Abstract
The goal of this study was to assess the quality and scope of the current cost-effectiveness analysis (CEA) literature in the field of hand and upper extremity orthopaedic surgery. We conducted a systematic review of MEDLINE and the CEA Registry to identify CEAs that were conducted on or after Jan 1, 1997; studied a procedure pertaining to the field of hand and upper extremity surgery; were clinical studies; and reported outcomes in terms of quality-adjusted life years. We identified a total of 33 studies that met our inclusion criteria. The quality of these studies was assessed using the Quality of Health Economic Analysis (QHES) scale. The average total QHES score for all 33 studies was 82 (high-quality). However, over time, a greater proportion of these studies have demonstrated poorer QHES quality (scores <75). Lower scoring studies demonstrated several deficits including failures in identifying reference perspectives; incorporating comparators and sensitivity analyses; discounting costs and utilities; and disclosing funding. It will be important to monitor the ongoing quality of CEA studies in orthopaedics.
Introduction
The cost-effectiveness analysis (CEA) is one of the most commonly utilized tools in economic evaluation of medical care and assesses the value of an intervention relative to a comparator by assessing differences in costs and subsequent quality of life1.
In the field of orthopaedics, prior systematic reviews have assessed the quality of CEAs across sports2 and trauma3, finding the overall quality of studies to be good in these orthopaedic subspecialties. However, there has been no systematic examination of CEAs in upper extremity surgery. Brauer and colleagues conducted two separate studies evaluating the quality of CEAs across all orthopaedic subspecialties from 1976 to 2001 and included a total of only five hand and upper extremity studies4,5. No subgroup analyses were conducted on these studies.
Cost-effectiveness plays an important role in the field of upper extremity surgery, where a patient’s level of function can have direct and indirect effects on quality of life6. There is very little known about the scope and quality of the CEA literature in hand and upper extremity surgery. Our goal was to conduct a systematic review to (1) assess the quality and scope of the current collection of hand and upper extremity CEAs, (2) identify areas for further economic evaluation in the field of hand and upper extremity surgery, and (3) identify opportunities for quality improvement.
Materials and Methods
Overview
This review focuses on cost-utility analyses, which measure health outcomes in quality-adjusted life-years (QALYs) that incorporate subjective valuations of health states by patients2,5,7. Cost-utility analyses utilize an incremental cost-effectiveness ratio (ICER) which represents the difference in costs divided by the difference in QALYs between two interventions1. ICERs are evaluated against a willingness-to-pay (WTP) threshold, which represents the maximum threshold cost that society is willing to spend for an additional QALY, commonly $50,000 or $100,000 per QALY8. If a procedure’s ICER falls below the WTP threshold, it can be considered a cost-effective alternative to its comparator.
Search and Inclusion Criteria
The systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist9. We employed a systematic search of MEDLINE as well as the Cost-Effectiveness Analysis (CEA) Registry by the Center for the Evaluation of Value and Risk in Health at Tufts University10.
This CEA registry is a well-established and high-quality repository of CEAs11,12. The registry possesses over 5600 cost-utility analyses and incorporates strict inclusion criteria for papers that are published in English, are CEAs, and measure health benefits in QALYs. The registry excludes reviews, editorials, and methodological articles. We used a supplementary MEDLINE search to capture any studies that met the above inclusion criteria but were not yet included within the CEA registry13.
We included studies from the CEA Registry with a keyword pertaining to upper extremity anatomy. Our MEDLINE search incorporated any study 1) pertaining to upper extremity anatomy; 2) conducted on or after Jan 1, 1997; and 3) utilizing the terms quality-adjusted life-years (QALYs) and costs. Following our preliminary searches of the CEA registry and MEDLINE, we included any study in this review that cleared the following exclusion criteria: 1) was conducted on or after Jan 1, 1997; 2) studied a procedure consistent with the field of orthopaedic upper extremity surgery; 3) was a clinical study; and 4) utilized methodology consistent with a cost-utility analysis.
Our rationale for including a time limit in our search was based on the recommendations of the First Panel on Cost-Effectiveness in Health and Medicine, published on October 16, 1996, which outlined the first consensus-based guidelines for the conduct of CEAs1. We restricted our search from the start of January 1997 to December 2016 to allow adequate time for studies to reflect the guidelines. We restricted our review to clinical studies, excluding any reviews, methodological studies, or studies focusing on non-operative interventions without including a surgical/procedural comparator. We excluded CEAs not incorporating QALYs, cost-benefit analyses, or cost-minimization analyses14. We restricted our search to studies conducted in humans and in English-only for ease of interpretation. Although a language restriction can possess bias, there is no evidence that it has any effects on resultant data in systematic reviews and meta-analyses15.
CEA Registry Search
The CEA Registry screening process was searched by “[Anatomic location].” Anatomic location terms included the broad term “upper extremity” and more specific anatomic regions pertinent to upper extremity clinical anatomy: shoulder, glenohumeral, labrum, humerus, elbow, wrist, radius, ulna, scaphoid, carpal, hand, thumb, finger, metacarpal, carpometacarpal, metacarpophalangeal, phalanx, phalanges, intermetacarpal, and interphalangeal. Additionally, we included terms specific to anatomic injuries: “rotator cuff” and “carpal tunnel syndrome.” The CEA Registry yielded a total of 134 studies.
MEDLINE search
We utilized the PubMed interface to search the MEDLINE database13. Search terms were grouped into three broad categories: [Anatomic location], [Procedure], and [Cost Study] (Appendix 1). The MEDLINE query resulted in 61 studies, of which 35 were not captured by the CEA Registry search.
