Abstract
During cell migration, the forces generated in the actin cytoskeleton are transmitted across transmembrane receptors to the extracellular matrix or other cells through a series of mechanosensitive, regulable protein–protein interactions termed the molecular clutch. In integrin-based focal adhesions, the proteins forming this linkage are organized into a conserved three-dimensional nano-architecture. Here we discuss how the physical interactions between the actin cytoskeleton and focal-adhesion-associated molecules mediate force transmission from the molecular clutch to the extracellular matrix.
Cell migration is important during embryonic development, immune responses and wound healing, and can lead to inflammation and cancer metastasis when misregulated1. Migration can occur through different mechanisms, including lamellipodia or pressure-driven bleb formation2, water permeation3 and other processes4,5, depending on the cell type and tissue environment — a plasticity that facilitates robust migration in many contexts1. However, the common feature of all these scenarios is that cells must be able to apply forces to generate traction against, and move themselves relative to, their immediate surroundings. The actin cytoskeleton is the major source of internally generated force that regulates cell shape and drives migration6. Actin-based cellular forces must somehow be transmitted through the cell membrane to generate friction that induces traction against the extracellular environment. Friction between the cell and its environment can either be non-specific or mediated by specific surface receptors that bind to the extracellular matrix (ECM) or other cells. Non-specific friction can be generated when cells are held under confinement, and is thought to drive non-haptotactic, bleb-based amoeboid motility during immune responses and cancer metastasis7,8. Specific interactions between cells and their surroundings, such as integrin–ECM and cadherin–cadherin receptor–ligand interactions, drive haptotactic ‘mesenchymal’ motility during wound healing and development. This Review will focus on the physical mechanisms of cell–ECM traction generation during lamellipodia- and integrin-dependent mesenchymal cell migration.
Mesenchymal cell migration involves coordinated lamellipodial protrusion at the cell leading edge in the direction of migration and adhesion of this protrusion to the ECM, usually in response to external chemical or physical guidance cues9. Lamellipodial protrusion is always associated with actin filament polymerization subjacent to the leading edge plasma membrane10,11, and filament end elongation is thought to push the plasma membrane forward12. This actin polymerization against the plasma membrane barrier, together with myosin II contraction of cortical actin filaments within the lamella, also generates a net rearward ‘retrograde flow’ of the F-actin network relative to the direction of cell movement10,11,13. The ‘molecular clutch hypothesis’ provides a mechanical metaphor to help explain how the cell converts this rearward actin flow into forward cell movement14. This hypothesis postulates that integrin-containing focal adhesions (FAs) act as a mechanical ‘clutch’ by mediating transient indirect interactions between the retrograde-moving actin cytoskeleton and ECM-bound integrins (Fig. 1) Here we outline the growing body of evidence supporting the notion that force transduction in integrin-based FAs at the leading edge of migrating cells is regulated by an organized 3D ‘molecular clutch’ consisting of the FA molecules talin and vinculin. We describe how forces originating in the leading edge actin cytoskeleton are transmitted to the ECM to generate the rearward traction forces needed for forward cell movement. Although it is well appreciated that force transmission regulates integrin and FA signalling15,16, we focus on how the physical linkages between actin and the ECM are formed and regulated, and how forces transmitted by the clutch impact FA molecules to allow individual FAs to act as mechanosensors.
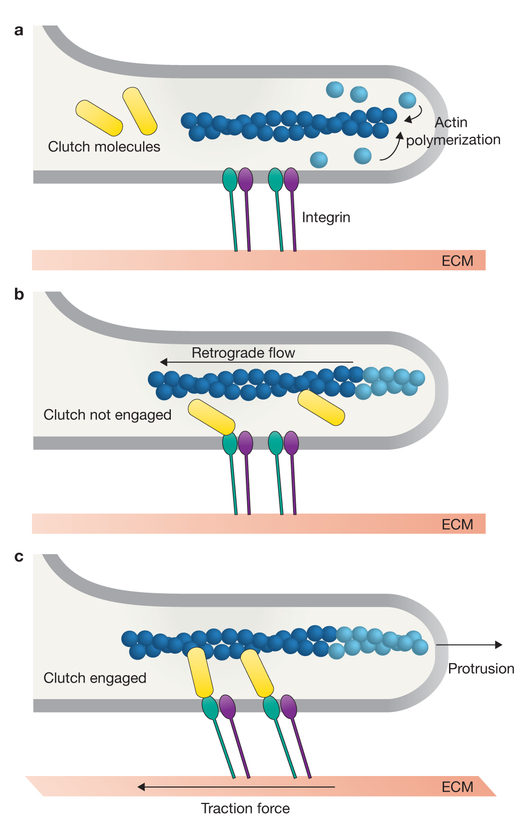
Figure 1.
The molecular clutch hypothesis. (a) New actin monomers (light blue) are incorporated on to the barbed end of a pre-existing actin filament (dark blue) facing the leading edge of the lamellipodia. Transmembrane integrin dimers (green and purple) are bound to the extracellular matrix (ECM). (b) If the clutch (yellow) is not engaged to connect actin to the ECM, then actin polymerization results in rapid retrograde cytoskeletal flow, no net leading edge protrusion and no traction force on the ECM. (c) If the clutch is engaged, the forces generated by polymerization of the actin cytoskeleton are physically transmitted to the ECM, resulting in slowing of retrograde flow, traction force on the ECM and a net edge protrusion.
The molecular clutch hypothesis
Cell movement has captivated scientists since the invention of the light microscope17, and microscopy continues to provide insight into the molecular regulation of mammalian cell migration. In the early 1960s, Abercrombie proposed that protruding and ruffling of a broad, thin membrane at the front of migrating chick fibroblasts was the main “locomotory organ” of the fibroblast18, and because of its lamellar structure and protrusive behaviour, dubbed it the lamellipodium. This was observed to undergo cycles of protrusion and retraction resulting in net forward movement19. Abercrombie and Harris observed that marker particles derived from ink or resin adhered to the cell surface moved centripetally from the leading edge along the dorsal and ventral cell membranes at a constant rate and with rearward direction relative to the direction of leading edge protrusion20,21. These first observations of retrograde flow were proposed to be related to forward edge protrusion and rearward traction forces, although a moving plasma membrane was initially thought to cause the flow of particles20–22. Wolpert and Allison later proposed that this rearward particle movement could be due to the movement of the filamentous network inside the cell pulling proteins in a fluid plasma membrane22. Using electron microscopy, Small and Abercrombie showed that the leading part of a migrating cell is enriched in filamentous actin that is organized into structurally distinct leading lamellipodia followed by thin lamellae, and that actin filaments within lamellipodia are polarized with their fast-growing ‘barbed’ ends facing the cell edge23,24. In 1985, Wang performed seminal fluorescence recovery after photobleaching (FRAP) experiments of fluorescently labelled actin to demonstrate that actin monomers were incorporated into filaments at the leading edge of lamellipodia and that they underwent a rearward movement away from the edge10. Forscher also observed retrograde flow in neuronal growth cones and showed that it depended on both actin polymerization and myosin II contractility13,25. Theriot and Mitchison demonstrated that actin polymerization in the lamellipodia was directly coupled to forward cell movement11, and retrograde flow was observed to be inversely related to cell speed10,13,26,27. Based on these observations, in 1988 Mitchison and Kirschner proposed that a ‘molecular clutch’ connected the retrograde-moving actin cytoskeleton to ECM receptors in the plasma membrane, allowing tension to be exerted on the substrate14.
In the subsequent decades, the evidence supporting the molecular clutch hypothesis has grown; however, the basic principles remain unchanged (Fig. 1). Actin is rapidly polymerized in lamellipodia, and actin polymerization and myosin II contraction drive the net rearward/retrograde movement of the actin network. Macromolecular FAs act as a regulable molecular clutch by mediating transient, indirect interactions between the retrograde-moving actin cytoskeleton and ECM-bound integrins. The clutch is ‘engaged’ when the actin cytoskeleton is indirectly connected to immobilized, ECM-bound integrins through the macro-molecular FA complex. If actin polymerization and myosin contraction remain constant, this engagement causes retrograde flow to slow down as forces from the actin cytoskeleton are propagated to the substrate, resulting in rearward traction, whereas continued polymerization at the membrane-facing barbed ends of actin filaments that are immobilized at the adhesion site drives forward membrane protrusion. Conversely, disengagement or slippage of the clutch would result in faster retrograde flow, decreased traction forces and cessation of membrane protrusion.
