Abstract
Over the past decade, mouse-tracking in choice tasks has become a popular method across psychological science. This method exploits hand movements as a measure of multiple response activations that can be tracked continuously over hundreds of milliseconds. Whereas early mouse-tracking research focused on specific debates, researchers have realized the methodology has far broader theoretical value. This more recent work demonstrates that mouse-tracking is a widely applicable measure across the field, capable of exposing the micro-structure of real-time decisions including their component processes and millisecond-resolution time-course in ways that inform theory. In the article, recent advances in the mouse-tracking approach are described, and comparisons with the gold standard measure of reaction time and other temporally-sensitive methodologies are provided. Future directions, including mapping to neural representations with brain-imaging and ways to improve our theoretical understanding of mouse-tracking methodology, are discussed.
Keywords: mouse-tracking, hand movement, decision-making, temporal dynamics, reaction time
Much of psychological science is concerned with understanding the underlying processes that drive particular behavioral responses. When such responses occur in only a few hundred milliseconds, as they often do, gaining insight into the cognitive processes that culminate in a given response has proven difficult. The most common solution to this problem is as alive as it was a century-and-a-half ago, when Donders (1868/1969) first measured the human reaction time (RT) to infer a dissociation between hypothetically distinct processes. Since then, the study of mental chronometry has unquestionably advanced, and using RTs or RT distributions to infer about the time-course of cognitive processes is a gold standard in the field. Measuring neural activity with high temporal resolution (e.g., EEG) or patterns of visual attention (eye-tracking) have also led to unprecedented insights into the temporal evolution of behavioral responses. However, while such techniques shed light into the cognitive and neural processes accompanying a given response, more direct measures of the real-time evolution of the response itself—and of potential activation of alternative responses—have been lacking.
Over the past decade, the measurement of hand trajectories en route to choices on a screen has opened up new avenues of investigation into the dynamics of a wide range of cognitive processes. Often obtained via computer-mouse movements, hand-tracking in choice tasks—and mouse-tracking more specifically—is now a popular method across many areas of the field, proving to be a temporally fine-grained measure by which participants’ tentative commitments to various choice alternatives can be tracked continuously over hundreds of milliseconds. Moreover, now that software specialized for running and analyzing mouse-tracking experiments is freely-available (Freeman & Ambady, 2010; Kieslich & Henninger, 2017), researchers need only a computer and mouse to use the methodology, making its availability on par with the common RT.
Neurophysiological research in both monkeys and humans supports the use of hand movement as a valid index of evolving decisions. Specifically, activity in neuronal populations of the premotor cortex is strongly linked to hand movement, and these neuronal populations are stimulated by the decision process in a dynamic fashion. For instance, single-cell recordings revealed that during tasks in which monkeys must use their hand to select one of two response options, directionally-tuned cells in the premotor cortex initially fire for both response options simultaneously. However, as the decision-making process evolves, neuronal activity for the selected option gradually increases while that for the unselected option is inhibited (Cisek & Kalaska, 2005). Such findings suggest that ongoing updates of a decision process are made immediately available to the premotor cortex, which continuously guides response-directed hand movement as a decision unfolds (Cisek & Kalaska, 2010; also see Freeman et al., 2011a).
In the most popular of mouse-tracking tasks, participants begin a trial by clicking a button at the bottom-center of the screen, after which they are presented with a stimulus. They then move the cursor to response alternatives in either top corners of the screen. Response alternatives may be presented prior to or coinciding with stimulus onset, or in some cases the alternatives are the stimuli themselves (Fig. 1a). The original and most common use of this paradigm is to measure the extent to which, although participants explicitly select one response, their mouse-trajectory reveals a conspicuous attraction toward other responses that are temporarily considered but not ultimately selected. To provide a few examples, participants’ mouse trajectories may simultaneously veer toward a ‘female’ response due to a male face’s feminine features (Freeman et al., 2008); toward a ‘candy’ response due to a spoken word’s overlapping phoneme, e.g., “candle” (Spivey, Grosjean, & Knoblich, 2005); toward an ‘angry’ response due to stereotypes linking Black faces to hostility (Hehman, Ingbretsen, & Freeman, 2014; Stolier & Freeman, 2016); or toward an image of a cupcake before selecting a banana, due to the inability to resist unhealthy food (Stillman, Medvedev, & Ferguson, 2017).
Figure 1.
