Abstract
Background
A prospective randomized trial was conducted to compare the impact of systemic chemotherapy vs. multi-modality therapy (complete cytoreductive surgery (CRS), hyperthermic intraperitoneal chemotherapy (HIPEC), and systemic chemotherapy) on overall survival (OS) in patients with gastric carcinomatosis.
Methods
Patients with measurable metastatic gastric adenocarcinoma involving the peritoneum, and resectable to ‘no evidence of disease’ were randomized to gastrectomy, metastasectomy, HIPEC, and systemic FOLFOXIRI (GYMS arm) or FOLFOXIRI alone (SA arm).
Results
Seventeen patients were enrolled (16 evaluable); 7 of 9 patients in the multi-modality GYMS arm achieved complete cytoreduction (CCR0). Median OS was 11.3 months in the GYMS arm and 4.3 months in the SA arm. Four patients in the GYMS arm survived >12 months, 2 patients close to 2 years at last follow-up, and 1 patient more than 4 years, with 2 of these patients still alive. No patient in the SA arm lived beyond 11 months. All patients surviving beyond 12 months in the surgery arm achieved complete cytoreduction and had an initial Peritoneal Cancer Index (PCI) of ≤15.
Conclusion
Maximal cytoreductive surgery combined with regional (HIPEC) and systemic chemotherapy in selected patients with gastric carcinomatosis and limited disease burden can achieve prolonged survival.
Keywords: metastatic gastric cancer, cytoreductive surgery, heated intraperitoneal chemotherapy (HIPEC), metastasectomy
Introduction
Despite a plethora of clinical and pre-clinical evidence, the adoption of maximal cytoreductive surgery (CRS) plus intraperitoneal chemotherapy into the management of peritoneal carcinomatosis of gastric origin has been met with considerable skepticism and resistance.[1, 2] Clinical data supporting a strategy of complete cytoreduction and heated intraperitoneal chemotherapy (HIPEC) include 5-year survival rates of 13 – 27 percent observed in selected patients with peritoneal carcinomatosis of gastric origin treated with CRS and HIPEC in several well-conducted retrospective series. This includes: (1) a recent French multi-institutional series of 159 patients, which reported 1-, 2-, and 5-year survival rates of 43, 18, and 13 percent, respectively; (2) a large single-institution matched cohort study, which reported 1- and 2-year survival rates of 45 percent in patients where an CCR0 or CCR1 resection could be achieved; (3) a comparison of outcome of patients where complete cytoreduction was possible versus patients who were left with residual peritoneal surface disease; (4) or a recent randomized study, which showed a small benefit of the adjunct of HIPEC following CRS in patients with advanced gastric cancer with synchronous presentation compared to cytoreduction alone.[3-8] These results are in stark contrast to outcomes reported in gastric cancer patients with spread to the peritoneum treated with chemotherapy only, which report a median overall survival of 7 to 15 months, and only ~10% of patients who live beyond two years.[9-11] The finding of an albeit small benefit indicating that regional chemotherapy can prolong survival in patients with peritoneal carcinomatosis of gastric origin is also consistent with results from adjuvant regional chemotherapy where several randomized studies in patients who undergo curative resection of gastric cancer have shown a survival benefit from the addition of HIPEC (reviewed in Tran et al.[12]).
A possible role of cytoreduction and regional chemotherapy in gastric cancer with peritoneal involvement is also supported by recent findings in other gastrointestinal cancers such as colorectal cancer with metastasis to the peritoneal surface. A large phase III clinical trial of patients with peritoneal carcinomatosis of colon and rectal origin showed a 9.7 months improvement in median overall survival in the CRS plus HIPEC group compared to the systemic chemotherapy alone control arm.[13] The authors have recently published an 8-year follow-up which confirmed superior outcomes in patients who underwent cytoreductive surgery plus HIPEC versus systemic chemotherapy (median disease-specific survival of 22.2 versus 12.6 months, respectively) with 5-year survival rates of 45 % for those patients in whom a CCR0/1 resection could be achieved.[14]
On the other hand, critiques of such an approach cite a (1) lingering concern for inherent selection bias for patients with a more favorable presentation and biology in retrospective series;[1, 2] (2) the lack of a convincing difference in patients with a high peritoneal surface disease burden, or in patients where no macroscopic cytoreduction (CCR2) could be achieved where outcomes were less favorable, and where any advantage attributable to CRS and HIPEC compared to matched historical controls appeared to fade;[3, 4, 7, 8, 14] (3) the well documented learning curve for safe and effective CRS and HIPEC, which carries - despite improved patient selection - an inherent treatment-related mortality of 5 – 10 percent;[6, 15, 16] or (4) a possible equivalent efficacy of modern chemotherapy and molecular therapy compared to morbid CRS plus HIPEC in patients with peritoneal carcinomatosis with low-volume disease.[11]
Within this peritoneal disease landscape, the GYMSSA trial was undertaken to address whether the addition of CRS plus HIPEC to a modified systemic FOLFOXIRI regimen (5-FU, leucovorin, oxaliplatin, and irinotecan) can improve overall survival in patients with metastatic gastric cancer. This study has also been designed to answer the question: what are the characteristics of patients who might benefit from the GYMS approach for metastatic gastric cancer, when metastases to the liver and lung are included.
Patients and Methods
Study Design – Patient Randomization
The GYMSSA Trial was approved by the Institutional Review Board and conducted under the protocol number 09-C-0189. The experimental design has been previously described.[11] Patients with a diagnosis of metastatic adenocarcinoma of the stomach either to the liver, the peritoneum, the lung or combinations thereof were eligible. Both, patients with synchronous or metachronous metastatic disease were eligible. Inclusion criteria were: (1) a diagnosis of histologically or cytologically confirmed gastric adenocarcinoma with metastatic disease measurable by computed tomography (CT), and/or magnetic resonance imaging (MRI); (2) that could be, in the opinion of the Principal Investigator, resected to ‘no evidence of disease’ based on imaging studies or staging laparoscopy on cross sectional imaging. Exclusion criteria were: (1) disease sites other than either peritoneum, lung or liver; brain metastases, evidence of extensive para-aortic/retro-pancreatic lymph node metastases, and/or significant ascites; (3) ECOG status of >2; (4) inability to tolerate any of the chemotherapeutic agents; (5) bone marrow suppression; (6) active systemic infections; or, (7) any concomitant medical problems that would place the patient at an unacceptable risk for a major surgical procedure or for administration of FOLFOXIRI.
Eligible patients underwent pathological re-review of their outside pathology and diagnostic laparoscopy with peritoneal washings for cytology. After laparoscopy confirmed metastatic disease, patients were randomized to systemic chemotherapy (SA arm) versus gastrectomy, metastasectomy, systemic chemotherapy (GYMS arm) using a computerized randomization algorithm.