These 169 total studies independently underwent a (1) combined title and abstract screening followed by a (2) full text screening separately by two authors (PVR and RAQ) to ensure full adherence to the inclusion criteria. This resulted in a final total of 33 studies to be included in this review (Figure 1).
Figure 1.
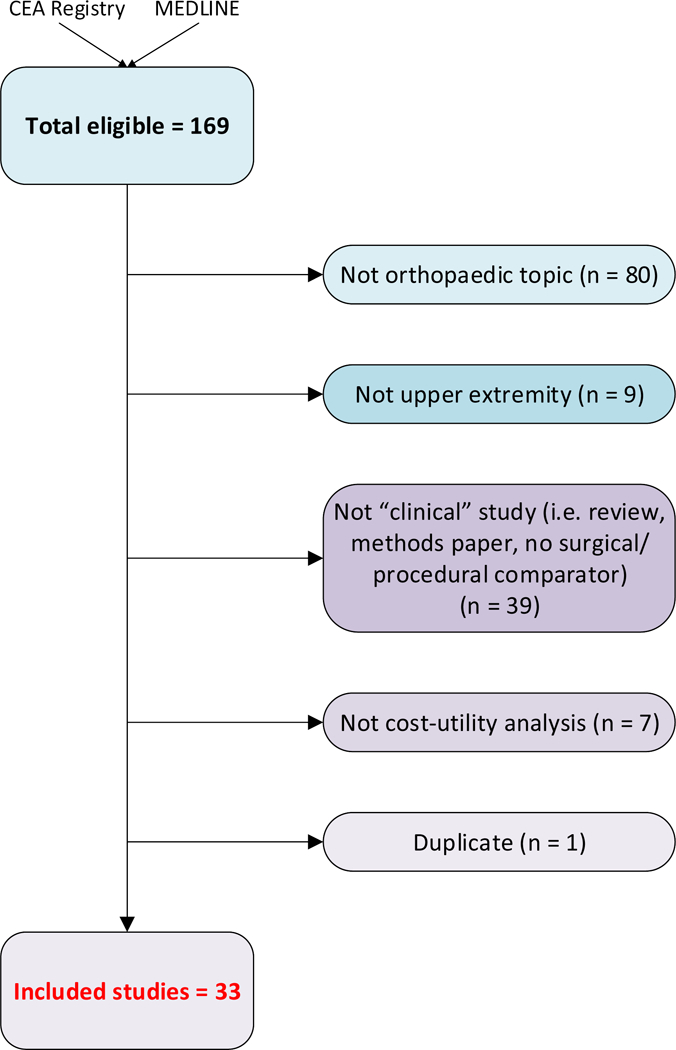
Overview of screening process used for systematic review.
Quality Scoring and Data Extraction
We assigned a quality score to each study using the Quality of Health Economic Analysis (QHES) scale16. The QHES scale ranges from 0 to 100; although there is no standard for the QHES score, a score above 75 is considered high-quality16. Each of the 33 studies underwent comprehensive paper review by the primary author (PVR), followed by independent review (RAQ) of a random sampling of 20% of these studies to ensure at least 90% inter-rater agreement.
We also recorded the following study characteristics: study design (CEA conducted alongside a randomized-controlled trial or a prospective cohort study, decision tree, Markov cohort, or microsimulation); lowest level of evidence used to derive health state transition probabilities (“level I” for randomized-controlled trials, “level II” for prospective cohort studies, “level III” for retrospective studies, or “level IV” for case series); whether the study reported an ICER; perspective (“health care payer” or “societal,” where societal perspective incorporates unpaid and informal health care costs); time horizon lengths; sensitivity analyses conducted (“deterministic” for parameter variations over a range or “probabilistic” for variations conducted over a distribution); and utility assessment (“direct” if the study elicited health-state preferences using tools such as time trade-off, standard gamble, or visual analog scales, or “indirect” if the study used tools such as EuroQol (EQ-5D)17,18 or SF-6D19,20).
Statistical Analyses
QHES scores extracted from each study were averaged across all studies, and by anatomical region and time frame. We used frequencies to describe the distributions of study characteristics, and we calculated averages for study characteristics expressed by continuous factors (e.g. time horizon). We conducted the analysis for the overall sample and stratified by anatomic region, time period, and geographic region to elucidate any trends. We extracted ICERs from studies, when available, and adjusted to 2016 US Dollars using the Consumer Price Index21 and 2016 United Kingdom Pounds using Bank of England inflation values22.
Results
Overview
The CEA Registry yielded a total of 134 studies. The MEDLINE query resulted in 61 studies, of which 35 were not captured by the CEA Registry search. Of these 169 studies, 33 studied met our inclusion criteria and were included in this review (Figure 1). Table I provides an overview of the 33 CEAs screened into this review.
Study characteristics
Table 2 provides the general study characteristics for the 33 studies. All anatomic categories have a collection of model-based and randomized controlled trial (RCT)-based study designs. All RCT-based studies have been conducted in the United Kingdom, Australia, or Europe whereas most (95%) decision tree, Markov cohort, and microsimulation designs have been conducted in the United States or Canada.
Table 2.