Forces generated in the actin cytoskeleton drive actin retrograde flow
The retrograde movement of the actin cytoskeleton is the basis of the molecular clutch hypothesis. In adherent migrating cells, the cortical actin cytoskeleton is organized into two structurally and functionally distinct regions: the lamellipodium and the lamellum23,28,29. Rapid Arp2/3-mediated F-actin polymerization at the tip of the lamellipodium generates a pushing force against the leading edge plasma membrane and has been proposed to drive its protrusion through a Brownian ratchet mechanism12,30,31. If membrane expansion is constrained, F-actin polymerization against the inextensible membrane barrier also results in a counterforce that is thought to push the entire F-actin network rearward relative to the membrane, with the majority of F-actin depolymerizing at the base of the lamellipodium10,25,28,32. Therefore, although new actin monomers are continuously incorporated at the lamellipodium tip, the lamellipodial actin network exhibits treadmilling behaviour and undergoes retrograde flow of ~0.5–1.5 μm min−1 (ref. 28). The flat lamellum region is located proximal to the lamellipodium (that is, closer to the cell centre) and contains many distinct F-actin structures including dorsal stress fibres, transverse arcs and ventral stress fibres33–35. In the lamellum, myosin II assembles into mini-filaments and contracts actin bundles to generate forces that reorganize and disassemble actin, and drive a slower retrograde flow of ~0.25–0.5 μm min−1 (refs28,33,36,37). Myosin II inhibition blocks slow lamella retrograde flow, but leaves rapid lamellipodial flow intact28,38,39, whereas blocking actin polymerization disrupts lamellipodial flow28. Thus, actin polymerization drives rapid retrograde flow in the lamellipodia, and myosin contraction drives slower retrograde flow in the lamella. However, in some cell types, such as fish skin keratocytes and neuronal growth cones, these two cellular regions are not well delineated, with both actin polymerization and myosin II activity partially contributing to a general leading edge retrograde flow40–42.
FAs are a 3D macromolecular complex that physically connects the actin cytoskeleton to the ECM
Evidence from diverse cell types indicates that the speed of actin retrograde flow is inversely correlated to edge protrusion11,13,28,41, suggesting that slowing actin retrograde flow can drive forward cell movement, a process that would require transmission of the forces generated in the actin cytoskeleton to the ECM. Cells in tissue culture generate much more force on the ECM than is necessary for translocation43, implying that actin flow is likely to power other processes such as ECM remodelling and FA disassembly in the cell rear. However, to understand how force transmission to the ECM drives cell movement, it is important to understand how the actin cytoskeleton is physically linked to the extra-cellular environment.
FAs are integrin-based adhesion organelles that physically connect the actin cytoskeleton to the ECM44–46 through a membrane-associated macromolecular complex. Integrins are transmembrane heterodimers of α and β subunits that use their large extracellular domain to specifically interact with different extracellular proteins such as fibronectin, collagen and laminin47,48, and must undergo a dramatic conformational change to become ‘activated’ and competent to tightly bind ligands48–50. The β-integrin cytoplasmic tail binds several proteins including talin51, α-actinin52 and kindlin53, but neither α- or β-integrin can interact with actin directly54–56. Therefore, the connection between integrins and actin must be mediated indirectly by the assembly of the macromolecular FA structure.
FAs are dynamic structures that can contain hundreds of different molecules including scaffolding and structural proteins, kinases and phosphatases, and their composition changes in response to diverse stimuli57–60. FAs form in the protruding lamellipodia as small puncta containing integrin, focal adhesion kinase (FAK) and paxillin61–63. Although most of these ‘nascent’ FAs have a lifetime of ~1 min, a subset are stabilized and undergo ‘maturation’ when they reach the lamellum62. FA maturation requires stress fibre assembly and myosin II activity39,62,64 as FAs elongate along an actin–α-actinin template in the direction of retrograde flow62 and undergo dramatic compositional changes58.
The proteins that localize to FAs are not homogenously distributed in the 3D FA structure. Recent advances in light microscopy have allowed the determination of protein localization in FAs at the nanoscale level, which has revealed that mature FAs are vertically stratified along the axis perpendicular to the ventral plasma membrane (Fig. 2)65–69. Paxillin and FAK localize with integrin cytoplasmic tails within ~30 nm of the plasma membrane (that is, low in the FA) in a region termed the inte-grin signalling layer67,69. In contrast, actin and the actin-associated proteins zyxin, VASP and α-actinin localize >50 nm above the plasma membrane (that is, high in the FA) in the ‘actin regulatory layer’67. Talin is a large protein that can directly interact with both integrin and actin70. The talin head, which binds β-integrin cytoplasmic tails, co-localizes with paxillin and FAK near the plasma membrane, whereas the talin tail, which binds actin, localizes ~30 nm higher51,67,69,71,72. Vinculin primarily co-localizes with the talin rod in the intermediary region, or ‘force transduction layer’67, but is initially recruited near the plasma membrane and is redistributed upwards as the FA matures69. This conserved layered organization of FA proteins is observed in diverse cell types67–69, suggesting that it arises from the self-assembly of protein–protein interactions at FAs.
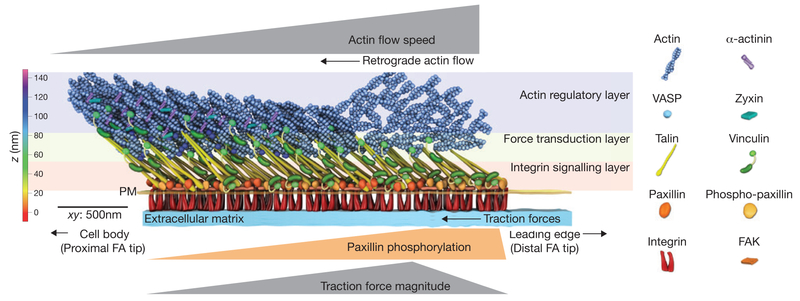
Figure 2.
Nano-scale architecture of the focal adhesion clutch. Focal adhesions (FAs) are organized into 3D ‘nano-domains’ with unique protein compositions and mechanical signatures. The distal tip of the FA facing the leading edgeis where lamellipodial dendritic actin interacts with the FA, and contains an enrichment of phosphorylated paxillin, rapid retrograde flow and high traction forces. The proximal tip of the FA interacts with the actin stress fibre and is enriched with the actin binding proteins α-actinin, zyxin and VASP, and is characterized by slow retrograde flow and low traction forces. Additionally, proteins are stratified in the axis perpendicular to the cell plasma membrane (PM). Paxillin, FAK and the talin head domain are co-localized with integrin cytoplasmic tails near the plasma membrane in the integrin signalling layer. Actin and actin-binding proteins are localized >50 nm above the plasma membrane in the actin regulatory layer. Talin and vinculin reside in the force transduction layer that spans between the integrin signalling and actin regulatory layers. Talin is oriented with the N-terminus near the plasma membrane and the C-terminus ~30 nm higher and extended towards the FA proximal tip. The colour bar shows the vertical distance from the extracellular matrix, whereas the scale bar denotes the distance across the xy plane.
Proteins are also organized along the length of individual FAs (that is, the axis parallel to the ventral plasma membrane; Fig. 2). FAs grow in the direction of actin retrograde flow, so mature FAs are oriented with their long axis perpendicular to the leading edge61,62. We will refer to the tip of the FA nearest the leading edge as the distal tip, and the tip closer to the cell body as the proximal tip. Actin stress fibres attach at the proximal tip of FAs, so actin-associated proteins are also concentrated at the proximal tip61,67,73,74. In contrast, FAK-dependent tyrosine phosphorylation of paxillin is highest at the distal tip of FAs, providing a mechanism for concentrating SH2-domain-containing proteins that bind to phosphotyrosine in this region75. Talin molecules are organized along the long axis of the FA, with their integrin-binding head localized closer to the distal tip of the adhesion and their actin-binding tail stretched rearward in the direction of F-actin flow76. It is likely that other proteins are also organized along the long axis of the FA by actin retrograde flow.