SA Arm – Systemic Chemotherapy
As patients with advanced gastric adenocarcinoma were likely to receive at least one chemotherapy regime either preoperatively or in the immediate adjuvant setting, a search was conducted for an effective chemotherapy regimen to serve as ‘standard therapy’ in the SA arm. that would be different from the commonly used standard ECF (epirubicin, cisplatin, 5-FU) or taxane/cisplatin doublet regimens.[9, 17-19] The FOLFOXIRI regimen (oxaliplatin, irinotecan, 5-FU) was chosen based on these criteria and because of promising survival results in early clinical trials in gastric adenocarcinoma and acceptable toxicity.[20-22] Moreso, phase III clinical trials have shown that irinotecan can yield similar survival results in metastatic gastric cancer much like a platinum-based agent, and the recent success of FOLFIRINOX in pancreatic cancer, a triplet regimen equally consisting of 5-FU, oxaliplatin, and irinotecan, further supported its use in this disease.[23, 24]
Within 14 days of study randomization patients began FOLFIXIRI treatment. Systemic chemotherapy was administered once every 14 days, and repeated for 12 cycles (approximately 6 months). On treatment Day #1 irinotecan was administered IV over 90 minutes followed by leucovorin and oxaliplatin, given concomitantly over 2 hours, followed by 5-FU given via continuous infusion (CIV) over 48 hours. Dosing schedule of the various agents is depicted in Fig. 1.
Figure 1.
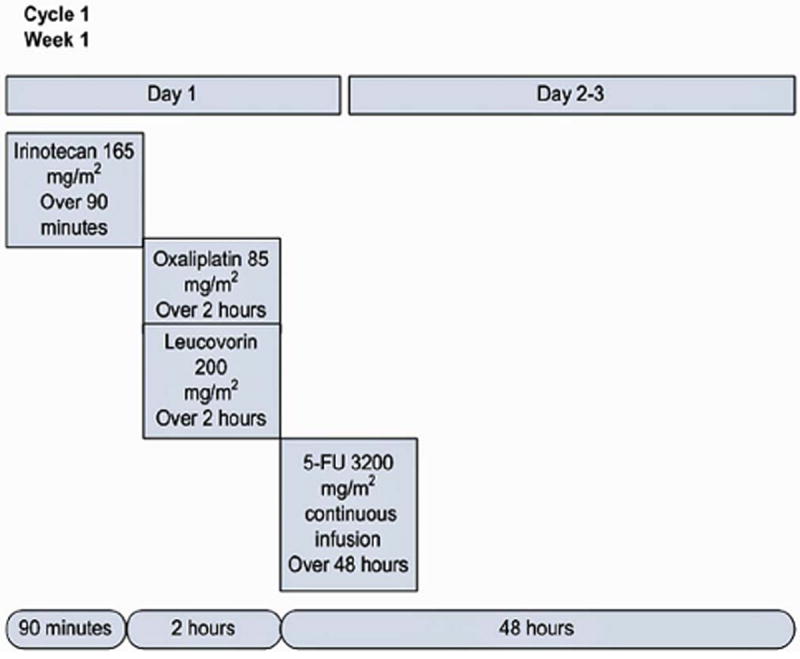
Dosing schedule of the FOLFOXIRI regimen used in the GYMSSA study. One cycle is shown which is repeated every two weeks for a total of 12 cycles.
GYMS Arm – Gastrectomy, Metastasectomy (CRS and HIPEC), Systemic Chemotherapy
Within 14 days of randomization patients randomized to GYMS arm underwent gastrectomy and/or metastasectomy with the intention to render the patient free of all gross disease. Patients with proximal lesions underwent total gastrectomy with a 2-4cm esophageal margin, those with distal lesions subtotal gastrectomy with ≥5cm proximal margins. Every gastrectomy included a total omentectomy as well as modified D2 lymphadenectomy (clearance of lymph nodes in porta hepatis, common hepatic, retro-pancreatic and supra-pancreatic, celiac axis, and splenic hilum locations without organ resections), and roux-en-Y gastro- or esophagojejunostomy. Completion of roux-en-Y reconstruction was delayed until after peritoneal perfusion (HIPEC). Prior to creation of intestinal anastomosis, an additional 1cm from the transected end was resected in order to yield fresh intestinal ends for anastomosis unaffected by heated regional chemotherapy. To accurately capture the extent of the peritoneal disease burden, both the Peritoneal Cancer Index (PCI), originally described by Sugarbaker,[25] and the Gilly classification were recorded immediately upon opening the abdomen [26]. Any visible peritoneal disease was removed by a complete stripping of the peritoneum of the entire involved quadrant. An example of a complete radical peritonectomy of the left upper quadrant is shown in Fig. 2. In half of cases a total abdominal peritonectomy was performed which included stripping of the parietal peritoneum of the abdominal walls, paracolic gutters, the diaphragm, the pelvis including upper rectum, and resections of affected small or large bowel mesentery including necessary visceral resection.
Figure 2.
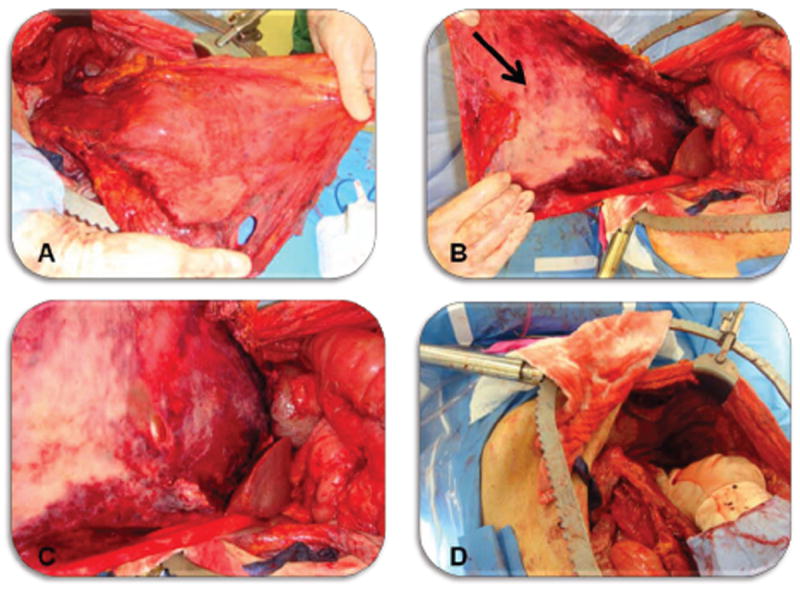
Radical peritonectomy of the left upper quadrant. A Non-visceral view of the stripped parietal peritoneum from left diaphragm, left paracolic gutter, and left upper abdominal wall stretched to the patients left lower extremity. The ligamentum falciparum and attached pre-peritoneal fat is attached to the medial aspect of the specimen. B Visceral view of stripped peritoneum, arrow marks peritoneal surface disease. C Close view of peritoneal disease burden. D Left upper quadrant status post radical peritonectomy (prior to perfusion).
Prior to the perfusion (HIPEC), a Completeness of Cytoreduction score (CCR) according to Sugarbaker’s criteria was recorded:[25] CCR- 0 (no residual tumor), CCR-1 (no residual nodules greater than 2.5 mm in diameter); CCR-2 (no residual nodules greater than 25 mm), and CCR-3 (residual nodules greater than 25 mm). Hyperthermic intraperitoneal chemotherapy (HIPEC) was administered using a closed circuit of oxaliplatin solution at 460mg/m2 in 5% dextrose in water (D5W) at 41°C for 30 minutes. Prior to perfusion a single dose each of fluorouracil (5-FU) 400 mg/m2 IV in 50 ml D5W and leucovorin 20 mg/m2 IV in 50 ml D5W were administered over 5 minutes to enhance the effect of regional oxaliplatin delivered IP. The perfusion flow rate was then maintained at ~2.0 L/min and a perfusate volume, which moderately distends the abdominal cavity, correlating with intra-abdominal pressures of 5 to 15 mm Hg (2.0 L/m2). During the peritoneal perfusion, constant physical manipulation of the abdomen (shaking) was maintained for the entire 30 minute perfusion period to assure even distribution of the perfusate. At completion of the perfusion, the abdomen was opened and copiously irrigated. Patients with hepatic metastases (≤5 lesions allowed) underwent liver resection ± radiofrequency ablation. All patients were scheduled to start FOLFOXIRI chemotherapy no later than 8 weeks after surgical resection.
