Abstract
Parkinson’s disease is a circuit-level disorder with clinically-determined motor subtypes. Despite evidence suggesting each subtype may have different pathophysiology, few neuroimaging studies have examined levodopa-induced differences in neural activation between tremor dominant and posterior instability/gait difficulty subtype patients during a motor task. The goal of this fMRI study was to examine task-induced activation and connectivity in the cortico-striatal-thalamo-cortical motor circuit in healthy controls, tremor dominant patients, and postural instability/gait difficulty patients before and after levodopa administration. Fourteen tremor dominant and 12 posterior instability/gait difficulty cognitively-intact patients and 21 age- and sex-matched healthy controls completed a right-hand, paced tapping fMRI paradigm. Collectively, Parkinson’s disease patients off medication (OFF) showed hypoactivation of the motor cortex relative to healthy controls, even when controlling for performance. After levodopa intake, the posterior instability/gait difficulty patients had significantly increased activation in the left putamen compared with tremor dominant patients and healthy controls. Psychophysiological interaction analysis revealed that levodopa increased effective connectivity between the posterior putamen and other areas of the motor circuit during tapping in tremor dominant patients, but not in the posterior instability/gait difficulty patients. This novel, levodopa-induced difference in the neural responses between Parkinson’s disease motor may have significant implications for elucidating the mechanisms underlying the distinct phenotypic manifestations and enabling the classification of motor subtypes objectively using fMRI.
Keywords: Parkinson’s disease, tremor dominant, postural instability/gait difficulty, psychophysiological interaction, functional MRI, RRID:SCR_007037, RRID:SCR_009489
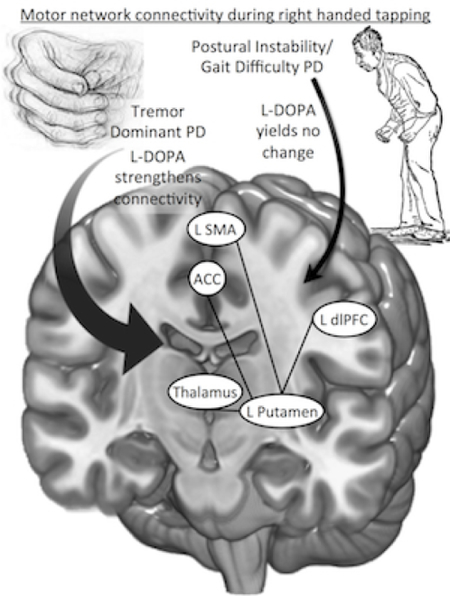
Graphical abstract
After levodopa administration, tremor dominant Parkinson’s patients show greater coupling between several motor regions required for a tapping task. Postural instability/gait difficulty Parkinson’s patients did not show the same medication effect, potentially reflecting different pathophysiologic mechanisms between subtypes.
Introduction
Motor impairments are the defining symptoms of Parkinson’s disease (PD), but these symptoms can be heterogeneous and manifest as different clinical subtypes. Two of the most commonly described motor subtypes are tremor dominant (TD) and postural instability/gait difficulty (PIGD; [Jankovic et al., 1990]. The TD subtype is characterized by predominant tremor symptoms and has been associated with cerebello-thalamo-cortical motor pathway alterations [Zhang et al., 2014]. In contrast, the PIGD subtype is chiefly associated with bradykinesia and rigidity along with early imbalance and gait impairment. Compared to TD, the PIGD phenotype has been associated with later onset, increased risk of cognitive decline [Alves et al., 2006], faster disease progression [Jankovic and Kapadia, 2001], and suboptimal response to dopamine replacement therapy [Vu et al., 2012]. Clinical manifestations of PIGD are thought to involve cortico-striato-thalamo-cortical (CSTC) circuits [Lewis et al., 2011; Prodoehl et al., 2013]. Despite clinical and potential pathophysiological distinctions between these motor subtypes, PD patients with tremor have often been excluded in fMRI studies in an attempt to avoid motion artifact [Wu et al., 2015], potentially biasing prior functional imaging studies in PD. By investigating the pathophysiological differences between PD motor subtypes, it may be possible to elucidate the mechanisms underlying the distinct phenotypic manifestations and allow for more tailored treatment strategies and improved therapeutic clinical trial designs.
Functional blood oxygen level-dependent (BOLD) activation differences involving the basal ganglia, supplementary motor area, primary and premotor motor cortices, and parietal lobes have been reported in PD patients compared to healthy controls during simple motor task functional MRI (fMRI) paradigms (for review, see [Herz et al., 2014]). However, the direction of activation differences in the premotor and primary motor cortices has been notably inconsistent, with reports of hyperactivation [Eckert et al., 2006; Haslinger et al., 2001; Lewis et al., 2011; Sabatini et al., 2000; Yu et al., 2007], hypoactivation [Buhmann et al., 2003; Burciu et al., 2015; Prodoehl et al., 2013; Tessa et al., 2010; Tessa et al., 2012], and no activation differences [Cerasa et al., 2006; Elsinger et al., 2003]. Disease stage and medication status at the time of scanning may explain some of these inconsistencies. For instance, hypoactivation of motor areas has been reported in de novo patients [Buhmann et al., 2003; Tessa et al., 2012], while introducing a dopamine agonist or replacement can lead to somewhat mitigating effects in these motor regions [Buhmann et al., 2003; Herz et al., 2014; Lucetti et al., 2014]. Another possible explanation for the inconsistencies is the practice of combining motor subtypes as a single, clinical sample (e.g., [Burciu et al., 2015; Spetsieris et al., 2009]), since the additional heterogeneity may influence outcomes.
Histology, SPECT, PET, and conventional BOLD activation fMRI studies have revealed time-averaged, striatal physiology differences that have implicated CSTC network regions in non-TD patient populations [Lewis et al., 2011; Prodoehl et al., 2013]. However, since PD can be considered a circuit disease [Eckert et al., 2006; Göttlich et al., 2013; Zhang et al., 2015] that can be modulated by dopaminergic stimulation [Lucetti et al., 2014] and external tasks [Wu et al., 2009], investigating the dynamic interaction between regions within the CSTC and dopaminergic medication effects could provide new insight into mechanisms of dysfunction during motor demands. Psychophysiological interaction (PPI) is a linear analysis technique that estimates task-induced, dynamic coupling between regions, often referred to as effective connectivity [Friston, 2011; O’Reilly et al., 2012]. Leveraging the PPI technique could provide novel information about whether CSTC circuit function during tapping differs between subtypes in response to levodopa administration.