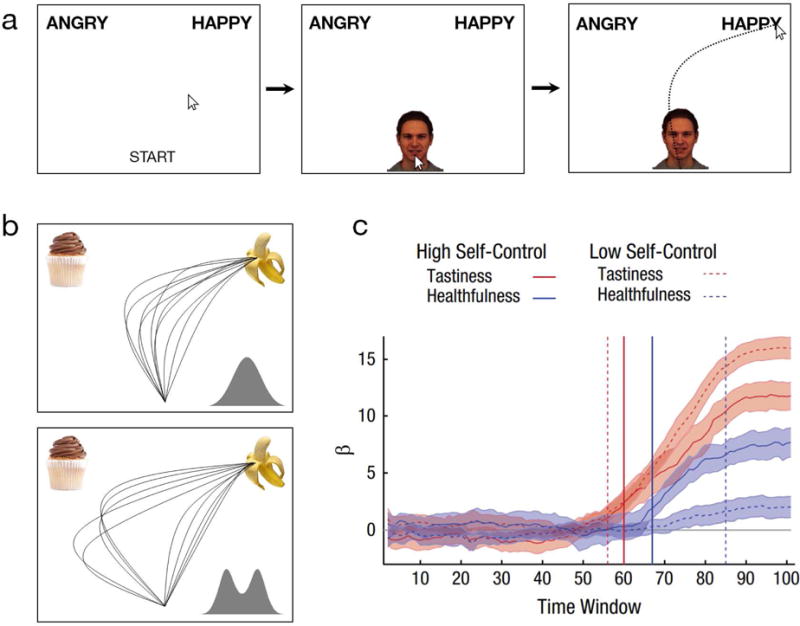
(a) A depiction of a standard two-choice mouse-tracking paradigm, where on each trial participants click a start button at the bottom-center, which reveals a stimulus. Participants then move the cursor and click on one of the two responses in either top corners. There are many variants, including multi-choice paradigms (e.g., four choices), sequences of stimuli (e.g., priming), or responses serving as stimuli themselves (as in b). (b) Mouse-tracking reveals decision micro-structure. In conditions of conflict, dynamic models tend to predict simultaneously active processes (e.g., impulse toward unhealthy food vs. long-term goal toward healthy food) that continuously self-organize into an explicit response. This leads to parallel attraction effects with a unimodal distribution. Dual-systems models tend to predict a System 1 process occurs automatically (e.g., automatic impulse) on certain trials, which is then intervened on by a System 2 process (e.g., controlled goal). This leads to two subpopulations of trials (extreme mid-flight correction trials and no-attraction trials) creating a bimodal distribution. (c) Example of mouse-tracking used as a time-course methodology from Sullivan et al. (2015). The strength of the relationship (regression coefficients) between trajectories’ angle-of-movement and the relative tastiness and healthfulness of one food option over another is plotted for each time window, separately for participants with low and high self-control ability. Vertical lines indicate onset of significant effects. Healthfulness was processed as early as tastiness for high self-control participants; for low self-control participants, healthfulness was processed considerably later.
A Hidden Attraction
The early days of mouse-tracking research focused on such parallel-attraction effects to advance various dynamic models of language (Dale, Kehoe, & Spivey, 2007; Farmer et al., 2007; Spivey et al., 2005), social cognition (Freeman & Ambady, 2009, 2011a; Freeman et al., 2008; Freeman et al., 2010; Wojnowicz et al., 2009), visual attention (Song & Nakayama, 2006, 2008), and decision-making (McKinstry, Dale, & Spivey, 2008), often opposing dual-systems or stage-based models. Researchers realized that the continuous nature of hand movement, as opposed to discrete RTs or ballistic eye-movements, was able to provide evidence for continuous cognitive dynamics in a way previously not possible, in turn helping rule out alternative models (for reviews, Freeman, Dale, & Farmer, 2011b; Song & Nakayama, 2009; Spivey & Dale, 2006).
For instance, in attitudes research, dual-systems models have long argued that people automatically activate an implicit attitude (e.g., Black people = bad), which may be subsequently intervened on and replaced by an explicit attitude (e.g., Black people = good) if they conflict (Devine, 1989). In contrast, dynamic models propose that both attitudes are simultaneously activated and self-organize into a coherent evaluation (Wojnowicz et al., 2009). Or, in language research, stage-based models argue that one syntactic structure may be initially activated during sentence processing, but in ambiguous cases this structure may be re-analyzed and replaced by a new syntactic structure if the first turns out to be inappropriate (van Gompel, Pickering, & Traxler, 2001). In contrast, dynamic constraint-based models propose that multiple syntactic structures compete over time to stabilize on a given interpretation, without any subsequent reanalysis mechanism (Farmer et al., 2007). Using mouse-tracking, researchers in these cases were able to provide evidence for a temporally-continuous attraction toward two responses in parallel (i.e., both ‘like’ and ‘dislike’ when evaluating Black people; both syntactic interpretations when processing ambiguous sentences), thereby supporting dynamic models. Specifically, at each moment during the decision process, mouse-trajectories always reflected some dynamically-weighted co-activation of both implicit and explicit attitudes, or both syntactic structures, which provided important challenges for dual-systems or stage-based models.
Several other early mouse-tracking studies adopted a similar logic, including in domains of spoken-word recognition (Spivey et al., 2005), social categorization (Freeman et al., 2008), and decision-making (McKinstry et al., 2008). Together, the early mouse-tracking research focused on the temporal continuity of trajectory-attraction effects to make claims about the continuous nature of underlying cognitive processes. Since then, over 100 studies have now exploited mouse-tracking to index such attraction effects, but they have adopted a theoretical plurality that is no longer squarely focused on continuous dynamics. Now far broader than a dynamic vs. dual-systems debate, a surge of mouse-tracking research has shown that the technique can be leveraged across a wide range of domains to measure covert activations of responses that do not manifest in explicit decisions, including: self-control (Sullivan et al., 2015), emotion (Mattek et al., 2016), memory (Papesh & Goldinger, 2012), group processes (Lazerus et al., 2016), ambivalence (Schneider & Schwarz, 2017), inter-temporal choice (Dshemuchadse, Scherbaum, & Goschke, 2013), theory-of-mind (van der Wel, Sebanz, & Knoblich, 2014), self-esteem (Leitner et al., 2014), moral cognition (Koop, 2013), subliminal perception (Xiao & Yamauchi, 2017), embodiment (Lepora & Pezzulo, 2015), deception (Duran, Dale, & McNamara, 2010), among countless others. Mouse-tracking has therefore become a powerful measure of multiple response activation with wide applicability across psychological science.