Follow-up – Data Collection
Clinical follow-up was every two months during chemotherapy, then every 3 months for the first two years, and every 6 months for 3 years. Follow-up examinations included cross-sectional imaging, physical examination, and laboratory studies. Because of the nature of peritoneal imaging and because cytoreductive surgery would likely eliminate all disease detectable by cross-sectional imaging, treatment response was measured in terms of radiographic or symptomatic disease-free survival and overall survival. In the SA arm, patients were followed with CT scans, and response was evaluated by radiological (RESIST) criteria.
The trial was monitored annually by the NCI Data and Safety Monitoring Board (DMSB) for toxicity and adverse events. After the first phase of accrual an assessment for futility was performed by the DMSB, was closed due to slow accrual.
Statistical Analysis
Overall survival was measured from time of randomization to death from any cause, and patients alive at the time of last analysis were censored. The GYMSSA trial’s intent-to-treat analysis has been described in detail previously.[11] In brief, the goal of this study was to determine if the use of gastrectomy, metastasectomy, HIPEC and systemic therapy would result in an 8-month increase in overall survival, from a median of 12 to 20 months when compared to systemic chemotherapy alone. Kaplan-Meier curves and a two-tailed log-rank test were the primary analysis methods. Assuming exponential overall survival curves, the hazard rate for the systemic therapy is 0.0578, or approximately a 5.8% probability of death each month when the median survival is 12 months. For the median overall survival of 20 months assumed in the GYMS arm, this corresponds to a hazard rate of 0.0347, and the resulting hazard ratio for the comparison of the two overall survival curves would be 1.67. To compare these overall survival curves and detect a difference with a 0.05 two-tailed log-rank test, a total of 68 evaluable subjects per arm (136 total) were needed to be enrolled over a 6-year period and followed for an additional 2 years from the date of entry of the last patient, with 121 total deaths, in order to have 80% power to compare the overall survival curves. Additionally, patients were stratified for site of metastases (liver, peritoneum, or lung), time to development of first metastasis following initial diagnosis (<1 year versus ≥1 year), and history of previous systemic therapy for gastric metastases.
Results
Between June 2009 and April 2012, 34 patients with a diagnosis of metastatic gastric cancer were screened at NCI Surgery Branch for the entry into the GYMSSA trial. Seventeen patients met eligibility criteria and were randomized to either the surgery and systemic chemotherapy (GYMS) or the systemic chemotherapy (SA) alone arm. One patient in the SA arm was found following enrollment to have a diagnosis other than metastatic adenocarcinoma of the stomach on re-evaluation of his pathology. This report describes the outcome of the 17 patients enrolled to date. Patients’ baseline characteristics were similar in both groups (Table 1). Patients in both the systemic chemotherapy plus surgery arm (GYMS) and the systemic chemotherapy alone arm (SA) had predominately poorly differentiated gastric adenocarcinoma with signet ring cell features. Patients in both groups were equally pretreated with several cycles of systemic chemotherapy. The majority (n= 14) of patients presented with synchronous metastatic disease, and had not undergone prior gastrectomy. Patients in the GYMS arm had predominantly distal cancers whereas GE junction/cardia locations were more common in the SA arm. Peritoneal cytology was equally frequently positive in both groups.
Table I.
Patients and systemic treatment characteristics
| Cytoreductive Surgery + HIPEC (N=9) | Systemic Chemotherapy alone (N=8) | ||
|---|---|---|---|
| Age (yrs/range) | 45 (31-45) | 52 (39-68) | |
| Gender (Female/Male) | 3 & 6 | 4 & 4 | |
| Differentiation | |||
| moderate | 1 | 2 | |
| poorly | 8 | 6 | |
| Signet ring cell | |||
| no | 1 | 1 | |
| yes | 8 | 7 | |
| Location of 1° tumor† | |||
| † Tumors involving multiple locations listed separately | GE junction/Cardia | 1 | 5 |
| Fundus | 1 | 2 | |
| Body | 3 | 1 | |
| Antrum/Pylorus | 4 | 0 | |
| Preoperative LNs involvement (CT scan, PET scan, EUS) : | |||
| no | 2 | 2 | |
| yes | 7 | 6 | |
| Prior systemic chemotherapy | |||
| no | 3 | 1 | |
| yes | 6 | 7 | |
| number of cycles | 6 - 8 | 2 - 8 | |
| Prior Gastrectomy | |||
| no | 7 | 7 | |
| yes | 2 | 1 | |
| Peritoneal washings at laparoscopy | |||
| negative | 0 | 1 | |
| positive | 8 | 6 | |
| unknown | 1 | 1 | |
| FOLFOXIRI chemotherapy | Postoperative FOLFOXIRI | FOLFOXIRI chemotherapy alone | |
| None (never started) | 4 | 2 | |
| Started late (>8 wks post-op) | 1 | N/A | |
| Completed 12 cycles | 3 | 2 | |
| Total # chemo cycles completed/patient receiving | 44/5 | 31/6 |
Gastrectomy, cytoreductive surgery plus HIPEC and systemic chemotherapy – GYMS arm
Median overall survival in the GYMS arm was 11.3 months. All patients randomized to the GYMS arm underwent gastrectomy except one subject who had subtotal gastrectomy 2 years before study entry, and who underwent liver resection only following randomization (Table 2). The median time between randomization and surgery was 7 days (range: 3 - 20 days). Median duration of cytoreduction surgery and HIPEC was 10.1 hours (range: 5 – 12 hours). Median hospital stay after initial surgery was 17 days (range: 7 – 42 days), including on average 5 ± 2 days in the intensive care unit. Median blood loss during the surgery was 650 ml (range: 400 – 2,800 ml).
Table II.
Operative characteristics and outcomes of patients randomized to the surgery plus chemotherapy arm (GYMS).