In this study, we investigated differences in brain activity between TD and PIGD motor subtypes and age- and sex-matched healthy controls during a paced, finger-tapping task. Given prior evidence that differences in nigrostriatal projection pathology could play a role in the manifestation of motor subtypes [Eggers et al., 2011; Jellinger, 2012; Spiegel et al., 2007], we hypothesized that task-induced activity within the downstream CSTC network would differ between TD and PIGD subtypes. Further, we examined the effect of levodopa on effective connectivity of the posterior putamen (pPut), a key node in the CSTC motor network [Haber, 2003], during the tapping task. Since PIGD symptoms are less responsive to levodopa and patients with the PIGD subtype generally require higher doses of levodopa therapy [Vu et al., 2012], we hypothesized that PIGD patients would show a diminished levodopa-induced effect on motor network connectivity compared to TD patients.
Materials and methods
2.1. Recruitment
Twenty-nine PD patients meeting the UK Brain Bank criteria for clinical diagnosis of idiopathic PD [Gibb and Lees, 1988] with Hoehn and Yahr stage I-III were recruited from the Movement Disorders Center at the University of Colorado Hospital. All patients were currently taking dopaminergic medications. We attempted to recruit TD and PIGD subtypes in equal number through phone interview pre-screening of symptoms. At a screening visit, PD patients had TD and PIGD scores calculated as well as motor subtype assignment using previously published methods [Jankovic et al., 1990]. One patient was classified as indeterminate and excluded from further analyses, leaving 14 classified as PIGD and 14 as TD. Twenty-one age- and sex-matched healthy controls were recruited from patient spouses and the local community through advertisements and electronic postings. Written informed consent was obtained from all participants as approved by the Colorado Multiple Institutional Review Board and complied with the Declaration of Helsinki.
2.2. Exclusion criteria
Exclusion criteria for all participants included: (1) untreated neurological or psychiatric conditions, (2) cognitive impairment (Montreal Cognitive Assessment < 26), (3) untreated general medical disorder (e.g., uncontrolled diabetes), and (4) any contraindications to MRI. Exclusion criteria for patients also included: (1) prior deep brain stimulation surgery, and (2) motor symptoms prohibitively severe for stable MRI examination. Controls were excluded if presently taking any medications that alter dopaminergic function.
2.3. Experimental Design
2.3.1. Clinical assessments
All study participants had their handedness evaluated using the Edinburgh Handedness Inventory and were screened for cognitive impairment using the Montreal Cognitive Assessment. PD patients had their motor and non-motor symptoms rated using the Movement Disorder Society-Revised Unified Parkinson Disease Rating Scale (MDS-UPDRS) [Goetz et al., 2008]. At a screening visit, patients underwent motor assessments (MDS-UPDRS Part III) in a practically defined off state (at least 12 hours following the last dose of any dopaminergic medication, “OFF”), and again one hour after ingestion of 200mg/50mg levodopa/carbidopa immediate release formulation. Patients were asked to not alter their medication regimens between the screening visit and the MR scanning visit, but nevertheless were re-assessed in the OFF and ON state on the day of scanning using the MDS-UPDRS Part III to account for day-to-day differences in their motor symptoms.
2.3.2. Data Acquisition
Imaging data for 38 of the participants were acquired on a 3.0T GE Signa HDx system with an 8-channel head coil. The imaging data for the remaining nine participants (2 healthy controls, 2 TD patients, and 5 PIGD patients) were acquired on a 3.0T Siemens Skyra system with a 20-channel head/neck coil due to replacement of the scanner. Structural, 3D T1-weighted scans (SPGR on GE Signa; MPRAGE on Siemens Skyra) were acquired for spatial normalization. Structural imaging parameters on both scanners were TR=2200 ms; TE=2.5ms; flip-angle = 8 deg; FOV = 220 mm2; 176 slices; 0.9 mm thickness; in-plane resolution = 0.9 mm2. BOLD-contrast fMRI scans were collected using a gradient-echo echo-planar imaging with the following parameters: TR = 2000ms; TE = 28ms; flip angle = 70 deg.; FOV = 220mm2; 32 interleaved slices; 4 mm slice thickness with 0 mm gap; axial plane angled at the AC-PC through the brain and cerebellum, in-plane resolution = 3.4 mm2; number of volumes = 184.
2.3.3. Functional Task
Six 30-second blocks of right handed tapping, paced (1 Hz) by auditory cues, were alternated with six 30-second blocks of passive visual fixation (crosshair). Each tapping sequence began with the right thumb, progressed in order to the little finger, and then reversed back to the thumb. Stimuli were controlled and tapping performance recorded using E-Prime and a Celeritas Series five-button Fiber Optic Button Response System (Psychology Software Tools, Sharpsburg, PA). PD patients performed the same task OFF and ON.
Participants’ task performance was monitored visually via a window in the MRI control room and by simultaneous electromyography (EMG) recordings during fMRI scanning using a BIOPAC EMG Amplifier and AcqKnowledge software (BIOPAC Systems, Inc., Goleta, CA, USA). A pair of small adhesive, MRI-compatible EMG electrodes were placed over the left and right first dorsal interosseous muscle and a reference ground electrode was placed over a bony protuberance at either the wrist or elbow. The EMG signal data was monitored in real time and also reviewed post-hoc to ensure participant compliance with the tapping task. One PIGD patient was excluded from the analysis due to movement detected in the left hand during the right hand tapping task, reducing the PIGD sample to 13.
2.4. Preprocessing
The first four volumes were discarded to account for saturation effects. The remaining functional images underwent slice-timing correction, realignment to the mean and linear coregistration to the individual’s T1-weighted anatomical image within SPM8. Following the Unified Segmentation protocol [Ashburner and Friston, 2005], forward deformations from the individual’s T1-weighted structural image normalization to MNI template space were applied to the coregistered functional scans before smoothing with a 6mm FWHM kernel. An autoregressive AR(1) model was used to account for serial correlation.
2.5. Movement analysis
Head movement within the scanner was minimized by foam padding. As a first pass, participants with scans containing movement ≥3 mm of translation or ≥3 degrees of rotation were excluded. One PIGD participant did not meet these criteria and was removed from any further analysis, leaving 12 PIGD patients. Subsequently, participants’ realignment parameters were evaluated using the Artifact Detection Toolkit (https://www.nitrc.org/projects/artifact_detect/) to identify any volume with greater than 1 mm of movement from the previous TR. Each of these identified outlier volumes was then censored via a nuisance covariate in the participant’s first-level analysis. All remaining participants had ≥75% of their respective volumes passing the censoring criteria. In total, 14 TD, 12 PIGD, and 21 healthy control participants passed all data quality standards.
2.6. First-level and random effects modeling
Brain activation during tapping > rest was evaluated after convolving 30s boxcar functions with a canonical hemodynamic response function for each block. Covariates of no interest included six rigid-body realignment parameters and any censored volume regressors. All first-level analyses were visually inspected to ensure general data quality and left hemisphere motor circuit activation. Group differences for tapping > rest were assessed using a factorial model with three factors: PD diagnosis (healthy controls or PD), subtype (none, TD, or PIGD), and medication status (OFF or ON). This model allowed us to test for activation differences between healthy controls and all PD patients without presuming identical patterns for the two PD subtypes. Scanner type and recorded tapping performance were entered as covariates of no interest. Voxels within a dilated gray matter mask were used in the whole brain analyses.
