, and 0.1% N-(1-naphthyl) ethylenediamine dihydrochloride. Absorbance was measured at 490 nm with background correction at 650 nm using a microplate reader. NO concentration was determined using a standard curve of sodium nitrite solution. Urea determination was performed using a kit from RANDOX (Crumlin, UK).
2.7. Arginase activity
Arginase activity was assessed as described previously [3]. Briefly, 200,000 macrophages were seeded in a 96-well plate. After the indicated times, cell cultures were washed with phosphate-buffered saline (PBS) and lysed with 100 μl of 0.1% Triton X-100 solution for 15 min. To measure total arginase activity, 10 μl of 100 mM MnCl2 solution was added and incubated at 56 °C for 7 min. One hundred microliters of 0.5 M arginine was added and incubated at 37 °C for 1 h. The reaction was stopped with an acid mixture, and the urea concentration was determined at 540 nm using a microplate reader.
2.8. Macrophage phagocytosis assay
Peritoneal macrophages were obtained as described above, and 1 × 106 cells in supplemented RPMI-1640 media were placed on glass coverslips in a 35 × 10-mm culture plate. After 2 h of incubation at 37 °C in humidified air containing 5% CO2, suspended cells were washed with PBS, and the remaining adherent macrophages were replenished with supplemented RPMI-1640 media. Macrophages were differentiated to the M1 phenotype with LPS/IFNγ (20 ng/ml, 1 μg/ml, respectively). After 12 or 24 h, cultures were washed with PBS, and incubated for 1 h in supplemented RPMI-1640 media containing 1 × 106 opsonized Saccharomyces cerevisiae yeast. The preparations were rinsed with saline solution and stained with 0.5% Safranina, and the number of yeast cells ingested by macrophages was assessed by microscopy. To calculate the number of phagocytic macrophages as well as the number of yeast cells ingested per macrophage, 200 cells per preparation were evaluated.
2.9. In vitro infection of macrophages with Leishmania major
Infection assay was performed as described before [21] with modifications. Briefly, promastigotes were obtained from macerates of infected tissue from WT mice and differentiated by culturing in complete Schneider’s media for 3 days at 26 °C. Promastigotes were added to peritoneal macrophages and incubated on coverslips in 24-well plates at a ratio of 70:1 for 2 h. The macrophage were then washed twice with complete Dulbecco’s Modified Eagle’s Medium to remove non-phagocytosed parasites and incubated at 37 °C for 3 days. Cells were then fixed with 2% formaldehyde for 20 min at room temperature, washed twice with PBS, and stained with 0.5–1 ml Giemsa for 5–10 min followed by two washes with distilled H2O. Coverslips were removed from the wells, air dried, and mounted onto slides with DPX (distyrene plasticizer and xylene) mounting medium, and microscopic examination of 100 cells per group was performed.
2.10. Statistical analysis
The statistical significance of the data was evaluated using the Student’s t-test. In all cases, differences between groups were considered to be statistically significant at P < 0.05.
3. Results
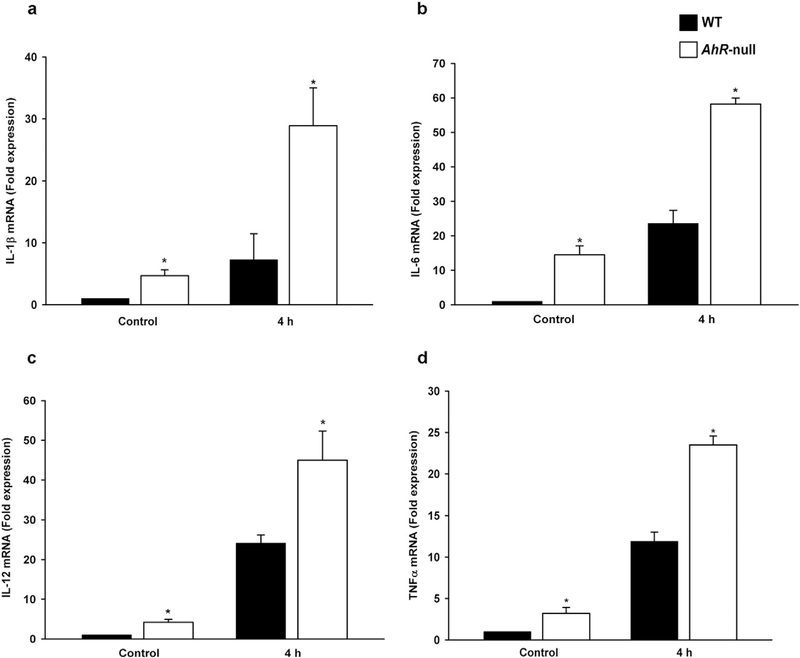
The M1 macrophage phenotype was characterized through the evaluation of M1-associated markers. WT and Ahr-null peritoneal macrophage were differentiated into M1 macrophage via IFNγ and LPS treatments, and several proinflammatory cytokines associated with M1 macrophage identified. After 4 h of activation, Ahr-null cells exhibited higher levels of Il1b, Il6, Il12, and Tnfa mRNA compared to WT macrophage (Fig. 1a–d). The levels of all cytokine mRNA present in Ahr-null cells were at least twice that of the control WT macrophages. Moreover, the basal expression of Il1b, Il6, Il12, and Tnfa (without IFNγ and LPS stimulation) was higher in Ahr-null macrophages compared to WT macrophages.