Micro-Structure of Decisions
From their beginning, mouse-tracking studies sought to rule out dual-systems or stage-based models by demonstrating the continuity of trajectory-attraction effects, advancing the claim of a co-activation of competing processes that together coalesce into a stable response. Evidence in support of such alternative models, instead, would be reflected by discrete mid-flight corrections (e.g., automatic impulse toward unhealthy food vs. controlled correction in favor of healthy food; Stillman et al., 2017), such that an initial movement straight to one response is followed by a discrete corrective movement straight to the opposing response (Freeman et al., 2008, Study 3). Most realistic models of this kind assume that such stage-based corrections are probabilistic to some degree, not necessarily taking place on every trial; however, they generally assume responses are being drawn from two subpopulations under conflict: some trials where an ‘inappropriate’ impulse must be squashed (e.g., “grab the cupcake—no, grab the banana!”) and other trials where it never activated in the first place (e.g., “grab the banana!”). Accordingly, it is the bimodal nature of trajectories’ response distribution that is often crucial in establishing a claim of dual-processing stages during mouse-tracking (Fig. 1b; Freeman & Dale, 2013).
Indeed, such systematic flip-flopping of mouse-trajectories has now been taken as evidence supporting dual-systems or stage-based accounts of several aspects of language processing (Barca & Pezzulo, 2015; Dale & Duran, 2011; Tomlinson, Bailey, & Bott, 2013) and ambivalence (Schneider & Schwarz, 2017). However, the question need not be either–or. For example, social categorization has been found to exhibit dynamic effects (e.g., a masculine female face: parallel attraction to ‘male’ before selecting ‘female’) but also dual-systems-like effects as well (e.g., initial attraction to ‘male’, followed by abrupt correction toward ‘female’), even within the same task (Freeman, 2014). Indeed, models that take a formal dynamical-systems approach (e.g., for social categorization, Freeman & Ambady, 2011a) in some cases may even predict such a trajectory-pattern that appears like dual systems in stage-like sequence but instead reflects a rapid ‘phase transition’ within a single dynamic system (Spivey, Anderson, & Dale, 2009). Thus, the more important question may not be which pattern is observed for a given cognitive process, but rather under what conditions these different patterns manifest.
Of course, dynamic and dual-systems models are only two, albeit popular, accounts of cognitive processing. In the context of mouse-tracking, another way to conceive of dual-systems models is that they predict two movement-components, which each inhabit their own spatiotemporal dynamics, e.g., early movement to top-left and late movement to top-right (but see Spivey et al., 2010). This logic can be broadened, however, to more complex models that predict the tandem operation of more than two systems or processes. For example, the Quad Model is a popular model of implicit social cognition that posits the existence of four distinct processes (Conrey et al., 2005); in certain tasks, one may expect four movement-components that inhabit different parts of the spatiotemporal sequence, with four factors biasing the decision process at different times. Recently, researchers have taken several approaches to characterize such trajectory components, including changes in trajectory direction or acceleration/deceleration (e.g., Dale & Duran, 2011; Dale et al., 2008), dimensionality-reduction approaches (Hehman, Stolier, & Freeman, 2015), and entropy analyses that identify high-speed movements and motor “breaks” (Calcagnì, Lombardi, & Sulpizio, 2017). Moreover, these and other velocity and acceleration analyses may be used to measure additional characteristics of a decision process, such as instability. For instance, individuals with less inter-racial exposure were shown to exhibit more unstable dynamics and abrupt race-categorization shifts when categorizing racially-ambiguous faces, an effect predicted by dynamic-computational models (Freeman, Pauker, & Sanchez, 2016).
Such recent work shows that mouse-tracking has the ability to uncover a micro-structure of real-time decisions, revealing dissociable dynamics and processing components that can inform theory. Further research is certainly needed to link trajectory components to the specific theoretical processes under study, but at this early stage it is clear is that even when explicit responses or RTs may be similar, mouse-tracking can qualitatively distinguish between vastly different dynamics before arriving there.
A Matter of Time
A critical advantage of mouse-tracking is that it can sensitively expose millisecond-resolution timing information. Time-course analyses can provide powerful information about when specific factors are computed during an evolving decision or how specific processes temporally unfold. In one study, participants were asked on every trial to indicate their preference between two food options, for which ratings of tastiness and healthfulness were also obtained. At each time-point, the relationships between mouse-trajectories’ angle-of-movement and the relative tastiness and healthfulness of one food option over another was examined. For people with high self-control ability, both tastiness and healthfulness began correlating with mouse-trajectories at the same time during the decision process; but for people with low self-control ability, healthfulness began correlating considerably later in time (Fig. 1c). These results suggest that food options’ tastiness has an early advantage in driving real-time preferences than healthfulness for people with a weak ability to control their impulses (Sullivan et al., 2015).