2.7. Region of interest (ROI) definition and analysis
Four ROIs, including bilateral pPut and caudate head (HCd), showing functional coactivation with the terms sensorimotor processes and action value, respectively, were derived from a prior, study with a meta-analytic framework [Pauli et al., 2016]. The masks were eroded by 1 mm in 3 dimensions to limit overlap with other striatal regions. Beta weights of right finger tapping activity were extracted from each ROI and tested for effect of diagnosis, subtype, and drug.
2.8. Psychophysiological Interaction (PPI)
Because our task involved unilateral right finger tapping, we used the left pPut as the seed region to determine the effect of levodopa on effective connectivity within the CSTC circuit. Briefly, effective connectivity within the context of PPI reflects experimentally-induced changes in functional connectivity (i.e., increased or decreased connectivity during tapping versus rest) between a seed and other brain regions [Friston et al., 1997]. Using the gPPI toolbox [McLaren et al., 2012], the first eigenvariate from each ROI was extracted, deconvolved [Kim and Horwitz, 2008], and used as the physiologic regressor in the PPI analysis. The PPI analysis also included a task regressor consisting of the tapping block design, and the interaction between the physiologic and task regressors [O’Reilly et al., 2012]. Nuisance regressors for the six realignment parameters, cerebrospinal fluid, and white matter signal were included in the first-level model. Contrasts from the first-level interaction terms, reflecting increased connectivity during tapping compared with rest, were entered into the factorial analysis subtype (TD vs. PIGD) and drug (OFF vs. ON). Scanner type and tapping performance were covariates of no interest. Parameter estimates from corrected clusters were extracted and plotted to determine magnitude and direction of the interaction.
2.9. Statistical analysis
We analyzed differences in age and Montreal Cognitive Assessment scores between the three groups (healthy controls, TD, and PIGD). We analyzed group differences in recorded taps using ANCOVA, adjusting for age. Chi-squared statistics were used to test for significantly different distribution of female participants, MRI scanner, or symptom onset side between groups. PD-specific clinical characterizations (Hoehn & Yahr, MDS-UPDRS scores, and levodopa equivalent daily dose) were compared via a two-sample t-test with subtype as the factor. Effects of levodopa on MDS-UPDRS symptoms and tapping behavior between the PD subtypes were assessed with repeated-measures ANOVA. Tukey’s HSD post-hoc testing was applied to characterize significant group differences. Correlations between clinical metrics and extracted parameter estimates (i.e., beta weights) from significant clusters in the random effects were compared using Spearman’s rho. Group differences of the mean parameter estimates for each ROI was conducted using ANOVA and Tukey’s HSD post-hoc test. All statistical analyses were thresholded at p = 0.05, unless otherwise specified, and completed in SPSS 23 (IBM Corp., NY, USA).
Whole brain statistical threshold contrasts compared all PD versus healthy controls for each medication state separately, followed by pairwise-comparisons between each subtypes and healthy controls for each medication state. Statistical thresholds for reporting clusters of BOLD activation were set at a voxel-level p < 0.001 followed by cluster-level α < 0.05, corrected for multiple comparisons using 3dClustSim as implemented in AFNI version 16.1.01. For the PPI whole-brain results, the motor subtype*medication status interaction was tested prior to any main effects. Subtype and medication status main effects were assessed in areas not showing a significant interaction. Significant effective connectivity changes were reported for voxel-wise peak p = 0.005, cluster-level corrected at α < 0.05.
Results
3.1. Demographics & Symptomatology
Twenty-six patients (14 TD and 12 PIGD) and 21 healthy controls were included in the final analysis. Healthy controls, TD patients, and PIGD patients did not differ in age, sex, handedness, Montreal Cognitive Assessment scores or scans acquired on different MR systems (Table 1). Between the PD motor subtypes, disease duration, levodopa equivalent daily dose, types of prescribed medications, and MDS-UPDRS subscales did not significantly differ. Hoehn & Yahr scores were significantly higher for PIGD compared with TD (F1, 26 = 5.0, p = 0.035). Neither total MDS-UPDRS nor MDS-UPDRS motor symptoms changed significantly between ON and OFF conditions either between subtype (total: p = 0.37 ; III: p = 0.79) or within patient (total: p = 0.38; III: p = 0.38).
Table 1.
Demographic and clinical characteristics
| Parkinson’s disease patients | |||||
|---|---|---|---|---|---|
| Controls (n = 21) | All (n = 26) | PIGD (n = 12) | TD (n = 14) | p-values | |
| Age (y) | 61.6+/−8.8 | 62.2+/−7.5 | 63.3+/−8.2 | 60.9+/−7.1 | 0.67 |
| Gender (M/F) | 13/8 | 17/9 | 7/5 | 10/4 | 0.53 |
| Handedness (R/L) | 19/2 | 24/2 | 11/1 | 13/1 | 0.96 |
| Montreal Cognitive Assessment | 28+/−2 | 28+/−2 | 28+/−2 | 28+/−1 | 0.85 |
| Hoehn & Yahr Stage | -- | 2.2+/−0.7 | 2.6+/−0.7 | 1.9+/−0.4 | 0.035* |
| Predominant onset side (R/L) | -- | 16/10 | 7/5 | 9/5 | 0.77 |
| Disease duration (y) | -- | 5.5+/−3.0 | 5.1+/−2.3 | 5.3+/−2.5 | 0.64 |
| Levodopa Equivalent Daily Dose (mg) | -- | 500+/−300 | 530+/−340 | 470+/−260 | 0.93 |
| # taking levodopa (n) | -- | 16 | 7 | 9 | 0.77 |
| # taking dopamine agonist (n) | -- | 20 | 9 | 11 | 0.83 |
| # taking MAOB-I (n) | -- | 13 | 8 | 5 | 0.13 |
| # taking Amantadine (n) | -- | 2 | 1 | 1 | 0.92 |
| MDS-UPDRS I | -- | 9+/−5 | 9+/−4 | 10+/−7 | 0.16 |
| MDS-UPDRS II | -- | 11+/−7 | 8+/−4 | 14+/−8 | 0.07 |
| MDS-UPDRS III-OFF | -- | 33+/−9 | 32+/−9 | 34+/−10 | 0.61 |
| MDS-UPDRS III-ON | -- | 24+/−8 | 24+/−10 | 24+/−8 | 0.45 |
| MDS-UPDRS IV | -- | 2+/−2 | 2+/−2 | 2+/−3 | 0.15 |
Group difference p-values are reported for a three-way ANOVA (Controls and subtypes); Parkinson’s disease-specific measures were compared by t-test; Chi-squared tests were used for categorical comparisons.