Although FAs have a conserved nanoscale architecture, proteins within FAs are highly dynamic. Inactive integrins in FAs can diffuse within the plasma membrane and are immobilized by activation77,78. Most FA proteins, including paxillin, vinculin, α-actinin, talin, kind-lin, FAK, zyxin, VASP and ILK, exchange rapidly with the cytoplasmic pool (FRAP t1/2 measured to be less than 30 seconds)73,79–81. Additionally, FRAP for FA proteins is rarely approximated by a single exponential curve73, suggesting that subpopulations of molecules within the FA have different dynamics. Indeed, both paxillin and vinculin have at least four distinct sub-populations in the FA and surrounding cytoplasm, and paxillin and vinculin have different dynamics at the distal and proximal tip of the FA73. Thus, even when an FA seems stable for tens of minutes, the molecules within the adhesion are rapidly turning over. Furthermore, the types of interactions occuring within the FA can change over time. Fluorescence fluctuation correlation methods suggest that talin–vinculin complexes form before the integrin–talin complex in nascent FAs, whereas α-actinin clusters periodically enter nascent FAs and transiently interact with integrins82. Results from fluoresecence cross-correlation spectroscopy studies have suggested that molecules can enter and leave the stable FA as preformed cytoplasmic complexes corresponding to the different FA nanoscale layers. For example, paxillin and FAK co-localize in FAs near the plasma membrane67 and diffuse together in the cytoplasm83. Thus, it is possible that the self-organization of protein–protein interactions dictating FA architecture initiates from interactions in the cytoplasm.
The actin cytoskeleton is a master regulator of Fas
The molecular clutch hypothesis proposes that the forces generated in the leading edge actin cytoskeleton are transmitted across FAs to generate rearward traction forces against the ECM39,84–86. Furthermore, actin polymerization and integrin adhesion are spatiotemporally coordinated87, and actin polymerization62,86, F-actin structural organization33,64,88 and myosin II contractility64,89 all contribute to the regulation of FAs in lamellipodia and lamella.
The lateral force of retrograde flow has been hypothesized to help drive integrin activation by separating α- and β-integrin cytoplasmic tails90. Integrin activation can be initiated by the binding of cytoplasmic proteins, such as talin, to the β-integrin tail (‘inside-out’ activation) or by the binding of integrins to their extracellular ligand (‘outside-in’ activation)50,91–93. However, integrin inactivation and constitutive activation with small molecules or antibodies both decrease migration speeds94, suggesting that a carefully regulated cycle of integrin activation and inactivation is required for proper cell migration. Integrin activation involves a dramatic conformational change between an inactive low-affinity conformation with the extracellular domain folded close to the plasma membrane and an activated high-affinity conformation with the extracellular domain extended away from the plasma membrane50,93,95. However, both inside-out and outside-in integrin activation correspond to a lateral separation of the α- and β-integrin cytoplasmic tails that can be measured by a loss in intermolecular fluorescence resonance energy transfer (FRET)96. Furthermore, introducing an artificial 14 nm separation between the α5 and β1 cytoplasmic domains is sufficient to induce high-affinity binding to fibronectin in vitro, and simulations of molecular dynamics suggest that the lateral force of actin retrograde flow linked to the β tail by a clutch molecule could pull the β tail away from the α tail to stabilize integrin heterodimers in an open, high-affinity conformation49,90. This force-dependent model of integrin activation predicts that integrin cytoplasmic tails would open in the direction of retrograde actin flow, resulting in a polarized and oriented population of active integrins in FAs.
Actin polymerization also controls the formation of initial macro-molecular nascent FAs. FA formation and stability in the lamellipodia requires active actin polymerization62,86, and loss of Arp2/3 complex activity reduces FA assembly and results in disorganized, abnormal adhesions that do not support haptotactic migration up a surface-bound gradient of ECM97,98. Both FAK and vinculin can bind directly to the Arp2/3 complex, suggesting a direct molecular link between Arp2/3 activity and FAs99,100. Nevertheless, more research is needed to understand precisely how Arp2/3 regulates nascent FA assembly.
Actin also regulates FA growth and maturation. Although most nascent FAs disassemble at the base of the lamellipodium, a subset stabilize and undergo maturation at the border between the lamellipodium and the lamellum62,101. Thus, a row of maturing FAs spatially defines the lamellipodium–lamellum border and contributes to the abrupt slowing of actin retrograde flow speeds in the lamellum28,84,86. During maturation, FAs undergo a compositional change as they grow and elongate in the direction of retrograde flow58,61,62,64,102. FAs grow at a rate directly proportional to actin flow, independently of specific molecular perturbations; thus, faster retrograde flow results in faster FA elongation103. This suggests that FA growth, and therefore local integrin activation, is limited by the distance of actin retrograde movement, in agreement with the lateral-force model of integrin activation90. FA maturation requires tension to be applied across FAs, either from intracellular myosin contractility or extracellular pulling104–109, and FA size correlates to the amount of applied force85. During FA maturation, α-actinin is recruited to cross-link actin filaments62. Mature FAs remain attached to actin stress fibres throughout their lifetime, and their maintenance requires association with contrac-tile F-actin bundles33,64,88. Disruption of dorsal stress fibres generated by mDia2 (mammalian diaphanous-related, a member of the formin family of proteins) leads to abnormal FA morphology and dynamics33,110, and several other formin family members have been found in biochemically isolated FAs58. Further work is needed to clarify the role of specific actin nucleators and F-actin structures in regulating the different stages of FA assembly, growth and disassembly in the lamellipodia and lamella.
Forces at FAs regulate protein–protein interactions and protein activity
Forces generated in the actin cytoskeleton are transmitted across the macromolecular FA to generate traction on the extracellular substrate. Individual FAs have been measured to apply traction forces to the extra-cellular substrate ranging from less than 1 kPa to greater than 10 kPa (1–10 nN μm−2), although these are the cumulative forces distributed across many thousands of molecules in the FA85,111. The development of fluorescence-based molecular tension sensors has allowed the direct measurement of forces applied across individual FA molecules112–114. The tension across individual vinculin molecules in FAs is estimated to be ~2.5 pN when measured with a FRET biosensor112. Single-molecule integrin tension sensors based on FRET114,115 or quenching113,116 measured ~1–40 pN of tension on individual integrins at FAs. However, both vinculin and integrin molecules were observed to experience a dynamic range of tensions at FAs. Although other molecules, including talin and p130Cas, are thought to bear tension at FAs, additional tools are needed to directly measure tension in other proteins of interest76,117.
Proteins respond to tension through diverse mechanisms, and in addition to generating traction for cell migration, forces transmitted across FAs can also significantly alter protein localization and activity at FAs. Some molecules form catch-bonds, characterized by an increase in the dissociation lifetime with increasing tensile force. For example, the lifetime of α5β1-integrin–fibronectin bonds is significantly prolonged when 10–30 pN of force is applied118, meaning that increasing forces stabilize the integrin–fibronectin interaction. Other proteins, such as talin, exhibit slip-bond behaviour119, and can also undergo a force-dependent extension120. Although talin contains many vinculin binding sites (VBSs) buried within the native structure, an unstretched talin molecule has only a single available VBS120. Application of 2 pN of force to stretch talin unmasks cryptic VBSs, allowing the recruitment of additional vinculin molecules120. Talin extension occurs at forces of more than 5 pN, and the binding of vinculin to talin prevents refolding of the talin rod in vitro121. However, at >25 pN force, vinculin dissociates from talin121. Force can also directly alter protein activity. The application of a 1–3 pN pulling force to the mDia2 formin bound to the end of an actin filament results in a significant increase in the actin polymerization rate122,123. The mechanosensitivity of a range of proteins in FAs suggests that the molecular clutch itself may be mechanosensitive.