In cultural psychology, research has long suggested that people from ‘high-context’ East Asian societies are more attuned to contextual associations than people from ‘low-context’ Western societies (Nisbett et al., 2001). In one study, American and native Chinese participants were presented with White and Asian faces embedded in scene environments more stereotypically associated White or Asian individuals. While categorizing a face’s race, incongruent contexts led trajectories to veer toward the context-associated response, while congruent contexts led trajectories to more directly approach the context-associated response. More importantly, the onset and peak of these contextual effects occurred earlier for native Chinese relative to American participants. Such results show that visual context exerts an earlier impact for individuals from ‘high-context’ societies, suggesting they may have a greater preparedness to integrate contextual information into real-time perceptions (Freeman et al., 2013). Additional studies have adopted a similar approach to explore how specific facial features drive gender, race, and age categorization with different temporal ordering as well (Freeman & Ambady, 2011b; Freeman et al., 2010). In the domain of subliminal perception, recent research showed that top-down attention both delays and prolongs the time-course of subliminal semantic processing, revealing novel information about how attention interacts with nonconscious perceptual processes (Xiao & Yamauchi, 2017). Such recent work shows that mouse-tracking is a powerful methodology able to dissociate the timing of different cognitive processes and, in some cases, link such timing to individual differences.
Compared to other time-sensitive measures, mouse-tracking has distinct advantages and limitations. Eye-tracking in choice tasks relies on discrete saccades (tracked as fast as they occur: ~3–4 times/second) whereas mouse-tracking relies on continuous hand motion (tracked as fast as possible: typically ~70 times/second) (Magnuson, 2005). Moreover, the eyes may only fixate on one response at a time, whereas the hand may inhabit in-between states amidst multiple responses. These qualities make mouse-tracking uniquely suited to measure how a response evolves continuously over time, including any tentative attraction to other possible responses. That said, eye-tracking may be more sensitive to pre-attentive processes prior to hand-movement initiation, and thus combining the two may be valuable (e.g., Quétard et al., 2016). ERPs, on the other hand, provide an index of when neural processing relevant to a decision process is modulated, whereas mouse-tracking provides a more direct measure of how multiple response alternatives accrue evidence to drive the decision over time. However, mouse-tracking timing information is most meaningfully interpreted in relative terms. For instance, finding that a given facial feature begins affecting mouse-trajectories at 432 ms during age categorization, but at 332 ms during gender categorization, suggests the facial feature starts playing a role in gender categorization 100 ms earlier (Freeman & Ambady, 2011b). However, placing meaning in 432 or 332 ms with respect to underlying cognitive processing is unwarranted. But because how long it takes for a cognitive change to manifest in hand movement should be uniform throughout the decision process (Cisek & Kalaska, 2005, 2010), relative differences in mouse-tracking timing can powerfully reveal how much earlier or later different factors reign over a decision process with millisecond-level precision. With ERP, however, timing can be interpreted in absolute terms more meaningfully.
The Trajectory Forward
In short, mouse-tracking has become a widely applicable measure of multiple response activation, capable of exposing component processes within real-time decisions and their time-course information. Indeed, compared to the gold standard of the RT, even the most straightforward mouse-tracking measures (e.g., deviation) are dissociable from RTs or general indecision. For instance, greater deviation effects predict stronger activation of conflict-monitoring regions even when statistically controlling for RT (Stolier & Freeman, 2017), and there are numerous cases where a deviation effect is observed without any RT effect (e.g., Stillman et al., 2017; Wojnowicz et al., 2009) or where a deviation effect is uniquely predictive independent of RT (e.g., O’Hora et al., 2016). While delayed RTs may suggest parallel activation of multiple response options, there are numerous alternative explanations as well, e.g., slower evidence-accumulation of a single response. Such dissociations become even clearer when considering three- or four-choice paradigms (e.g., Cloutier, Freeman, & Ambady, 2014; Tomlinson, Gotzner, & Bott, 2017), where mouse-tracking can reveal which specific response among multiple unselected alternatives is simultaneously attracting participants’ decision trajectory; a delayed RT, on the other hand, may potentially suggest that another response was activated in parallel but cannot distinguish which one it was. Moreover, as described earlier, mouse-tracking can detect qualitatively distinct decision micro-structure and temporal dynamics that may be wildly different even when two RTs are identical. A mouse-tracking methodology may therefore complement the traditional power of RTs and RT distributions to gain wholly new insights into a wide range of processes across the field.