3.2. Task performance
The number of recorded button presses while tapping during the OFF scan differed between the three groups, even after accounting for age (F3, 46 = 3.52, p = 0.038; PIGD OFF 156 +/−21; healthy controls 170 +/−9; TD OFF 169 +/−17, mean +/− SD). Tukey’s HSD post-hoc revealed that PIGD OFF had significantly fewer recorded taps than healthy controls (p = 0.029), but not TD OFF (p = 0.077). During the ON scan, the number of recorded taps did not differ between groups (F2, 45 = 2.19, p = 0.12). There was no significant interaction between drug and subtype (F1,23 = 0.040, p = 0.84) or main effect of drug (F1, 23 = 0.001, p = 0.99) or subtype (F1, 23 = 1.9, p = 0.18).
3.3. Whole brain BOLD activation analysis: OFF levodopa
During right hand finger tapping > rest, healthy controls (Fig. 1A) and all PD OFF (Fig. 1B) showed significant activation in motor areas including the contralateral sensorimotor cortex and thalamus, bilateral premotor cortex and supplementary motor area, and ipsilateral anterior cerebellum. PD OFF also showed activation in the left putamen (Fig. 1B). No areas showed significant activation during rest > task. Compared to healthy controls, all PD OFF had decreased activation in left primary motor cortex (BA 4; Fig. 1C, Table 2). There was decreased activation in left motor cortex in TD OFF relative to controls, but not PIGD OFF relative to controls (Table 2).
Fig. 1. Group activation and differences during tapping.
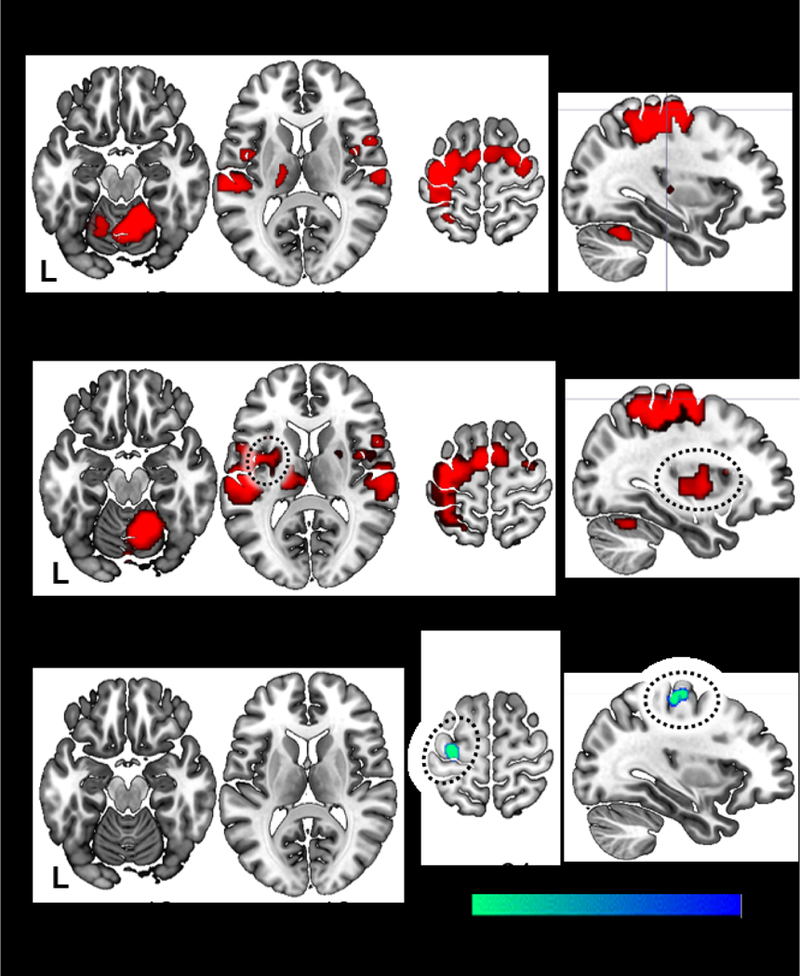
Healthy controls (A) and all Parkinson’s disease OFF (B) show similar patterns of functional activation in motor areas during a right-hand, paced finger tapping task relative to rest. All Parkinson’s disease OFF medication evidenced additional left putaminal activation (circled) and significantly decreased activation in the contralateral premotor cortex (BA 4) compared with controls (C).
Table 2.
Activation differences (Tapping > Rest) between groups
| Hemi | ROI | Brodmann Area | x | y | z | peak t-score | KE(mm3) | ||
|---|---|---|---|---|---|---|---|---|---|
| OFF levodopa | |||||||||
| All Parkinson’s disease < Controls | Left | Primary motor cortex | BA 4 | −32 | −24 | 60 | 4.9 | 746 | |
| TD < Controls | Left | Primary motor cortex | BA 4 | −32 | −25 | 60 | 4.4 | 567 | |
| ON levodopa | |||||||||
| PIGD > Controls | Left | Putamen | −28 | −6 | −5 | 4.5 | 1505 | ||
| TD < Controls | Right | Premotor cortex | BA 6 | 39 | −12 | 60 | 4.3 | 581 | |
| Superior Parietal Lobe | BA 7 | 26 | −54 | 43 | 3.8 | 583 | |||
| TD < PIGD | Right | Superior Temporal Lobe | BA 42 | 56 | −31 | 16 | 4.5 | 935 | |
| Right | Superior Temporal Lobe | BA 21 | 60 | −13 | −3 | 4.1 | 506 | ||
Peak p = 0.001, cluster-corrected α < 0.05; TD = tremor-dominant; PIGD = postural instability/gait difficulty; “ON” activations were observed one hour after 200mg levodopa.
3.4. Whole brain BOLD activation analysis: ON levodopa
No brain activation differences during the tapping task were observed between healthy controls and all PD ON; however, PD subtype comparisons showed several differences (Table 2). Compared with healthy controls, PIGD ON had greater activation within the left putamen, while TD ON had decreased activation in the right premotor cortex (BA 6) and superior parietal lobe (BA 7). PIGD ON also had increased activation in the right superior temporal gyrus (BA 42 and 21) compared to TD ON. Within-subject pairwise comparisons between ON and OFF states showed no effect of drug on brain activation within or between PD subtypes.
3.5. Correlation between clinical measures and brain activity during finger tapping
There were no significant correlations between parameter estimates from significant clusters in ON or OFF and clinical measures.
3.5. ROI analysis
During the ON state, there was a significant group difference in mean parameter estimates in the left pPut ROI (F2, 44 = 5.2, p = 0.009). Tukey’s HSD showed PIGD ON > healthy controls (p = 0.009) and PIGD ON > TD ON (p = 0.035; Fig. 2). During the OFF state, there was no group difference in mean parameter estimates. Parameter estimates for the control ROIs, right pPut, left HCd, and right HCd did not differ significantly, regardless of levodopa status.