Talin, vinculin and α-actinin regulate the FA clutch
If FAs represent a molecular clutch, actin retrograde flow will be specifically engaged to the ECM at FAs. Thus, a key prediction of the molecular clutch hypothesis is that actin flow slows down locally at FAs as traction forces increase. Indeed, actin retrograde flow speeds have been experimentally observed to decrease at FAs39,78,86,103,124, and traction forces are observed to be highest at FAs39,85,125. Since actin does not directly interact with integrins, actin retrograde flow must be transmitted across a molecular ‘transmission interface’ of actin-and integrin-binding proteins. Many molecules in FAs, including integrins, paxillin, FAK, α-actinin, talin and vinculin, undergo retrograde flow at rates equal to or slower than the local actin retrograde flow39,78,86,103,124,126, suggesting that forces driving retrograde flow are transmitted across many of the molecules within FAs.
Molecules that directly bind integrins and/or actin are thought to regulate force transmission across FAs by modulating the efficiency of the integrin–actin connection. In the simplest clutch scenario, force transmission at FAs will be at a maximum when actin is tightly associated with integrins. However, if there is ‘slippage’ between actin retrograde flow and the ECM, less force would be expected to be transmitted4,39,127,128.
There is growing evidence that talin, vinculin and α-actinin can directly engage actin retrograde flow to increase force transmission at FAs. Talin is one of the few known molecules that binds directly to both β-integrin tails and F-actin51,71, and when talin is depleted, cells have decreased traction forces and fail to stiffen in response to tension129,130. Additionally, talin depletion causes slippage between actin retrograde flow and the ECM, resulting in a much wider region of fast lamellipodial actin flow at the cell edge119,130. Vinculin binds simultaneously to talin and F-actin, and helps to mechanically reinforce the talin–actin linkage131,132. When vinculin is depleted, cells have decreased traction forces, widened lamellipodia and faster retrograde flow rates103,111,132,133, and both actin-and talin-binding domains are required to restore WT retrograde flow and traction forces103,132. Furthermore, vinculin is required for myosin-dependent traction forces132, suggesting that the talin–vinculin–actin linkage directly regulates myosin-dependent FA clutch engagement103,132. The talin–vinculin interaction is highly sensitive to force changes120,121, suggesting that clutch engagement has a positive feedback effect. As force transmission increases across talin, additional vinculin molecules are recruited to bind actin, slow retrograde flow and increase force transmission. α-actinin, which can bind integrin and actin, is also required for maximum force transmission across β3 integrins, and α-actinin recruitment correlates with force generation134, suggesting that α-actinin could regulate the molecular clutch in addition to cross-linking actin.
Mechanosensitivity of the molecular clutch
Since the FA clutch can be dynamically regulated, force transmission could be variable over time127. Furthermore, the level of clutch engagement is predicted to be dependent on the stiffness of the substrate, suggesting that the clutch is mechanosensitive127. Modelling the ensemble behaviour of substrate-bound linkages to an actin cytoskeleton undergoing a constant rate of retrograde flow predicts that if the FA is unable to significantly deform a stiff substrate, then there will be stochastic, weak clutch engagement and constant traction forces in the FA127. Actin and the substrate form many linkages that rapidly reach their breaking strength as tension builds against the stiff ECM127. However, on a softer, deformable substrate, cooperative engagement of many linkages is increased because stretching of the substrate allows time for more linkages to form before any one reaches its breaking strength127. As more linkages form, retrograde flow slows and traction forces increase until the entire ensemble of linkages breaks under the increased tension127. Thus, on softer substrates, the ensemble behaviour of molecules within an FA are predicted to exhibit a ‘tugging’ behaviour, with the position of peak traction and the magnitude of traction across individual FAs fluctuating over time127. In line with these predictions, high-resolution traction force microscopy has shown that traction forces fluctuate by several thousand Pa within individual FAs, and a single FA can undergo several transitions between low and high traction regimes each minute111. Furthermore, the ability for cells to fluctuate between low and high forces requires the presence of vinculin, supporting the importance of vinculin in regulating the FA clutch111. FA ‘tugging’ is mechanosensitive, as it only occurs on soft substrates. On stiff substrates, individual FAs have stable traction forces, in contrast to the tugging traction forces observed on softer substrates111. Furthermore, this tugging behaviour is required for cells to differentiate between soft and stiff substrates as they migrate111. The ability of individual FAs to dynamically sample substrate stiffness by applying tugging pulling forces and to act as mechanosensors to guide cell migration111,127 is in agreement with a simple dynamic molecular clutch model127. Further experiments are required to determine whether oscillations in myosin II activity135,136 or actin polymerization28,137 could also contribute to the tugging behaviour of FAs.
The 3D molecular clutch
The 3D organization of proteins in FAs is likely to impose physical limitations on the molecular clutch. As actin flows directionally from the distal FA tip to the proximal tip along the xy axis of FAs, forces applied to molecules within FAs are biased in this direction, resulting in molecules becoming aligned within the FA. For example, talin molecules are oriented with the actin-binding tail domain higher and further from the leading edge than the integrin-binding head67,76. Actin retrograde flow is likely to polarize other molecules, such as integrins, and results in FA elongation away from the leading edge during maturation62. There is also growing evidence that the clutch is differentially engaged along the length of the FA, as both actin retrograde flow speeds and traction forces are highest towards the distal tip of mature FA39,111.
Along the z-axis of the FA, more than 50 nm separates the actin cytoskeleton in the upper FA layer from integrin cytoplasmic tails in the lowest FA layer at the ventral plasma membrane67, indicating that forces must be transmitted from actin vertically down the FA. If forces dissipate as they propagate from actin downwards toward the ECM, then differences would exist in the retrograde flow of proteins in the different FA layers. Indeed, α-actinin has a fast retrograde flow speed of ~2.5 μm min−1 and co-localizes with actin more than 60 nm above the ventral plasma membrane67,124. In contrast, paxillin and integrin, which localize close to the plasma membrane, have retrograde flow speeds below 0.1 μm min−1. Talin and vinculin, which localize in an intermediary layer between the plasma membrane and the actin cytoskeleton, have flow speeds of between 0.1–0.3 μm min−1. Additionally, both the direction and speed of actin flow are most closely coupled with α-actinin flow, whereas vinculin and talin have slightly less coupling with actin124. Integrin and paxillin flow have the lowest observed coupling with actin flow speed and direction124. This suggests that the forces of actin flow dissipate as they are transmitted through a series of slipping or viscous linkages mediated by specific proteins organized along the vertical axis of the FA.
In addition to the conserved 3D organization of FA proteins, spatial differences also exist in retrograde flow and traction forces in FAs. Within a single FA, there are 3D nano-domains with varying protein composition and mechanical signatures (Fig. 2). However, further work is needed to understand how this spatial organization influences FA protein activity. The spatial compartmentalization of different protein groups might help to limit the types of interaction that can occur at FAs. For example, talin-binding regulates the redistribution of vinculin into the upper FA layers as the FA matures69, possibly allowing for increased clutch engagement. As proteins move up, down or rearward with actin retrograde flow, they could come into contact with different groups of binding partners, vary the magnitude of mechanical tension, or change downstream signalling.
