Abstract
Purpose
Multiparametric-MRI (MP-MRI) may be beneficial in the search for rational ways to decrease prostate cancer interventions in patients on active surveillance (AS). The goal of this study was to apply a previously generated nomogram based on MP-MRI to predict AS eligibility repeat biopsy outcomes.
Materials and Methods
85 patients were reviewed who met AS criteria at entry based on their initial biopsy and then underwent 3.0T MP-MRI with subsequent MRI/Ultrasound (US) fusion guided prostate biopsy between 2007–2012. The accuracy of a previously published nomogram was assessed in patients on AS prior to confirmatory biopsy. For each cut-point value, the number of “biopsies avoided” (i.e. reliance on MRI alone without re-biopsy) was determined over the full range of nomogram cutoffs.
RESULTS
The performance of the MP-MRI AS nomogram was assessed based on a decision to biopsy at various nomogram generated probabilities. Based on cutoff probabilities ranging from 19% to 32% on the nomogram, the number of patients that could be spared a repeat biopsy ranged from 27% to 68% of the AS cohort. The sensitivity of the test in this interval ranged from 97% to 71% and negative predictive value ranged from 91% to 81%.
CONCLUSIONS
MP-MRI based nomograms may reasonably decrease the number of repeat biopsies in patients on AS by as much as 68%. Analysis over the full range of nomogram generated probabilities allows patient and caregiver preference based decision on the risk assumed for the benefit of fewer repeat biopsies.
Keywords: prostate neoplasms, active surveillance, magnetic resonance imaging, early detection of cancer
Introduction
The majority of prostate cancer (PCa) diagnosed in the United States prove to be low-grade, low-volume disease1. For these men, American Urologic Association clinical guidelines have recommended active surveillance (AS) as a management option2. Practice is somewhat at variance with these guidelines as many patients and their fear that there is unrecognized higher-grade tumor present3.
Multiparametric prostate MRI (MP-MRI) combined with MRI/Ultrasound (US) fusion-guided biopsy is highly sensitive for high-grade PCa and allows for more reliable characterization of PCa with decreased risk of upgrading4, 5. A predictive model was designed and tested to determine AS eligibility for men with PCa that relies solely on MP-MRI to avoid the subjectivity associated with clinical staging, the risk of understaging of prostate biopsy, and the variability in serum PSA often observed in clinical practice6. This nomogram uses the 1) number of lesions on MRI, 2) lesion suspicion level based on MP-MRI results (low, moderate, high), and 3) lesion density to predict patients’ AS eligibility based on repeat biopsy AS criteria with excellent discriminative ability (AUC = 0.71).
While nomograms provide a user-friendly bedside model to estimate objectively a patient’s probability of having indolent cancer, converting a probability into a concrete and practical recommendation can often be challenging. The patient faces a decision which is binary: Do they continue AS or opt for treatment? Yet, a nomogram provides a continuous estimate of the probability that their tumor is safely amenable to AS. In this study, we aimed to optimize the utility of our previously described MRI-based nomogram to predict repeat biopsy to confirm AS eligibility for clinical decision-making and enhanced patient-clinician communication.
Materials and methods
Study population
A retrospective review was performed of an Institutional Review Board-approved study at the National Cancer Institute, National Institutes of Health (ClinicalTrials.gov identifier: NCT00102544). Study cohort enrollment was from August 2007 through August 2012. The initial standard extended-sextant 12-core biopsy pathology was reviewed by a single pathologist and patients were included if they met the Johns Hopkins AS criteria (PSA density ≤ 0.15, ≤ 2 positive cores, ≤ 50% tumor in any core, Gleason score ≤ 6, and stage T1c).7 All patients who had not already had PSA progression underwent baseline pre-biopsy MP-MRI. If targetable lesions were identified on MP-MRI, the patients subsequently underwent confirmatory TRUS-guided 12-core extended-sextant prostate biopsy and targeted MRI/US fusion-guided biopsies with electromagnetic tracking, as previously described.8, 9 Candidacy for continued active surveillance was based on repeat biopsy results. The targeted biopsy results were also incorporated into the assessment of continued candidacy with the criteria of ≤ 50% tumor in any core, Gleason score ≤ 6.