Fig. 2. Postural instability/gait difficulty patients show increased activation within putamen after levodopa.
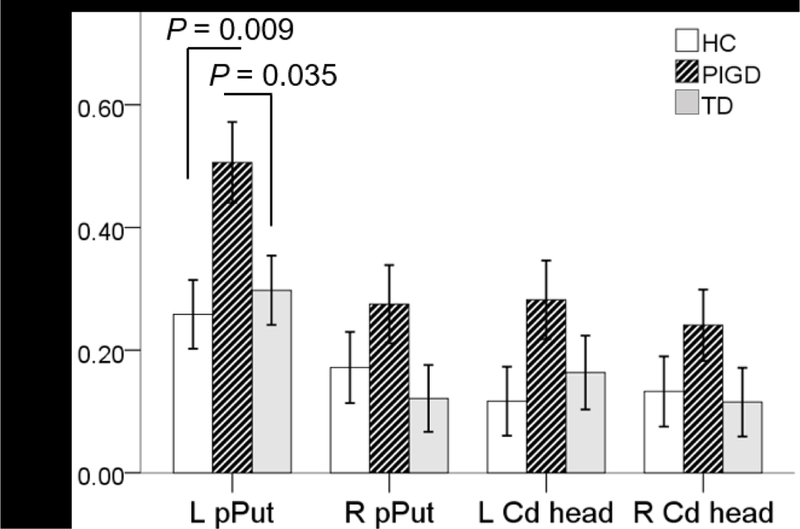
Extracted beta-weights from four striatal regions reflect BOLD activation during tapping > rest in each group. Compared to controls and TD ON, PIGD ON had significantly greater estimates from the posterior putamen contralateral to the tapping. None of the control, striatal regions showed significant differences. Error bars represent standard error of the mean. L = left; R = Right; pPut = posterior putamen; HCd = head of the caudate
3.6. PPI analysis
The subtype*medication status interaction term was significant in multiple motor regions (Table 3, Fig. 3). Effective connectivity differed between subtype and medication between the left pPut seed and the left dorsolateral prefrontal cortex (BA 9), bilateral thalamus, bilateral supplementary motor area (BA 6), and bilateral middle cingulate gyrus (BA 24; Fig. 3A). Extracted parameter estimates indicated that in TD patients, levodopa administration led to increasing connectivity between the left pPut seed and these regions while such an effect was not seen in the PIGD patients (Fig. 3B). These interaction results were not associated with disease duration. Outside of the regions showing interaction effects, no main effect of subtype or medication status yielded significant results.
Table 3.
Task-based effective connectivity interactions between Parkinson’s disease subtypes OFF and ON levodopa
| Hemisphere | ROI | Brodmann Area | x | y | z | peak t-score | kE (mm3) |
|---|---|---|---|---|---|---|---|
| Left | Dorsolateral prefrontal cortex | BA 9 | -29 | 38 | 29 | 4.69 | 2160 |
| Bilateral | Thalamus (VLN) | 7 | -10 | 5 | 4.33 | 3348 | |
| Bilateral | Supplemental motor area | BA 6 | 7 | 5 | 65 | 4.32 | 3699 |
| Bilateral | Middle cingulate gyrus | BA 24 | -8 | 11 | 35 | 3.80 | 3375 |
Seed region = left posterior putamen; peak p = 0.005, cluster-corrected α < 0.05; “ON” connectivity was observed one hour after 200mg levodopa. VLN = ventrolateral nucleus
Fig. 3. PD motor subtype by drug interaction on connectivity between putamen and motor network.
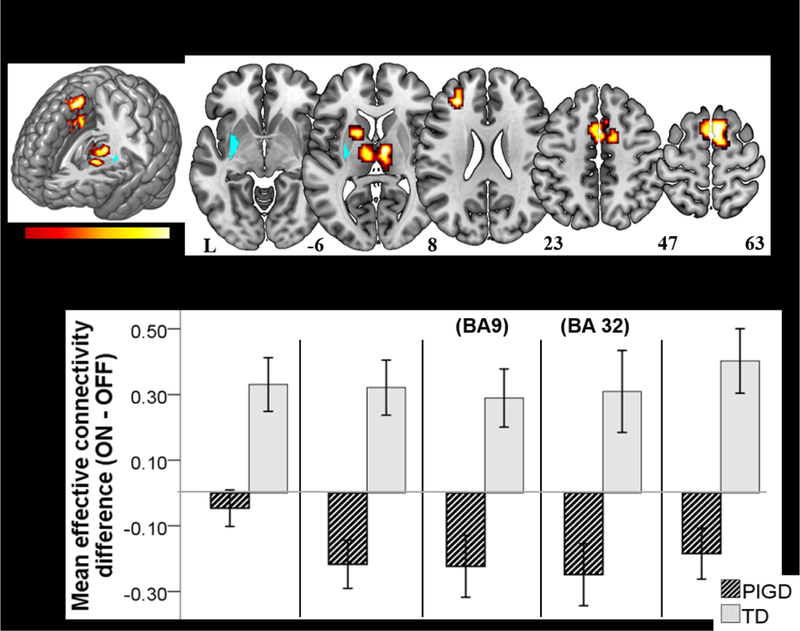
(A) Effective connectivity between left posterior putamen seed (cyan) and motor network areas during right hand finger tapping was affected by levodopa differently in the TD and PIGD subtypes. Maps reflect voxel-wise P = 0.005, cluster-corrected α = 0.05. (B) The magnitude and direction of connectivity changes ON minus OFF levodopa for both motor subtypes are displayed for five regions showing a subtype by medication effect. Overall, connectivity with the left posterior putamen increased in TD ON vs. OFF, but was relatively unchanged or decreased in PIGD ON vs. OFF. Error bars represent +/− one standard error of the mean.
Discussion
During a paced, right-hand finger-tapping task, we found that PD patients of the PIGD subtype on levodopa showed significantly increased activation solely within the left putamen relative to TD patients on levodopa as well as to healthy controls. The levodopa-induced hyperactivation within the left putamen in PIGD patients was not accompanied by an increase in effective connectivity between left posterior putamen seed and a distributed motor network. In contrast, the TD motor subtype patients did show a levodopa-induced increase in effective connectivity between the left posterior putamen and CSTC motor network during the finger-tapping task. Together, these results support the presence of an underlying pathophysiological difference between PD motor subtypes that can be elicited using a dopaminergic medication challenges during a motor task.
