Abstract
The transition from oocyte to embryo marks the onset of development. This process requires complex regulation to link developmental signals with profound changes in mRNA translation, cell cycle control, and metabolism. This control is beginning to be understood for most organisms, and research in the fruit fly Drosophila melanogaster has generated new insights. Recent findings have increased our understanding of the roles played by hormone and Ca2+ signaling events as well as metabolic remodeling crucial for this transition. Specialized features of the structure and assembly of the meiotic spindle have been identified. The changes in protein levels, mRNA translation, and polyadenylation that occur as the oocyte becomes an embryo have been identified together with key aspects of their regulation. Here we highlight these important developments and the insights they provide on the intricate regulation of this dramatic transition.
Keywords: Oocyte-to-embryo transition, Oocyte maturation, Meiosis, Maternal mRNA translation, Fertilization
1. Introduction/overview
In nearly every animal the early embryonic divisions occur in the absence of transcription of the zygotic genome. In human embryos, transcription is not thought to initiate until after two or three embryonic divisions. In organisms in which the embryo develops outside of the mother, rapid embryogenesis is needed to produce motile progeny, necessitating an accelerated division cycle that precludes growth and transcription. Maternal stockpiles of mRNAs, translational machinery components, and nutrients permit early embryogenesis to occur in the absence of zygotic transcription.
This developmental strategy requires that maternal stores be deposited into the oocyte during oogenesis. This is accomplished during an arrest in meiosis, when the immature oocyte is arrested in prophase I of meiosis. Release from this arrest and meiotic progression then occur during oocyte maturation, leading to a secondary meiotic arrest. In most vertebrates, the oocyte completes the first meiotic division and has a secondary arrest at metaphase of meiosis II. In insects, this secondary arrest is in metaphase I. The secondary meiotic arrest is thought to facilitate coupling of the completion of meiosis to fertilization, and it is released by egg activation and fertilization. Thus, developmental cues must impact meiotic progression and regulate deposition of maternal products during oogenesis.
The control of the oocyte-to-embryo transition in Drosophila parallels that of other animals, but Drosophila offers experimental advantages as a model to decipher the regulation of this key developmental event. In addition to the ease of genetic analysis and tools for reverse genetics, the stages of oocyte maturation are morphologically distinct, permitting sufficient quantities to be isolated for biochemical analyses and imaging. Release of the secondary meiotic arrest and promotion of the changes in the proteome and mRNA translation can be triggered by in vitro activation of mature oocytes.
Here we detail recent advances from Drosophila in our understanding of the onset of meiotic division in meiosis, the formation of the specialized meiotic spindle, the metabolic and signaling events accompanying oocyte maturation, the control of egg activation by Ca2+ fluxes, and the consequences of fertilization to activate embryogenesis. Global approaches are discussed that have defined the changes in protein levels, mRNA translation, and polyadenylation that accompany oocyte maturation and egg activation. Ultimately the oocyte-to-embryo transition leads to the activation of expression of the zygotic genome, a step called the maternal-to-zygotic transition (MZT). Many advances have been made in defining the control of this developmental hand off, but these have been extensively reviewed recently, so the maternal-to-zygotic transition is not covered in this review [1–5].
Prior to discussing oocyte maturation, an overview of the late stages of Drosophila oogenesis is warranted. The oocyte develops within an egg chamber in a series of fourteen morphologically distinct stages, and the events of oocyte maturation occur in stages 12–14 (Fig. 1). A single cell out of a group of 16 sister cells, which remain attached by ring canals to share the same cytoplasm, becomes an oocyte, while the other fifteen become supporting nurse cells. The oocyte arrests in prophase I, after homologous chromosomes have paired and are attached physically by chiasmata (the product of a crossover recombination event) or heterochromatin links [6,7]. These locked homolog pairs are called bivalents, and they contain four DNA duplexes, due to the two sister chromatids of each homolog.
Fig. 1.

Overview of Drosophila oogenesis and early development. Drosophila has two ovaries, each of which contains 20–30 ovarioles. Each ovariole is like a production line, where the oocyte develops within an egg chamber in a series of fourteen morphologically distinct stages. A single cell out of a group of 16 sister cells, which remain attached by ring canals to share the same cytoplasm, becomes an oocyte, while the other fifteen become supporting nurse cells (NCs). The developing oocyte is surrounded by a layer of polyploid follicle cells (FCs), which play a role in patterning in early stages and are responsible for depositing the egg shell during late oogenesis. The oocyte arrests in prophase I, and this arrest is released upon oocyte maturation (stages 12–14), with meiosis progressing to a secondary arrest point in metaphase I in mature oocytes. As oocyte maturation proceeds, NCs dump their content into the oocyte and then proceed to undergo cell death. Upon ovulation, the mature oocyte descends into the oviduct, where mechanical forces and hydration induce egg activation. After fertilization in the uterus, the maternal and paternal haploid nuclei fuse and start to undergo thirteen division cycles, characterized by rapid S- and M phases, in a common cytoplasm. If egg activation occurs but the egg is not fertilized, then development is arrested after completion of meiosis, and the four female meiotic products congregate as a polar body. In addition to completion of meiosis, the changes in translation of maternal mRNAs occur during egg activation, independent of fertilization. DNA is represented in blue, spindles in green.
2. Meiosis during oocyte maturation
Oocyte maturation is the process by which the oocyte enters the first meiotic division, releasing the primary meiotic arrest in prophase I. During oocyte maturation, meiosis resumes, with nuclear envelope breakdown (also referred to as germinal vesicle breakdown, GVBD). A specialized meiotic spindle is assembled, and on each homolog of the bivalent the sister-chromatid kinetochores are mono-oriented towards the same pole of the meiotic spindle. Because the two homologs are held together but are attached to microtubules (MTs) from opposite poles, this is a stable configuration present at the metaphase I arrest in the mature stage 14 oocyte.
2.1. Meiotic progression
Progression from the prophase I arrest to metaphase I requires cell cycle regulation to activate the Cyclin B/CDK1 mitotic kinase. The role of conserved regulators in activating Cyclin B (CycB)/CDK1 during Drosophila oocyte maturation has been reviewed [8]. Briefly, the onset of maturation and GVBD are dictated by the timing of CDK1 activation, as in vertebrates, in which active CycB/CDK1 is critical. In Drosophila two other mitotic Cyclins, A and B3, also contribute to oocyte maturation [9]. This requires the Cdc25 phos-phatase TWINE, whose activation is dependent on POLO kinase, whose activity is in turn inhibited in the prophase I-arrested oocyte by MATRIMONY (MTRM) [10,11]. As levels of POLO protein increase [12], it is thought that it exceeds the pool of MTRM by stage 13 of oogenesis, thus triggering maturation. Consistent with this, mutations in Mtrm dominantly cause premature GVBD. Maturation additionally is controlled by Endosulfine (ENDOS) and the Great-wall kinase [11,13]. When phosphorylated by Greatwall, ENDOS can inhibit the PP2A phosphatase [14,15], and this could promote the onset of meiosis. In fact, mutations in endos result in delayed GVBD and failure to progress to metaphase I. In other organisms Greatwall is activated in a feed-forward mechanism by CycB/CDK1, but it is not known whether this is also true in Drosophila oocytes.
The developmental signals responsible for triggering oocyte maturation and how these lead to the activation of POLO and ultimately CycB/CDK1 remains unknown. Technical hurdles have hindered evaluation of known cell cycle components, as it is difficult to inactivate functions specifically in stage 13 or 14 oocytes.Additionally, once the eggshell is deposited in stage 13, the oocyte cannot successfully be treated with many inhibitors.
2.2. Assembly of the oocyte meiotic spindle
The meiotic spindle forms as GVBD occurs, and it is localized near the dorsal surface of the oocyte. This spindle is distinguished from the meiotic spindle in spermatocytes or from mitotic spindles. The microtubule organizing center (MTOC), the centrosome, is composed of a pair of centrioles surrounded by pericentriolar material (PCM) [16]. In most animal oocytes, however, centrioles are lost and the oocyte assembles an acentrosomal spindle, which forms in the absence of centrioles. During Drosophila oogenesis, the centrioles from the nurse cells migrate into the oocyte to form a large MTOC with 60 centrioles that organizes the MTs necessary for localization of patterning proteins and RNAs [17], but all of these centrioles are ultimately lost as oogenesis proceeds.
Recent advances in imaging technologies, in combination with fluorescently tagged protein fusions for key centriole and PCM components, have facilitated analysis of the events leading to centriole loss during Drosophila oogenesis [17]. The PCM starts shrinking during mid-oogenesis (stages 7/8), prior to oocyte maturation, and this correlates with a reduction of POLO levels at the MTOC. Centriole number decreases once oocyte maturation begins, presumably as a consequence of PCM loss [17]. POLO plays a key role in centriole loss in oocytes, as reduction of POLO protein levels by RNAi led to premature loss of centriole markers during stage 10, whereas tethering of POLO to the centriole by fusing it to the PACT centriole protein led to centriole retention even in mature oocytes. Notably, apart from demonstrating POLO protects centrioles, this permitted testing the requirement of centriole loss in the oocyte. Retention of centrioles in mature oocytes resulted in defects during early embryonic divisions [17], supporting the idea that centriole elimination in the oocyte is crucial for ensuring that there are only two centrioles in the fertilized embryo, both derived from the sperm (Fig. 2).