Molecular clutches at diverse adhesive interactions
Although there is mounting experimental evidence to indicate that FAs act as bona fide molecular clutches during integrin-mediated cell migration, other sites of cell contact or adhesion could act analogously to transmit forces driving cortical actin motion to the extracellular milieu in diverse cellular processes (Fig. 3). In theory, any transmembrane protein capable of non-specific frictional or specific binding interactions with the extracellular environment and of indirectly binding the actin cytoskeleton could facilitate force transmission in a similar manner to integrins at FAs. In the simplest scenario, cell motility could be driven by friction between any actin-clutch-associated transmembrane protein complex and a surface, if the protein and the surface were pressed against each other by physical confinement7. Integrins themselves are involved in various structures other than FAs and dynamic processes besides cell migration that could involve molecular clutches. For example, integrin LFA−1 and its ligand ICAM−1 mediate leukocyte–endothelial cell adhesion during leukocyte transmigration, which can occur via transcellular or paracellular routes138. The leukocyte actin cytoskeleton can connect to LFA−1 through talin55, and the endothelial actin cytoskeleton connects to ICAM−1 through α-actinin139, suggesting that both cells could actively transmit force across the adhesion through indirect protein interactions with actin to drive cells through endothelia. Furthermore, repair of endothelial cells following leukocyte trans-migration involves integrin-dependent ventral lamellipodia that may form a molecular clutch involved in maintaining endothelial barrier function140. Rapid actin polymerization is essential for proper synapse formation between T cells and antigen-presenting cells to mediate the adaptive immune response, and the centripetal flow of actin organizes both T-cell receptors and LFA−1 at the immunological synapse141. Bacterial and viral pathogens frequently exploit cell adhesion molecules, including integrins and cadherins142, to gain entry into host cells, and virulence factors can activate key clutch regulators, such as vinculin143. The ability of cadherins to indirectly connect to the actin cytoskeleton through α- and β-catenin allows tension to be applied across cell–cell adhesions144. These adhesions are similar to FAs in many ways, as force transduction across cadherins is regulated by interactions between α-catenin, vinculin and actin144. Furthermore, N-cadherin engages actin retrograde flow to promote neuronal growth cone migration145, and VE-cadherin undergoes actin-dependent basal to apical flow146. Thus, force transduction at cell–cell junctions via a cadherin-based molecular clutch is likely to be critical for remodelling epithelia during morphogenesis147.
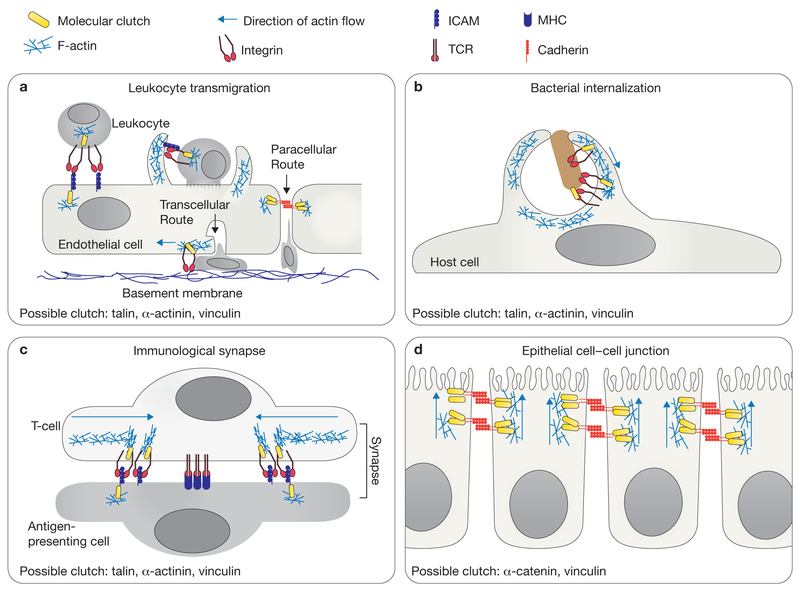
Figure 3.
Molecular clutches may mediate diverse cell adhesive interactions. (a) During leukocyte diapedesis, initial cell–cell adhesion is mediated by the interactions of the LFA−1 integrin and its ligand ICAM−1. Paracellular migration occurs when the endothelial cells temporarily disassemble cell–cell junctions, allowing the leukocyte to migrate between two endothelial cells. Transcellular migration occurs when the leukocyte migrates through a single endothelial cell. The migrating leukocyte extends invasive protrusions into the endothelial cell, and the endothelial cell forms a transmigratory cup around the leukocyte. Following successful transmigration, the transmigratory pore is closed by integrin-dependent ventral lamellipodia to restore endothelial barrier integrity. (b) Pathogens often seek entry into host cells by co-opting the integrin or cadherin adhesion machinery. Bacteria can bind to these adhesion receptors, stimulate actin polymerization and activate clutch molecules to promote the formation of a phagocytic cup. (c) The T-cell immunological synapse requires centripetal actin flow to organize adhesion receptors into distinct domains. Rapid retrograde flow organizes and potentially activates LFA−1 integrins in the actin-rich regions. In contrast, the T-cell receptors (TCR) cluster in the actin-free centre. MHC, major histocompatibility complex. (d) Cadherins mediate cell–cell adhesion and connect indirectly to the actin cytoskeleton through β-catenin, α-catenin and vinculin. Cadherins have been observed to undergo actin-dependent basal-to-apical flow that could generate force for epithelial morphogenesis. Active polymerization of the actin cytoskeleton is depicted as a blue mesh and the direction of actin flow is indicated with a blue arrow (a–d).
In conclusion, it is possible that forces generated in the actin cytoskeleton are harnessed by molecular clutches at a variety of adhesive interactions to regulate force transmission during diverse cellular functions. The insights gained from studying force transmission at integrin-based FAs could be applied to better understand these processes.
ACKNOWLEDGEMENTS
The authors thank Michelle Baird and Michael Davidson (Florida State University) for assistance with figure design and members of the Waterman Lab for helpful discussions. Funding was provided by the Division of Intramural Research, NHLBI (L.B.C. and C.M.W.).
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Friedl P Prespecification and plasticity: shifting mechanisms of cell migration. Curr. Opin. Cell Biol 16, 14–23 (2004). [DOI] [PubMed] [Google Scholar]
- 2.Paluch EK & Raz E The role and regulation of blebs in cell migration. Curr. Opin. Cell Biol 25, 582–590 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stroka KM et al. Water permeation drives tumor cell migration in confined microenvironments. Cell 157, 611–623 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Renkawitz J et al. Adaptive force transmission in amoeboid cell migration. Nat. Cell Biol 11, 1438–1443 (2009). [DOI] [PubMed] [Google Scholar]
- 5.Petrie RJ, Koo H & Yamada KM Generation of compartmentalized pressure by a nuclear piston governs cell motility in a 3D matrix. Science 345, 1062–1065 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pollard TD & Cooper JA Actin, a central player in cell shape and movement. Science 326, 1208–1212 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y-J et al. Confinement and low adhesion induce fast amoeboid migration of slow mesenchymal cells. Cell 160, 659–672 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Ruprecht V et al. Cortical contractility triggers a stochastic switch to fast amoeboid cell motility. Cell 160, 673–685 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petrie RJ, Doyle AD & Yamada KM Random versus directionally persistent cell migration. Nat. Rev. Mol. Cell Biol 10, 538–549 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang YL Exchange of actin subunits at the leading edge of living fibroblasts: possible role of treadmilling. J. Cell Biol 101, 597–602 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Theriot JA & Mitchison TJ Actin microfilament dynamics in locomoting cells. Nature 352, 126–131 (1991). [DOI] [PubMed] [Google Scholar]
- 12.Mogilner A & Oster G Cell motility driven by actin polymerization. Biophys. J 71, 3030–3045 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin CH & Forscher P Growth cone advance is inversely proportional to retrograde F-actin flow. Neuron 14, 763–771 (1995). [DOI] [PubMed] [Google Scholar]
- 14.Mitchison T & Kirschner M Cytoskeletal dynamics and nerve growth. Neuron 1, 761–772 (1988). [DOI] [PubMed] [Google Scholar]
- 15.Marjoram RJ, Lessey EC & Burridge K Regulation of RhoA activity by adhesion molecules and mechanotransduction. Curr. Mol. Med 14, 199–208 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rose DM, Alon R & Ginsberg MH Integrin modulation and signaling in leukocyte adhesion and migration. Immunol. Rev 218, 126–134 (2007). [DOI] [PubMed] [Google Scholar]
- 17.Dunn GA & Jones GE Cell motility under the microscope: Vorsprung durch Technik. Nat. Rev. Mol. Cell Biol 5, 667–672 (2004). [DOI] [PubMed] [Google Scholar]
- 18.Abercrombie M The bases of the locomotory behaviour of fibroblasts. Exp. Cell Res 8, 188–198 (1961). [DOI] [PubMed] [Google Scholar]