Fig. 1.
mRNA levels of proinflammatory cytokines inWT and Ahr-null macrophages duringM1 polarization. Peritoneal macrophages (1 × 106 cells/ml) from WT and Ahr-null mice were activated with LPS/IFNγ (20 ng/ml, 1 μg/ml, respectively) for 4 h, and Il1b (a), Il6 (b), Il12 (c), and Tnfa (d) mRNA levels were determined by qPCR. mRNA levels were normalized to 18S ribosomal RNA. Results are expressed as the mean ± standard deviation (S.D.) of three independent experiments. *P < 0.05,WT vs. Ahr-null, Student’s t-test.
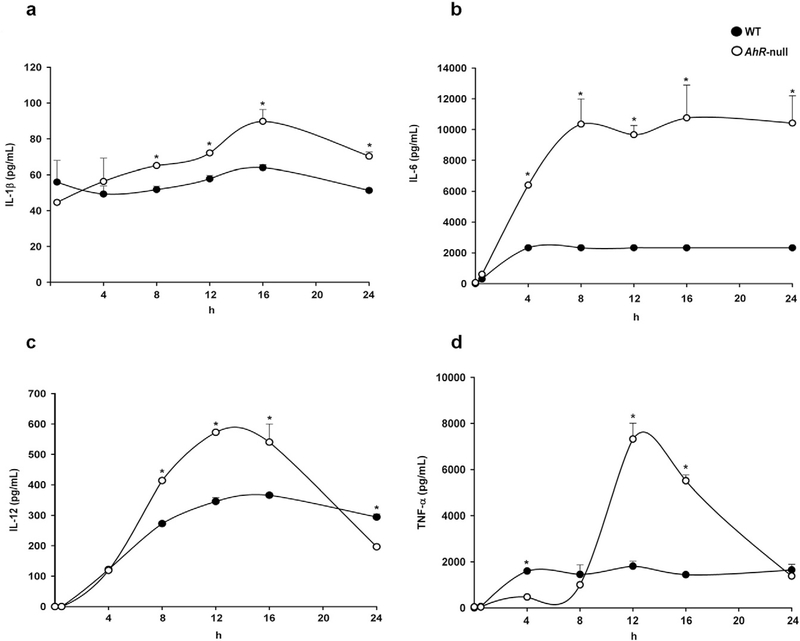
The differences in mRNA levels between M1 Ahr-null and M1 WT macrophage affect cytokine protein levels were next examined. As expected, Ahr-null macrophages secreted higher amounts of IL-1β, IL-6, IL-12p40, and TNFα than WT cells, consistent with the differences in transcript levels observed (Fig. 2a–d).
Fig. 2.
Protein levels of proinflammatory cytokines in WT and Ahr-null macrophage during M1 polarization. Peritoneal macrophages (1 × 106 cells/ml) from WT and Ahr-null mice were activated with LPS/IFNγ (20 ng/ml, 1 μg/ml, respectively), and IL-1β (a), IL-6 (b), IL-12 (c), and TNFα (d) protein levels from supernatant cultures were determined by ELISA at the indicated times. Results are expressed as the mean ± S.D. of three independent experiments. *P < 0.05,WT vs. Ahr-null, Student’s t-test.