Fig. 2.
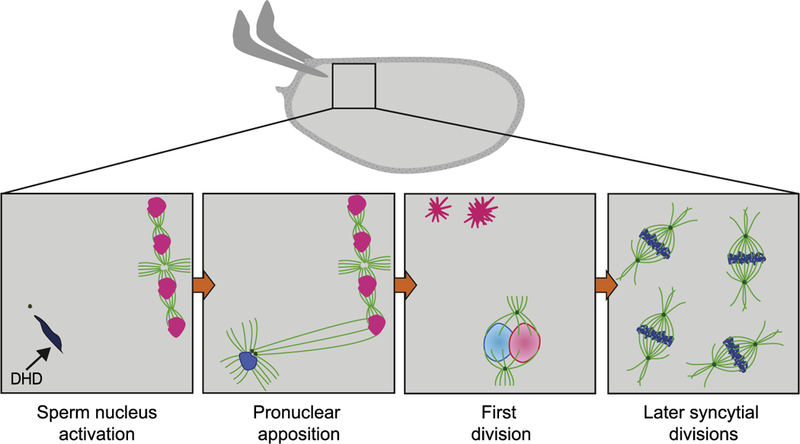
Fertilization and the start of embryonic divisions. The activated egg is fertilized once it reaches the uterus. Insets represent progression of cytoplasmic events following fertilization. First, a single sperm enters the egg through the micropyle, and remains at the anterior of the oocyte (dark blue), as female meiosis is completed in the egg and four meiotic products (pink) are produced. The sperm contributes the centrosomes (green) and paternal haploid genomic content (dark blue) to the embryo. Initially the male pronucleus is in a highly-condensed chromatin state due to the presence of protamines. The sperm nuclear membrane breaks down, and protamines are evicted from the sperm DNA and replaced by histones during sperm nucleus activation, in a process that requires the histone chaperone HIRA (not shown) and the maternally provided thioredoxin DHD (shown). Afterwards, the paternal centrioles and MTOCs (green dots) initiate the formation of a mitotic aster (bright green), which increases in size until it reaches the female meiotic product in the closest proximity. The female pronucleus is then pulled towards the male pronucleus during pronuclear apposition. The first division follows, in which there is no intermixing of the maternal chromosomes (light pink) and paternal chromosomes (light blue) until telophase. The remaining female meiotic products undergo chromosome condensation and remain arrested as polar bodies (dark pink) at the cortex of the egg. Subsequent mitotic divisions occur in a common cytoplasm, or syncytia, starting at the middle of the embryo and then expanding towards the surface with increased nuclear number. The syncytial divisions are driven by maternally provided proteins and mRNAs.
As GVBD occurs in the oocyte, the chromosomes can be observed to bind MTs, serving as the center for spindle organization. This requires a mechanism to produce a bipolar spindle with poles and to ensure bipolar attachment of the homologs to the spindle. The Chromosome Passenger Complex (CPC) plays an important role in meiotic spindle formation and correct chromosome attachment [18,19], with components of the CPC localizing to the middle of the forming spindle. The MT-bundling activity of the kinesin SUBITO also is required for spindle formation [20], and it may act to recruit and stabilize other MT-bundling proteins to the spindle. Some PCM proteins present at the poles may act to taper the poles.
Depletion and mutation of kinetochore proteins have provided critical insights into kinetochore function in meiotic spindle formation [21]. Although the initial formation of the spindle is kinetochore-independent, both the central spindle and the kinetochores become necessary to stabilize the spindle. It is proposed that initial lateral attachments of the MTs to the kinetochore are followed by end-on attachments as homologs attain stable bipolar kinetochore attachments on the spindle. The CPC and other central spindle proteins are thought to facilitate kinetochore-MT binding in the prometaphase belt model, which speculates that the central spindle acts as a belt to concentrate the plus ends of MTs near the kinetochores [19].
2.3. Regulation of gene expression during oocyte maturation
Oocyte maturation occurs in the absence of transcription with stable levels of mRNAs [22]. Hence, the events of maturation must be regulated posttranscriptionally. The enigma of how maternal mRNAs can be loaded into the oocyte and stably maintained for prolonged periods, yet not translated, was explained by the proposal that maternal mRNAs are masked by short poly(A) tails, which both stabilize mRNAs and prevent their translation. Thus, an initiating event of oocyte maturation would be cytoplasmic polyadenylation and translation of selected mRNAs. This indeed has been shown to be the case in Xenopus and mouse, in which polyadenylation leads to translation of mitotic Cyclins that activate Cyclin/CDK and promote the onset of meiosis [23,24].
Recent global analyses have identified the changes in poly(A) tail length and mRNA translation during maturation, from stage 11 through stage 14 oocytes, scoring about 4500 mRNAs [22,25]. These studies revealed that regulation of translation in this developmental window is more complex than simple polyadenylation and unmasking of maternal mRNAs. Hundreds of mRNAs are translationally upregulated, but hundreds are translationally downregulated. These changes in translation are dynamic, with some mRNAs, such as cyclin B and fizzy (Cdc20) mRNAs, being progressively translationally upregulated in stages 11–14, others not upregulated until stage 14, and others being translated in stages 11 and 12 and then downregulated. Poly(A) tail length also dynamically changes during oocyte maturation [22,25], with some mRNAs exhibiting increased poly(A) tail length but others undergoing poly(A) tail shortening. In general, for both up and down regulated mRNAs, translational efficiency correlates with poly(A) length changes. The GLD-2 like cytoplasmic poly(A) polymerase encoded by wispy (wisp) is responsible for poly(A) tail lengthening during oocyte maturation and egg activation [22,25–28].
A complementary study employed quantitative mass spectrometry to measure changes in the levels of each protein between stage 11 and 14 [12]. Approximately 30% of 3400 scored proteins significantly change in levels, with hundreds of proteins increasing and hundreds decreasing. The weak concordance between changes in protein levels and changes in translation efficiency for their mRNAs indicated that protein levels are affected both by mRNA translation and posttranslationally, likely by degradation. Proteins that increase in abundance are those expected to act during the meiotic divisions, as is the case for several MT-associated proteins. Proteins whose activity is not required until the onset of embryogenesis also increase during oocyte maturation, which suggests that it may be necessary to stockpile these proteins prior to egg activation to ensure sufficient quantities for the rapid early embryonic divisions. Components of the DNA replication machinery, centrosomal proteins, and embryonic patterning regulators are examples of proteins in this class.
3. Oocyte metabolism prior to and during maturation
Oogenesis results in the oocyte being stockpiled not only with mRNAs but also with nutrients and organelles. Recent advances have shed light on the regulation of nutrient deposition and uncovered active regulation of mitochondria during Drosophila oogenesis (Fig. 3).
Fig. 3.

Signaling events and metabolic changes during oocyte maturation. Lipids and carbohydrate stores that will serve as energy sources accumulate in a stepwise manner during late oogenesis. At the onset of maturation, lipids are present in the form of droplets (yellow circles) in the cytoplasm. This lipid accumulation is promoted by ecdysone signaling. The insulin signaling pathway is active at this stage, leading to the inactivation of GSK3β . As oocyte maturation proceeds, insulin signaling is no longer present, and GSK3β now is able to regulate changes in carbohydrate metabolism, particularly the accumulation of glycogen (green stars) during maturation. In addition, GSK3β mediates the entry of mitochondria into a quiescent state, characterized by inactivation of the electron transport chain, mitochondrial membrane depolarization, and a change in mitochondrial morphology (depicted). GSK3β also is responsible for activating phosphorylation of the calcipressin SRA (not shown), adding a level of coordination between insulin and the subsequent Ca2+ signaling events at egg activation. Ecdysone signaling acts once again on mature oocytes, this time to stimulate ovulation, which will result in the oocyte proceeding through egg activation. The supporting nurse cells (dark gray) also provide stockpiles of maternal mRNAs, while the oocyte progresses in meiosis to a metaphase I arrest (spindle in green, chromosomes in blue).
3.1. Lipid storage
Accumulation of triglycerides and sterols in the oocyte has been observed in other organisms as well as in Drosophila. These are stored in the form of cytoplasmic lipid droplets in the oocyte [29], and apart from their use in ATP production and membrane lipid biosynthesis during embryogenesis [30], these lipid droplets can have other roles, such as anchoring and storing histones [31]. Accumulation of lipids starts as early as stage 10 of oogenesis, and is regulated in part by the transcription factor SREBP [32]. SREBP regulates the expression of the LDL receptor homolog LpR2, which is required for lipid uptake in the germline [33], in addition to other target genes involved in the uptake and storing of lipids. The stores of lipids accumulated during oogenesis serve as an energy source during embryonic development.