General study characteristics of N = 33 cost-effectiveness studies of upper extremity orthopaedic surgery. *
| Study characteristic | n (%) |
|---|---|
| Study design/model | |
| RCT | 9 (27%) |
| Prospective cohort | 5 (15%) |
| Decision tree | 14 (42%) |
| Markov model, cohort | 4 (12%) |
| Microsimulation | 1 (3%) |
| Level of evidence ** | |
| Level I | 12 (36%) |
| Level II | 3 (9%) |
| Level III | 4 (12%) |
| evel IV | 14 (42%) |
| Perspective | |
| Health care payer | 10 (30%) |
| Societal | 16 (48%) |
| Both | 2 (6%) |
| None | 5 (15%) |
| Time Horizon | |
| Lifetime | 8 (24%) |
| 20 years | 5 (15%) |
| 20 years | 20 (61%) |
| Country of origin | |
| United States | 17 (52%) |
| United Kingdom | 9 (27%) |
| Canada | 3 (9%) |
| Others‡ | 4 (12%) |
| Utility Assessment | |
| Direct | 11 (33%) |
| Indirect | 22 (67%) |
| Sensitivity analysis | |
| Deterministic only | 17 (52%) |
| Probabilistic only | 1 (3%) |
| Both | 11 (33%) |
| None | 4 (12%) |
| ICER reported? | |
| Yes | 26 (79%) |
| No | 7 (21%) |
| Cost methodology explained? | |
| Yes | 29 (88%) |
| No | 4 (12%) |
| Statement of funding? | |
| Yes | 28 (85%) |
| No | 5 (15%) |
RCT = cost-effectiveness analysis conducted alongside a randomized controlled trial; Prospective cohort = cost-effectiveness analysis conducted alongside a cohort or series of patients followed prospectively for data on outcomes; ICER = incremental cost-effectiveness ratio.
Refers to lowest level of evidence the study used to derive health state transition probabilities
Other countries include Australia, Norway, Netherlands, and Italy.
Studies from the United States predominantly use a societal perspective (70.6%) whereas studies from the United Kingdom predominantly use a health care payer perspective (55.6%). When stratified by time period, there is a trend towards shorter time horizons in recent years; for example, CEAs from 2015-2016 demonstrate a range of only 1 to 2 years. 47% and 100% of the studies in United States and Canada, respectively, use direct utility measurements such as time trade-off, standard gamble, or visual analog scales. However, all studies from the United Kingdom, Australia, and Europe utilize indirect utility measurement tools such as EQ-5D, SF-36, or health utility index (HUI).
77% of studies from the United States use deterministic analyses only, whereas 67% of studies from the United Kingdom utilize both deterministic and probabilistic sensitivity analyses. Of the 28 studies with a statement of funding, 12 are funded by public funds, 5 by private funds, 2 by both, and 9 did not specify. We found studies conducted in the United States use a wide range of WTP thresholds ($4,836 per QALY to $100,000 per QALY) while studies conducted in the United Kingdom tend to use standard WTP thresholds of £20,000-£30,000 per QALY.
Study quality
The average total QHES score for all 33 studies is 82 (high-quality). When stratified according to anatomic group, the QHES scores are: 77 shoulder; 87 arm; 78 elbow; 85 forearm; 86 hand and wrist; and 91 general upper limb. Figure 2 demonstrates an increase in the number of upper-extremity CEAs published since 1997. Over time, a greater proportion of these studies have demonstrated poorer QHES quality (scores <75), with scores as low as 41.
Figure 2.
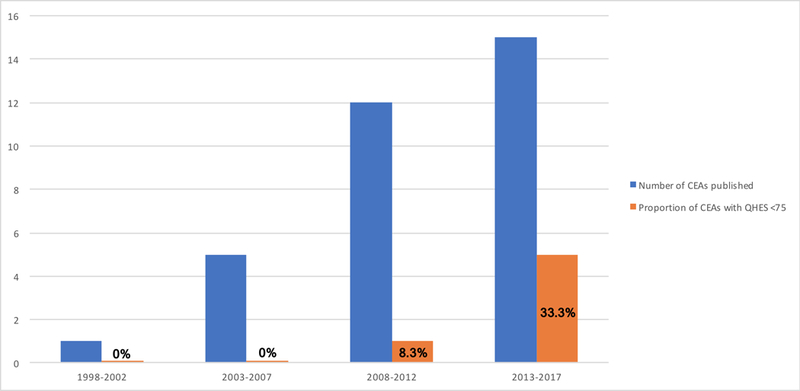
Bar graph illustrating the number of cost-effectiveness analyses published over time, coupled with the proportion of cost-effectiveness analyses for each time grouping that demonstrated quality QHES scores <75.
Discussion
Since the First Panel Recommendations were published in 1997, the overall quality of cost-effectiveness literature within the field of upper extremity orthopaedic surgery has been high. When stratified by each anatomic region of study, the average QHES score is still >75. Over time, however, there has been a greater proportion of studies demonstrating lower quality scores in recent years. Lower scores <75 tend to demonstrate several deficits including failures in identifying reference case perspectives; incorporating comparators, ICERs, and sensitivity analyses; discounting costs and utilities; and including a statement of funding or support.
Reviews within the subspecialties of sports medicine2 and trauma3 have found the quality to be strong, with QHES averages of 81.8 and 79.25, respectively. Brauer and colleagues have noted more orthopaedic studies complying with the recommendations of the First Panel after 19985. Our study, however, seems to indicate a divergence in the quality of the upper extremity orthopaedic cost-effectiveness literature since then. Given this increasing variability, the new recommendations from the Second Panel on Cost-Effectiveness in Health and Medicine published in October 2016 are timely to re-establish standard methodological practices23.