An important direction underway is mapping mouse-tracking data to neural representation. Recent research has synchronized in-scanner mouse-tracking with multivariate fMRI to map the covert activation of specific responses with underlying neural representations. For instance, on a given trial, the extent to which the mouse-trajectory was simultaneously attracted to the opposite category-response (e.g., ‘male’ for a masculine female face) predicted the extent to which the neural-representational pattern in face-processing regions was more similar to that opposite category (Fig. 2; Stolier & Freeman, 2017). Or, due to automatic stereotype-driven expectations, the extent to which a participant was attracted to the ‘angry’ category even for a Black face displaying no anger predicted the extent to which face-processing regions’ neural-representational pattern was more similar to the ‘angry’ category (Stolier & Freeman, 2016). Combining mouse-tracking with fMRI decoding approaches has tremendous potential, as this paradigm can identity which levels of neural representation are impacted by specific changes in a decision trajectory. In addition, combining mouse-tracking with ERPs could provide unprecedented information about decision-related timing. However, an important challenge for future work aiming to synchronize two such high-resolution time series would be to provide sufficient timing precision. ERP artifacts could also easily arise due to motor movement, which would need to be minimized (see also Fischer & Hartmann, 2014). TMS has also been usefully combined with mouse-tracking, allowing a causal test of a brain region’s role in resolving competitive dynamics, such as in semantic categorization (Hindy et al., 2009).
Figure 2.
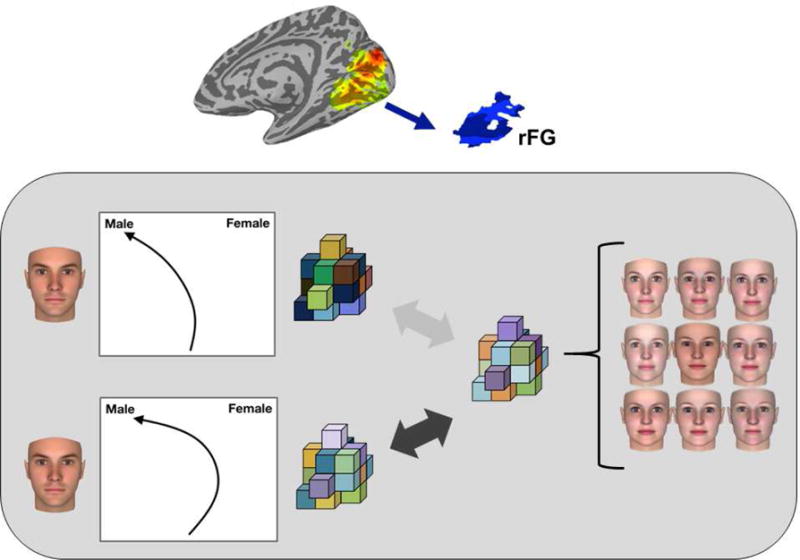
Schematic illustration of results from Stolier and Freeman (2017). Sets of cubes are meant to illustrate neural-representational multi-voxel patterns. During synchronized mouse-tracking and fMRI, participants categorized the gender or race of typical and atypical exemplar faces. On a given atypical trial (e.g., feminine male face), the extent to which participants were attracted to the opposite category response (e.g., ‘female’) predicted an increased similarity in the face’s neural-representational pattern to that opposite category in the right fusiform gyrus (rFG), a face-processing region. The opposite category’s neural-representational pattern was measured as the average pattern of all typical trials for that category (e.g., average of typical female trials). This paradigm can therefore identity which levels of neural representation are impacted by specific dynamics of a decision trajectory.
Mouse-tracking has often been referred to as an implicit measure, but future research should better establish the implicit nature of specific effects. Although there is some theoretical dispute regarding what constitutes an implicit measure, investigations into whether mouse-tracking effects are resistant to social desirability and reflect nonconscious or introspectively inaccessible representations would be important, as well as what roles automaticity vs. control or activation vs. validation processes play (e.g., Gawronski, LeBel, & Peters, 2007). But the question itself may be regarded as problematic, in that it is akin to asking whether the RT method broadly is implicit. The answer, of course, is that it depends on how one uses it. An RT effect in the context of semantic or evaluative priming, or an implicit association test, may be referred to as implicit, yet an RT effect in a time-unconstrained discrimination task may not. Further work is needed to rigorously test the implicit nature of mouse-tracking effects in particular task contexts, including the roles of methodological factors.
Once upon a time, research on motor control was dubbed the ‘Cinderella of psychology’ (Rosenbaum, 2005), because the broader field neglects it, it was argued, believing motor processes have little to do with the cognitive processes of interest (but see, e.g., Wolpert & Landy, 2012). Beyond pushing the fact that cognitive and motor processes are far more coextensive, researchers’ move from the discrete response to continuous hand movement is opening the door to new avenues of investigation into a wide range of cognitive processes. The famous Milner and Goodale (1995) finding that a lesion patient could not report visual attributes of a bar in front of her, but when her hand reached for the bar its trajectory clearly reflected knowledge of those attributes, tells us that the moving hand may reveal more than we think. This may be an extreme example, but it makes crystal-clear that in hand movement lies novel—sometimes covert—information about cognition. Indeed, this notion is only becoming increasingly clear as the mouse-tracking approach to psychological science grows and evolves. Further work is certainly needed to more deeply understand the link between specific hand-movement parameters and theoretical constructs, but if the past decade is any example, the trajectory looks on the rise.