3.2. Glycogen accumulation
Carbohydrate reserves also are amassed during oogenesis and maternally provided to the egg. Together with lipids accumulated earlier in oogenesis, these carbohydrate stores are the energy sources for embryogenesis [34]. The accumulation of carbohydrates occurs in the form of an increase in glycogen levels during late oogenesis, coinciding with the onset of oocyte maturation [35]. The increase in glycogen is accompanied by increases in glycolytic and citric acid cycle intermediates [35], suggesting that flow through these catabolic pathways is redirected towards glycogen anabolism. Consistent with this, mutant egg chambers for the gluconeogenesis component pepck exhibit decreased glycogen levels [35]. However, as the membrane of the oocyte does not become impermeable to small solutes until egg activation, it remains possible that extracellular metabolites might be transported into the oocyte. Thus, remodeling of carbohydrate metabolism could be accompanied by the transport of metabolites for proper glycogen accumulation. The consequences of interfering with the glycogen pathway specifically and its effects on early embryogenesis remain to be determined.
3.3. Mitochondria selection and quiescence
Mitochondria produce most of the ATP in eukaryotic cells and are the exclusive site of oxidative phosphorylation in the cell. Mitochondria contain their own genome or mitochondrial DNA (mtDNA), and mutations in mtDNA can lead to mitochondrial dysfunction and to deleterious effects on organismal health [36]. Mitochondria, and therefore mtDNA, are inherited maternally [36], as paternally derived mitochondria are degraded either during spermatogenesis or, such as in Drosophila, shortly after fertilization [37,38]. Therefore, the state, respiratory activity and quality of mitochondria deposited during oogenesis determine the mitochondria population inherited by the future embryo.
Mitochondrial selection occurs early in oogenesis, so the mitochondrial diversity inherited by the egg has already been established in mature oocytes. Competition between different mtDNAs occurs after germline stem cell (GSC) divisions [39], coinciding with mtDNA replication [40]. Although there is purifying selection against defective mtDNA via competition, some defective genomes are not completely eliminated [39], being presumably maintained by complementation with other mtDNAs in the same organelle or in the population.
Another step of selection determines which mitochondria form part of the pole cells, and thus the future germline. Mitochondria accumulate at the posterior of the oocyte, the future site of primordial germ cell (PGC) formation, following stage 10 and continuing through maturation [41], a process that requires the germ plasm component oskar [41]. Mitochondria from this posteriorly accumulated pool will be later, during embryogenesis, incorporated into the emerging pole cells. Whether specific mitochondrial genomes are selected for this localization based on fitness or if they are selected non-specifically from the population subjected to purifying selection earlier in oogenesis remains unclear.
The respiratory activity of mitochondria also is subject to regulation. It was observed that mitochondria enter a state of quiescence during late oogenesis, and do not regain activity until later in embryonic development [35]. In early oogenesis, when selection occurs, mitochondria exhibit a perinuclear localization and have a strong membrane potential, as measured using the mitochondrial membrane potential dye TMRE. In contrast, following stage 10, mitochondria become disperse, are structurally different, and their membrane becomes depolarized, seemingly through downregulation of most electron transport chain (ETC) complex activity during late oogenesis. The entry of mitochondria into this quiescencent state appears to be regulated by insulin signaling [35], and coincides with the glycogen accumulation and metabolic remodeling occurring during oocyte maturation. Thus, quiescence of mitochondria may be a means to modify the metabolic or oxidative state of oocytes to support the energy requirements of the early embryo.
4. Signaling pathways affecting late oogenesis
Although the cues that trigger the onset of oocyte maturation in Drosophila remain to be identified, steroid and insulin signaling have been demonstrated to affect nutrient deposition into maturing oocytes. Steroid signaling plays a parallel role in Drosophila as in mammals in promoting rupture of the follicle cell layer during ovulation (Fig. 3).
4.1. Ecdysone signaling of late oogenesis events
Signaling through the ecdysone receptor (EcR) and its ligand, the active form of the steroid hormone ecdysone, 20-hydroxyecdysone (20E), is involved in multiple processes during late oogenesis. The ovary is the main source of ecdysone production, with 20E being produced by the follicle cell (FC) layer in the adult ovary [42]. In addition to acting earlier in oogenesis, ecdysone promotes lipid accumulation beginning at stage 10 of oogenesis by activating SREBP. In addition to this local signaling, genetic analysis of a dominant negative EcR gene driven to be expressed solely in neurons revealed that 20E produced by the germline might also modulate feeding behavior in female flies [43]. This has been proposed to be a mechanism through which local and systemic metabolic modulation by ecdysone creates a homeostatic metabolic state, whereby behavioral changes and directed lipid uptake by the germline ensure optimal lipid storage in the oocyte [43].
Apart from this earlier role in lipid storage, ecdysone acts also during ovulation. During ovulation, a mature oocyte from one of the ovarioles enters the lateral oviduct, causing the rupture of the FC layer [44], and coinciding with egg activation [45]. The rupture of the FC layer requires the action of SHADE, which converts ecdysone into the 20E form in the posterior FCs of the egg chamber [46]. The resulting increase in 20E production leads to autocrine activation of a form of EcR, which in turn activates expression of genes in the posterior follicle cells that promote rupture of the FC layer [46]. Additional control of this process comes from activation of OAMB in posterior FCs [47], a receptor for the neurotransmitter octopamine known to regulate ovulation, egg laying and response to male accessory proteins [48,49]. Together, these findings suggest that ecdysone and octopamine signaling are coordinated to control oocyte release during ovulation.
4.2. Insulin signaling
Insulin signaling regulates germline stem cell divisions and many other aspects of egg chamber development [50,51]. Although active throughout early oogenesis, the insulin signaling pathway is shut off in maturing egg chambers to allow for glycogen accumulation. In Drosophila, eight insulilike peptides (dILPs) serve as ligands for the insulin receptors, InR and Lgr3, in target tissues, and the effects of these dILPs vary between tissues [52]. In the ovary, dilp8 and dilp5 are reportedly expressed [52], with dilp5 mRNA detected by in situ hybridization in FCs during mid-oogenesis [53]. The effector kinase AKT, downstream of InR, initially antagonizes Glycogen synthase kinase 3 β (GSK3β ), another downstream component of insulin signaling that promotes glycogen accumulation [35]. In maturing oocytes, AKT activity is decreased and GSK3β becomes active. Premature inactivation of insulin, by InR or akt RNAi, leads to premature glycogen accumulation, whereas GSK3β RNAi leads to decreased glycogen levels in mature oocytes [35], suggesting that insulin signaling is antagonistic to glycogen accumulation. Not surprisingly, GSK3β knockdown during oogenesis leads to defects in early embryogenesis, although it remains unclear whether these defects are due to improper glycogen accumulation or to glycogen-independent GSK3β functions, such as its ability tophosphorylate and activate SRA (see below) [54]. Insulin signaling can interact with other endocrine signaling pathways [55], so it remains possible that the coordinated activities of multiple pathways are exerting tight control on the events underlying oocyte maturation.
5. Egg activation
The mature Drosophila oocyte is arrested at metaphase I. CycB/CDK1 activity is high and necessary for the metaphase I arrest [9]. Mature oocytes can be held in the ovary for prolonged periods if the female has not mated or if environmental conditions are not good. Such held oocytes become dehydrated. As the oocyte passes into the oviduct, dramatic changes occur that are grouped under the heading of egg activation [56]. These include the release of the metaphase I arrest and the completion of meiosis, promoted by the inactivation of CycB/CDK1 through CycB destruction by activation of the APC/C [57,58]. Swelling and hydration of the egg in addition to changes in the cytoskeleton at the cortex occur [56]. As egg activation resets the egg for embryogenesis, it is accompanied by massive changes in gene expression, all controlled posttranscriptionally [59]. This is associated with disassembly of large cytoplasmic RNP granules [60]. In the next sections, we focus on recent advances in our understanding of the molecular regulation of the events of egg activation (Fig. 4).
Fig. 4.