Our review identified the shoulder and wrist to be the anatomic areas of considerable cost-effectiveness research, with emphasis on rotator cuff tears and carpal tunnel syndrome given their procedural prevalence. Studies in this review have supported single-row, arthroscopic rotator cuff repairs to be more cost-effective alternatives to nonoperative, open, or double-row procedures24-26, and endoscopic carpal tunnel release to be a more cost-effective option than open release when both techniques are performed in outpatient settings27,28. There is, however, a paucity of cost-utility analyses of the elbow and of pediatric upper extremity pathologies.
Studies conducted in the United States tend to utilize decision tree or Markov models with societal perspectives. All analyses conducted alongside an RCT came from the United Kingdom, Australia, or Europe; these studies tend to use health care payer perspectives. Studies conducted in the United States were observed to utilize a range of WTP thresholds. These differences reflect the experience of CEAs in each country. Decision-making bodies in Europe, Australia, and Canada have more formally integrated CEAs into coverage decisions, opting to use third-party payer perspectives as opposed to the United States-based First Panel’s recommendation for societal perspectives23,29. Likewise, health technology assessment bodies in Europe have emphasized funding for RCT-based research to assess clinical evidence alongside cost-effectiveness30,31. The United States experience has been far more limited, with political resistance to incorporation of CEAs in federal coverage decisions32. The Second Panel’s efforts represent an effort to standardize CEAs across countries for ease of comparability.
This review should be viewed in light of its limitations. Given the stringent inclusion criteria of the CEA Registry, we may have biased our results towards a higher quality score. However, similar reviews have observed equally high qualities when primarily using databases such as MEDLINE2,3. The QHES tool values the design and reporting of cost-effectiveness studies; therefore, it is possible that a study lost points for not being explicit in its reporting of study design and results. The quality scores are certainly susceptible to the biases of the individual grader; however, we ensured at least a 90% inter-rater agreement across two reviewers.
Cost-effectiveness is an area of increasing interest in upper extremity orthopaedic surgery, especially in the shoulder and the wrist. We observe a high quality of CEA studies in upper extremity surgery since 1997, but a growing proportion of lower quality studies in recent years. It will be important to monitor the ongoing quality of CEA studies in orthopaedics and ensure proper peer-review of future studies based on the Second Panel recommendations to ensure standards of reporting and comparability.
Supplementary Material
Table I.
Main findings and study designs of cost-effectiveness studies included in this review, organized by anatomic region.*
| ANATOMY | AUTHOR | YEAR | COUNTRY | STUDY DESIGN | ICER (2016 values) | WTP | MAIN FINDINGS |
|---|---|---|---|---|---|---|---|
| Shoulder | Butt33 | 2015 | UK | Prospective cohort | NA | NA | Arthroscopic decompression for subacromial impingement provides 0.23 QALYs gained for £5,683 |
| Jowett34 | 2013 | UK | RCT | Dominant | £20,000/QALY | Corticosteroid injection and exercise therapy may be cheaper and more effective (i.e. dominant) to exercise-alone in moderate to severe subacromial impingement syndrome | |
| Carr24 | 2015 | UK | RCT | £32,510/QALY | £20,000/QALY | Open rotator cuff repair was not a cost-effective alternative to arthroscopic rotator cuff repair in the base case, intention-to-treat analysis | |
| Crall25 | 2012 | USA | Microsimulation | Multiple; all <WTP or dominant | $25,000/QALY | Primary arthroscopic stabilization was a cost-effective alternative to nonoperative treatment for first-time anterior glenohumeral dislocation with ICERs <$25,000/QALY across majority of age groups | |
| Coe35 | 2012 | USA | Markov cohort | $103,668/QALY** | $100,000/QALY | Reverse shoulder arthroplasty was a cost-effective alternative to humeral head replacement for rotator cuff tear arthropathy | |
| Genuario26 | 2012 | USA | Decision tree | Tears <3 cm: $638,601/QALY; Tears ≥3 cm: $514,233/QALY | $100,000/QALY | Double-row rotator cuff repair is not a cost-effective alternative for any size rotator cuff tears | |
| Mather36 | 2013 | USA | Markov cohort | Dominant | $50,000/QALY | Surgical rotator cuff repair is a cost-effective alternative for the U.S. populations compared to nonoperative treatment with lifetime savings of $13,771 and 0.62 QALY improvement | |
| Renfree37 | 2013 | USA | Prospective cohort | NA | NA | Reverse shoulder arthroplasty for rotator cuff arthropathy provides 2-year gain of 1.02 (SF-36) and 1.97 (EQ-5D) QALYs for a cost of $21,536 | |
| Vavken38 | 2015 | USA | Decision tree | $132,009/QALY | $100,000/QALY | Platelet-rich plasma after arthroscopic rotator cuff repair is not a cost-effective alternative to repair without platelet-rich plasma, assuming a 5% revision rate | |
| Vitale39 | 2007 | USA | Prospective cohort | NA | NA | Surgical rotator cuff repair provides a mean lifetime gain of 0.81 (HUI) and 3.43 (EQ-5D) QALYs for total cost of $10,605 | |
| Dattani40 | 2013 | UK | Prospective cohort | NA | NA | Arthroscopic capsular release for contracture of the shoulder provides 0.50 QALYs gained for £2204 | |
| Mather41 | 2010 | USA | Markov cohort | Dominant | $50,000/QALY | Total shoulder arthroplasty is a cost-effective alternative to hemiarthroplasty for glenohumeral osteoarthritis with $1,970 less costs and 0.77 more QALYs | |
| Pearson42 | 2010 | USA | Decision tree | Base case: $80,546/QALY; >9 years: $34,883/QALY |