Acknowledgments
This work was supported in part by research grants NSF BCS-1423708, NSF BCS-1654731, and NIH R01-MH112640 to J.B.F.
References
- Barca L, Pezzulo G. Tracking second thoughts: continuous and discrete revision processes during visual lexical decision. PloS one. 2015;10(2):e0116193. doi: 10.1371/journal.pone.0116193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcagnì A, Lombardi L, Sulpizio S. Analyzing spatial data from mouse tracker methodology: An entropic approach. Behavior Research Methods. 2017:1–19. doi: 10.3758/s13428-016-0839-5. [DOI] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF. Neural Correlates of Reaching Decisions in Dorsal Premotor Cortex: Specification of Multiple Direction Choices and Final Selection of Action. Neuron. 2005;45(5):801–814. doi: 10.1016/j.neuron.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF. Neural mechanisms for interacting with a world full of action choices. Annual Review of Neuroscience. 2010;33:269–298. doi: 10.1146/annurev.neuro.051508.135409. [DOI] [PubMed] [Google Scholar]
- Cloutier J, Freeman JB, Ambady N. Investigating the early stages of person perception: The asymmetry of social categorization by sex vs. age. PloS one. 2014;9(1):e84677. doi: 10.1371/journal.pone.0084677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrey FR, Sherman JW, Gawronski B, Hugenberg K, Groom CJ. Separating multiple processes in implicit social cognition: The Quad Model of implicit task performance. Journal of personality and social psychology. 2005;89:469–487. doi: 10.1037/0022-3514.89.4.469. [DOI] [PubMed] [Google Scholar]
- Dale R, Duran ND. The Cognitive Dynamics of Negated Sentence Verification. Cognitive science. 2011;35:983–996. doi: 10.1111/j.1551-6709.2010.01164.x. [DOI] [PubMed] [Google Scholar]
- Dale R, Kehoe C, Spivey MJ. Graded motor responses in the time course of categorizing atypical exemplars. Memory & Cognition. 2007;35(1):15–28. doi: 10.3758/bf03195938. [DOI] [PubMed] [Google Scholar]
- Dale R, Roche J, Snyder K, McCall R. Exploring action dynamics as an index of paired-associate learning. PloS one. 2008;3:e1728. doi: 10.1371/journal.pone.0001728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine P. Stereotypes and prejudice: Their automatic and controlled components. Journal of personality and social psychology. 1989;56:5–18. [Google Scholar]
- Donders FC. On the speed of mental processes. Acta psychologica. 1868/1969;30:412–431. doi: 10.1016/0001-6918(69)90065-1. [DOI] [PubMed] [Google Scholar]
- Dshemuchadse M, Scherbaum S, Goschke T. How decisions emerge: Action dynamics in intertemporal decision making. Journal of Experimental Psychology: General. 2013;142(1):93. doi: 10.1037/a0028499. [DOI] [PubMed] [Google Scholar]
- Duran ND, Dale R, McNamara DS. The action dynamics of overcoming the truth. Psychonomic Bulletin and Review. 2010;17:486–491. doi: 10.3758/PBR.17.4.486. [DOI] [PubMed] [Google Scholar]
- Farmer TA, Cargill S, Hindy N, Dale R, Spivey MJ. Tracking the continuity of language comprehension: Computer-mouse trajectories suggest parallel syntactic processing. Cognitive science. 2007;31:889–909. doi: 10.1080/03640210701530797. [DOI] [PubMed] [Google Scholar]
- Fischer MH, Hartmann M. Pushing forward in embodied cognition: may we mouse the mathematical mind? Frontiers in psychology. 2014;5 doi: 10.3389/fpsyg.2014.01315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JB. Abrupt category shifts during real-time person perception. Psychonomic bulletin & review. 2014;21(1):85–92. doi: 10.3758/s13423-013-0470-8. [DOI] [PubMed] [Google Scholar]
- Freeman JB, Ambady N. Motions of the hand expose the partial and parallel activation of stereotypes. Psychological Science. 2009;20:1183–1188. doi: 10.1111/j.1467-9280.2009.02422.x. [DOI] [PubMed] [Google Scholar]
- Freeman JB, Ambady N. MouseTracker: Software for studying real-time mental processing using a computer mouse-tracking method. Behavior Research Methods. 2010;42:226–241. doi: 10.3758/BRM.42.1.226. An in-depth description of freely-available software specialized for conducting and analyzing mouse-tracking studies, including discussion of measures and methodological validation. [DOI] [PubMed] [Google Scholar]
- Freeman JB, Ambady N. A dynamic interactive theory of person construal. Psychological review. 2011a;118:247–279. doi: 10.1037/a0022327. [DOI] [PubMed] [Google Scholar]
- Freeman JB, Ambady N. Hand movements reveal the time-course of shape and pigmentation processing in social categorization. Psychonomic Bulletin and Review. 2011b;18:705–712. doi: 10.3758/s13423-011-0097-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JB, Ambady N, Midgley KJ, Holcomb PJ. The real-time link between person perception and action: Brain potential evidence for dynamic continuity. Social Neuroscience. 2011a;6:139–155. doi: 10.1080/17470919.2010.490674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JB, Ambady N, Rule NO, Johnson KL. Will a category cue attract you? Motor output reveals dynamic competition across person construal. Journal of Experimental Psychology: General. 2008;137(4):673–690. doi: 10.1037/a0013875. [DOI] [PubMed] [Google Scholar]