- 19.Abercrombie M, Heaysman JEM & Pegrum SM The locomotion of fibroblasts in culture: I. Movements of the leading edge. Exp. Cell Res 59, 393–398 (1970). [DOI] [PubMed] [Google Scholar]
- 20.Abercrombie M, Heaysman JEM & Pegrum SM The locomotion of fibroblasts in culture: III. Movements of particles on the dorsal surface of the leading lamella. Exp. Cell Res 62, 389–398 (1970). [DOI] [PubMed] [Google Scholar]
- 21.Harris A & Dunn G Centripetal transport of attached particles on both surfaces of moving fibroblasts. Exp. Cell Res 73, 519–523 (1972). [DOI] [PubMed] [Google Scholar]
- 22.Harris AK Cell surface movements related to cell locomotion. Ciba Found. Symp 14, 3–26 (1973). [DOI] [PubMed] [Google Scholar]
- 23.Abercrombie M, Heaysman JEM & Pegrum SM The locomotion of fibroblasts in culture: IV. Electron microscopy of the leading lamella. Exp. Cell Res 67, 359–367 (1971). [DOI] [PubMed] [Google Scholar]
- 24.Small JV, Isenberg G & Celis JE Polarity of actin at the leading edge of cultured cells. Nature 272, 638–639 (1978). [DOI] [PubMed] [Google Scholar]
- 25.Forscher P Actions of cytochalasins on the organization of actin filaments and microtubules in a neuronal growth cone. J. Cell Biol. 107, 1505–1516 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee J, Ishihara A, Theriot JA & Jacobson K Principles of locomotion for simple-shaped cells. Nature 362, 167–171 (1993). [DOI] [PubMed] [Google Scholar]
- 27.Henson JH et al. Two components of actin-based retrograde flow in sea urchin coelomocytes. Mol. Biol. Cell 10, 4075–4090 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ponti A, Machacek M, Gupton SL, Waterman-Storer CM & Danuser G Two distinct actin networks drive the protrusion of migrating cells. Science 305, 1782–1786 (2004). [DOI] [PubMed] [Google Scholar]
- 29.Cramer LP Molecular mechanism of actin-dependent retrograde flow in lamellipodia of motile cells. Front. Biosci 2, 260–70 (1997). [DOI] [PubMed] [Google Scholar]
- 30.Cortese JD, Schwab B, Frieden C & Elson EL Actin polymerization induces a shape change in actin-containing vesicles. Proc. Natl Acad. Sci. USA 86, 5773–5777 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alberts JB & Odell GM In silico reconstitution of Listeria propulsion exhibits nano-saltation. PLoS Biol. 2, e412 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iwasa JH & Mullins RD Spatial and temporal relationships between actin-filament nucleation, capping, and disassembly. Curr. Biol 17, 395–406 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hotulainen P & Lappalainen P Stress fibers are generated by two distinct actin assembly mechanisms in motile cells. J. Cell Biol 173, 383–394 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Small JVV, Rottner K, Kaverina I & Anderson KII Assembling an actin cytoskeleton for cell attachment and movement. Biochim. Biophys. Acta 1404, 271–281 (1998). [DOI] [PubMed] [Google Scholar]
- 35.Kovac B, Teo JL, Mäkelä TP & Vallenius T Assembly of non-contractile dorsal stress fibers requires α-actinin−1 and Rac1 in migrating and spreading cells. J. Cell Sci 126, 263–73 (2013). [DOI] [PubMed] [Google Scholar]
- 36.Verkhovsky AB, Svitkina TM & Borisy GG Myosin II filament assemblies in the active lamella of fibroblasts: their morphogenesis and role in the formation of actin filament bundles. J. Cell Biol 131, 989–1002 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilson CA et al. Myosin II contributes to cell-scale actin network treadmilling through network disassembly. Nature 465, 373–377 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waterman-Storer CM & Salmon ED Actomyosin-based retrograde flow of microtubules in the lamella of migrating epithelial cells influences microtubule dynamic instability and turnover and is associated with microtubule breakage and treadmilling. J. Cell Biol 139, 417–434 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gardel ML et al. Traction stress in focal adhesions correlates biphasically with actin retrograde flow speed. J. Cell Biol 183, 999–1005 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Medeiros NA, Burnette DT & Forscher P Myosin II functions in actin-bundle turnover in neuronal growth cones. Nat. Cell Biol 8, 215–226 (2006). [DOI] [PubMed] [Google Scholar]
- 41.Jurado C, Haserick JR & Lee J Slipping or gripping? Fluorescent speckle microscopy in fish keratocytes reveals two different mechanisms for generating a retrograde flow of actin. Mol. Biol. Cell 16, 507–518 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vallotton P, Danuser G, Bohnet S, Meister J-J & Verkhovsky AB Tracking retrograde flow in keratocytes: news from the front. Mol. Biol. Cell 16, 1223–1231 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Del Alamo JC et al. Spatio-temporal analysis of eukaryotic cell motility by improved force cytometry. Proc. Natl Acad. Sci. USA 104, 13343–13348 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hynes RO & Destree AT Relationships between fibronectin (LETS protein) and actin. Cell 15, 875–886 (1978). [DOI] [PubMed] [Google Scholar]
- 45.Singer II The fibronexus: a transmembrane association of fibronectin-containing fibers and bundles of 5 nm microfilaments in hamster and human fibroblasts. Cell 16, 675–685 (1979). [DOI] [PubMed] [Google Scholar]
- 46.Singer II Association of fibronectin and vinculin with focal contacts and stress fibers in stationary hamster fibroblasts. J. Cell Biol. 92, 398–408 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tamkun JW et al. Structure of integrin, a glycoprotein involved in the transmembrane linkage between fibronectin and actin. Cell 46, 271–282 (1986). [DOI] [PubMed] [Google Scholar]
- 48.Hynes RO Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687 (2002). [DOI] [PubMed] [Google Scholar]
- 49.Takagi J, Erickson HP & Springer TA C-terminal opening mimics “inside-out” activation of integrin α5β1. Nat. Struct. Biol 8, 412–416 (2001). [DOI] [PubMed] [Google Scholar]
- 50.Takagi J, Petre BM, Walz T & Springer TA Global conformational rearrangements in integrin extracellular domains in outside-in and inside-out signaling. Cell 110, 599–611 (2002). [DOI] [PubMed] [Google Scholar]
- 51.Calderwood DA et al. The talin head domain binds to integrin subunit cytoplasmic tails and regulates integrin activation. J. Biol. Chem 274, 28071–28074 (1999). [DOI] [PubMed] [Google Scholar]
- 52.Otey CA, Pavalko FM & Burridge K An interaction between α-actinin and the β1 integrin subunit in vitro. J. Cell Biol 111, 721–729 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harburger DS, Bouaouina M & Calderwood DA Kindlin−1 and −2 directly bind the C-terminal region of β integrin cytoplasmic tails and exert integrin-specific activation effects. J. Biol. Chem 284, 11485–11497 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Calderwood DA et al. Integrin β cytoplasmic domain interactions with phosphotyrosine-binding domains: a structural prototype for diversity in integrin signaling. Proc. Natl Acad. Sci. USA 100, 2272–2277 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Legate KR & Fässler R Mechanisms that regulate adaptor binding to β-integrin cytoplasmic tails. J. Cell Sci 122, 187–198 (2009). [DOI] [PubMed] [Google Scholar]
- 56.Morse EM, Brahme NN & Calderwood DA Integrin cytoplasmic tail interactions. Biochemistry 53, 810–820 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zaidel-Bar R, Itzkovitz S, Ma’ayan A, Iyengar R & Geiger B Functional atlas of the integrin adhesome. Nat. Cell Biol 9, 858–867 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kuo J-CC, Han X, Hsiao C-TT, Yates JR Iii& Waterman CM Analysis of the myosin-II-responsive focal adhesion proteome reveals a role for β-Pix in negative regulation of focal adhesion maturation. Nat. Cell Biol 13, 383–393 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Byron A, Humphries JD, Bass MD, Knight D & Humphries MJ Proteomic analysis of integrin adhesion complexes. Sci. Signal 4, 2 (2011). [DOI] [PubMed] [Google Scholar]
- 60.Schiller HB, Friedel CC, Boulegue C & Fässler R Quantitative proteomics of the integrin adhesome show a myosin II-dependent recruitment of LIM domain proteins. EMBO Rep. 12, 259–266 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zaidel-Bar R, Ballestrem C, Kam Z & Geiger B Early molecular events in the assembly of matrix adhesions at the leading edge of migrating cells. J. Cell Sci 116, 4605–4613 (2003). [DOI] [PubMed] [Google Scholar]