| Patient | Extent of Resection | Peritonectomy locations |
Lymph Node involvement |
Peritoneal Carcinomatosis Index |
Gilly’s Stage | CCR Score |
Operative Blood loss (ml) |
Operative time (hrs) |
Reoperation | Postoperative Length of Stay (days) |
Intensive Care Unit Length of Stay (days) |
Number of Readmissions |
Total Number of Hospital Days |
Cycles of Adjuvant FOLFOXIRI (post operative weeks to therapy) |
Overall Survival (m) |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Evaluated |
Total Involved |
Before Surgery |
After Surgery |
Before Surgery |
After Surgery |
Residual Disease Status |
Median 650 |
Median 10.2 |
Median 17 | Median 5 | Median 2.5 | Median | Median 8 |
|||||
| 1 | Liver Seg. III&VI, Seg. VII-microwave ablation; R. adrenalectomy | Partial Peritonectomy | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 400cc | 5 | no | 11 | 2 | 6 | 121 | None | 19 |
| 2 | Total Gastrectomy; Liver Seg. II &III, LOA | Complete left & right upper quadrants | 23 | 16 | 2 | 0 | 1 | 0 | 0 | 650 cc | 8.40 | yes | 15 | 1 | 4 | 71 | 12 cycles; 6 weeks | 11.2 |
| 3 | Total Gastrectomy; Peritonectomy; L. Salpingo-oophorectomy | Complete left & right upper quadrants | 17 | 12 | 10 | 0 | 0 | 0 | 0 | 700 cc | 10.58 | no | 14 | 6 | 4 | 34 | 12 cycles; 6 weeks | 11 |
| 4 | Total Gastrectomy; Pancreatectomy, Omentectomy, Splenectomy, Right hemicolectomy | Total radical abdominal peritonectomy | 29 | 17 | 17 | 1 | 3 | 1 | 2 | 2800 cc | 11.16 | yes | 17 | 6 | 2 | 82 | None | 4 |
| 5 | Total Gastrectomy; Omentectomy; Transverse colon partial resection | Partial Peritonectomy | 12 | 0 | 0 | 0 | 0 | 0 | 0 | 500 cc | 8.25 | no | 24 | 2 | 3 | 62 | None | 24+ |
| 6 | Total Gastrectomy; Transverse colectomy; peritonectomy | Retro peritoneum | 204 | 188 | 0 | 0 | 0 | 0 | 0 | 400 cc | 10.15 | yes | 29 | 24 | 2 | 38 | 5 cycles; 10 weeks | 5 |
| 7 | Total Esophago-Gastrectomy; | Four-quadrant peritonectomy | 14 | 6 | 5 | 1 | 1 | 0 | 0 | 400 cc | 7.07 | no | 12 | 1 | 2 | 25 | 12 cycles; 4.5 weeks | 18+ |
| 8 | Total Gastrectomy; Liver resection seg. III & border of VIII; Cecectomy; Jejunal resection time two; Transverse colectomy; cholecystectomy | Four-quadrant and pelvic peritonectomy | 26 | 3 | 21 | 0 | 3 | 0 | 0 | 400 cc | 12.04 | yes | 49 | 49 | - | 49 | None | 1.8 |
| 9 | Total Gastrectomy; Omentectomy; Right hemicolectomy; small bowel resection; | Radical peritonectomy | 6 | 0 | 15 | 2 | 3 | 1 | 1 | 27000 cc | 11.31 | no | 23 | 5 | 0 | 23 | 3 cycles | 9+ |
CCR, Completeness of Cytoreduction; HIPEC, Heated Intraperitoneal Chemotherapy.
Most patients had total gastrectomy either due to location or extent of the primary tumor. Patients underwent a median of 2 (range: 1-5) concomitant organ resections, the most common, large and small bowel resections (Table 2). Most patients had a pre-surgery PCI of ≤20 (89%, n=8/9), three of which were zero (33%, n=3/9) and two of which were less than five (22%, n=2/9), and only one patient had a PCI of 21 (11%, n=1/9). Complete cytoreduction to microscopic level (CCR0) could be achieved in the majority of patients (Table 2), and all patients received the intended HIPEC following cytoreduction and peritonectomy.
Most patients had a pre-surgery PCI of ≤20 (89%, n=8/9), three of which were zero (33%, n=3/9). Patient 1 was operated on for liver metastases, had no carcinomatosis, and thus a PCI of 0. Two patients had a PCI of less than five (22%, n=2/9), and one patient had a PCI of 21 (11%, n=1/9). Patient 2 had a preoperative PCI of 2 and suffered a very early and rapid progression following multimodality treatment and died due to a complications related to progression of disease. With regards to the PCI of patients in the systemic therapy arm, an estimate of the peritoneal disease burden was captured from exploratory laparoscopy. Laparoscopic assessment was performed in all patients that included evaluation of all 4 quadrants. There was only 1 patient (12.5%, n=1/8) who had peritoneal tumor nodules > 2.5cm found on exploratory laparoscopy. Thus, the extent of peritoneal burden in the chemotherapy only arm appeared to be similar, and perhaps less, than that of multimodality cohort.
There were no intra-operative mortalities in the GYMS arm. Overall 90-day mortality in the surgical arm was 11% (n=1). Patient#8 died of infectious complications and septic shock 49 days after surgery. Major surgical complications and early postoperative morbidity included anastomotic leak in 2 patients, endoscopic esophageal perforation in one, pleural effusion in 3 patients, and wound infection in 2 patients. In total, four patients (44%) in the GYMS arm required reoperation. Only 3 patients required reoperation within 30-days of the initial surgery and were related to perioperative complications. One patient suffered intraperitoneal hemorrhage on postoperative day 2 requiring surgical repair and multiple blood transfusions, however, never recovered and died two months later because of development of coagulopathy and septic complications. Both patients, with esophagojejunal leaks required a second operation (Table 3). The remaining patient underwent went reoperation 11 months after the initial surgery due to hemoperitoneum and upon emergent reexploration was found to have disease progression. Median number of hospital re-admissions in the surgery arm was 3 (range: 2 – 6) and the median number of in-hospital days counting all admissions was 38 days (range: 9 – 110 days). Four patients were unable to start systemic chemotherapy (44%, n=4/9), three of them due to failure to thrive. Five patients went on to receive systemic therapy (56%, n=5/9). Three patients (33%, n=3/9) completed 12 cycles of FOLFOXIRI, one patient started 10 weeks later and only completed 5 cycles.
Table III.
Surgical outcome of patients randomized to the GYMS arm (90-day perioperative morbidity and mortality)
| 90 day outcome including grade of complication | ||||
|---|---|---|---|---|
| Serious adverse events (SAE) / Post-operative complications (re-operation, anastomotic leak, bleeding, wound infection) | Grade 3 | Grade 4 | Grade 5 | |
| Patient | ||||
| #1 | No Post-operative complications | none | none | none |
| #2 | Hemorrhage, GI∷Peritoneal cavity Gr.4 Perforation: Esophagus Gr. 5 (Post Endoscopy). | Intraperitoneal hemorrhage | Esphageal perforation post-endoscopy (for esophageal stenosis) and neutropenic sepsis | |
| #3 | Pleural effusion Gr 3; Infection :Pelvis Gr.3 Thrombosis/thrombus/embolism Gr4 | Pleural effusion and Intraabdominal abscess | Pulmonary embolus | |
| #4 | Pleural effusion Gr 3. Leak (including anastomotic), GI∷Pancreas Gr. 3 | Pancreatic leak; esophago-jejunal anastomotic leak; Pleural effusion | ||
| #5 | Infection :Wound Gr. 3 | Wound infection | ||
| #6 | Leak, GI∷EsophagusLeak (including anastomotic)- Gr. 4 Febrile neutropenia Gr. 5 | Esophago-jejunal anastomotic leak | Esophago-jejunal anastomotic leak and neutropenic sepsis | |
| #7 | Febrile neutropenia Gr.3 | Febrile neutropenia | ||
| #8 | Hemorrhage, GI∷Peritoneal cavity G. 4 Infection with Septic shock. Gr.5 | Intraperitoneal hemorrhage | Septic shock (non-neutropenic) | |
| #9 | Pleural effusion; Pneumonia; Depression | Pleural effusion; Pneumonia; Depression | ||
Systemic chemotherapy alone – SA arm
Median OS in the SA arm of 7 patients was 4.3 months. One of the initial 8 patients was removed from the analysis after re-review of his pathology did not confirm a diagnosis of metastatic gastric adenocarcinoma. The median time between randomization and start of chemotherapy was 19 days (range: 8 – 25 days). Two patients declined to participate after being randomized to the “systemic chemotherapy only” arm. One of the patients underwent gastrectomy with cytoreduction at an outside institution after completing five cycles of FOLFOXIRI; one patient withdrew consent after completing 2 cycles and was pursuing gastrectomy outside the NIH. No objective radiological responses were observed in SA arm. The best response to FOLFOXIRI chemotherapy was stabilization of disease for 4 months in the only patient who completed 12 cycles of systemic chemotherapy. One patient progressed after 5 cycles of therapy, and one after only 1 cycle of chemotherapy; both required hospital admission due to progressive disease.