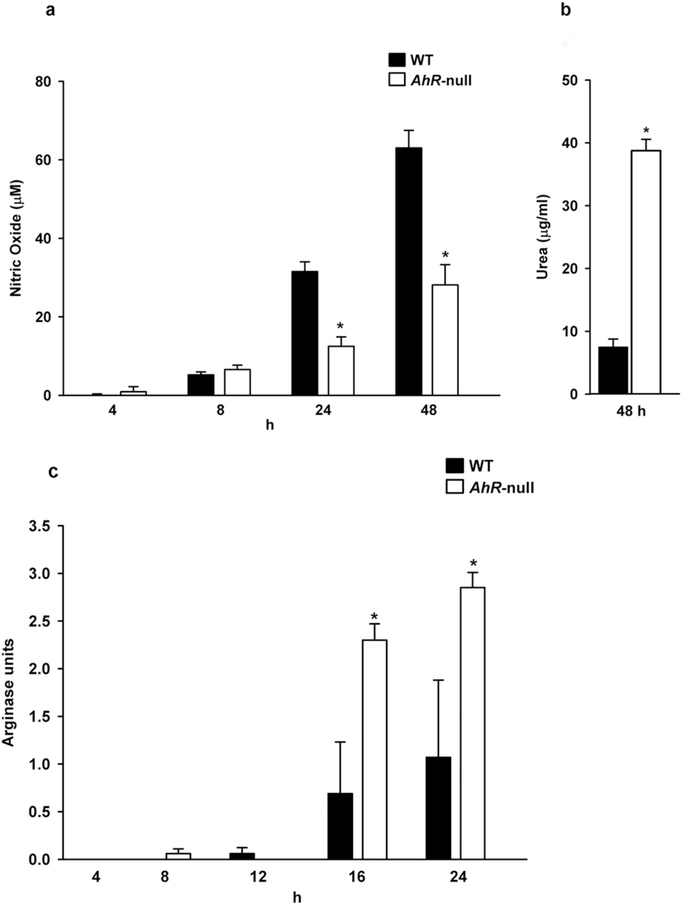
Activation of macrophage to differentiate to the M1 phenotype also leads to the production of ROS and nitrogen intermediates. Therefore, NO levels were determined. In both cell types, an increase in NO production was observed in a time-dependent manner after polarization to the M1 phenotype. However, Ahr-null macrophage showed a reduced capacity to produce NO compared to WT cells (Fig. 3a). Moreover, after IFNγ and LPS treatments iNOS mRNA levels are lower on Ahr-null macrophages when compared to WT cells (data not shown). As expected, Ahr-null cells produced higher amounts of urea at 48 h (Fig. 3b). No significant differences between WT and Ahr-null macrophages were observed in urea levels the first 24 h (data not shown). In contrast, arginase activity was higher in Ahr-null cells when compared to WT macrophages (Fig. 3c).
Fig. 3.
Nitric oxide and urea levels, and arginase activity inWT and Ahr-null macrophage afterM1 polarization. Peritoneal macrophages (1 × 106 cells/ml) from WT and Ahr-null mice were activated with LPS/IFNγ (20 ng/ml, 1 μg/ml, respectively), and NO (a) and urea (b) levels, and arginase activity (c) were measured in the supernatants at the indicated times. Results are expressed as the mean ± S.D. of three independent experiments. *P < 0.05,WT vs. Ahr-null, Student’s t-test.
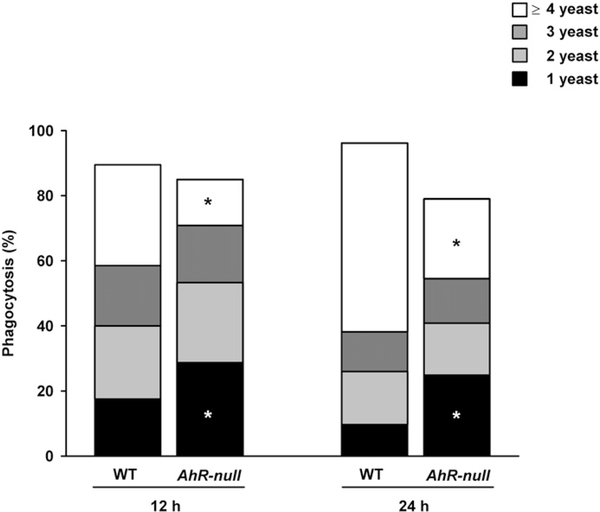
The phagocytic capacity of M1 macrophages in WT and Ahr-null mice were determined and Ahr-null macrophage exhibited a reduced capacity to phagocytose yeast (Fig. 4). Moreover, Ahr-null cells are not only deficient, but also less efficient, at phagocytosing yeast, as more WT macrophage phagocytose four or more yeast cells compared to Ahr-null cells.
Fig. 4.
Phagocytosis of S. cerevisiae by WT and Ahr-null macrophage. Peritoneal macrophages (1 × 106 cells/ml) from WT and Ahr-null mice were activated with LPS/IFNγ (20 ng/ml, 1 μg/ml, respectively) for 12 or 24 h and incubated with 1 × 106 S. cerevisiae cells per well for 1 h. Results are expressed as the mean number of ingested yeast cells per macrophage of three independent experiments. *P < 0.05, WT vs. Ahr-null, Student’s t-test.