$50,000/QALY | ORIF of displaced, midshaft clavicular fractures can be a cost-effective alternative to nonoperative treatment if the incremental QALYs gained (0.014) persists beyond 9 years. At the base case, ORIF is not a cost-effective alternative | |
| Arm | Corbacho43 | 2016 | UK | RCT | Dominated | £20,000/QALY | Surgical treatment for displaced, proximal humerus fractures in adults is not a cost-effective alternative to non-operative treatment with greater total costs and lower total QALYs (i.e. dominated) |
| Fjalestad44 | 2010 | Norway | RCT | £315,922/QALY | NA | Surgical treatment for displaced, proximal humerus fractures in adults did not produce statistically significant different costs or QALYs from nonoperative treatment. | |
| Elbow | Coombes45 | 2016 | Australia | RCT | $30,287/QALY | $50,000/QALY | Physiotherapy-alone was a cost-effective alternative to corticosteroid injection with or without physiotherapy for chronic lateral epicondylalgia |
| Giannicola46 | 2013 | Italy | Prospective cohort | NA | NA | Open surgical treatment of elbow stiffness produces 0.1539 annual increases in QALYs with an average cost of £3565 | |
| Song47 | 2012 | USA | Decision tree | $2,265/QALY | $100,000/QALY | Simple decompression was the most cost-effective initial procedure for ulnar neuropathy of the elbow when compared to anterior subcutaneous and submuscular transpositions and medial epicondylectomy | |
| Forearm | Karantana48 | 2015 | UK | RCT | £44,814/QALY | £10,000-£50,000/QALY | Volar locking plating was not a cost-effective alternative to percutaneous wire fixation for distal radius fractures |
| Rockwell49 | 2004 | Canada | Markov cohort | Dominated | NA | Prophylactic plating of the donor radius after harvest of radial osteocutaneous flap is not a cost-effective alternative to treatment of fractures when they occur, producing higher cost ($2071 vs. $140) with lower QALYS (8.55 vs. 9.92) | |
| Shauver50 | 2011 | USA | Decision tree | $17,130/QALY | $50,000/QALY | ORIF dominated wire fixation and external fixation and was a cost-effective alternative to casting for distal radius fractures in the elderly | |
| Tubeuf51 | 2015 | UK | RCT | £96,793/QALY | £30,000/QALY | Volar locking plating was not a cost-effective alternative to percutaneous Kirschner wire fixation for dorsally displaced distal radius fractures | |
| Hand & Wrist | Baltzer52 | 2013 | Canada | Decision tree | Collagenase: $303,654/QALY; Fasciectomy: Dominated | $50,000-$100,000/QALY | Collagenase was not a cost-effective alternative to percutaneous needle aponeurotomy for Dupuytren’s contracture; partial fasciectomy was dominated by aponeurotomy |
| Chen53 | 2011 | USA | Decision tree | Fasciectomy: $916,405/QALY; Collagenase: $55,865/QALY**; Aponeurotomy: $55,458/QALY** | $50,000/QALY | All compared to no treatment for Dupuytren’s contracture, open partial fasciectomy is not a cost-effective alternative; collagenase can be a cost-effective alternative if priced <$945; aponeurotomy can be cost-effective if success rate is 100% | |
| Chung27 | 1998 | USA | Decision tree | 25-year-old: $293/QALY; 65-year-old: $1,042/QALY |
$4836/QALY to $13,508/QALY | Endoscopic carpal tunnel release is a cost-effective alternative to open release for carpal tunnel syndrome | |
| Korthals-de Bos54 | 2006 | Netherlands | RCT | £469/QALY | £2,500/QALY | Surgery is a cost-effective alternative to splinting for carpal tunnel syndrome | |
| Thoma28 | 2006 | Canada | Decision tree | Main OR: $147,665/QALY; Day unit: dominant |
$100,000/QALY | Endoscopic carpal tunnel release is not a cost-effective alternative to open release for carpal tunnel syndrome when endoscopic release is performed in the main operating room and when open release is performed in the day surgery unit. When both are performed in the day surgery unit, endoscopic release dominates open release. | |
| Cavaliere55 | 2010 | USA | Decision tree | TWA vs. nonsurgical: $2,512/QALY; TWA vs. arthrodesis: $2,564/QALY | $50,000/QALY | Total wrist arthroplasty (TWA) was a cost-effective alternative to both nonsurgical management and to total wrist arthrodesis for the rheumatoid wrist | |
| Chung56 | 2010 | USA | Decision tree | Prosthesis vs. transplant: dominant; Double-hand transplant vs. prosthesis: $426,808/QALY |
$50,000-$100,000/QALY | Prosthetic use dominated hand transplantation for unilateral hand amputation. Double hand transplantation was preferred over prostheses (26.73 vs. 25.20 QALYs) for double hand amputation, but was not cost-effective. | |
| Davis57 | 2006 | USA | Decision tree | $7,135/QALY | $20,000/QALY | ORIF is a cost-effective treatment alternative to cast immobilization for acute nondisplaced mid-waist scaphoid fractures | |
| Sears58 | 2014 | USA | Decision tree | Single-digit: $145,643/QALY; 3-digit: $28,963/QALY; 4-digit: $25,413/QALY |
$100,000/QALY | Replantation had greater costs and QALYs compared with revision amputation in all injury scenarios. Replantation of single-digit injuries was not a cost-effective alternative to revision amputation, whereas replantation of 3- or 4-digit amputations were cost-effective alternatives | |
| General | Doan59 | 2013 | UK | Markov cohort | £11,130/QALY | £30,000/QALY | OnabotulinumtoxinA along with usual care was a cost-effective alternative to usual care for upper-limb post-stroke spasticity |
| Shaw60 | 2010 | UK | RCT | £119,049/QALY | £20,000/QALY | Addition of botulinum toxin A to a therapy program for upper-limb post-stroke spasticity was not cost-effective | |
RCT = cost-effectiveness analysis conducted alongside a randomized controlled trial; Prospective cohort = cost-effectiveness analysis conducted alongside a cohort or series of patients followed prospectively for data on outcomes; ICER = incremental cost-effectiveness ratio; QALY = quality-adjusted life-year; WTP = willingness-to-pay threshold; HUI = health utility index; ORIF = open reduction internal fixation.