Survival
Median survival in the GYMS arm was 11.3 months and in the SA arm 4.3 months, respectively. No patient in the SA arm lived beyond 12 months. Four patients in the surgery arm lived beyond 12 months, three close or longer than 2 years, and one beyond 4 years. Two patients are still alive in the GYMS arm. All patients in the systemic chemotherapy arm had succumbed to their disease within 12 months (Fig. 3). All patients surviving beyond 12 months in the surgery arm (GYMS) achieved a CCR score of 0 and had an initial PCI of ≤15.
Figure 3.
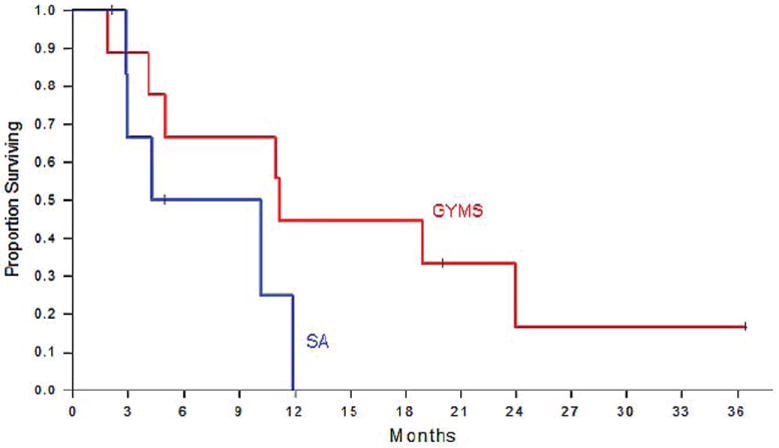
Kaplan Meier analysis of overall survival of patients randomized to multimodality treatment including CRS and HIPEC (GYMS arm) versus patients receiving chemotherapy only (SA arm)
Discussion
The acceptance of CRS plus HIPEC in CRC into clinical practice evolved over more than a decade.[27] Initially, several small, well conducted retrospective series revealed that some patients treated with CRS and HIPEC can live for multiple years, and some, in fact ~15%, are cured with this approach, an outcome previously not observed in patients treated with systemic chemotherapy only.[26, 28] This led to the conduct of a phase III clinical trial which randomized patients to CRS plus HIPEC followed by chemotherapy versus chemotherapy only, a study, which in patients with low volume peritoneal surface disease burden, unequivocally proved the superiority of the multi-modality approach, which incorporated cytoreductive surgery.[13, 14]
In metastatic gastric cancer involving the peritoneum, several retrospective series have reported cases where patients survive considerably longer than two years despite peritoneal carcinomatosis involving all four quadrants at time of CRS.[2, 3] Among those, the most impactful series is a well-conducted matched case control study from a high-volume peritoneal surface malignancy center where patients with limited peritoneal carcinomatosis of gastric origin treated with CRS and HIPEC achieved remarkable 1- and 2-year survival rates of 45 and 45 percent, respectively; this survival did not differ from those matched patients who underwent radical gastric resection with curative intent (absent peritoneal carcinomatosis).[4] The observation that select stage IV patients can achieve similar survival outcomes as those with stage III gastric adenocarcinoma, strongly suggests a positive treatment effect of this multi-modality approach. Additionally, investigators from France compared outcome of gastric cancer patients with peritoneal carcinomatosis treated with CRS and HIPEC from a single institution database and found that patients in whom a complete cytoreduction could be achieved (CCR-0) lived substantially longer than a matched cohort where complete cytoreduction was not achievable (CCR-2).[6] However, despite these and other retrospective data, no definitive phase III trial in advanced gastric cancer as in CRC has yet to be initiated to determine if the addition of CRS and HIPEC to systemic chemotherapy is beneficial in patients with this aggressive malignancy. Such a study is very well justified not only because of the urgent need to develop new therapeutic approaches to gastric cancer, but also because of the unique cytoarchitecture of metastatic gastric cancer having preferential spread to the peritoneal surface.
The lack of systemically administered drug penetration into peritoneal surface tumor deposits has long been recognized as one of the main reasons for the ineffectiveness of systemic chemotherapy for this indication.[1, 29, 30] Peritoneal surface deposits from metastatic gastric cancer, and in particular from its variant linitis plastica, or the diffuse form of gastric cancer, have a unique abundance of stroma.[31, 32] The tumor compartment in these lesions can be as small as 20 percent, a microenvironmental cytoarchitecture only seen in pancreatic adenocarcinoma.[31, 33] Recently, aberrations in transforming growth factor beta (TFGβ) signaling, such as loss of the downstream effector SMAD4, have been implicated in the cause of this unique tumor microenvironment formation, which is characterized by a paucity of vessels, high intra-tumoral pressures, and high collagen I deposition - all features impeding perfusion and effective cytotoxic drug delivery to the tumor cells [29, 32-34]. Thus, regional chemotherapy, which achieves much greater intra-tumoral drug concentrations at the peritoneal surface could be a promising tool to overcome this unique impediment to systemic drug delivery within this tumor entity.[1, 30, 35] This should be particularly the case when peritoneal chemotherapy perfusion is applied in the presence of local hyperthermia, a means of dose dense drug delivery, which has elegantly been shown to increase drug penetration as well as cytotoxicity of the delivered agent.[36, 37]
The GYMSSA trial was conducted in an effort to confirm these clinical and pre-clinical findings by testing the hypothesis, multi-modality therapy with CRS plus HIPEC, in addition to systemic chemotherapy, can achieve improved outcome compared to systemic chemotherapy alone in patients with metastatic gastric cancer. Results of this preliminary study are in line with early results from peritoneal surface malignancy studies in CRC. While the limited number of enrolled patients does not allow any statistical comparisons, the fact that four patients with carcinomatosis survived longer than one year, two close to two years, and one beyond four year with two patients still alive at time of last follow-up should be noted as such a ‘tail of the curve’ of the Kaplan Meier survival plots was only present in the GYMS, and not in the SA, arm. The longest survivor in the systemic chemotherapy only (SA) arm lived less than 12 month, a patient with a solitary mass in the hepatogastric ligament who completed 12 cycles of FOLFIRINOX chemotherapy. No objective responses were observed in patients receiving chemotherapy alone. The outcome of patients treated with chemotherapy was reflective of the previously published results in patients with stage IV gastric cancer treated with 1st and 2nd line systemic chemotherapy. The fact that some patients in the CRS plus HIPEC arm are surviving significantly longer than patients treated with systemic chemotherapy alone only is a common observation of most studies of patients treated with cytoreductive surgery plus regional chemotherapy. One of the most compelling evidence-based arguments in support of this multi-modality treatment strategy are apparent in the best results published to date of ‘long term survivors’ with advanced CRC who underwent CRS plus HIPEC in a randomized trial comparing this multi-modality therapy to systemic therapy alone. The 8-year follow-up of the large Phase III clinical trial in CRC from the Netherlands reported a 5-year survival rate of 45% in the multi-modality treatment arm for patients undergoing CCR1 cytoreduction [13, 14]. The median cancer-specific survival was 22.2 months in the CRS/HIPEC arm and 12.6 months in the control arm of that study (p = 0.028). The 5-year survival was 45% for those patients in whom a R1 resection was achieved.