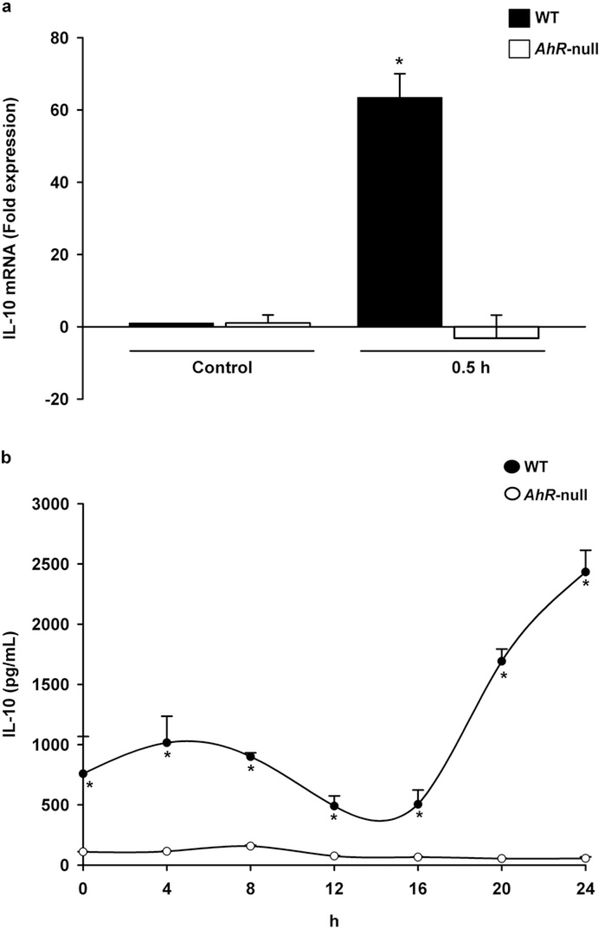
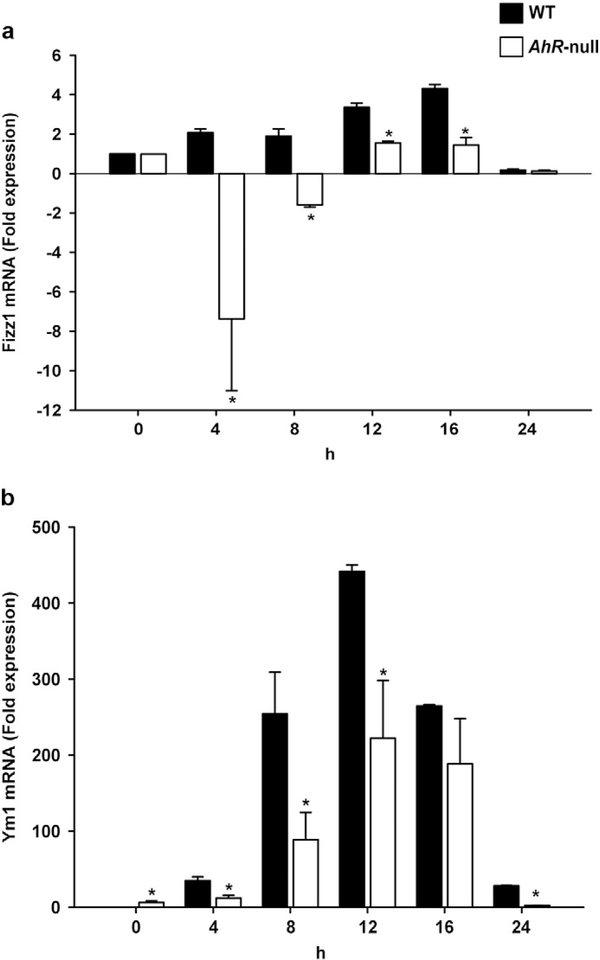
We then explored whether lack of AHR modifies M2 polarization. WT and Ahr-null macrophage were polarized to the M2 phenotype via IL-4 stimulation, and several markers were characterized. First, IL-10, a cytokine associated with the M2 phenotype was identified. IL-4 treatment upregulated mRNA encoding IL-10 in WT macrophage but not in Ahr-null cells (Fig. 5a). This induction was observed only at 30 min suggesting that it is enough to sustain the IL-10 protein levels. Accordingly, IL-10 secretion was only observed in the supernatants recovered from WT cells (Fig. 5b). Fizz1 and Ym1 are additional M2 markers; after M2 polarization, WT macrophage demonstrated higher levels of both markers in a time-dependent manner compared to Ahr-null macrophage (Fig. 6a, b). In contrast, downregulation of Fizz1 mRNA in Ahr-null cells was observed at 4 and 8 h after IL-4-mediated stimulation (Fig. 6a).
Fig. 5.
Levels of IL-10 in WT and Ahr-null macrophage during M2 polarization. Peritoneal macrophages (1 × 106 cells/ml) from WT and Ahr-null mice were treated with IL-4 (20 ng/ml), and IL-10 mRNA (a) and protein (b) levels were determined by qPCR and ELISA, respectively, at the indicated times. mRNA levels were normalized to 18S ribosomal RNA. Results are expressed as the mean ± S.D. of three independent experiments. *P < 0.05, WT vs. Ahr-null, Student’s t-test.
Fig. 6.
Expression levels of Fizz1 and Ym1 in WT and Ahr-null macrophage during M2 polarization. Peritoneal macrophages (1 × 106 cells/ml) from WT and Ahr-null mice were treated with IL-4 (20 ng/ml), and Fizz1 (a) and Ym1 (b) mRNA levels were determined by qPCR at the indicated times. mRNA levels were normalized to 18S ribosomal RNA. Results are expressed as the mean ± S.D. of three independent experiments. *P < 0.05, WT vs. Ahr-null, Student’s t-test.