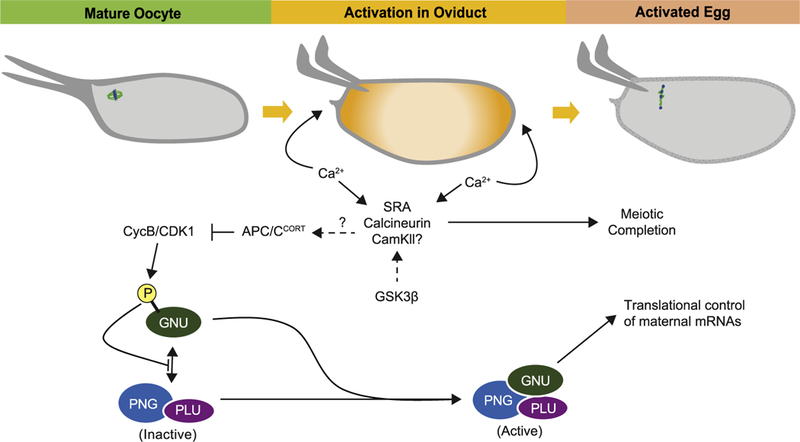
Overview of egg activation in Drosophila. Passage of the oocyte through the oviduct triggers egg activation. During activation, a single wave of calcium moves through the cytoplasm, starting from the poles and moving towards the middle of the egg. The increase in intracellular Ca2+ requires an influx of extracellular Ca2+, presumably via activation of mechanosensitive channels and a release of Ca2+ from ER stores. The increase in intracellular Ca2+ leads to activation in Ca2+ signaling components at egg activation, such as SRA, calcineurin, and potentially CAMKII, which then regulate downstream targets. The preparation of the oocyte for the consequent Ca2+ signaling at egg activation appears to be set up during oocyte maturation, as phosphorylation of SRA by GSK3β during late oogenesis is required for proper egg activation. Completion of meiosis is linked to the activation of Ca2+ signaling in the oocyte, and a potential target of this pathway is the APC/C, whose activation leads to a decrease in CycB/Cdk1 activity and meiotic progression. As high CycB/Cdk1 in the mature oocyte leads to inhibitory phosphorylation of GNU, an activating subunit of the PNG complex, the decrease of CycB/Cdk1 levels at activation allows for dephosphorylation of GNU, which then binds and activates the PNG complex. The PNG complex then mediates translational control of many maternal mRNAs during egg activation. At activation, changes to the cytoskeleton and crosslinking of the vitelline membrane also ensue (not depicted).
5.1. Calcium signaling
As in other organisms, an increase in intracellular Ca2+ occurs during egg activation in Drosophila melanogaster. Many aspects of Ca2+ signaling and roles for Ca2+ effectors, such as Drosophila calcipressin encoded by the sarah (sra) gene, have been previously reviewed [61], so we focus our discussion on recent insights into this event of egg activation. Briefly, as in many other metazoans, an intracellular Ca2+ increase occurs at activation, and this activates a signaling cascade that controls many aspects of egg activation. An influx of exogenous Ca2+ occurs in vivo as the oocyte passes into the oviduct during ovulation [62], coinciding with the time when activation is occurring [45]. In vitro activation of oocytes expressing the intracellular Ca2+ sensor GCAMP revealed that this rise in intracellular Ca2+ occurs in the form of a single wave during activation [62,63]. Moreover, consistent with previous studies showing that mechanical stimuli can elicit activation of Drosophila oocytes [64], the influx of extracellular Ca2+ can be blocked by pharmacologically inhibiting transient receptor potential (TRP) mechanosensitive channels [62]. However, blocking the Ca2+ wave by various manipulations does not impede oocyte swelling, suggesting that hydration of the oocyte is either parallel to or precedes the Ca2+ wave.
Internal stores of Ca2+ released from the endoplasmic reticulum (ER) and the actin cytoskeleton also contribute to the Ca2+ wave. The release of Ca2+ from ER stores occurs through activation of inositol triphosphate receptors (IP3R), and seems to be important for wave propagation instead of initiation. IP3R RNAi oocytes are able to initiate the wave but are unable to sustain it [62]. Interestingly, a study on the dynamics of the ER using a GFP-tagged reporter of the ER resident protein PDI found that ER organization in embryos differs from that in mature oocytes [65]. In contrast to the sheet-like organization in early embryos, the ER in late oogenesis exhibits a reticulated organization that increases in volume as the oocyte matures. The prevalence of different ER structures in other cell types has been suggested to relate to specialized functions of this organelle [66]. Whereas ER organization has been implicated in creating Ca2+ microdomains during embryonic mitosis [67], its structural organization during oogenesis may reflect roles of the ER in protein synthesis, metabolism, and a preparation for the Ca2 wave at activation.
Additionally, a role for the actin cytoskeleton in the Ca2+ flux also has been proposed. Treating oocytes with cytochalasin-D, a potent actin polymerization inhibitor, results in a stuttered Ca2+ increase during in vitro activation [63]. This suggests that the actin cytoskeleton also could act to sustain the propagating Ca2+ wave, although the mechanism by which this occurs is still unclear. It was suggested, however, that the actin cytoskeleton acts in Ca2+ release through interactions with TRP or IP3Rs [63], which could represent a way of coupling the mechanical stimulation that occurs during ovulation to the induction of the Ca2+ wave. This idea is consistent with observations that TRP channels can be modulated by mechanical signals from the cytoskeleton through interactions with MT-and actin-binding proteins [68], or that actin interactions with IP3Rs mediate Ca2+ transients in mammalian cells [69,70].
5.2. Completion of meiosis
In vertebrates, the Ca2+ flux at fertilization leads to inactivation of the APC/C inhibitor Emi2, thus promoting degradation of Securin and CycB to release the meiotic arrest. Although the role of APC/C inhibitors in meiotic arrest in the Drosophila oocyte has not yet been evaluated, one possible target for regulation by Ca2+ is activation of the APC/C via CORTEX (CORT). CORT is a member of the Cdc20 family of APC/C activators that is oocyte specific [57,58]. Whereas both Cdc20s, FIZZY and CORT, are redundant for the metaphase I/anaphase I transition, only CORT is uniquely required for the metaphase II/anaphase II transition [57]. CORT protein is present in mature oocytes, yet apparently inactive until egg activation [58]. Eggs laid by females with amorphic mutations in cort arrest in metaphase II of meiosis and show a failure to reduce CycB levels [58]. This is similar to the phenotype observed in mutants for the Ca2+ effector sra, indicating that CORT could indeed be a Ca2+ target. In addition to triggering the meiotic divisions, APC/CCORT binds and targets many proteins for degradation, which could contribute to regulation of many indirect targets by Ca2+ signaling [12,71].
After meiotic completion, the most internal of the four meiotic products becomes the female pronucleus. The unused meiotic products, the polar bodies, are not budded off through cytokinesis as in vertebrate oocytes (Fig. 2). Thus, in Drosophila an asymmetrical positioning of the meiotic spindle with respect to the oocyte cytoplasm is not required. The first meiotic division occurs parallel to the oocyte plasma membrane, whereas the second occurs perpendicular to the membrane.
5.3. Changes in gene expression at egg activation
The changes in protein levels, mRNA translation, and polyadenylation during egg activation have been globally mapped by comparing mature oocytes to laid, activated eggs. Changes in maternal mRNA translation at egg activation were analyzed by both ribosome footprinting and polysome analysis [59], uncovering extensive changes in mRNA translation. The mature oocyte was found to be translationally active, not quiescent, and at egg activation, hundreds of mRNAs become translationally repressed, while hundreds of other mRNAs become translationally activated.
The translation of approximately 90% of these mRNAs is controlled by the PAN GU (PNG) kinase complex, whose activity recently has been found to be linked to completion of meiosis in the oocyte [59,72]. The PNG complex is composed of a ser/thr kinase catalytic subunit PNG and two activating subunits, GIANT NUCLEI (GNU) and PLUTONIUM (PLU). Although all three subunits are present in mature oocytes, GNU is phosphorylated by CycB/CDK1, and thereby inhibited from binding and activating PNG. Upon egg activation and degradation of CycB, GNU becomes dephosphorylated, and active PNG kinase complex assembles. The activation is only transient, however, as GNU becomes degraded after egg acti-vation in a PNG-dependent manner [72]. PNG phosphorylates GNU and this may target it for degradation. This level of developmental control temporarily restricts PNG complex activity and permits massive translational changes over a narrow time window. This regulation is coordinated with meiotic completion via the influence of CycB/CDK1 on PNG complex activation.
Pronounced changes in poly(A) tail lengths also occur, and egg activation is the developmental period when poly(A) tail length is most tightly coupled to translational efficiency [22]. Coupling persists during the first three hours of embryogenesis, but the two processes become unlinked as zygotic transcription initiates and gene expression is no longer controlled posttranscriptionally.
Polyadenylation at egg activation is WISP-dependent, with nearly every mRNA showing a reduction of poly(A) tail length in wisp mutants [22,25,27]. Exceptions are the mRNAs for ribosomal proteins. Strikingly, the relationship between translational efficiency and poly(A) tail length is not different between wild-type and wisp mutant laid eggs, implying that selective poly(A)-tail shortening is what primarily specifies translational changes during egg activation. Given that wisp mutant eggs have defective meiotic divisions and arrest following activation it cannot, however, be the case that poly(A) tail lengthening is completely dispensable [26,28].
At egg activation, many proteins increase in abundance, whereas many others decrease [59]. Among the proteins whose levels increase are those needed for early embryonic patterning, division, and zygotic gene expression. Proteins whose levels decrease do not fall into specific GO classes, but one interesting group (Fig. 5) is proteins whose levels increase at maturation but then decrease at activation [12]. CORT belongs to this group of proteins, pointing to a hand-off of regulated proteolysis to other APC/C forms or to SCF. Another example is the oocyte-specific thioredoxin encoded by the deadhead (dhd) gene [73], hinting that changes in redox regulation may be critical at this time. Like DHD and CORT, many proteins in this group are likely needed for meiosis or other aspects of late oogenesis, and continued presence of these proteins could be detrimental to embryogenesis. This has been shown to be the case for MTRM, the POLO inhibitor, because if MTRM is not degraded at egg activation defects in the embryonic mitotic divisions result [71].