Note: adjusting ICER to 2016 values raised the ratio above the corresponding willingness-to-pay threshold
Acknowledgments
ROLE OF THE FUNDING SOURCE: Research reported in this publication was supported by a T32 training grant (AR055885) from the National Institutes of Health. EL receives funding support from a K24 National Institute of Arthritis and Musculoskeletal and Skin Diseases grant (AR057827). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA. 1996;276(15):1253–8. [PubMed] [Google Scholar]
- 2.Nwachukwu BU, Schairer WW, Bernstein JL, Dodwell ER, Marx RG, Allen AA. Cost-effectiveness analyses in orthopaedic sports medicine: A systematic review. Am J Sports Med. 2015;43(6):1530–7. [DOI] [PubMed] [Google Scholar]
- 3.Nwachukwu BU, Schairer WW, O’Dea E, McCormick F, Lane JM. The quality of cost-utility analyses in orthopedic trauma. Orthopedics. 2015;38(8):e673–80. [DOI] [PubMed] [Google Scholar]
- 4.Brauer CA, Rosen AB, Olchanski NV, Neumann PJ. Cost-utility analyses in orthopaedic surgery. J Bone Joint Surg Am. 2005;87(6):1253–9. [DOI] [PubMed] [Google Scholar]
- 5.Brauer CA, Neumann PJ, Rosen AB. Trends in cost effectiveness analyses in orthopaedic surgery. Clin Orthop Relat Res. 2007;457:42–8. [DOI] [PubMed] [Google Scholar]
- 6.Hudak PL, Amadio PC, Bombardier C. Development of an upper extremity outcome measure: the DASH (disabilities of the arm, shoulder and hand) [corrected]. The Upper Extremity Collaborative Group (UECG). Am J Ind Med. 1996;29(6):602–8. [DOI] [PubMed] [Google Scholar]
- 7.Bozic KJ, Saleh KJ, Rosenberg AG, Rubash HE. Economic evaluation in total hip arthroplasty: Analysis and review of the literature. J Arthroplasty. 2004;19(2):180–9. [DOI] [PubMed] [Google Scholar]
- 8.King JT Jr, Tsevat J, Lave JR, Roberts MS Willingness to pay for a quality-adjusted life year: Implications for societal health care resource allocation. Med Decis Making. 2005;25(6):667–77. [DOI] [PubMed] [Google Scholar]
- 9.Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). Accessed 15 Dec 2015. http://prisma-statement.org/Default.aspx.
- 10.Cost-Effectiveness Analysis Registry. Accessed 15 Dec 2016. http://healtheconomics.tuftsmedicalcenter.org/cear4/Home.aspx.
- 11.Alvin MD, Miller JA, Lubelski D, Rosenbaum BP, Abdullah KG, Whitmore RG, et al. Variations in cost calculations in spine surgery cost-effectiveness research. Neurosurg Focus. 2014;36(6):E1. [DOI] [PubMed] [Google Scholar]
- 12.Baptista A, Teixeira I, Romano S, Carneiro AV, Perelman J. The place of DPP-4 inhibitors in the treatment algorithm of diabetes type 2: A systematic review of cost-effectiveness studies. Eur J Health Econ. 2017;8(8):937–965. [DOI] [PubMed] [Google Scholar]
- 13.MEDLINE. Accessed 15 Dec 2016. https://www.ncbi.nlm.nih.gov/pubmed.
- 14.Briggs AH, O’Brien BJ. The death of cost-minimization analysis? Health Econ. 2001;10(2):179–84. [DOI] [PubMed] [Google Scholar]
- 15.Morrison A, Polisena J, Husereau D, Moulton K, Clark M, Fiander M, et al. The effect of English-language restriction on systematic review-based meta-analyses: A systematic review of empirical studies. Int J Technol Assess Health Care. 2012;28(2):138–44. [DOI] [PubMed] [Google Scholar]
- 16.Ofman JJ, Sullivan SD, Neumann PJ, Chiou CF, Henning JM, Wade SW, et al. Examining the value and quality of health economic analyses: implications of utilizing the QHES. J Manag Care Pharm. 2003;9(1):53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Group EuroQol. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. [DOI] [PubMed] [Google Scholar]
- 18.Brooks R EuroQol: the current state of play. Health Policy. 1996;37(1):53–72. [DOI] [PubMed] [Google Scholar]
- 19.Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ. 2002;21(2):271–92. [DOI] [PubMed] [Google Scholar]
- 20.Brazier J, Usherwood T, Harper R, Thomas K. Deriving a preference-based single index from the UK SF-36 Health Survey. J Clin Epidemiol. 1998;51(11):1115–28. [DOI] [PubMed] [Google Scholar]
- 21.Bureau of Labor Statistics. Consumer Price Index. Accessed 21 Sep 2016. http://www.bls.gov/data/inflation_calculator.htm/.