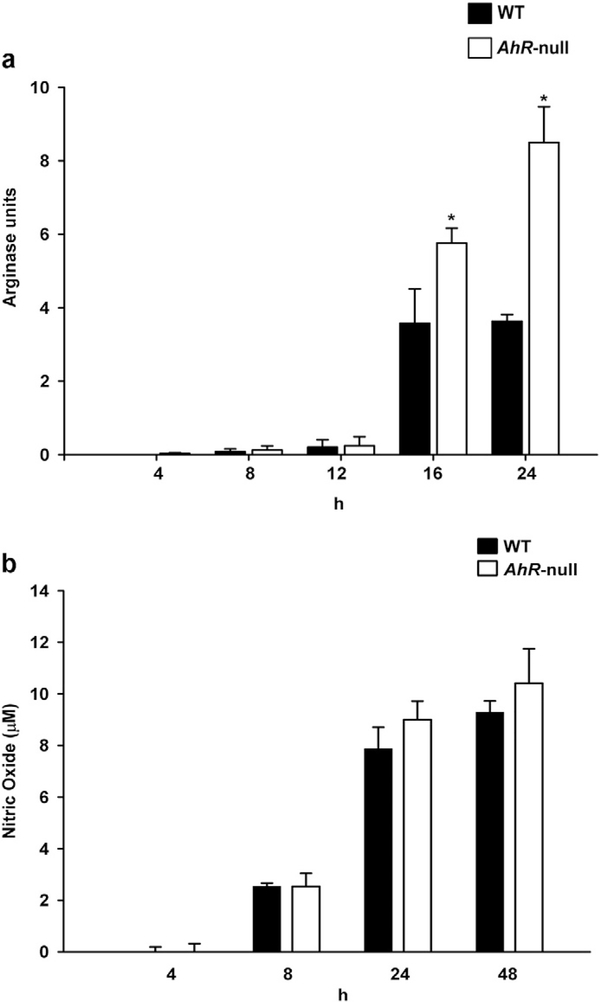
When macrophages are activated to differentiate to the alternative M2 phenotype, arginase I is induced [25]. Therefore, potential differences in arginase activity between WT and Ahr-null macrophages were investigated following IL-4-mediated activation. A time-dependent increase in arginase activity was observed in both cell types. However, after 16 h, Ahr-null macrophages exhibited higher levels of arginase activity compared to WT cells (Fig. 7a), and it was at least twice when compared to M1 cells (Fig. 3c). No differences were observed in nitric oxide levels between WT and Ahr-null macrophages (Fig. 7b).
Fig. 7.
Arginase activity and nitric oxide levels in WT and Ahr-null macrophage after M2 polarization. Peritoneal macrophages (1 × 106 cells/ml) from WT and Ahr-null mice were treated with IL-4 (20 ng/ml), and arginase activity (a) and nitric oxide levels (b) were measured at the indicated times as described in the Materials and methods section. Results are expressed as the mean ± S.D. of three independent experiments. *P < 0.05, WT vs. Ahr-null, Student’s t-test.
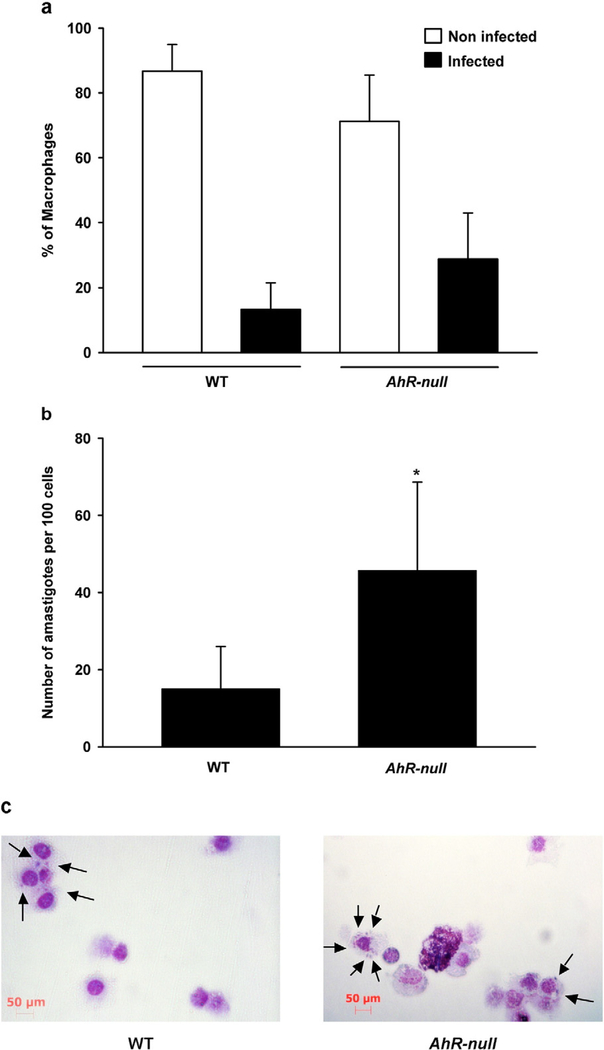
Finally, the ability of WT and Ahr-null macrophages to respond to L. major infection in vitro was compared. Peritoneal macrophages were infected with L. major promastigotes and no differences were observed in the percentage of infected cells between WT and Ahr-null macrophage (Fig. 8a). However, there was a higher number of amastigotes per cell in Ahr-null macrophages compared to WT cells, suggesting that the macrophages from Ahr-null mice are more susceptible to L. major infections (Fig. 8b, c).