- 62.Choi CK et al. Actin and α-actinin orchestrate the assembly and maturation of nascent adhesions in a myosin II motor-independent manner. Nat. Cell Biol 10, 1039–1050 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lawson C et al. FAK promotes recruitment of talin to nascent adhesions to control cell motility. J. Cell Biol. 196, 223–232 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oakes PW, Beckham Y, Stricker J & Gardel ML Tension is required but not sufficient for focal adhesion maturation without a stress fiber template. J. Cell Biol 196, 363–374 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shroff H et al. Dual-color superresolution imaging of genetically expressed probes within individual adhesion complexes. Proc. Natl Acad. Sci. USA 104, 20308–20313 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shtengel G et al. Interferometric fluorescent super-resolution microscopy resolves 3D cellular ultrastructure. Proc. Natl Acad. Sci. USA 106, 3125–3130 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kanchanawong P et al. Nanoscale architecture of integrin-based cell adhesions. Nature 468, 580–584 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Paszek MJ et al. Scanning angle interference microscopy reveals cell dynamics at the nanoscale. Nat. Methods 9, 825–827 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Case LB et al. Molecular mechanism of vinculin activation and nanoscale spatial organization in focal adhesions. Nat. Cell Biol 17, 880–892 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Critchley DR Biochemical and structural properties of the integrin-associated cytoskeletal protein talin. Annu. Rev. Biophys 38, 235–254 (2009). [DOI] [PubMed] [Google Scholar]
- 71.Horwitz A, Duggan K, Buck C, Beckerle MC & Burridge K Interaction of plasma membrane fibronectin receptor with talin — a transmembrane linkage. Nature 320, 531–533 (1986). [DOI] [PubMed] [Google Scholar]
- 72.Goldmann WH et al. Examining F-actin interaction with intact talin and talin head and tail fragment using static and dynamic light scattering. Eur. J. Biochem 250, 447–450 (1997). [DOI] [PubMed] [Google Scholar]
- 73.Wolfenson H et al. A role for the juxtamembrane cytoplasm in the molecular dynamics of focal adhesions. PLoS One 4, e4304 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zamir E et al. Molecular diversity of cell-matrix adhesions. J. Cell Sci 112, 1655–1669 (1999). [DOI] [PubMed] [Google Scholar]
- 75.Zaidel-Bar R, Milo R, Kam Z & Geiger B A paxillin tyrosine phosphorylation switch regulates the assembly and form of cell-matrix adhesions. J. Cell Sci 120, 137–148 (2007). [DOI] [PubMed] [Google Scholar]
- 76.Margadant F et al. Mechanotransduction in vivo by repeated talin stretch-relaxation events depends upon vinculin. PLoS Biol. 9, e1001223 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shibata ACE et al. Archipelago architecture of the focal adhesion: membrane molecules freely enter and exit from the focal adhesion zone. Cytoskeleton 69, 380–392 (2012). [DOI] [PubMed] [Google Scholar]
- 78.Rossier O et al. Integrins β1 and β3 exhibit distinct dynamic nanoscale organizations inside focal adhesions. Nat. Cell Biol 14, 1057–1067 (2012). [DOI] [PubMed] [Google Scholar]
- 79.Von Wichert G, Haimovich B, Feng G-S & Sheetz MP Force-dependent integrincytoskeleton linkage formation requires downregulation of focal complex dynamics by Shp2. EMBO J. 22, 5023–5035 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lele TP, Thodeti CK, Pendse J & Ingber DE Investigating complexity of protein-protein interactions in focal adhesions. Biochem. Biophys. Res. Commun 369, 929–934 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lavelin I et al. Differential effect of actomyosin relaxation on the dynamic properties of focal adhesion proteins. PLoS One 8, e73549 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bachir AI et al. Integrin-associated complexes form hierarchically with variable stoichiometry in ascent adhesions. Curr. Biol 24, 1845–1853 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hoffmann J-E, Fermin Y, Stricker RL, Ickstadt K & Zamir E Symmetric exchange of multi-protein building blocks between stationary focal adhesions and the cytosol. eLife 3, e02257 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shemesh T, Verkhovsky AB, Svitkina TM, Bershadsky AD & Kozlov MM Role of focal adhesions and mechanical stresses in the formation and progression of the lamellipodium-lamellum interface [corrected]. Biophys. J 97, 1254–1264 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Balaban NQ et al. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat. Cell Biol. 3, 466–472 (2001). [DOI] [PubMed] [Google Scholar]
- 86.Alexandrova AY et al. Comparative dynamics of retrograde actin flow and focal adhesions: formation of nascent adhesions triggers transition from fast to slow flow. PLoS One 3, e3234 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gupton SL & Waterman-Storer CM Spatiotemporal feedback between actomyosin and focal-adhesion systems optimizes rapid cell migration. Cell 125, 1361–1374 (2006). [DOI] [PubMed] [Google Scholar]
- 88.Chrzanowska-Wodnicka M & Burridge K Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J. Cell Biol 133, 1403–1415 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Webb DJ et al. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat. Cell Biol 6, 154–161 (2004). [DOI] [PubMed] [Google Scholar]
- 90.Zhu J et al. Structure of a complete integrin ectodomain in a physiologic resting state and activation and deactivation by applied forces. Mol. Cell 32, 849–861 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tanentzapf G & Brown NH An interaction between integrin and the talin FERM domain mediates integrin activation but not linkage to the cytoskeleton. Nat. Cell Biol 8, 601–606 (2006). [DOI] [PubMed] [Google Scholar]
- 92.O’Toole TE et al. Integrin cytoplasmic domains mediate inside-out signal transduction. J. Cell Biol 124, 1047–1059 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Anthis NJ et al. The structure of an integrin/talin complex reveals the basis of inside-out signal transduction. EMBO J. 28, 3623–3632 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Huttenlocher A, Ginsberg MH & Horwitz AF Modulation of cell migration by integrin-mediated cytoskeletal linkages and ligand-binding affinity. J Cell Biol 134, 1551–1562 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chigaev A, Buranda T, Dwyer DC, Prossnitz ER & Sklar LA FRET detection of cellular α4-integrin conformational activation. Biophys. J 85, 3951–3962 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim M, Carman CV & Springer TA Bidirectional transmembrane signaling by cytoplasmic domain separation in integrins. Science 301, 1720–1725 (2003). [DOI] [PubMed] [Google Scholar]
- 97.Wu C et al. Arp2/3 is critical for lamellipodia and response to extracellular matrix cues but is dispensable for chemotaxis. Cell 148, 973–987 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Beckham Y et al. Arp2/3 inhibition induces amoeboid-like protrusions in MCF10A epithelial cells by reduced cytoskeletal-membrane coupling and focal adhesion assembly. PLoS One 9, e100943 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.DeMali KA, Barlow CA & Burridge K Recruitment of the Arp2/3 complex to vinculin: coupling membrane protrusion to matrix adhesion. J. Cell Biol 159, 881–891 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Serrels B et al. Focal adhesion kinase controls actin assembly via a FERM-mediated interaction with the Arp2/3 complex. Nat. Cell Biol 9, 1046–1056 (2007). [DOI] [PubMed] [Google Scholar]