- 22.Bank of England. Inflation Calculator. Accessed 21 Sep 2016. http://www.bankofengland.co.uk/education/Pages/resources/inflationtools/calculator/default.aspx. [Google Scholar]
- 23.Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, et al. Recommendations for Conduct, Methodological Practices, and Reporting of Cost-effectiveness Analyses: Second Panel on Cost-Effectiveness in Health and Medicine. JAMA. 2016;316(10):1093–103. [DOI] [PubMed] [Google Scholar]
- 24.Carr AJ, Cooper CD, Campbell MK, Rees JL, Moser J, Beard DJ, et al. Clinical effectiveness and cost-effectiveness of open and arthroscopic rotator cuff repair [the UK Rotator Cuff Surgery (UKUFF) randomised trial]. Health Technol Assess. 2015;19(80):1–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crall TS, Bishop JA, Guttman D, Kocher M, Bozic K, Lubowitz JH. Cost-effectiveness analysis of primary arthroscopic stabilization versus nonoperative treatment for first-time anterior glenohumeral dislocations. Arthroscopy. 2012;28(12):1755–65. [DOI] [PubMed] [Google Scholar]
- 26.Genuario JW, Donegan RP, Hamman D, Bell JE, Boublik M, Schlegel T, et al. The cost-effectiveness of single-row compared with double-row arthroscopic rotator cuff repair. J Bone Joint Surg Am. 2012;94(15):1369–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chung KC, Walters MR, Greenfield ML, Chernew ME. Endoscopic versus open carpal tunnel release: A cost-effectiveness analysis. Plast Reconstr Surg. 1998;102(4):1089–99. [DOI] [PubMed] [Google Scholar]
- 28.Thoma A, Wong VH, Sprague S, Duku E. A cost-utility analysis of open and endoscopic carpal tunnel release. Can J Plast Surg. 2006;14(1):15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neumann PJ, Sanders GD, Russell LB, Siegel JE, Ganiats TG. Cost Effectiveness in Health and Medicine. Second ed. New York: Oxford University Press, 2017. [Google Scholar]
- 30.National Institute for Health Research. Health Technology Assessment (HTA) Programme. Accessed 22 February 2017. http://www.nets.nihr.ac.uk/programmes/hta.
- 31.Velasco-Garrido MB R Health technology assessment: An introduction to objectives, role of evidence, and structure in Europe. European Observatory on Health Systems and Policies, World Health Organization (WHO). Accessed 22 February 2016. http://www.euro.who.int/__data/assets/pdf_file/0018/90432/E87866.pdf. [Google Scholar]
- 32.Garber AM, Phelps CE. Future costs and the future of cost-effectiveness analysis. J Health Econ. 2008;27(4):819–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Butt U, Whiteman A, Wilson J, Paul E, Roy B. Does arthroscopic subacromial decompression improve quality of life? Ann R Coll Surg Engl. 2015;97(3):221–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jowett S, Crawshaw DP, Helliwell PS, Hensor EM, Hay EM, Conaghan PG. Cost-effectiveness of exercise therapy after corticosteroid injection for moderate to severe shoulder pain due to subacromial impingement syndrome: A trial-based analysis. Rheumatology (Oxford). 2013;52(8):1485–91. [DOI] [PubMed] [Google Scholar]
- 35.Coe MP, Greiwe RM, Joshi R, Snyder BM, Simpson L, Tosteson AN, et al. The cost-effectiveness of reverse total shoulder arthroplasty compared with hemiarthroplasty for rotator cuff tear arthropathy. J Shoulder Elbow Surg. 2012;21(10):1278–88. [DOI] [PubMed] [Google Scholar]
- 36.Mather RC, Koenig L, Acevedo D, Dall TM, Gallo P, Romeo A, et al. The societal and economic value of rotator cuff repair. J Bone Joint Surg Am. 2013;95(22):1993–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Renfree KJ, Hattrup SJ, Chang YH. Cost utility analysis of reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(12):1656–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vavken P, Sadoghi P, Palmer M, Rosso C, Mueller AM, Szoelloesy G, et al. Platelet-rich plasma reduces retear rates after arthroscopic repair of small- and medium-sized rotator cuff tears but is not cost-effective. Am J Sports Med. 2015;43(12):3071–6. [DOI] [PubMed] [Google Scholar]
- 39.Vitale MA, Vitale MG, Zivin JG, Braman JP, Bigliani LU, Flatow EL. Rotator cuff repair: An analysis of utility scores and cost-effectiveness. J Shoulder Elbow Surg. 2007;16(2):181–7. [DOI] [PubMed] [Google Scholar]
- 40.Dattani R, Ramasamy V, Parker R, Patel VR. Improvement in quality of life after arthroscopic capsular release for contracture of the shoulder. Bone Joint J. 2013;95-b(7):942–6. [DOI] [PubMed] [Google Scholar]
- 41.Mather RC, Watters TS, Orlando LA, Bolognesi MP, Moorman CT. Cost effectiveness analysis of hemiarthroplasty and total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(3):325–34. [DOI] [PubMed] [Google Scholar]