Fig. 8.
Macrophages from Ahr-null mice are more susceptible to L. major infection. Peritoneal macrophages (1 × 105 cells) from WT or Ahr-null mice were activated with LPS/IFNγ (20 ng/ml, 1 μg/ml, respectively) for 2 h and incubated with 7 × 106 promastigotes of L. major. After 72 h, cells were fixed and stained, and the percentage of macrophages non-infected and infected (a) and amastigote numbers per 100 cells (b) were determined. (c) Microscopic analysis of Ahr-null and WT macrophages after L. major infection. Data represent the mean value ± S.D. of four independent experiments. *P < 0.05, WT vs. Ahr-null, Student’s t-test.
4. Discussion
AHR is a ligand-activated transcription factor that regulates the expression of several genes, including those encoding metabolic enzymes. Recently, growing evidence indicates that AHR regulates of the immune system function. It was established that AHR acts as a negative regulator of the immune response by inhibiting the expression of inflammatory cytokines (reviewed in: [10]). Compared to WT cells, Ahr-null macrophage over produce several proinflammatory cytokines after LPS-mediated stimulation, suggesting that AHR may affect the balance between the inflammatory M1 phenotype and the alternative anti-inflammatory M2 phenotype. Therefore, the goal of the present study was to characterize the effect of the loss of AHR on macrophage polarization.
When macrophage was activated to differentiate to the M1 phenotype, important differences in pro-inflammatory cytokines were observed between WT and Ahr-null cells. The mRNAs encoding IL-1β, IL-6, IL-12, and TNFα were higher in Ahr-null macrophage than in WT cells. These increased levels of cytokine transcripts correlated with increased protein levels. Additionally, time course experiments indicated that Ahr-null peritoneal macrophage produce higher amounts of IL-1β and IL-6 proteins at all time points tested, whereas IL-12 and TNFα protein levels reach a maximum at 12 h and subsequently decrease to levels similar to those observed in WT cells at 24 h. These data are consistent with previous results, which showed that, after 24 h of LPS treatment, Ahr-null macrophages produce higher IL-6, IL-12, and TNFα protein levels compared to WT cells [11]. It was proposed that the mechanism by which the AHR negatively regulates pro-inflammatory cytokines is through its interaction with STAT1, which suppresses nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) transcriptional activity [11]. Additionally, the AHR-Sp1 transcription factor complex represses histidine decarboxylase expression, which results in a decrease in histamine production and negatively regulates LPS-induced production of pro-inflammatory cytokines, such as IL-6 [18]. In contrast, NO production was decreased in Ahr-null macrophage compared to WT cells, which is consistent with recent data that shows that inducible nitric oxide synthase (iNOS) expression is regulated by AHR [30] and that the overexpression of AHR in macrophage enhances the generation of ROS [1]. Since ROS production is essential for killing bacteria, the lower production of NO by Ahr-null peritoneal macrophages suggests that these cells may have reduced microbicide capacity. Besides, Ahr-null macrophages produced higher levels of urea than WT cells, indicating that arginine metabolism via arginase is increased.
In addition to proinflammatory cytokine production, an important hallmark of the host defense is receptor-mediated phagocytosis. In the present study, the phagocytic capacity of M1 macrophages was evaluated through the use of opsonized yeast, which is mediated by mannose and Fcγ receptors [28]. The results from the phagocytic assays indicated that Ahr-null macrophages demonstrated a reduced capacity to phagocytose yeast compared to WT cells. Intracellular signaling pathways triggered by several receptors, including Fcγ receptors, induce actin polymerization. These processes depend on Cdc42, which interacts with the Wiskott-Aldrich Syndrome protein (WASP), which, in turn, activates actin-related protein 2/3 and initiates actin polymerization [23]. Furthermore, it was previously shown that overexpression of AHR in Neuro2a cells results in an induction of Cdc42 expression [2]. Therefore, it is tempting to speculate that Ahr-null macrophages have a decreased capacity to express Cdc42, which affects phagocytosis.
The above data indicate that, after inducing macrophages to differentiate to the M1 phenotype, Ahr-null cells present a mixed response. Ahr-null cells exhibit a stronger M1 polarization as revealed by cytokine production, and demonstrate decreased NO production and phagocytic capacity. Functionally, this may lead to a stronger activation of adaptive immunity, but it may impair the microbicidal potential of the cells.
When macrophages were polarized to the M2 phenotype, we found differences between WT and Ahr-null cells in all M2 markers evaluated. Both Fizz1 and Ym1 mRNA levels were lower in Ahr-null macrophages. Additionally, the mRNA and protein levels of IL-10 were lower, and almost undetectable, in Ahr-null cells compared to WT macrophage. IL-10 inhibits pro-inflammatory cytokine synthesis from T cells and leads to the development of Th2 immunity. Therefore, M2 macrophages from Ahr-null mice may be unable to resolve inflammation or heal wounds.