Fig. 5.

Developmentally regulated protein level changes during the Drosophila oocyte-to-embryo transition. The oocyte is transcriptionally silent during oocyte maturation and egg activation, so control of gene regulation occurs at a posttranscriptional level. Changes to the proteome can result from changes in mRNA translation or protein degradation. A comprehensive quantitative analysis of protein level changes during oocyte maturation and egg activation uncovered subsets of proteins whose changes suggest developmental regulation. 421 proteins, Group I (purple), accumulate during maturation and remain constant in levels after activation. This group includes the translational regulators PNG and PLU, in addition to most MCMs (MCM2–3 and MCM5–7, with the exception of MCM4) and YA, a protein necessary for the first mitosis. A similar group of 43 proteins (not shown), which includes DNA polymerase and ORC subunits, accumulates during maturation and shows a further increase in levels after egg activation. Both of these groups suggest that the drivers of the early mitotic divisions are set up during oocyte maturation. Group II (red) includes 66 proteins that are upregulated during maturation and then decrease in levels at egg activation, a pattern consistent with roles in late oogenesis, maturation, or egg activation. These proteins likely require downregulation at egg activation because they may be detrimental to mitosis and early embryogenesis. This group includes the PNG complex subunit GNU, the thioredoxin DHD and GEMININ. Another group of 117 proteins, Group III (blue), remains constant in levels during maturation but is upregulated at egg activation, implying a role for these proteins in events post-activation. This group includes STIM, the SCF subunit LIN19 and the transcription factor STAT92E. The Y-axis represents the relative protein levels, and the X-axis represents developmental stage in each representative diagram. The dotted line shows when egg activation occurs.
A comparison of the proteome and mRNA translation datasets revealed that increased mRNA translation can account for increased protein levels, but translational inhibition fails to account for most decreases in protein abundance [59]. These discrepancies between changes in protein levels and mRNA translation highlight the importance of posttranslational control during egg activation and point to a crucial role for proteolysis. As there also are proteins that are robustly activated for translation yet whose protein levels do not increase, it appears that increased translation is needed to replace proteins that were present in oocytes but became degraded at egg activation, possibly as a mechanism to reset the proteome.
6. Fertilization and early development
Fertilization marks the final phase of the oocyte-to-embryo transition, as the maternal and paternal DNA contributions are combined to generate a diploid zygotic genome. The merger of the maternal and paternal chromosomes occurs in telophase of the first mitotic division, and this is followed by rapid, synchronous mitotic divisions. As expression from the zygotic genome does not begin until the maternal-to-zygotic transition, the early embryonic divisions are controlled by maternal stockpiles of mRNAs and proteins.
6.1. Redox state and activation of sperm chromatin
The completion of female meiosis and other egg activation events occur independently of sperm entry, but the onset of embryonic divisions and development is dependent on fertilization. Many aspects of fertilization and early development have been previously reviewed [74], so we focus on recent insights into this intricate process. While egg activation occurs during the descent of the oocyte through the oviduct, fertilization occurs in the uterus, with the release of a single sperm from the spermatheca and its entry into the egg cytoplasm through the micropyle at the anterior of the egg. The sperm provides two exclusive elements to the new embryo: the paternal haploid DNA content and a single centriole (Fig. 2). Sperm entry is coordinated with completion of meiosis II, such that the most proximal female pronucleus migrates towards the male pronucleus, leading to pronuclear apposition and the start of the first mitotic division.
Following sperm entry, the male pronucleus is activated, the protamines are evicted from sperm chromatin and exchanged for histones in a process that depends on maternally provided factors, such as the histone chaperone HIRA [74] and DHD. Eggs laid by dhd mutant mothers arrest with sperm chromatin that fails to decondense [75,76], the result of a delay in protamine eviction and exchange for histones. Additionally, the female pronucleus fails to migrate towards the male pronucleus, and the embryos begin to develop as haploid [75]. The sperm decondensation phenotype can be rescued with wild-type DHD but not with a mutant DHD in which one of two catalytic cysteines is mutated to create a substrate trap [75], consistent with redox function being required for sperm decondensation. Moreover, whereas oxidation of protamines can lead to their oligomerization, adding DHD leads to protamine reduction and their eviction from DNA [76]. Thus, DHD may regulate sperm chromatin decondensation via reduction of sulfide-bridges between protamines, though this regulation may be indirect, either by activation of a disulfide reductase specialized for protamine reduction or regulation of a global redox state by DHD during egg activation.
6.2. Start of embryonic divisions
After fertilization and pronuclear apposition, the embryo is set to start development. During Drosophila early embryogenesis, the embryo undergoes 13 rounds of rapid, synchronized nuclear division cycles in a common cytoplasm, leading to the formation of a syncytial blastoderm that contains 6000 nuclei prior to cellular blastoderm formation. These early divisions lack gap phases, instead being cycles of S-and M-phases driven by fluctuations of high CDK1 activity and relying on maternal mRNAs and proteins [5]. These divisions occur without significant zygotic transcription, as the embryo is largely transcriptionally silent until the first wave of zygotic transcription at the onset of the maternal-to-zygotic tran-sition (MZT) [4]. S-phase is gradually lengthened during cycles 11 through 13, slowing the overall time for each nuclear division, with a pronounced G2 phase being added during cellularization, when the zygotic genome becomes fully activated for transcription and maternal mRNAs are degraded. The following three divisions after cellularization occur in mitotic domains regulated by patterning genes [5].
The start of the embryonic divisions depends on specialized regulators, provided maternally and activated at egg activation. One example is YOUNG ARREST (YA), a nuclear protein of unknown molecular function, which is activated after egg activation and is necessary for the first mitotic division [77]. Another example is the PNG complex. Loss-of-function mutations for any of the subunits of the complex result in arrested embryos in which DNA replication occurs without nuclear division [78]. This reflects the requirement for PNG activity to promote cycB mRNA translation, thereby restoring CycB protein levels after meiotic completion to restart mitosis in the embryo [79]. PNG also affects the later MZT, as it is required for translation of smaug mRNA [80]. SMAUG protein then promotes deadenylation and degradation of maternal mRNAs as zygotic transcription initiates [59,81]. PNG function addition-ally is needed for degradation at the MZT of several maternally deposited RNA-binding proteins that repress translation, although it is not known whether the effect of PNG is direct [82]. At the oocyte-to-embryo transition, the coordinated activity of YA, PNG and other known and yet to be uncovered regulators, together with their downstream targets, ensure a proper transition into embryogenesis.
Recent work points to the importance of metabolic regulation during early Drosophila development. The SHMT enzyme, required for synthesis of purines, thymidine nucleotides, and S-adenosyl methionine, is necessary for divisions beyond cycle 13 [83]. It is postulated that this is the time when maternal stock- piles of these metabolites become depleted. Analysis of dNTP levels in early embryos, controlled by Ribonucleotide Reductase (RNR), demonstrated that maternal stockpiles are not sufficient to support accurate divisions beyond cycle 11. It appears that RNR becomes allosterically activated as levels of maternally-provided dNTPs begin to drop as DNA replication proceeds, thereby providing the dNTPs needed for additional divisions [84]. Future studies of metabolism during early embryogenesis are likely to uncover additional regulatory mechanisms.
7. Parallels to other organisms
Hormone signaling is a conserved featured of oocyte development. Gonadotrophins and sex steroids in mammals and frogs, and the steroid hormone ecdysone in Drosophila control various aspects of oogenesis. In Drosophila, apart from acting in early oogenesis [85], ecdysone also acts later in oogenesis to control changes in lipid metabolism [43,86] and ovulation [46]. In contrast to vertebrates, where a surge in luteinizing hormone induces oocyte maturation, the signal for maturation in flies has not been deter- mined and a role for hormone signaling in this process is unclear [8]. Similarly to mammalian ovulation, Drosophila ovulation requires the rupture of the FC layer [46] and generation of a corpus luteum [44]. In both mammals and flies, hormone signaling may serve to modulate oogenesis by an organismal regulation of metabolism and behavior.
Signaling by insulin has been proposed as an evolutionary regulator of female reproduction. Components of this pathway are highly conserved, and aspects controlled by insulin, such as early germline stem cell divisions and local and systemic metabolic regulation, are similar in both fly and vertebrate oocyte development [50,86,87]. Control of glycogen accumulation during late oogenesis by insulin also was described in Xenopus oocytes [35]. Interestingly, in frogs, fish and worms, the ERK branch of the insulin signaling pathway can induce oocyte maturation independently of hormone signaling [50]. ERK is a downstream target of MOS, and although the fly homolog dmos is not essential in Drosophila [88] and the RAS/ERK branch downstream of insulin has not been evaluated in flies [50], it remains possible that components of this branch may act to control oocyte maturation. The insulin and ecdysone pathways can interact, and cooperation between insulin and gonadotrophin signaling to modulate oocyte maturation has been documented in mammals [50]. Hence, it remains possible that cooperation between insulin and ecdysone, perhaps synergistically with another signaling pathway, could be underlying oocyte maturation in Drosophila.