In another phase III randomized clinical trial, of patients that underwent cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis of gastric origin, 30% survived longer than two years.[7] These results are consistent with multiple retrospective series of cytoreductive surgery and regional chemotherapy, which consistently report on ‘an improved tail of the survival curve’ in patients treated with CRS/HIPEC for peritoneal carcinomatosis of gastric origin.[3, 4, 6] Despite our study’s strength of randomized trial design with accurate pre- and intra-operative laparoscopic staging, eliminating the inherent selection bias of retrospective series, its main weakness is the limited number of patients enrolled. While the limited number of accrued patients prevented statistically valid survival comparisons between study groups, it is intriguing, as a descriptive finding, to recognize that patient survival in the GYMSSA trial was longer than what has been reported for stage IV gastric cancer patients with peritoneal carcinomatosis treated with either multi-modality therapy or with chemotherapy alone.
Over the three year period there were 34 evaluable patients referred who were screened for the trial. Reasons for low accrual was related to the referral patterns and were because of three limitations: Some patients were not keen on taking the risk being randomized to a second-line chemotherapy regimen when they had practicing surgical oncologists who would offer HIPEC for gastric cancer; the multimodality approach with its anticipated high complication rate requiring a prolonged hospital stay, and despite offering accommomadations for families out of state, for some families this created prohibitive logistical issues; lastly, some medical oncologists were reluctant to refer patients with peritoneal carcinomatosis for a surgical option. Of the 34 evaluable patients, only 17 met inclusion criteria and were randomized (50%). The most common reason patients were ineligible after screening was the patients’ performance status. These were predominatly patients with more than moderate amounts of ascites, or multiple sites of disease initially not present at their imaging at the time of referral.
The findings of the GYMSSA trial underscore the importance of clinical equipoise, particularly considering the benefits and effectiveness of the multi-modality treatment approach tested, and its related risks and burdens, compared to systemic treatment alone for advanced gastric adenocarcinoma. Of note, the morbidity of cytoreductive surgery and HIPEC (GYMS) for Stage IV gastric adenocarcinoma seen thus far in this trial is comparatively higher than that previously reported in the few studies of CRS/HIPEC for peritoneal surface malignancy of gastric origin, as well as other epithelial cancer origin.[6, 7, 13] One explanation might be that there are considerable technical differences in the management of the involved and diseased peritoneal surface in gastric cancer compared to peritoneal mesothelioma, colorectal cancer, or other cancers. One important differentiating factor is the frequent need for total gastrectomy as a part of indicated organ resections in order to achieve clearance of the primary tumor. Peritoneal debulking with routine concomitant gastrectomy has been previously described in other peritoneal surface malignancies.[38] A previous retrospective database review did not report an increase in intestinal leaks in patients who had a concomitant gastrectomy as part of their cytoreductive surgery; however, less than half of patients (15 out of 37) who had gastric resection in that study underwent total gastrectomy with esophago-jejunal anastomosis. It is conceivable that esophago-jejunal anastomoses with their more restricted blood supply are more prone to breakdown than gastro-esophageal anastomoses in the setting of cytotoxic hyperthermic peritoneal chemotherapy. What is more, proximal gastrointestinal anastomotic leaks in the setting of a stripped parietal peritoneum coupled with cytotoxic therapy-related immunosuppression might lead more frequently to septic complications than leaks from other areas of the GI tract. The more virulent bacterial flora in the oropharyngeal tract and the loss of the ‘stomach barrier’ to contain these pathogens compared to leaks occurring from say the relatively ‘sterile’ small intestinal anastomoses in patients who have an intact stomach might therefore increase the risk of post-operative sepsis and septic complications. That might have been the case in patient 6 who succumbed to neutropenic fever and septic shock after FOLFOXIRI cycle 5, and who had an esophagojejunal anastomotic leak treated non-operatively prior to initiation of systemic therapy. However, the low frequency leak rate makes this an unlikely event. Equally so, slow bone marrow recovery due to HIPEC or FOLFOXIRI chemotherapy leading to thrombocytopenia, bleeding, and the need for re-exploration only occurred in patient 8, and is a rare event. Secondly, the added modified D2 lymphadenectomy with its prolongation of operative time, increased blood loss, and additional exposure of peritoneal surfaces has been shown to substantially increase operative morbidity and mortality in randomized series of patients with gastric cancer.[39] The unproven benefit of D2 lymphadenectomy in patients with gastric cancer from Western countries raises, in retrospect, the question of its value when it is added to an already lengthy and morbid cytoreductive procedure. Another difference to previous peritoneal surface management trials in mesothelioma and colorectal cancer is the choice of the regional chemotherapy agent - oxaliplatin compared to the previously more frequently used mitomycin C. Both agents have ample pharmacokinetic data available: when comparing maximum systemic exposure of both agents after HIPEC using standard 20mg/m2 mitomycin C or 400mg/m2 oxaliplatin, oxaliplatin reaches systemic peak PK levels, which are closer to its described maximum peak levels when given systemically when compared to mitomycin C.[40] That might, in addition to the differing mechanisms of action of the two agents, translate into increased bone marrow toxicity, and might cause more serious sequelae from septic complications as suggested by the findings of small retrospective series comparing the two HIPEC agents in the management of colorectal cancer peritoneal surface involvement.[41-43]
Most patients (5 out of 9) in the GYMS arm of the trial succumbed to disease progression. Death due to immunosuppression from either systemic FOLFOXIRI or regional chemotherapy occurred infrequently; patient#6 recovered from CRS and HIPEC but died of septic shock in the presence of profound neutropenia 3 months after start of FOLFOXIRI. Cytoreduction as a contributing factor to that patient’s mortality remains speculative. Patient #2 died of hemoperitoneum 11 months after CRS and HIPEC, but was diagnosed with progressive disease upon explorative laparotomy, likely the major contributing factor to his demise. One patient (#8) died of infectious complications and septic shock resulting in multi-system organ failure on post-operative day 49. Contributing factors to that patient’s demise had been a slow recovery of bone marrow function, production of platelets in particular, necessitating re-exploration and massive blood product transfusion. Overall, death due to disease progression in both the GYMS as well as SA arm was the most frequent cause of mortality in this study.