One of the most important findings in the present study, together with the impairment of NO production, is the difference observed between Ahr-null and WT macrophage regarding arginase activity, one of the hallmarks of the alternatively activated macrophages (M2). Cells deficient in AHR exhibited a 2-fold greater arginase activity compared to WT cells. In macrophage, arginine is metabolized either by iNOS to produce NO and citrulline or by arginase to produce urea and ornithine [25]. Arginase induction has been observed in several diseases. In particular, a high replication of L. major correlates with an increase in arginase activity [12]. This association is due to the presence of high ornithine levels, which is the precursor for the polyamines that are essential for cell proliferation, tissue repair, and growth of L. major. Consistent with these results, 72 h after L. major infection, Ahr-null macrophage exhibited a higher number of amastigotes per cell compared to WT cells. This results contrast to the lower capacity of Ahr-null cells to phagocyte yeast. The differences may be explained in terms of the parasite recognition. While promastigotes requires multiple receptor ligation for initiating the appropriate response, mannose receptor plays an important role in the yeast recognition [9]. Besides, since Ahr-null macrophages present a decrease in NO levels, which plays a major role in killing L. major (reviewed in: [14]), these cells may present a highly susceptible L. major infection despite mounting a strong Th1 response.
In addition to the role of arginase activity in L. major proliferation, arginase also plays a role in the development of cancer [19], asthma [17], and other infections, such as schistosomiasis [8]. Therefore, the increased arginase activity observed in Ahr-null macrophages could alter the course of these diseases. Alternatively, arginase also regulates the activity of iNOS by modulating the availability of L-arginine [13], which may contribute to the low levels of NO production observed in Ahr-null macrophages.
Similar to the effects observed following the induction of M1 polarization, a mixed response was observed when Ahr-null macrophages were polarized to the M2 phenotype. Some M2 markers, such as FIZZ, YM1, and IL-10, were downregulated, whereas arginase activity was upregulated.
In conclusion, disruption of the Ahr gene alters macrophage polarization to either the M1 or M2 phenotype compared to WT macrophage. These changes may affect the development and resolution of several diseases, such as bacterial or parasitic infections. Finally, before proposing that the AHR could be a therapeutic target, a great deal of additional research is needed to understand the mechanisms by which the AHR modifies several components of macrophages not only in vitro, but also in vivo, particularly in the context of disease.
Acknowledgments
We thank Dr. Leopoldo Flores for his helpful comments. This work was supported by CONACYT grant number 153377.
References
- [1].Abe H, Kimura A, Tsuruta S, Fukaya T, Sakaguchi R, Morita R, et al. , Aryl hydrocarbon receptor plays protective roles in ConA-induced hepatic injury by both suppressing IFN-gamma expression and inducing IL-22, Int. Immunol 26 (2014) 129–137. [DOI] [PubMed] [Google Scholar]
- [2].Akahoshi E, Yoshimura S, Ishihara-Sugano M, Over-expression of AhR (aryl hydrocarbon receptor) induces neural differentiation of Neuro2a cells: neurotoxicology study, Environ. Health 5 (24) (2006) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Classen A, Lloberas J, Celada A, Macrophage activation: classical vs. alternative, in: Reiner NE (Ed.), Macrophages and Dendritic Cells, Humana Press, New York, 2009. (368 pp.). [Google Scholar]
- [4].Elizondo G, Rodriguez-Sosa M, Estrada-Muniz E, Gonzalez FJ, Vega L, Deletion of the aryl hydrocarbon receptor enhances the inflammatory response to Leishmania major infection, Int. J. Biol. Sci 7 (2011) 1220–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Fernandez-Salguero P, Pineau T, Hilbert DM, McPhail T, Lee SS, Kimura S, et al. , Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor, Science 268 (1995) 722–726. [DOI] [PubMed] [Google Scholar]
- [6].Fernandez-Salguero PM, Hilbert DM, Rudikoff S, Ward JM, Gonzalez FJ, Aryl-hydrocarbon receptor-deficient mice are resistant to 2,3,7,8-tetrachlorodibenzo-pdioxin-induced toxicity, Toxicol. Appl. Pharmacol 140 (1996) 173–179. [DOI] [PubMed] [Google Scholar]
- [7].Gonzalez FJ, Fernandez-Salguero P, The aryl hydrocarbon receptor: studies using the AHR-null mice, Drug Metab. Dispos 26 (1998) 1194–1198. [PubMed] [Google Scholar]