- 101.Laukaitis CM, Webb DJ, Donais K & Horwitz AF Differential dynamics of α5 integrin, paxillin, and α-actinin during formation and disassembly of adhesions in migrating cells. J. Cell Biol 153, 1427–1440 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vicente-Manzanares M, Zareno J, Whitmore L, Choi CK & Horwitz AF Regulation of protrusion, adhesion dynamics, and polarity by myosins IIA and IIB in migrating cells. J. Cell Biol 176, 573–580 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Thievessen I et al. Vinculin-actin interaction couples actin retrograde flow to focal adhesions, but is dispensable for focal adhesion growth. J. Cell Biol 202, 163–177 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Riveline D et al. Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J. Cell Biol 153, 1175–1186 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Galbraith CG, Yamada KM & Sheetz MP The relationship between force and focal complex development. J. Cell Biol 159, 695–705 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Helfman DM et al. Caldesmon inhibits nonmuscle cell contractility and interferes with the formation of focal adhesions. Mol. Biol. Cell 10, 3097–3112 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shemesh T, Geiger B, Bershadsky AD & Kozlov MM Focal adhesions as mechanosensors: a physical mechanism. Proc. Natl Acad. Sci. USA 102, 12383–12388 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang N, Butler JP & Ingber DE Mechanotransduction across the cell surface and through the cytoskeleton. Science 260, 1124–1127 (1993). [DOI] [PubMed] [Google Scholar]
- 109.Choquet D, Felsenfeld DP & Sheetz MP Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell 88, 39–48 (1997). [DOI] [PubMed] [Google Scholar]
- 110.Gupton SL, Eisenmann K, Alberts AS & Waterman-Storer CM mDia2 regulates actin and focal adhesion dynamics and organization in the lamella for efficient epithelial cell migration. J. Cell Sci 120, 3475–3587 (2007). [DOI] [PubMed] [Google Scholar]
- 111.Plotnikov SV, Pasapera AM, Sabass B & Waterman CM Force fluctuations within focal adhesions mediate ECM-rigidity sensing to guide directed cell migration. Cell 151, 1513–1527 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Grashoff C et al. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature 466, 263–266 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu Y, Yehl K, Narui Y & Salaita K Tension sensing nanoparticles for mechano-imaging at the living/nonliving interface. J. Am. Chem. Soc 135, 5320–5323 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Morimatsu M, Mekhdjian AH, Adhikari AS & Dunn AR Molecular tension sensors report forces generated by single integrin molecules in living cells. Nano Lett. 13, 3985–3989 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang X & Ha T Defining single molecular forces required to activate integrin and notch signaling. Science 340, 991–994 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jurchenko C, Chang Y, Narui Y, Zhang Y & Salaita KS Integrin-generated forces lead to streptavidin-biotin unbinding in cellular adhesions. Biophys. J 106, 1436–1446 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sawada Y et al. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell 127, 1015–1026 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kong F, García AJ, Mould AP, Humphries MJ & Zhu C Demonstration of catch bonds between an integrin and its ligand. J. Cell Biol 185, 1275–1284 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jiang G, Giannone G, Critchley DR, Fukumoto E & Sheetz MP Two-piconewton slip bond between fibronectin and the cytoskeleton depends on talin. Nature 424, 334–337 (2003). [DOI] [PubMed] [Google Scholar]
- 120.Del Rio A et al. Stretching single talin rod molecules activates vinculin binding. Science 323, 638–641 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yao M et al. Mechanical activation of vinculin binding to talin locks talin in an unfolded conformation. Sci. Rep 4, 4610 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kozlov MM & Bershadsky AD Processive capping by formin suggests a force-driven mechanism of actin polymerization. J. Cell Biol 167, 1011–1017 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jégou A, Carlier M-F & Romet-Lemonne G Formin mDia1 senses and generates mechanical forces on actin filaments. Nat. Commun 4, 1883 (2013). [DOI] [PubMed] [Google Scholar]
- 124.Hu K, Ji L, Applegate KT, Danuser G & Waterman-Storer CM Differential transmission of actin motion within focal adhesions. Science 315, 111–115 (2007). [DOI] [PubMed] [Google Scholar]
- 125.Sabass B, Gardel ML, Waterman CM & Schwarz US High resolution traction force microscopy based on experimental and computational advances. Biophys. J 94, 207–220 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Brown CM et al. Probing the integrin-actin linkage using high-resolution protein velocity mapping. J. Cell Sci 119, 5204–5214 (2006). [DOI] [PubMed] [Google Scholar]
- 127.Chan CE & Odde DJ Traction dynamics of filopodia on compliant substrates. Science 322, 1687–1691 (2008). [DOI] [PubMed] [Google Scholar]
- 128.Shemesh T, Bershadsky AD & Kozlov MM Physical model for self-organization of actin cytoskeleton and adhesion complexes at the cell front. Biophys. J 102, 1746–1756 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Giannone G, Jiang G, Sutton DH, Critchley DR & Sheetz MP Talin1 is critical for force-dependent reinforcement of initial integrin-cytoskeleton bonds but not tyrosine kinase activation. J. Cell Biol 163, 409–419 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang X et al. Talin depletion reveals independence of initial cell spreading from integrin activation and traction. Nat. Cell Biol 10, 1062–1068 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chen H, Choudhury DM & Craig SW Coincidence of actin filaments and talin is required to activate vinculin. J. Biol. Chem 281, 40389–40398 (2006). [DOI] [PubMed] [Google Scholar]
- 132.Dumbauld DW et al. How vinculin regulates force transmission. Proc. Natl Acad. Sci. USA 110, 9788–9793 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Diez G, Auernheimer V, Fabry B & Goldmann WH Head/tail interaction of vinculin influences cell mechanical behavior. Biochem. Biophys. Res. Commun 406, 85–88 (2011). [DOI] [PubMed] [Google Scholar]
- 134.Roca-Cusachs P et al. Integrin-dependent force transmission to the extracellular matrix by α-actinin triggers adhesion maturation. Proc. Natl Acad. Sci. USA 110, 1361–1370 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Giannone G et al. Periodic lamellipodial contractions correlate with rearward actin waves. Cell 116, 431–443 (2004). [DOI] [PubMed] [Google Scholar]
- 136.Vasquez CG, Tworoger M & Martin AC Dynamic myosin phosphorylation regulates contractile pulses and tissue integrity during epithelial morphogenesis. J. Cell Biol 206, 435–450 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Giannone G et al. Lamellipodial actin mechanically links myosin activity with adhesion-site formation. Cell 128, 561–575 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Muller WA Mechanisms of leukocyte transendothelial migration. Annu. Rev. Pathol 6, 323–344 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Celli L, Ryckewaert J-J, Delachanal E & Duperray A Evidence of a functional role for interaction between ICAM−1 and nonmuscle α-actinins in leukocyte diapedesis. J. Immunol 177, 4113–4121 (2006). [DOI] [PubMed] [Google Scholar]
- 140.Martinelli R et al. Release of cellular tension signals self-restorative ventral lamellipodia to heal barrier micro-wounds. J. Cell Biol 201, 449–465 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dustin ML Cell adhesion molecules and actin cytoskeleton at immune synapses and kinapses. Curr. Opin. Cell Biol 19, 529–533 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hauck CR, Agerer F, Muenzner P & Schmitter T Cellular adhesion molecules as targets for bacterial infection. Eur. J. Cell Biol 85, 235–242 (2006). [DOI] [PubMed] [Google Scholar]
- 143.Hamiaux C, van Eerde A, Parsot C, Broos J & Dijkstra BW Structural mimicry for vinculin activation by IpaA, a virulence factor of Shigella flexneri. EMBO Rep. 7, 794–799 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Leckband DE & de Rooij J Cadherin adhesion and mechanotransduction. Annu. Rev. Cell Dev. Biol 30, 291–315 (2014). [DOI] [PubMed] [Google Scholar]
- 145.Bard L et al. A molecular clutch between the actin flow and N-cadherin adhesions drives growth cone migration. J. Neurosci 28, 5879–5890 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kametani Y & Takeichi M Basal-to-apical cadherin flow at cell junctions. Nat. Cell Biol 9, 92–98 (2007). [DOI] [PubMed] [Google Scholar]
- 147.Locascio A & Nieto MA Cell movements during vertebrate development: integrated tissue behaviour versus individual cell migration. Curr. Opin. Genet. Dev 11, 464–469 (2001). [DOI] [PubMed] [Google Scholar]