The findings of the GYMSSA trial differ from a recently published series of Yang XJ et al., which reported 34 patients who underwent CRS and HIPEC for peritoneal carcinomatosis of gastric origin.[7] Twenty-four of these patients had synchronous carcinomatosis at time of initial presentation, 10 were diagnosed with peritoneal carcinomatosis after the initial diagnosis (metachronous), and presumably surgical management of gastric cancer. No peri-operative deaths were reported in the 24 patients who underwent predominantly subtotal gastrectomy as the management of primary tumor plus CRS and HIPEC.[7] HIPEC was performed utilizing the open technique with cisplatin and mitomycin C, and the patients did not receive mandatory post-operative multi-agent chemotherapy.[7] Whether this improved outcomes was due to the general better outcomes of patients with gastric cancer in the Far East or the greater familiarity of surgeons with gastric cancer, the avoidance of total gastrectomy with esophagojejunal anastomosis, or the omission of adjuvant administration of toxic chemotherapy early in the post-operative recovery following surgery, remains unknown. Our preliminary results support further study of a surgical approach combined with regional chemotherapy for patients with gastric cancer and limited peritoneal surface disease burden. Concerted efforts should be undertaken to reduce the operative morbidity of this multi-modality approach. Factors to consider in an effort to attain clinical equipoise include avoidance of extensive lymph node dissection, delayed initiation of systemic multi-agent chemotherapy, or in the absence of an objective disease response, selection of a less toxic chemotherapy regimen than the FOLFOXIRI triplet.
Maximal cytoreductive surgery combined with regional and systemic chemotherapy in selected patients with gastric carcinomatosis and limited disease burden can achieve prolonged survival. These preliminary results support further pursuit of multi-modality (surgical plus regional and systemic chemotherapy) therapy for patients with gastric cancer and limited peritoneal surface disease burden. In view of the recently published financial burden caused by these procedures, selection of patients will be a key factor to consider in study design.[44] On-going study of this multi-modality therapeutic approach is warranted.
Acknowledgments
Funding: Supported by the NIH intramural grant.
Disclaimer: The views expressed in this manuscript are those of the authors and do not reflect the official policy of the United States Government.
Footnotes
Contributing Author Declaration: We certify that all individuals who qualify as authors have been listed; each has participated in one or more of the following areas: conception and design of this work, the acquisition and/or analysis of data, the writing, and/or critical revision of the document, and supervision of this cooperative research effort. All contributing authors approve of the submission of this version of the manuscript and assert that the document represents valid work. If information derived from another source was used in this manuscript, we obtained all necessary approvals to use it and made appropriate acknowledgements in the document. All contributing authors take public responsibility for this work.
References
- 1.Sugarbaker PH, Yu W, Yonemura Y. Gastrectomy, peritonectomy, and perioperative intraperitoneal chemotherapy: the evolution of treatment strategies for advanced gastric cancer. Semin Surg Oncol. 2003;21(4):233–48. doi: 10.1002/ssu.10042. [DOI] [PubMed] [Google Scholar]
- 2.Glehen O, Gilly FN, Cotte E. Hyperthermic intraperitoneal chemotherapy in advanced gastric cancer: the end of skepticism? Ann Surg Oncol. 18(6):1524–6. doi: 10.1245/s10434-011-1632-4. [DOI] [PubMed] [Google Scholar]
- 3.Glehen O, Gilly FN, Arvieux C, et al. Peritoneal carcinomatosis from gastric cancer: a multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann Surg Oncol. 17(9):2370–7. doi: 10.1245/s10434-010-1039-7. [DOI] [PubMed] [Google Scholar]
- 4.Hall JJ, Loggie BW, Shen P, et al. Cytoreductive surgery with intraperitoneal hyperthermic chemotherapy for advanced gastric cancer. J Gastrointest Surg. 2004;8(4):454–63. doi: 10.1016/j.gassur.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 5.Yonemura Y, Kawamura T, Bandou E, et al. Treatment of peritoneal dissemination from gastric cancer by peritonectomy and chemohyperthermic peritoneal perfusion. Br J Surg. 2005;92(3):370–5. doi: 10.1002/bjs.4695. [DOI] [PubMed] [Google Scholar]
- 6.Scaringi S, Kianmanesh R, Sabate JM, et al. Advanced gastric cancer with or without peritoneal carcinomatosis treated with hyperthermic intraperitoneal chemotherapy: a single western center experience. Eur J Surg Oncol. 2008;34(11):1246–52. doi: 10.1016/j.ejso.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Yang XJ, Huang CQ, Suo T, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol. 18(6):1575–81. doi: 10.1245/s10434-011-1631-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glehen O, Schreiber V, Cotte E, et al. Cytoreductive surgery and intraperitoneal chemohyperthermia for peritoneal carcinomatosis arising from gastric cancer. Arch Surg. 2004;139(1):20–6. doi: 10.1001/archsurg.139.1.20. [DOI] [PubMed] [Google Scholar]
- 9.Webb A, Cunningham D, Scarffe JH, et al. Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer. J Clin Oncol. 1997;15(1):261–7. doi: 10.1200/JCO.1997.15.1.261. [DOI] [PubMed] [Google Scholar]
- 10.Sarela AI, Miner TJ, Karpeh MS, et al. Clinical outcomes with laparoscopic stage M1, unresected gastric adenocarcinoma. Ann Surg. 2006;243(2):189–95. doi: 10.1097/01.sla.0000197382.43208.a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kerkar SP, Kemp CD, Duffy A, et al. The GYMSSA trial: a prospective randomized trial comparing gastrectomy, metastasectomy plus systemic therapy versus systemic therapy alone. Trials. 2009;10:121. doi: 10.1186/1745-6215-10-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan TD, Black D, Sugarbaker PH, et al. A systematic review and meta-analysis of the randomized controlled trials on adjuvant intraperitoneal chemotherapy for resectable gastric cancer. Ann Surg Oncol. 2007;14(10):2702–13. doi: 10.1245/s10434-007-9487-4. [DOI] [PubMed] [Google Scholar]
- 13.Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21(20):3737–43. doi: 10.1200/JCO.2003.04.187. [DOI] [PubMed] [Google Scholar]
- 14.Verwaal VJ, Bruin S, Boot H, et al. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol. 2008;15(9):2426–32. doi: 10.1245/s10434-008-9966-2. [DOI] [PubMed] [Google Scholar]
- 15.Verwaal VJ, van Tinteren H, Ruth SV, Zoetmulder FA. Toxicity of cytoreductive surgery and hyperthermic intra-peritoneal chemotherapy. J Surg Oncol. 2004;85(2):61–7. doi: 10.1002/jso.20013. [DOI] [PubMed] [Google Scholar]
- 16.Smeenk RM, Verwaal VJ, Zoetmulder FA. Learning curve of combined modality treatment in peritoneal surface disease. Br J Surg. 2007;94(11):1408–14. doi: 10.1002/bjs.5863. [DOI] [PubMed] [Google Scholar]