- Freeman JB, Dale R. Assessing bimodality to detect the presence of a dual cognitive process. Behavior Research Methods. 2013;45:83–97. doi: 10.3758/s13428-012-0225-x. [DOI] [PubMed] [Google Scholar]
- Freeman JB, Dale R, Farmer TA. Hand in motion reveals mind in motion. Frontiers in psychology. 2011b;2:59. doi: 10.3389/fpsyg.2011.00059. An early review of how hand movements may be informative for understanding cognitive processes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JB, Ma Y, Han S, Ambady N. Influences of culture and visual context on real-time social categorization. Journal of Experimental Social Psychology. 2013;49(2):206–210. doi: 10.1016/j.jesp.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JB, Pauker K, Apfelbaum EP, Ambady N. Continuous dynamics in the real-time perception of race. Journal of Experimental Social Psychology. 2010;46:179–185. doi: 10.1016/j.jesp.2009.10.002. [DOI] [Google Scholar]
- Freeman JB, Pauker K, Sanchez DT. A perceptual pathway to bias: Interracial exposure reduces abrupt shifts in real-time race perception that predict mixed-race bias. Psychological Science. 2016;27:502–517. doi: 10.1177/0956797615627418. [DOI] [PubMed] [Google Scholar]
- Gawronski B, LeBel EP, Peters KR. What do implicit measures tell us?: Scrutinizing the validity of three common assumptions. Perspectives on Psychological Science. 2007;2(2):181–193. doi: 10.1111/j.1745-6916.2007.00036.x. [DOI] [PubMed] [Google Scholar]
- Hehman E, Ingbretsen ZA, Freeman JB. The neural basis of stereotypic impact on multiple social categorization. Neuroimage. 2014;101:704–711. doi: 10.1016/j.neuroimage.2014.07.056. [DOI] [PubMed] [Google Scholar]
- Hehman E, Stolier RM, Freeman JB. Advanced mouse-tracking analytic techniques for enhancing psychological science. Group Processes & Intergroup Relations. 2015;18(3):384–401. A guide for understanding and conducting advanced mouse-tracking analyses that are theoretically informative for psychological science. [Google Scholar]
- Hindy NC, Hamilton R, Houghtling AS, Coslett HB, Thompson-Schill SL. Computer mouse-tracking reveals TMS disruptions of prefrontal function during semantic retrieval. Journal of Neurophysiology. 2009;102:3405–3413. doi: 10.1152/jn.00516.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieslich PJ, Henninger F. Mousetrap: An integrated, open-source mouse-tracking package. Behavior Research Methods. 2017:1–16. doi: 10.3758/s13428-017-0900-z. [DOI] [PubMed] [Google Scholar]
- Koop GJ. An assessment of the temporal dynamics of moral decisions. Judgment and Decision Making. 2013;8(5):527. [Google Scholar]
- Lazerus T, Ingbretsen ZA, Stolier RM, Freeman JB, Cikara M. Positivity bias in judging ingroup members’ emotional expressions. Emotion. 2016;16(8):1117. doi: 10.1037/emo0000227. [DOI] [PubMed] [Google Scholar]
- Leitner JB, Hehman E, Deegan MP, Jones JM. Adaptive disengagement buffers self-esteem from negative social feedback. Personality and Social Psychology Bulletin. 2014;40(11):1435–1450. doi: 10.1177/0146167214549319. [DOI] [PubMed] [Google Scholar]
- Lepora NF, Pezzulo G. Embodied choice: how action influences perceptual decision making. PLoS computational biology. 2015;11(4):e1004110. doi: 10.1371/journal.pcbi.1004110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnuson JS. Moving hand reveals dynamics of thought. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9995–9996. doi: 10.1073/pnas.0504413102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattek AM, Whalen PJ, Berkowitz JL, Freeman JB, Mattek AM, Freeman JB. Differential effects of cognitive load on subjective versus motor responses to ambiguously valenced facial expressions. 2016 doi: 10.1037/emo0000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinstry C, Dale R, Spivey MJ. Action dynamics reveal parallel competition in decision making. Psychological Science. 2008;19:22–24. doi: 10.1111/j.1467-9280.2008.02041.x. [DOI] [PubMed] [Google Scholar]
- Milner AD, Goodale MA. The Visual Brain in Action. New York: Oxford University Press; 1995. [Google Scholar]
- Nisbett RE, Peng K, Choi I, Norenzayan A. Culture and systems of thought: Holistic versus analytic cognition. Psychological review. 2001;108:291–310. doi: 10.1037/0033-295x.108.2.291. [DOI] [PubMed] [Google Scholar]