- 42.Pearson AM, Tosteson AN, Koval KJ, McKee MD, Cantu RV, Bell JE, et al. Is surgery for displaced, midshaft clavicle fractures in adults cost-effective?: Results based on a multicenter randomized, controlled trial. J Orthop Trauma. 2010;24(7):426–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corbacho B, Duarte A, Keding A, Handoll H, Chuang LH, Torgerson D, et al. Cost effectiveness of surgical versus non-surgical treatment of adults with displaced fractures of the proximal humerus: Economic evaluation alongside the PROFHER trial. Bone Joint J. 2016;98-b(2):152–9. [DOI] [PubMed] [Google Scholar]
- 44.Fjalestad T, Hole MO, Jorgensen JJ, Stromsoe K, Kristiansen IS. Health and cost consequences of surgical versus conservative treatment for a comminuted proximal humeral fracture in elderly patients. Injury. 2010;41(6):599–605. [DOI] [PubMed] [Google Scholar]
- 45.Coombes BK, Connelly L, Bisset L, Vicenzino B. Economic evaluation favours physiotherapy but not corticosteroid injection as a first-line intervention for chronic lateral epicondylalgia: Evidence from a randomised clinical trial. Br J Sports Med. 2016;50(22):1400–05. [DOI] [PubMed] [Google Scholar]
- 46.Giannicola G, Bullitta G, Sacchetti FM, Scacchi M, Polimanti D, Citoni G, et al. Change in quality of life and cost/utility analysis in open stage-related surgical treatment of elbow stiffness. Orthopedics. 2013;36(7):e923–30. [DOI] [PubMed] [Google Scholar]
- 47.Song JW, Chung KC, Prosser LA. Treatment of ulnar neuropathy at the elbow: Cost-utility analysis. J Hand Surg Am. 2012;37(8):1617–29 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karantana A, Scammell BE, Davis TR, Whynes DK. Cost-effectiveness of volar locking plate versus percutaneous fixation for distal radial fractures: Economic evaluation alongside a randomised clinical trial. Bone Joint J. 2015;97-b(9):1264–70. [DOI] [PubMed] [Google Scholar]
- 49.Rockwell GM, Thoma A. Should the donor radius be plated prophylactically after harvest of a radial osteocutaneous flap? A cost-effectiveness analysis. J Reconstr Microsurg. 2004;20(4):297–306. [DOI] [PubMed] [Google Scholar]
- 50.Shauver MJ, Clapham PJ, Chung KC. An economic analysis of outcomes and complications of treating distal radius fractures in the elderly. J Hand Surg Am. 2011;36(12):1912–8 e1-3. [DOI] [PubMed] [Google Scholar]
- 51.Tubeuf S, Yu G, Achten J, Parsons NR, Rangan A, Lamb SE, et al. Cost effectiveness of treatment with percutaneous Kirschner wires versus volar locking plate for adult patients with a dorsally displaced fracture of the distal radius: analysis from the DRAFFT trial. Bone Joint J. 2015;97-B(8):1082–9. [DOI] [PubMed] [Google Scholar]
- 52.Baltzer H, Binhammer PA. Cost-effectiveness in the management of Dupuytren’s contracture: A Canadian cost-utility analysis of current and future management strategies. Bone Joint J. 2013;95-B(8):1094–100. [DOI] [PubMed] [Google Scholar]
- 53.Chen NC, Shauver MJ, Chung KC. Cost-effectiveness of open partial fasciectomy, needle aponeurotomy, and collagenase injection for dupuytren contracture. J Hand Surg Am. 2011;36(11):1826–34 e32. [DOI] [PubMed] [Google Scholar]
- 54.Korthals-de Bos IB, Gerritsen AA, van Tulder MW, Rutten-van Molken MP, Ader HJ, de Vet HC, et al. Surgery is more cost-effective than splinting for carpal tunnel syndrome in the Netherlands: Results of an economic evaluation alongside a randomized controlled trial. BMC Musculoskelet Disord. 2006;7:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cavaliere CM, Chung KC. A cost-utility analysis of nonsurgical management, total wrist arthroplasty, and total wrist arthrodesis in rheumatoid arthritis. J Hand Surg Am. 2010;35(3):379–91 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chung KC, Oda T, Saddawi-Konefka D, Shauver MJ. An economic analysis of hand transplantation in the United States. Plast Reconstr Surg. 2010;125(2):589–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Davis EN, Chung KC, Kotsis SV, Lau FH, Vijan S. A cost/utility analysis of open reduction and internal fixation versus cast immobilization for acute nondisplaced mid-waist scaphoid fractures. Plast Reconstr Surg. 2006;117(4):1223–35; discussion 36-8. [DOI] [PubMed] [Google Scholar]
- 58.Sears ED, Shin R, Prosser LA, Chung KC. Economic analysis of revision amputation and replantation treatment of finger amputation injuries. Plast Reconstr Surg. 2014;133(4):827–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Doan QV, Gillard P, Brashear A, Halperin M, Hayward E, Varon S, et al. Cost-effectiveness of onabotulinumtoxinA for the treatment of wrist and hand disability due to upper-limb post-stroke spasticity in Scotland. Eur J Neurol. 2013;20(5):773–80. [DOI] [PubMed] [Google Scholar]
- 60.Shaw L, Rodgers H, Price C, van Wijck F, Shackley P, Steen N, et al. BoTULS: a multicentre randomised controlled trial to evaluate the clinical effectiveness and cost-effectiveness of treating upper limb spasticity due to stroke with botulinum toxin type A. Health Technol Assess. 2010;14(26):1–113, iii-iv. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