- 17.Roth AD, Fazio N, Stupp R, et al. Docetaxel, cisplatin, and fluorouracil; docetaxel and cisplatin; and epirubicin, cisplatin, and fluorouracil as systemic treatment for advanced gastric carcinoma: a randomized phase II trial of the Swiss Group for Clinical Cancer Research. J Clin Oncol. 2007;25(22):3217–23. doi: 10.1200/JCO.2006.08.0135. [DOI] [PubMed] [Google Scholar]
- 18.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 19.Vanhoefer U, Rougier P, Wilke H, et al. Final results of a randomized phase III trial of sequential high-dose methotrexate, fluorouracil, and doxorubicin versus etoposide, leucovorin, and fluorouracil versus infusional fluorouracil and cisplatin in advanced gastric cancer: A trial of the European Organization for Research and Treatment of Cancer Gastrointestinal Tract Cancer Cooperative Group. J Clin Oncol. 2000;18(14):2648–57. doi: 10.1200/JCO.2000.18.14.2648. [DOI] [PubMed] [Google Scholar]
- 20.Masi G, Allegrini G, Cupini S, et al. First-line treatment of metastatic colorectal cancer with irinotecan, oxaliplatin and 5-fluorouracil/leucovorin (FOLFOXIRI): results of a phase II study with a simplified biweekly schedule. Ann Oncol. 2004;15(12):1766–72. doi: 10.1093/annonc/mdh470. [DOI] [PubMed] [Google Scholar]
- 21.Cao W, Yang W, Lou G, et al. Phase II trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) as first-line treatment for advanced gastric cancer. Anticancer Drugs. 2009;20(4):287–93. doi: 10.1097/CAD.0b013e3283273509. [DOI] [PubMed] [Google Scholar]
- 22.Lee J, Kang WK, Kwon JM, et al. Phase II trial of irinotecan plus oxaliplatin and 5-fluorouracil/leucovorin in patients with untreated metastatic gastric adenocarcinoma. Ann Oncol. 2007;18(1):88–92. doi: 10.1093/annonc/mdl317. [DOI] [PubMed] [Google Scholar]
- 23.Bouche O, Raoul JL, Bonnetain F, et al. Randomized multicenter phase II trial of a biweekly regimen of fluorouracil and leucovorin (LV5FU2), LV5FU2 plus cisplatin, or LV5FU2 plus irinotecan in patients with previously untreated metastatic gastric cancer: a Federation Francophone de Cancerologie Digestive Group Study--FFCD 9803. J Clin Oncol. 2004;22(21):4319–28. doi: 10.1200/JCO.2004.01.140. [DOI] [PubMed] [Google Scholar]
- 24.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 364(19):1817–25. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 25.Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res. 1996;82:359–74. doi: 10.1007/978-1-4613-1247-5_23. [DOI] [PubMed] [Google Scholar]
- 26.Beaujard AC, Glehen O, Caillot JL, et al. Intraperitoneal chemohyperthermia with mitomycin C for digestive tract cancer patients with peritoneal carcinomatosis. Cancer. 2000;88(11):2512–9. doi: 10.1002/1097-0142(20000601)88:11<2512::aid-cncr12>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 27.Avital I, Brucher BL, Nissan A, Stojadinovic A. Randomized clinical trials for colorectal cancer peritoneal surface malignancy. Surg Oncol Clin N Am. 21(4):665–88. doi: 10.1016/j.soc.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Elias D, Blot F, El Otmany A, et al. Curative treatment of peritoneal carcinomatosis arising from colorectal cancer by complete resection and intraperitoneal chemotherapy. Cancer. 2001;92(1):71–6. doi: 10.1002/1097-0142(20010701)92:1<71::aid-cncr1293>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 29.Pilati P, Rossi CR, Mocellin S, et al. Multimodal treatment of peritoneal carcinomatosis and sarcomatosis. Eur J Surg Oncol. 2001;27(2):125–34. doi: 10.1053/ejso.2000.1021. [DOI] [PubMed] [Google Scholar]
- 30.Yonemura Y, Ninomiya I, Kaji M, et al. Prophylaxis with intraoperative chemohyperthermia against peritoneal recurrence of serosal invasion-positive gastric cancer. World J Surg. 1995;19(3):450–4. doi: 10.1007/BF00299188. discussion 455. [DOI] [PubMed] [Google Scholar]
- 31.Kano MR, Bae Y, Iwata C, et al. Improvement of cancer-targeting therapy, using nanocarriers for intractable solid tumors by inhibition of TGF-beta signaling. Proc Natl Acad Sci U S A. 2007;104(9):3460–5. doi: 10.1073/pnas.0611660104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Komuro A, Yashiro M, Iwata C, et al. Diffuse-type gastric carcinoma: progression, angiogenesis, and transforming growth factor beta signaling. J Natl Cancer Inst. 2009;101(8):592–604. doi: 10.1093/jnci/djp058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neesse A, Michl P, Frese KK, et al. Stromal biology and therapy in pancreatic cancer. Gut. 60(6):861–8. doi: 10.1136/gut.2010.226092. [DOI] [PubMed] [Google Scholar]
- 34.Kim JY, Park DY, Kim GH, et al. Smad4 expression in gastric adenoma and adenocarcinoma: frequent loss of expression in diffuse type of gastric adenocarcinoma. Histol Histopathol. 2005;20(2):543–9. doi: 10.14670/HH-20.543. [DOI] [PubMed] [Google Scholar]
- 35.Spratt JS, Adcock RA, Muskovin M, et al. Clinical delivery system for intraperitoneal hyperthermic chemotherapy. Cancer Res. 1980;40(2):256–60. [PubMed] [Google Scholar]
- 36.Sayag-Beaujard AC, Francois Y, Glehen O, et al. Intraperitoneal chemohyperthermia with mitomycin C for gastric cancer patients with peritoneal carcinomatosis. Anticancer Res. 1999;19(2B):1375–82. [PubMed] [Google Scholar]
- 37.Teicher BA, Kowal CD, Kennedy KA, Sartorelli AC. Enhancement by hyperthermia of the in vitro cytotoxicity of mitomycin C toward hypoxic tumor cells. Cancer Res. 1981;41(3):1096–9. [PubMed] [Google Scholar]
- 38.Piso P, Slowik P, Popp F, et al. Safety of gastric resections during cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis. Ann Surg Oncol. 2009;16(8):2188–94. doi: 10.1245/s10434-009-0478-5. [DOI] [PubMed] [Google Scholar]
- 39.Hartgrink HH, van de Velde CJ, Putter H, et al. Extended lymph node dissection for gastric cancer: who may benefit? Final results of the randomized Dutch gastric cancer group trial. J Clin Oncol. 2004;22(11):2069–77. doi: 10.1200/JCO.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 40.Elias D, Bonnay M, Puizillou JM, et al. Heated intra-operative intraperitoneal oxaliplatin after complete resection of peritoneal carcinomatosis: pharmacokinetics and tissue distribution. Ann Oncol. 2002;13(2):267–72. doi: 10.1093/annonc/mdf019. [DOI] [PubMed] [Google Scholar]
- 41.Rouers A, Laurent S, Detroz B, Meurisse M. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for colorectal peritoneal carcinomatosis: higher complication rate for oxaliplatin compared to Mitomycin C. Acta Chir Belg. 2006;106(3):302–6. doi: 10.1080/00015458.2006.11679897. [DOI] [PubMed] [Google Scholar]
- 42.McConnell YJ, Mack LA, Francis WP, et al. HIPEC + EPIC versus HIPEC-alone: Differences in major complications following cytoreduction surgery for peritoneal malignancy. J Surg Oncol. doi: 10.1002/jso.23276. [DOI] [PubMed] [Google Scholar]
- 43.Glockzin G, von Breitenbuch P, Schlitt HJ, Piso P. Treatment-related morbidity and toxicity of CRS and oxaliplatin-based HIPEC compared to a mitomycin and doxorubicin-based HIPEC protocol in patients with peritoneal carcinomatosis: A matched-pair analysis. J Surg Oncol. doi: 10.1002/jso.23228. [DOI] [PubMed] [Google Scholar]
- 44.Hultman B, Lundkvist J, Glimelius B, et al. Costs and clinical outcome of neoadjuvant systemic chemotherapy followed by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in peritoneal carcinomatosis from gastric cancer. Acta Oncol. 51(1):112–21. doi: 10.3109/0284186X.2011.594809. [DOI] [PubMed] [Google Scholar]