- O’Hora D, Carey R, Kervick A, Crowley D, Dabrowski M. Decisions in Motion. Decision Dynamics during Intertemporal Choice reflect Subjective Evaluation of Delayed Rewards. 2016;6:20740. doi: 10.1038/srep20740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papesh MH, Goldinger SD. Memory in motion: Movement dynamics reveal memory strength. Psychonomic bulletin & review. 2012;19(5):906–913. doi: 10.3758/s13423-012-0281-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quétard B, Quinton JC, Mermillod M, Barca L, Pezzulo G, Colomb M, Izaute M. Differential effects of visual uncertainty and contextual guidance on perceptual decisions: Evidence from eye and mouse tracking in visual search. Journal of vision. 2016;16(11):28–28. doi: 10.1167/16.11.28. [DOI] [PubMed] [Google Scholar]
- Rosenbaum DA. The Cinderella of psychology: The neglect of motor control in the science of mental life and behavior. American Psychologist. 2005;60:308–317. doi: 10.1037/0003-066X.60.4.308. [DOI] [PubMed] [Google Scholar]
- Schneider IK, Schwarz N. Mixed feelings: the case of ambivalence. Current Opinion in Behavioral Sciences. 2017;15:39–45. [Google Scholar]
- Song JH, Nakayama K. Role of focal attention on latencies and trajectories of visually guided manual pointing. Journal of vision. 2006;6:982–995. doi: 10.1167/6.9.11. [DOI] [PubMed] [Google Scholar]
- Song JH, Nakayama K. Target selection in visual search as revealed by movement trajectories. Vision research. 2008;48:853–861. doi: 10.1016/j.visres.2007.12.015. [DOI] [PubMed] [Google Scholar]
- Song JH, Nakayama K. Hidden cognitive states revealed in choice reaching tasks. Trends in cognitive sciences. 2009;13:360–366. doi: 10.1016/j.tics.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Spivey MJ, Anderson S, Dale R. The phase transition in human cognition. Journal of New Mathematics and Natural Computing. 2009;5:197–220. [Google Scholar]
- Spivey MJ, Dale R. Continuous dynamics in real-time cognition. Current Directions in Psychological Science. 2006;15(5):207–211. An outline of a dynamical-systems perspective of cognition including the role mouse-tracking could play in this perspective. [Google Scholar]
- Spivey MJ, Dale R, Knoblich G, Grosjean M. Do curved reaching movements emerge from competing perceptions? A reply to van der Wel et al. (2009) Journal of Experimental Psychology: Human Perception and Performance. 2010;36:251–254. doi: 10.1037/a0017170. [DOI] [PubMed] [Google Scholar]
- Spivey MJ, Grosjean M, Knoblich G. Continuous attraction toward phonological competitors. Proceedings of the National Academy of Sciences. 2005;102(29):10393–10398. doi: 10.1073/pnas.0503903102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman PE, Medvedev D, Ferguson MJ. Resisting Temptation: Tracking How Self-Control Conflicts Are Successfully Resolved in Real Time. Psychological Science. 2017:0956797617705386. doi: 10.1177/0956797617705386. [DOI] [PubMed] [Google Scholar]
- Stolier RM, Freeman JB. Neural pattern similarity reveals the inherent intersection of social categories. Nature Neuroscience. 2016;19:795–797. doi: 10.1038/nn.4296. [DOI] [PubMed] [Google Scholar]
- Stolier RM, Freeman JB. A neural mechanism of social categorization. Journal of Neuroscience. 2017;37(23):5711–5721. doi: 10.1523/JNEUROSCI.3334-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan N, Hutcherson C, Harris A, Rangel A. Dietary self-control is related to the speed with which attributes of healthfulness and tastiness are processed. Psychological Science. 2015;26(2):122–134. doi: 10.1177/0956797614559543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson JM, Bailey TM, Bott L. Possibly all of that and then some: Scalar implicatures are understood in two steps. Journal of Memory and Language. 2013;69(1):18–35. [Google Scholar]
- Tomlinson JM, Gotzner N, Bott L. Intonation and Pragmatic Enrichment: How Intonation Constrains Ad Hoc Scalar Inferences. Language and Speech. 2017;60(2):200–223. doi: 10.1177/0023830917716101. [DOI] [PubMed] [Google Scholar]
- van der Wel RP, Sebanz N, Knoblich G. Do people automatically track others’ beliefs? Evidence from a continuous measure. Cognition. 2014;130(1):128–133. doi: 10.1016/j.cognition.2013.10.004. [DOI] [PubMed] [Google Scholar]
- van Gompel RPG, Pickering MJ, Traxler MJ. Reanalysis in sentence processing: Evidence against current constraint-based and two-stage models. Journal of Memory and Language. 2001;45:225–258. [Google Scholar]
- Wojnowicz MT, Ferguson MJ, Dale R, Spivey MJ. The self-organization of explicit attitudes. Psychological Science. 2009;20:1428–1435. doi: 10.1111/j.1467-9280.2009.02448.x. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Landy MS. Motor control is decision-making. Current opinion in neurobiology. 2012;22(6):996–1003. doi: 10.1016/j.conb.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao K, Yamauchi T. The role of attention in subliminal semantic processing: A mouse tracking study. PloS one. 2017;12(6):e0178740. doi: 10.1371/journal.pone.0178740. [DOI] [PMC free article] [PubMed] [Google Scholar]