Compared to healthy controls, PD OFF across subtype demonstrated hypoactivation of the left primary motor cortex during the right handed motor task (BA 4; Fig. 1B). A number of prior studies have reported increased activation within contralateral primary motor cortex in PD [Eckert et al., 2006; Haslinger et al., 2001; Sabatini et al., 2000; Yu et al., 2007]; however, the patient cohorts included in these reports were largely non-TD patients. To investigate whether our observation could be attributable to one or both subtypes, we separately compared TD OFF and PIGD OFF to controls and found that the motor cortex hypoactivation relative to healthy controls was largely driven by the TD OFF patients. Our results are, therefore, in line with a recent study of 18 TD and 2 non-TD patients reporting motor cortex hypoactivation in PD after dopamine replacement therapy washout relative to controls [Burciu et al., 2015]. Notably, our Parkinson’s patients ON did not evidence any activation changes within primary or secondary motor cortices compared with controls, potentially reflecting a restoration of motor network function in the TD ON group and consistent with the “normalizing” effects of dopamine replacement therapy noted in other functional imaging studies where activation changes were no longer detectable after dopaminergic medication (reviewed in [Prodoehl et al., 2014; Tessitore et al., 2014].
The pathological hallmark in PD is the progressive loss of dopaminergic neurons in the substantia nigra resulting in a deficiency of dopamine within the striatum—the major input structure for the basal ganglia [Braak et al., 2006]. The putamen in particular has been implicated in motor pathology (reviewed by [Jellinger, 2012]) in PD, with specific emphasis on its posterior aspects due to the corticotopic organization of the basal ganglia [Haber, 2003] and the posterior to anterior, caudal to rostral pattern of disease progression [Nandhagopal et al., 2009; Spiegel et al., 2007; Sterling et al., 2013]. Changes in the putamen have been linked with symptomatology that defines PD motor subtypes [Bernheimer et al., 1973; Rosenberg-Katz et al., 2013; Vervoort et al., 2016]. Specifically, SPECT studies found evidence of an association between putaminal uptake of DaT tracer and worse rigidity and more pronounced dopaminergic loss in the posterior putamen in non-TD and mixed subtype compared with TD [Eggers et al., 2011; Spiegel et al., 2007]. These findings, together with our subtype comparisons that revealed that left putamen activity during finger tapping with the right hand was significantly increased by levodopa only in PIGD patients, provide further evidence of a role for the putamen in phenotypic subtype manifestations.
A key, novel finding in our study is that levodopa lead to divergent tapping-induced coupling responses within the motor network in Parkinson’s patients with PIGD and TD motor subtypes that could not be accounted for by differences in disease stage or duration. TD ON relative to TD OFF showed significantly increased effective connectivity between the left pPut seed and other motor network regions while PIGD ON relative to PIGD OFF showed no significant changes (Fig. 3B). This finding provides evidence of an underlying pathophysiological difference in the way distinct motor subtypes in PD respond to levodopa. Clinically, symptoms of the PIGD subtype are known to respond more poorly to levodopa than other motor symptoms. It is possible that differences in levodopa responsiveness between PD motor subtypes could stem from less amino-acid decarboxylase (AADC) bioavailability, and subsequently lower conversion of levodopa to dopamine, in PIGD patients [Iacono, 1997]. This, however, is unlikely to have significantly confounded our results because our patients were all in early to mid-stages of disease, there was no significant difference found in LED between subtypes, and there was no significant difference in the levodopa-induced changes in MDS-UPDRS motor scores between our cohorts. We therefore speculate that this poor response may be due to a functional disconnection of the posterior putamen, as evidenced in our study by increased putaminal activation without concomitant increases in motor network connectivity in PIGD ON. In other words, neural signals propagating to or originating from the putamen in PIGD patients are not being relayed to the major output pathways of the basal ganglia (globus pallidus interna and substantia nigra pars reticulate), on to the thalamus, and back to the cortex [Redgrave et al., 2010].
TD ON relative to TD OFF, in contrast to PIGD ON relative to PIGD OFF, showed increased effective connectivity between the left pPut seed and the distributed motor network, including bilateral thalami, left dorsolateral prefrontal cortex, middle cingulate gyrus, and supplementary motor area. This finding parallels findings of decreased resting-state functional connectivity measures in the posterior putamen in non-tremor dominant patients [Hacker et al., 2012], but not in TD patients [Chen et al., 2015; Zhang et al., 2014]. Additionally, our results coincide with the putamen signaling being less affected in TD than PIGD [Chen et al., 2015; Spiegel et al., 2007; Sterling et al., 2013], and suggest a more responsive putamen enables CSTC connectivity to be more modulated by levodopa.
Our study is limited by a modest sample size, though the groups are larger than many other prior reports (e.g., [Lewis et al., 2011; Sabatini et al., 2000; Tessa et al., 2012; Yu et al., 2007]. To mitigate possible false positives, we used high statistical significance thresholds as recently suggested for fMRI activation studies [Eklund et al., 2016; Woo et al., 2014]. Despite our careful efforts to match performance through experimental design, PIGD patients OFF had fewer recorded taps than TD OFF and healthy controls. It is unclear whether the difference is due to insufficient pressure on the button or slowness in release [Yu et al., 2007], but EMG recordings during tapping support that all participants were fully compliant with the task. Nevertheless, we included recorded taps as a covariate in the second-level analyses to minimize the effects that any performance differences may have had on brain activations related to motor output. An additional limitation is that all patients were scanned in a practical OFF state at a minimum of 12 hours after their last dose of medication. While the longer half-lives of dopamine agonists and the known long duration responses to anti-parkinsonian medications could have different lingering effects, we did not find a significant difference between the types of dopaminergic medications taken by the different subtypes (Table 1). Nevertheless, it remains to be investigated whether PD motor subtypes differ in their short and long duration withdrawal responses. Lastly, disease stage as measured by Hoehn and Yahr score was significantly higher in our PIGD cohort. It is possible that this disease stage difference could have impacted our findings. We, however, suspect this difference stems from a limitation in the Hoehn and Yahr scale in that it is weighted heavily toward the symptoms of postural instability and gait disability as the primary index of disease severity [Goetz 2004]. In our study cohorts, the other indices of disease severity including disease duration, LED requirements, MDS-UPDRS motor scores, and MDS-UPDRS total scores were not significantly different. Thus, the differences in Hoehn and Yahr scores is unlikely to have contributed significantly to our findings.
In conclusion, we provide novel evidence of divergent motor network effective connectivity responses to levodopa in two different Parkinson’s motor subtypes. Our findings extend our understanding of distinct pathophysiological changes underlying PD motor subtypes. Specifically, levodopa produced significantly increased activation in the contralateral putamen in PIGD patients relative to TD patients during right hand finger tapping without altering effective connectivity within the motor network as it did in the TD patients. This supports the presence of greater altered motor circuit dynamics in the PIGD subtype than in the TD subtypes. Together, our results suggest that PD motor subtypes may be identified by their divergent motor network effective connectivity responses to levodopa during a tapping task. Greater characterization of mechanisms underlying the distinct phenotypes could result in better targeted treatments and clinical trials.