Parallels between Drosophila and C. elegans highlight a crucial role for protein kinases in controlling mRNA translation and the proteome at the oocyte-to-embryo transition. The MBK-2 kinase in C. elegans controls proteolysis through the SCF E3 ubiquitin ligase to degrade oocyte proteins that could impede embryogenesis [89]. It also affects RNP granule dynamics and thus could influence maternal mRNA translation [90]. Interestingly, activation of MBK- 2 depends on the activation of APC/C and is therefore linked to the completion of meiosis [91]. The conserved strategy of linking alterations in the proteome necessary for the oocyte-to-embryo transition to the meiotic cell cycle via the activation of protein kinases raises the question of whether such a mechanism may also be employed in vertebrates.
8. Conclusions
The advances in research on the oocyte-to-embryo transition in Drosophila in the past few years have significantly enhanced our understanding while opening new areas and questions that merit further investigation. The mechanisms of nutrient deposition during Drosophila oogenesis and the influence of insulin and ecdysone signaling now are more fully understood. Recent studies have uncovered both selection mechanisms for mitochondria and regulation of mitochondrial activity. The discoveries of the dependence of activation and meiotic completion on a Ca2+wave and signaling pathway are a major advance in our understanding of the oocyte-to-embryo transition in Drosophila. The global delineation of mRNA translation changes accompanying egg activation revealed the central role that the PNG complex plays in controlling mRNA translation and how developmental control of this complex restricts this regulation of mRNA translation to a narrow developmental window. Comparison of the proteome and translatome changes at maturation and activation implicate a major role for proteolysis in influencing these developmental events.
Important areas still to be explored include: 1) finding the signals that lead to oocyte maturation and the onset of the meiotic divisions; 2) defining the role, regulation and mRNA components of RNP granules during maturation and egg activation; 3) identifying the key targets of Ca2+signaling responsible for the myriad of events occurring at egg activation; 4) elucidating the mechanism through which PNG can globally alter the translation of hundreds of mRNAs; and 5) delineating the control of metabolic changes in early embryogenesis. Future work in the field to address these issues promises a deeper understanding into the complexity of the Drosophila oocyte-to-embryo transition and insights into regulation of this transition in other animals.
Acknowledgments
We apologize to our colleagues whose work could not be cited due to space limitations. We thank Jessica Von Stetina, Peter Nicholls and Gabriel Neurohr for their helpful comments on the manuscript. This work was supported by NIH grant GM118098 to TO-W. TO-W is an American Cancer Society Research Professor.
References
- [1].Laver JD, Marsolais AJ, Smibert CA, Lipshitz HD, Regulation and function of maternal gene products during the maternal-to-zygotic transition in Drosophila, Curr. Top. Dev. Biol 113 (2015) 43–84, 10.1016/bs.ctdb.2015.06.007. [DOI] [PubMed] [Google Scholar]
- [2].Harrison MM, Eisen MB, Transcriptional activation of the zygotic genome in Drosophila, Curr. Top. Dev. Biol 113 (2015) 85–112, 10.1016/bs.ctdb.2015.07.028. [DOI] [PubMed] [Google Scholar]
- [3].Blythe SA, Wieschaus EF, Coordinating cell cycle remodeling with transcriptional activation at the Drosophila MBT, Curr. Top. Dev. Biol 113 (2015) 113–148, 10.1016/bs.ctdb.2015.06.002. [DOI] [PubMed] [Google Scholar]
- [4].Tadros W, Lipshitz HD, The maternal-to-zygotic transition: a play in two acts, Development 136 (2009) 3033–3042, 10.1242/dev.033183. [DOI] [PubMed] [Google Scholar]
- [5].Farrell JA, O’Farrell PH, From egg to gastrula: how the cell cycle is remodeled during the Drosophila mid-blastula transition, Annu. Rev. Genet 48 (2014) 269–294, 10.1146/annurevgenet-111212-133531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].McKim KS, Jang JK, Theurkauf WE, Hawley RS, Mechanical basis of meiotic metaphase arrest, Nature 362 (1993) 364–366, 10.1038/362364a0. [DOI] [PubMed] [Google Scholar]
- [7].Hughes SE, Gilliland WD, Cotitta JL, Takeo S, Collins KA, Hawley RS, Heterochromatic threads connect oscillating chromosomes during prometaphase I in Drosophila oocytes, PLoS Genet 5 (2009) e1000348, 10.1371/journal.pgen.1000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Von Stetina JR, Orr-Weaver TL, Orr Weaver TL, Developmental control of oocyte maturation and egg activation in metazoan models, Cold Spring Harb. Perspect. Biol 3 (2011) a005553, 10.1101/cshperspect.a005553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bourouh M, Dhaliwal R, Rana K, Sinha S, Guo Z, Swan A, Distinct and overlapping requirements for cyclins A, B and B3 in Drosophila female meiosis, G3 (Bethesda) 58 (2016) 3711–3724, 10.1534/g3.116.033050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Xiang Y, Takeo S, Florens L, Hughes SE, Huo LJ, Gilliland WD, Swanson SK, Teeter K, Schwartz JW, Washburn MP, Jaspersen SL, Hawley RS, The inhibition of polo kinase by matrimony maintains G2 arrest in the meiotic cell cycle, PLoS Biol 5 (2007) 2831–2846, 10.1371/journal.pbio.0050323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Von Stetina JR, Tranguch S, Dey SK, Lee LA, Cha B, Drummond-Barbosa D, a-Endosulfine is a conserved protein required for oocyte meiotic maturation in Drosophila, Development 135 (2008) 3697–3706, 10.1242/dev.025114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kronja I, Whitfield ZJ, Yuan B, Dzeyk K, Kirkpatrick J, Krijgsveld J, Orr-Weaver TL, Quantitative proteomics reveals the dynamics of protein changes during Drosophila oocyte maturation and the oocyte-to-embryo transition, Proc. Natl. Acad. Sci. U. S. A 111 (2014) 16023–16028, 10.1073/pnas.1418657111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Archambault V, Zhao X, White-Cooper H, Carpenter ATC, Glover DM, Mutations in Drosophila Greatwall/scant reveal its roles in mitosis and meiosis and interdependence with polo kinase, PLoS Genet 3 (2007) 2163–2179, 10.1371/journal.pgen.0030200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Williams BC, Filter JJ, Blake-Hodek KA, Wadzinski BE, Fuda NJ, Shalloway D, Goldberg ML, Greatwall-phosphorylated endosulfine is both an inhibitor and a substrate of PP2A-B55 heterotrimers, eLife 2014 (2014) e01695, 10.7554/eLife.01695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Vigneron S, Robert P, Hached K, Sundermann L, Charrasse S, Labbé JC, Castro A, Lorca T, The master greatwall kinase, a critical regulator of mitosis and meiosis, Int. J. Dev. Biol 60 (2016) 245–254, 10.1387/ijdb.160155tl. [DOI] [PubMed] [Google Scholar]
- [16].Fu J, Hagan IM, Glover DM, The centrosome and its duplication cycle, Cold Spring Harb. Perspect. Biol 7 (2015) a015800, 10.1101/cshperspect.a015800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Pimenta-Marques A, Bento I, Lopes CAM, Duarte P, Jana SC, Bettencourt-Dias M, A mechanism for the elimination of the female gamete centrosome in Drosophila melanogaster, Science 353 (2016), 10.1126/science.aaf4866(80)aaf4866-aaf4866. [DOI] [PubMed] [Google Scholar]
- [18].Radford SJ, Jang JK, McKim KS, The chromosomal passenger complex is required for meiotic acentrosomal spindle assembly and chromosome biorientation, Genetics 192 (2012) 417–429, 10.1534/genetics.112.143495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Radford SJ, Nguyen AL, Schindler K, McKim KS, The chromosomal basis of meiotic acentrosomal spindle assembly and function in oocytes, Chromosoma 126 (2017) 351–364, 10.1007/s00412-016-0618-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Radford SJ, Go AMM, McKim KS, Cooperation between kinesin motors promotes spindle symmetry and chromosome organization in oocytes, Genetics 205 (2017) 517–527, 10.1534/genetics.116.194647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Radford SJ, Hoang TL, Głuszek AA, Ohkura H, McKim KS, Lateral and end-on kinetochore attachments are coordinated to achieve Biorientation in Drosophila oocytes, PLoS Genet 11 (2015) e1005605, 10.1371/journal.pgen.1005605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Eichhorn SW, Subtelny AO, Kronja I, Kwasnieski JC, Orr-Weaver TL, Bartel DP, mRNA poly(A)-tail changes specified by deadenylation broadly reshape translation in Drosophila oocytes and early embryos, eLife 5 (2016) 714–724, 10.7554/eLife.16955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Richter JD, CPEB: a life in translation, Trends Biochem. Sci 32 (2007) 279–285, 10.1016/j.tibs.2007.04.004. [DOI] [PubMed] [Google Scholar]