- [8].Herbert DR, Orekov T, Roloson A, Ilies M, Perkins C, O’Brien W, et al. , Arginase I suppresses IL-12/IL-23p40-driven intestinal inflammation during acute schistosomiasis, J. Immunol 184 (2010) 6438–6446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Keppler-Ross S, Douglas L, Konopka JB, Dean N, Recognition of yeast by murine macrophage requires mannan but not glucan, Eukaryot. Cell 9 (2010) 1776–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kerkvliet NI, AHR-mediated immunomodulation: the role of altered gene transcription, Biochem. Pharmacol 77 (2009) 746–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kimura A, Naka T, Nakahama T, Chinen I, Masuda K, Nohara K, et al. , Aryl hydrocarbon receptor in combination with Stat1 regulates LPS-induced inflammatory responses, J. Exp. Med 206 (2009) 2027–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kropf P, Fuentes JM, Fahnrich E, Arpa L, Herath S, Weber V, et al. , Arginase and polyamine synthesis are key factors in the regulation of experimental leishmaniasis in vivo, FASEB J. 19 (2005) 1000–1002. [DOI] [PubMed] [Google Scholar]
- [13].Lee J, Ryu H, Ferrante RJ, Morris SM Jr., R.R. Ratan, Translational control of inducible nitric oxide synthase expression by arginine can explain the arginine paradox, Proceedings of the National Academy of Sciences of the United States of America, 100 2003, pp. 4843–4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Liu D, Uzonna JE, The early interaction of Leishmania with macrophages and dendritic cells and its influence on the host immune response, Front. Cell. Infect. Microbiol 2 (83) (2012) 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Liu YC, Zou XB, Chai YF, Yao YM, Macrophage polarization in inflammatory diseases, Int. J. Biol. Sci 10 (2014) 520–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lopez-Castejon G, Baroja-Mazo A, Pelegrin P, Novel macrophage polarization model: from gene expression to identification of new anti-inflammatory molecules, Cell. Mol. Life Sci 68 (2011) 3095–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Maarsingh H, Dekkers BG, Zuidhof AB, Bos IS, Menzen MH, Klein T, et al. , Increased arginase activity contributes to airway remodelling in chronic allergic asthma, Eur. Respir. J 38 (2011) 318–328. [DOI] [PubMed] [Google Scholar]
- [18].Masuda K, Kimura A, Hanieh H, Nguyen NT, Nakahama T, Chinen I, et al. , Aryl hydrocarbon receptor negatively regulates LPS-induced IL-6 production through suppression of histamine production in macrophages, Int. Immunol 23 (2011) 637–645. [DOI] [PubMed] [Google Scholar]
- [19].Mills CD, Shearer J, Evans R, Caldwell MD, Macrophage arginine metabolism and the inhibition or stimulation of cancer, J. Immunol 149 (1992) 2709–2714. [PubMed] [Google Scholar]
- [20].Mosser DM, Edwards JP, Exploring the full spectrum of macrophage activation, Nat. Rev. Immunol 8 (2008) 958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mylonas KJ, Nair MG, Prieto-Lafuente L, Paape D, Allen JE, Alternatively activated macrophages elicited by helminth infection can be reprogrammed to enable microbial killing, J. Immunol 182 (2009) 3084–3094. [DOI] [PubMed] [Google Scholar]
- [22].Okey AB, Riddick DS, Harper PA, The Ah receptor: mediator of the toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and related compounds, Toxicol. Lett 70 (1994) 1–22. [DOI] [PubMed] [Google Scholar]
- [23].Prehoda KE, Lim WA, How signaling proteins integrate multiple inputs: a comparison of N-WASP and Cdk2, Curr. Opin. Cell Biol 14 (2002) 149–154. [DOI] [PubMed] [Google Scholar]
- [24].Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, et al. , Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor, Nature 453 (2008) 65–71. [DOI] [PubMed] [Google Scholar]
- [25].Rath M, Muller I, Kropf P, Closs EI, Munder M, Metabolism via arginase or nitric oxide synthase: two competing arginine pathways in macrophages, Front. Immunol 5 (532) (2014) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rodriguez-Sosa M, Elizondo G, Lopez-Duran RM, Rivera I, Gonzalez FJ, Vega L, Over-production of IFN-gamma and IL-12 in AhR-null mice, FEBS Lett. 579 (2005) 6403–6410. [DOI] [PubMed] [Google Scholar]
- [27].Sanchez Y, Rosado Jde D, Vega L, Elizondo G, Estrada-Muniz E, Saavedra R, et al. , The unexpected role for the aryl hydrocarbon receptor on susceptibility to experimental toxoplasmosis, J. Biomed. Biotechnol 2010 (2010) 505694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Stuart LM, Ezekowitz RA, Phagocytosis: elegant complexity, Immunity 22 (2005) 539–550. [DOI] [PubMed] [Google Scholar]
- [29].Wang N, Liang H, Zen K, Molecular mechanisms that influence the macrophage m1-m2 polarization balance, Front. Immunol 5 (614) (2014) 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wheeler JL, Martin KC, Lawrence BP, Novel cellular targets of AhR underlie alterations in neutrophilic inflammation and inducible nitric oxide synthase expression during influenza virus infection, J. Immunol 190 (2013) 659–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Whitlock JP Jr., Induction of cytochrome P4501A1, Annu. Rev. Pharmacol. Toxicol 39 (1999) 103–125. [DOI] [PubMed] [Google Scholar]