Acknowledgements
We thank Deb Singel for scanning the participants. We also thank Drs. Maureen Leehey and Jason Tregellas for valuable discussions and guidance, and Dr. Chris Kennel for assisting with data analysis.
Footnotes
Conflict of Interest Statements
Dr. Mohl and Ms. Shelton have no relevant conflicts of interest concerning the research related to this manuscript.
Dr. Berman has no relevant conflicts of interest concerning the research related to this manuscript. He has received research grant support from the NIH, Dystonia Coalition, Dystonia Medical Research Foundation, and Benign Essential Blepharospasm Research Foundation. He also serves on the Medical Advisory Boards for the Benign Essential Blepharospasm Research Foundation and the National Spasmodic Torticollis Association.
Dr. Tanabe has no relevant conflicts of interest concerning the research related to this manuscript. She has received research grant support from the NIH.
References
- Alves G, Larsen J, Emre M (2006): Changes in motor subtype and risk for incident dementia in Parkinson’s disease. Mov Disord 10.1002/mds.20897. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ (2005): Unified segmentation. Neuroimage 26:839–51. [DOI] [PubMed] [Google Scholar]
- Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F (1973): Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci 20:415–455. [DOI] [PubMed] [Google Scholar]
- Braak H, Bohl JR, Müller CM, Rüb U, de Vos RAI, Del Tredici K (2006): Stanley Fahn Lecture 2005: The staging procedure for the inclusion body pathology associated with sporadic Parkinson’s disease reconsidered. Mov Disord 21:2042–2051. [DOI] [PubMed] [Google Scholar]
- Buhmann C, Glauche V, Stürenburg HJ, Oechsner M, Weiller C, Büchel C (2003): Pharmacologically modulated fMRI--cortical responsiveness to levodopa in drug-naive hemiparkinsonian patients. Brain 126:451–461. [DOI] [PubMed] [Google Scholar]
- Burciu RG, Ofori E, Shukla P, Planetta PJ, Snyder AF, Li H, Hass CJ, Okun MS, McFarland NR, Vaillancourt DE (2015): Distinct patterns of brain activity in progressive supranuclear palsy and Parkinson’s disease. Mov Disord 30:1248–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerasa A, Hagberg GE, Peppe A, Bianciardi M, Gioia MC, Costa A, Castriota-Scanderbeg A, Caltagirone C, Sabatini U (2006): Functional changes in the activity of cerebellum and frontostriatal regions during externally and internally timed movement in Parkinson’s disease. Brain Res Bull 71:259–269. [DOI] [PubMed] [Google Scholar]
- Chen H, Wang Z, Fang J, Gao L, Ma L, Wu T, Hou Y, Zhang J, Feng T (2015): Different Patterns of Spontaneous Brain Activity between Tremor‐Dominant and Postural Instability/Gait Difficulty Subtypes of Parkinson’s Disease: A Resting‐State fMRI Study. CNS Neurosci Ther 21:855–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert T, Peschel T, Heinze H-J, Rotte M (2006): Increased pre-SMA activation in early PD patients during simple self-initiated hand movements. J Neurol 253:199–207. [DOI] [PubMed] [Google Scholar]
- Eggers C, Kahraman D, Fink GR, Schmidt M, Timmermann L (2011): Akinetic-rigid and tremor-dominant Parkinson’s disease patients show different patterns of FP-CIT Single photon emission computed tomography. Mov Disord 26:416–423. [DOI] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H (2016): Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci 113:7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsinger C, Rao S, Zimbelman J (2003): Neural basis for impaired time reproduction in Parkinson’s disease: an fMRI study. J Int Neuropsychol Soc 10.1017/S1355617703970123. [DOI] [PubMed] [Google Scholar]
- Friston KJ (2011): Functional and effective connectivity: a review. Brain Connect 1:13–36. [DOI] [PubMed] [Google Scholar]
- Friston K, Buechel C, Fink G, Morris J, Rolls E, Dolan R (1997): Psychophysiological and modulatory interactions in neuroimaging. Neuroimage 6:218–29. [DOI] [PubMed] [Google Scholar]
- Gibb WR, Lees AJ (1988): The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry 51:745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz CG, Poewe W, Rascol O, Sampaio C, Stebbins GT, Counsell C, Giladi N, Holloway RG, Moore CG, Wenning GK and Yahr MD (2004). Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations the Movement Disorder Society Task Force on rating scales for Parkinson’s disease. Movement disorders, 19(9), 1020–1028. [DOI] [PubMed] [Google Scholar]
- Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stern MB, Dodel R, Dubois B, Holloway R, Jankovic J, Kulisevsky J, Lang AE, Lees A, Leurgans S, LeWitt PA, Nyenhuis D, Olanow CW, Rascol O, Schrag A, Teresi JA, van Hilten JJ, LaPelle N, Movement Disorder Society UPDRS Revision Task Force (2008): Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 23:2129–2170. [DOI] [PubMed] [Google Scholar]
- Göttlich M, Münte TF, Heldmann M, Kasten M, Hagenah J, Krämer UM (2013): Altered resting state brain networks in Parkinson’s disease. PloS One 8:e77336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber S (2003): The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat [DOI] [PubMed] [Google Scholar]
- Haslinger B, Erhard P, Kämpfe N, Boecker H, Rummeny E, Schwaiger M, Conrad B, Ceballos-Baumann A (2001): Event-related functional magnetic resonance imaging in Parkinson’s disease before and after levodopa. Brain 124:558–570. [DOI] [PubMed] [Google Scholar]
- Herz DM, Eickhoff SB, Løkkegaard A, Siebner HR (2014): Functional neuroimaging of motor control in Parkinson’s disease: a meta-analysis. Hum Brain Mapp 35:3227–3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono RP, Kuniyoshi SM, Ahlman JR, Zimmerman GJ, Maeda G, & Pearlstein RD (1997). Concentrations of indoleamine metabolic intermediates in the ventricular cerebrospinal fluid of advanced Parkinson’s patients with severe postural instability and gait disorders. Journal of neural transmission, 104(4–5), 451–459. [DOI] [PubMed] [Google Scholar]
- Jankovic J, M M, Carter J, Gauthier S, Goetz C, Golbe L, Huber S, Koller W, Olanow C, Shoulson I (1990): Variable expression of Parkinson’s disease: a base-line analysis of the DATATOP cohort. The Parkinson Study Group 40:1529–34. [DOI] [PubMed] [Google Scholar]
- Jankovic J, Kapadia AS (2001): Functional Decline in Parkinson Disease. Arch Neurol 58:1611. [DOI] [PubMed] [Google Scholar]