- [24].Weill L, Belloc E, Bava F-A, Méndez R, Translational control by changes in poly(A) tail length: recycling mRNAs, Nat. Struct. Mol. Biol 19 (2012) 577–585, 10.1038/nsmb.2311. [DOI] [PubMed] [Google Scholar]
- [25].Lim J, Lee M, Son A, Chang H, Kim VN, mTAIL-seq reveals dynamic poly(A) tail regulation in oocyte-to-embryo development, Genes Dev 30 (2016) 1671–1682, 10.1101/gad.284802.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Benoit P, Papin C, Kwak JE, Wickens M, Simonelig M, PAP- and GLD-2-type poly(A) polymerases are required sequentially in cytoplasmic polyadenylation and oogenesis in Drosophila, Development 135 (2008) 1969–1979, 10.1242/dev.021444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Cui J, Sartain CV, Pleiss JA, Wolfner MF, Cytoplasmic polyadenylation is a major mRNA regulator during oogenesis and egg activation in Drosophila, Dev. Biol 383 (2013) 121–131, 10.1016/j.ydbio.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Cui J, Sackton KL, Horner VL, Kumar KE, Wolfner MF, Wispy, the Drosophila homolog of GLD-2, is required during oogenesis and egg activation, Genetics 178 (2008) 2017–2029, 10.1534/genetics.107.084558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Welte MA, Proteins under new management: lipid droplets deliver, Trends Cell Biol 17 (2007) 363–369, 10.1016/j.tcb.2007.06.004. [DOI] [PubMed] [Google Scholar]
- [30].Sieber MH, Thummel CS, The DHR96 nuclear receptor controls triacylglycerol homeostasis in Drosophila, Cell Metab 10 (2009) 481–490, 10.1016/j.cmet.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Li Z, Thiel K, Thul PJ, Beller M, Kühnlein RP, Welte MA, Lipid droplets control the maternal histone supply of Drosophila embryos, Curr. Biol 22 (2012) 2104–2113, 10.1016/j.cub.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sieber MH, Thummel CS, Coordination of triacylglycerol and cholesterol homeostasis by DHR96, Cell Metab 10 (2012) 481–490, 10.1016/j.cmet.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Parra-Peralbo E, Culi J, Drosophila lipophorin receptors mediate the uptake of neutral lipids in oocytes and imaginal disc cells by an endocytosis-independent mechanism, PLoS Genet 7 (2011) e1001297, 10.1371/journal.pgen.1001297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tennessen JM, Bertagnolli NM, Evans J, Sieber MH, Cox J, Thummel CS, Coordinated metabolic transitions during Drosophila embryogenesis and the onset of aerobic glycolysis, G3 (Bethesda) 4 (2014) 839–850, 10.1534/g3.114.010652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sieber MH, Thomsen MB, Spradling AC, Electron transport chain remodeling by GSK3 during oogenesis connects nutrient state to reproduction, Cell 164 (2016) 420–432, 10.1016/j.cell.2015.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Stewart JB, Chinnery PF, The dynamics of mitochondrial DNA heteroplasmy: implications for human health and disease, Nat. Rev. Genet 16 (2015) 530–542, 10.1038/nrg3966. [DOI] [PubMed] [Google Scholar]
- [37].Sato M, Sato K, Maternal inheritance of mitochondrial DNA by diverse mechanisms to eliminate paternal mitochondrial DNA, Biochim. Biophys. Acta—Mol. Cell Res 1833 (2013) 1979–1984, 10.1016/j.bbamcr.2013.03.010. [DOI] [PubMed] [Google Scholar]
- [38].Politi Y, Gal L, Kalifa Y, Ravid L, Elazar Z, Arama E, Paternal mitochondrial destruction after fertilization is mediated by a common endocytic and autophagic pathway in Drosophila, Dev. Cell 29 (2014) 305–320, 10.1016/j.devcel.2014.04.005. [DOI] [PubMed] [Google Scholar]
- [39].Ma H, Xu H, O’Farrell PH, Transmission of mitochondrial mutations and action of purifying selection in Drosophila melanogaster, Nat. Genet 46 (2014) 393–397, 10.1038/ng.2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hill JH, Chen Z, Xu H, Selective propagation of functional mitochondrial DNA during oogenesis restricts the transmission of a deleterious mitochondrial variant, Nat. Genet 46 (2014) 389–392, 10.1038/ng.2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hurd TR, Herrmann B, Sauerwald J, Sanny J, Grosch M, Lehmann R, Long oskar controls mitochondrial inheritance in Drosophila melanogaster, Dev. Cell 39 (2016) 560–571, 10.1016/j.devcel.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Uryu O, Ameku T, Niwa R, Recent progress in understanding the role of ecdysteroids in adult insects: germline development and circadian clock in the fruit fly Drosophila melanogaster, Zool. Lett 1 (2015) 32, 10.1186/s40851-015-0031-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Sieber MH, Spradling AC, Steroid signaling establishes a female metabolic state and regulates SREBP to control oocyte lipid accumulation, Curr. Biol 25 (2015) 993–1004, 10.1016/j.cub.2015.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Deady LD, Shen W, Mosure SA, Spradling AC, Sun J, Matrix metalloproteinase 2 is required for ovulation and corpus luteum formation in Drosophila, PLoS Genet 11 (2015) e1004989, 10.1371/journal.pgen.1004989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Heifetz Y, Yu J, Wolfner MF, Ovulation triggers activation of Drosophila oocytes, Dev. Biol 234 (2001) 416–424, 10.1006/dbio.2001.0246. [DOI] [PubMed] [Google Scholar]
- [46].Knapp E, Sun J, Steroid signaling in mature follicles is important for Drosophila ovulation, Proc. Natl. Acad. Sci. U. S. A 114 (2017) 699–704, 10.1073/pnas.1614383114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Deady LD, Sun J, A follicle rupture assay reveals an essential role for follicular adrenergic signaling in Drosophila ovulation, PLoS Genet 11 (2015) e1005604, 10.1371/journal.pgen.1005604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Rubinstein CD, Wolfner MF, Drosophila seminal protein ovulin mediates ovulation through female octopamine neuronal signaling, Proc. Natl. Acad. Sci. U. S. A 110 (2013) 17420–17425, 10.1073/pnas.1220018110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Lee HG, Seong CS, Kim YC, Davis RL, Han KA, Octopamine receptor OAMB is required for ovulation in Drosophila melanogaster, Dev. Biol 264 (2003) 179–190, 10.1016/j.ydbio.2003.07.018. [DOI] [PubMed] [Google Scholar]
- [50].Das D, Arur S, Conserved insulin signaling in the regulation of oocyte growth, development, and maturation, Mol. Reprod. Dev 84 (2017) 444–459, 10.1002/mrd.22806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Drummond-Barbosa D, Spradling AC, Stem cells and their progeny respond to nutritional changes during Drosophila oogenesis, Dev. Biol 231 (2001) 265–278, 10.1006/dbio.2000.0135. [DOI] [PubMed] [Google Scholar]
- [52].Nässel DR, Kubrak OI, Liu Y, Luo J, Lushchak OV, Factors that regulate insulin producing cells and their output in Drosophila, Front. Physiol 4 (September) (2013) 252, 10.3389/fphys.2013.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Ikeya T, Galic M, Belawat P, Nairz K, Hafen E, Nutrient-dependent expression of insulin-like peptides from neuroendocrine cells in the CNS contributes to growth regulation in Drosophila, Curr. Biol 12 (2002) 1293–1300, 10.1016/S0960-9822(02)01043-6. [DOI] [PubMed] [Google Scholar]
- [54].Takeo S, Swanson SK, Nandanan K, Nakai Y, Aigaki T, Washburn MP, Florens L, Hawley RS, Shaggy/glycogen synthase kinase 3 and phosphorylation of Sarah/regulator of calcineurin are essential for completion of Drosophila female meiosis, Proc. Natl. Acad. Sci. U. S. A 109 (2012) 6382–6389, 10.1073/pnas.1120367109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Nässel DR, Vanden Broeck J, Insulin/IGF signaling in Drosophila and other insects: factors that regulate production, release and post-release action of the insulin-like peptides, Cell. Mol. Life Sci 73 (2016) 271–290, 10.1007/s00018-015-2063-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Horner VL, Wolfner MF, Transitioning from egg to embryo: triggers and mechanisms of egg activation, Dev. Dyn 237 (2008) 527–544, 10.1002/dvdy.21454. [DOI] [PubMed] [Google Scholar]