- Jellinger KA (2012): Neuropathology of sporadic Parkinson’s disease: evaluation and changes of concepts. Mov Disord 27:8–30. [DOI] [PubMed] [Google Scholar]
- Kim J, Horwitz B (2008): Investigating the neural basis for fMRI-based functional connectivity in a blocked design: application to interregional correlations and psycho-physiological interactions. Magn Reson Imaging 26:583–593. [DOI] [PubMed] [Google Scholar]
- Lewis MM, Du G, Sen S, Kawaguchi A, Truong Y, Lee S, Mailman RB, Huang X (2011): Differential involvement of striato- and cerebello-thalamo-cortical pathways in tremor-and akinetic/rigid-predominant Parkinson’s disease. Neuroscience 177:230–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucetti C, Diciotti S, Baldacci F, Tessa C, Ginestroni A, Cecchi P, Paoli L, Del Dotto P, Ceravolo R, Mascalchi M, Bonuccelli U (2014): Dopamine Agonist Modifies Cortical Activity in Parkinson Disease: A Functional Neuroimaging Study. Clin Neuropharmacol 37:166–172. [DOI] [PubMed] [Google Scholar]
- McLaren DG, Ries ML, Xu G, Johnson SC (2012): A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. NeuroImage 61:1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandhagopal R, Kuramoto L, Schulzer M, Mak E, Cragg J, Lee CS, McKenzie J, McCormick S, Samii A, Troiano A, Ruth TJ, Sossi V, de la Fuente-Fernandez R, Calne DB, Stoessl AJ (2009): Longitudinal progression of sporadic Parkinson’s disease: a multi-tracer positron emission tomography study. Brain 132:2970–2979. [DOI] [PubMed] [Google Scholar]
- O’Reilly JX, Woolrich MW, Behrens T, Smith SM, Heidi J-B (2012): Tools of the trade: psychophysiological interactions and functional connectivity. Soc Cogn Affect Neur 7:604–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauli WM, Randall CO, Yarkoni T, Wager TD (2016): Regional specialization within the human striatum for diverse psychological functions. Proc Natl Acad Sci USA 113:1907–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prodoehl J, Burciu RG, Vaillancourt DE (2014): Resting state functional magnetic resonance imaging in Parkinson’s disease. Curr Neurol Neurosci Rep 14:448. [DOI] [PubMed] [Google Scholar]
- Prodoehl J, Planetta PJ, Kurani AS, Comella CL, Corcos DM, Vaillancourt DE (2013): Differences in brain activation between tremor- and nontremor-dominant Parkinson disease. JAMA Neurol 70:100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgrave P, Rodriguez M, Smith Y, Rodriguez-Oroz MC, Lehericy S, Bergman H, Agid Y, DeLong MR, Obeso JA (2010): Goal-directed and habitual control in the basal ganglia: implications for Parkinson’s disease. Nat Rev Neurosci 11:760–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg-Katz K, Herman T, Jacob Y, Giladi N, Hendler T, Hausdorff JM (2013): Gray matter atrophy distinguishes between Parkinson disease motor subtypes. Neurology 80:1476–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini U, Boulanouar K, Fabre N, Martin F, Carel C, Colonnese C, Bozzao L, Berry I, Montastruc JL, Chollet F, Rascol O (2000): Cortical motor reorganization in akinetic patients with Parkinson’s disease: a functional MRI study. Brain 123 ( Pt 2):394–403. [DOI] [PubMed] [Google Scholar]
- Spetsieris PG, Ma Y, Dhawan V, Eidelberg D (2009): Differential diagnosis of parkinsonian syndromes using PCA-based functional imaging features. NeuroImage 45:1241–1252. [DOI] [PubMed] [Google Scholar]
- Spiegel J, Hellwig D, Samnick S, Jost W, Möllers M-O, Fassbender K, Kirsch C-M, Dillmann U (2007): Striatal FP-CIT uptake differs in the subtypes of early Parkinson’s disease. J Neural Transm 114:331–335. [DOI] [PubMed] [Google Scholar]
- Sterling NW, Du G, Lewis MM, Dimaio C, Kong L, Eslinger PJ, Styner M, Huang X (2013): Striatal shape in Parkinson’s disease. Neurobiol Aging 34:2510–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessa C, Lucetti C, Diciotti S, Paoli L, Cecchi P (2012): Hypoactivation of the primary sensorimotor cortex in de novo Parkinson’s disease. Neuroradiology http://link.springer.com/article/10.1007/s00234-011-0955-y. [DOI] [PubMed] [Google Scholar]
- Tessa C, Lucetti C, Diciotti S, Baldacci F, Paoli L, Cecchi P, Giannelli M, Ginestroni A, Del Dotto P, Ceravolo R, Vignali C, Bonuccelli U, Mascalchi M (2010): Decreased and increased cortical activation coexist in de novo Parkinson’s disease. Exp Neurol 224:299–306. [DOI] [PubMed] [Google Scholar]
- Tessitore A, Giordano A, De Micco R, Russo A, Tedeschi G (2014): Sensorimotor connectivity in Parkinson’s disease: the role of functional neuroimaging. Front Neurol 5:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vervoort G, Alaerts K, Bengevoord A, Nackaerts E, Heremans E, Vandenberghe W, Nieuwboer A (2016): Functional connectivity alterations in the motor and fronto-parietal network relate to behavioral heterogeneity in Parkinson’s disease. Parkinsonism Relat Disord 24:48–55. [DOI] [PubMed] [Google Scholar]
- Vu TC, Nutt JG, Holford NHG (2012): Progression of motor and nonmotor features of Parkinson’s disease and their response to treatment. Br J Clin Pharmacol 74:267–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo C-W, Krishnan A, Wager TD (2014): Cluster-extent based thresholding in fMRI analyses: Pitfalls and recommendations. NeuroImage 91:412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Ma Y, Zheng Z, Peng S, Wu X, Eidelberg D, Chan P (2015): Parkinson’s disease-related spatial covariance pattern identified with resting-state functional MRI. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab 35:1764–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Wang L, Chen Y, Zhao C, Li K, Chan P (2009): Changes of functional connectivity of the motor network in the resting state in Parkinson’s disease. Neurosci Lett 460:6–10. [DOI] [PubMed] [Google Scholar]
- Yu H, Sternad D, Corcos DM, Vaillancourt DE (2007): Role of hyperactive cerebellum and motor cortex in Parkinson’s disease. Neuroimage 35:222–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Liu X, Chen J, Liu B (2014): Distinguishing patients with Parkinson’s disease subtypes from normal controls based on functional network regional efficiencies. PloS One 9:e115131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Wang J, Liu X, Chen J, Liu B (2015): Aberrant Brain Network Efficiency in Parkinson’s Disease Patients with Tremor: A Multi-Modality Study. Front Aging Neurosci 7:169. [DOI] [PMC free article] [PubMed] [Google Scholar]