- [57].Swan A, Schupbach T, The Cdc20 (Fzy)/Cdh1-related protein, Cort, cooperates with Fzy in cyclin destruction and anaphase progression in meiosis I and II in Drosophila, Development 134 (2007) 891–899, 10.1242/dev.02784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Pesin JA, Orr-Weaver TL, Developmental role and regulation of cortex, a meiosis-specific anaphase-promoting complex/cyclosome activator, PLoS Genet 3 (2007) e202, 10.1371/journal.pgen.0030202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Kronja I, Yuan B, Eichhorn SW, Dzeyk K, Krijgsveld J, Bartel DP, Orr-Weaver TL, Widespread changes in the posttranscriptional landscape at the Drosophila oocyte-to-embryo transition, Cell Rep 7 (2014) 1495–1508, 10.1016/j.celrep.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Weil TT, Parton RM, Herpers B, Soetaert J, Veenendaal T, Xanthakis D, Dobbie IM, Halstead JM, Hayashi R, Rabouille C, Davis I, Drosophila patterning is established by differential association of mRNAs with P bodies, Nat. Cell Biol 14 (2012) 1305–1315, 10.1038/ncb2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Sartain CV, Wolfner MF, Calcium and egg activation in Drosophila, Cell Calcium 53 (2013) 10–15, 10.1016/j.ceca.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Kaneuchi T, Sartain CV, Takeo S, Horner VL, Buehner NA, Aigaki T, Wolfner MF, Calcium waves occur as Drosophila oocytes activate, Proc. Natl. Acad. Sci. U. S. A 112 (2015) 791–796, 10.1073/pnas.1420589112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].York-Andersen AH, Parton RM, Bi CJ, Bromley CL, Davis I, Weil TT, A single and rapid calcium wave at egg activation in Drosophila, Biol. Open 4 (2015) 553–560, 10.1242/bio.201411296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Horner VL, Wolfner MF, Mechanical stimulation by osmotic and hydrostatic pressure activates Drosophila oocytes in vitro in a calcium-dependent manner, Dev. Biol 316 (2008) 100–109, 10.1016/j.ydbio.2008.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Bobinnec Y, Marcaillou C, Morin X, Debec A, Dynamics of the endoplasmic reticulum during early development of Drosophila melanogaster, Cell Motil. Cytoskelet 54 (2003) 217–225, 10.1002/cm.10094. [DOI] [PubMed] [Google Scholar]
- [66].Schwarz DS, Blower MD, The endoplasmic reticulum: structure, function and response to cellular signaling, Cell. Mol. Life Sci 73 (2016) 79–94, 10.1007/s00018-015-2052-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Parry H, McDougall A, Whitaker M, Endoplasmic reticulum generates calcium signalling microdomains around the nucleus and spindle in syncytial Drosophila embryos, Biochem. Soc. Trans 34 (2006) 385–388, 10.1042/BST0340385. [DOI] [PubMed] [Google Scholar]
- [68].Smani T, Dionisio N, López JJ, Berna-Erro A, Rosado JA, Cytoskeletal and scaffolding proteins as structural and functional determinants of TRP channels, Biochim. Biophys. Acta—Biomembr 1838 (2014) 658–664, dx.doi.org/10.1016/j.bbamem.2013.01.009. [DOI] [PubMed] [Google Scholar]
- [69].Turvey MR, Fogarty KE, Thorn P, Inositol (1,4,5)-trisphosphate receptor links to filamentous actin are important for generating local Ca2+ signals in pancreatic acinar cells, J. Cell Sci 118 (2005) 971–980, 10.1242/jcs.01693. [DOI] [PubMed] [Google Scholar]
- [70].Foskett JK, White C, Cheung K-H, Mak D-OD, Inositol trisphosphate receptor Ca2++ release channels, Phys. Rev 87 (2007) 593–658, 10.1152/physrev.00035.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Whitfield ZJ, Chisholm J, Hawley RS, Orr-Weaver TL, A meiosis-specific form of the APC/C promotes the oocyte-to-embryo transition by decreasing levels of the Polo kinase inhibitor Matrimony, PLoS Biol 11 (2013) e1001648, 10.1371/journal.pbio.1001648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Hara M, Petrova B, Orr-Weaver TL, Control of PNG kinase, a key regulator of mRNA translation, is coupled to meiosis completion at egg activation, eLife 6 (2017) e22219, 10.7554/eLife.22219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Salz HK, Flickinger TW, Mittendorf E, Pellicena-Palle A, Petschek JP, Albrecht EB, The Drosophila maternal effect locus deadhead encodes a thioredoxin homolog required for female meiosis and early embryonic development, Genetics 136 (1994) 1075–1086, doi:PMC1205864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Loppin B, Dubruille R, Horard B, The intimate genetics of Drosophila fertilization, Open Biol 5 (2015) 150076, 10.1098/rsob.150076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Tirmarche S, Kimura S, Dubruille R, Horard B, Loppin B, Unlocking sperm chromatin at fertilization requires a dedicated egg thioredoxin in Drosophila, Nat. Commun 7 (2016) 13539, 10.1038/ncomms13539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Emelyanov AV, Fyodorov DV, Thioredoxin-dependent disulfide bond reduction is required for protamine eviction from sperm chromatin, Genes Dev 30 (2016) 2651–2656, 10.1101/gad.290916.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Sackton KL, Lopez JM, Berman CL, Wolfner MF, YA is needed for proper nuclear organization to transition between meiosis and mitosis in Drosophila, BMC Dev. Biol 9 (2009) 43, 10.1186/1471-213X-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Fenger DD, Carminati JL, Burney-Sigman DL, Kashevsky H, Dines JL, Elfring LK, Orr-Weaver TL, PAN GU: a protein kinase that inhibits S-phase and promotes mitosis in early Drosophila development, Development 127 (2000) 4763–4774. [DOI] [PubMed] [Google Scholar]
- [79].Vardy L, Orr-Weaver TL, The Drosophila PNG kinase complex regulates the translation of cyclin B, Dev. Cell 12 (2007) 157–166, 10.1016/j.devcel.2006.10.017. [DOI] [PubMed] [Google Scholar]
- [80].Tadros W, Goldman AL, Babak T, Menzies F, Vardy L, Orr-Weaver T, Hughes TR, Westwood JT, Smibert CA, Lipshitz HD, SMAUG is a major regulator of maternal mRNA destabilization in Drosophila and its translation is activated by the PAN GU kinase, Dev. Cell 12 (2007) 143–155, 10.1016/j.devcel.2006.10.005. [DOI] [PubMed] [Google Scholar]
- [81].Chen L, Dumelie JG, Li X, Cheng MH, Yang Z, Laver JD, Siddiqui NU, Westwood JT, Morris Q, Lipshitz HD, Smibert CA, Global regulation of mRNA translation and stability in the early Drosophila embryo by the Smaug RNA-binding protein, Genome Biol 15 (2014) R4, 10.1186/gb-2014-15-1-r4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Wang M, Ly M, Lugowski A, Laver JD, Lipshitz HD, Smibert CA, Rissland OS, ME31B globally represses maternal mRNAs by two distinct mechanisms during the Drosophila maternal-to-zygotic transition, eLife 6 (2017) e27891, 10.7554/eLife.27891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Winkler F, Kriebel M, Clever M, Gröning S, Großhans J, Essential function of the serine hydroxymethyl transferase (SHMT) gene during rapid syncytial cell cycles in Drosophila, G3 (Bethesda) 7 (2017) 2305–2314, 10.1534/g3.117.043133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Song Y, Marmion RA, Park JO, Biswas D, Rabinowitz JD, Shvartsman SY, Dynamic control of dNTP synthesis in early embryos, Dev. Cell 42 (2017) 301–308, 10.1016/j.devcel.2017.06.013,e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Belles X, Piulachs MD, Ecdysone signalling and ovarian development in insects: from stem cells to ovarian follicle formation, Biochim. Biophys. Acta—Gene Regul. Mech 1849 (2015) 181–186, 10.1016/j.bbagrm.2014.05.025. [DOI] [PubMed] [Google Scholar]
- [86].Sieber MH, Spradling AC, The role of metabolic states in development and disease, Curr. Opin. Genet. Dev 45 (2017) 58–68, 10.1016/j.gde.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Dupont J, Scaramuzzi RJ, Insulin signalling and glucose transport in the ovary and ovarian function during the ovarian cycle, Biochem. J 473 (2016) 1483–1501, 10.1042/BCJ20160124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Ivanovska I, Lee E, Kwan KM, Fenger DD, Orr-Weaver TL, The Drosophila MOS ortholog is not essential for meiosis, Curr. Biol 14 (2004) 75–80, 10.1016/j.cub.2003.12.031. [DOI] [PubMed] [Google Scholar]
- [89].Robertson S, Lin R, The oocyte-to-embryo transition, Adv. Exp. Med. Biol 757 (2013) 351–372, 10.1007/978-1-4614-4015-4-12. [DOI] [PubMed] [Google Scholar]
- [90].Wang JT, Smith J, Chen BC, Schmidt H, Rasoloson D, Paix A, Lambrus BG, Calidas D, Betzig E, Seydoux G, Regulation of RNA granule dynamics by phosphorylation of serine-rich, intrinsically disordered proteins in C. elegans, eLife 3 (2014) e04591, 10.7554/eLife.04591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Parry JM, Singson A, EGG molecules couple the oocyte-to-embryo transition with cell cycle progression, Springer Berlin Heidelberg, Results Probl. Cell Differ 53 (2011) 135–151, 10.1007/978-3-642-19065-07. [DOI] [PMC free article] [PubMed] [Google Scholar]