Imaging
MP-MRI was performed using the combination of an endorectal coil (BPX-30, Medrad, Pittsburgh, PA, USA) tuned to 127.8 MHz and a cardiac coil (16-channel) (SENSE, Philips Healthcare, Cleveland, OH) on a 3T magnet (Achieva, Philips Healthcare, Cleveland, OH). The endorectal coil balloon was distended with perfluorocarbon (3 mol/L-Fluorinert, 3M, St. Paul, MN, USA) to a volume of approximately 50 mL to reduce susceptibility artifacts induced by air in the coil’s balloon. MRI sequence parameters were detailed in a prior study, and included triplanar T2 weighted (T2W) turbo-spin-echo (TSE), diffusion weighted (DW) MRI, 3 dimensional MR Spectroscopic Imaging, axial pre-contrast T1 weighted (T1W) MRI, and axial 3D fast field echo dynamic contrast-enhanced (DCE) MRI.10
For multi-parametric MRI analysis on T2W MR and apparent diffusion coefficient (ADC) maps of DW MRI, the criterion for a “visible” lesion was a well circumscribed, round-ellipsoid low-signal-intensity region within the prostate gland.10 The 3D-MR spectroscopy analysis evaluated choline/citrate (Cho/Cit) ratios. Voxels were considered abnormal when the Cho/Cit ratio was 3 or more standard deviations (SD) above the mean healthy Cho/Cit ratio value (≥0.373), which was defined as 0.13+/−0.081 based on prior results.10 DCE MR images were evaluated by direct visual interpretation of raw dynamic enhanced T1W images and the diagnostic criteria for PCa included a focus of asymmetric, early and intense enhancement with rapid wash out compared to the background.10 The MP-MRI score assigned to each lesion is based on the number of positive sequences (e.g. positive T2W, Diffusion weighted, MR spectroscopy and DCE-MRI is indicative of a high score). In such a way, every lesion was analyzed using a non-weighted scoring system where lesion scores are categorized as low, moderate and high.11 The PIRADS system was not yet in use at the NCI during the period in which patients were accrued for this study. Although not an exact relationship, the low, and high suspicion on the NIH scale do seem to correlate with PIRADS 1–2 and PIRADS 5 scores. The largest diameter of each lesion was calculated manually on a picture archiving and communication system (PACS) workstation (Carestream, Inc, USA). Again, each visible lesion was manually segmented on a research software platform (iCAD, Nashua, NH, USA) blinded to the clinical and histopathologic data. The tumor volume was determined with the same software after manual segmentation on MRI. For segmenting tumors on MRI, T2W MRI, ADC maps of DW MRI and DCE MRI sequences were used in conjunction although final regions of interest were drawn on T2W MRI for targeting during fusion biopsy. Total MRI prostate volumes were manually obtained for each patient using a semi-automated software.12
Data Analysis
A previously described nomogram to predict disqualification from AS based on MP-MRI characteristics was utilized in this study6. To further evaluate the nomogram’s performance, the nomogram-generated probability was calculated for every patient in that original cohort. Cut-points were then assessed for the nomogram-generated probability of disqualification from AS candidacy based on repeat biopsy. Negative predicted values (NPV) and sensitivity were calculated by comparing the nomogram-generated recommendation against the confirmatory biopsy results. Decision curve analysis was performed utilizing R code made publicly available by Andrew Vickers (http://www.mskcc.org/research/epidemiology-biostatistics/health-outcomes/decision-curve-analysis-0). Leave-one-out cross-validation analyses of the logistic regression model development were performed to evaluate potential data overfitting.
Results
Eighty-five patients were reviewed of whom 25 were no longer eligible for AS on repeat biopsy. Disqualification from AS occurred in 6 men based on target biopsy alone, 6 men on standard biopsy alone, and in 13 men on both biopsy types. The mean age of the population was 60.2 years, mean PSA of 4.8ng/ml, and mean PSA density of 0.09. The mean time from first biopsy to confirmatory targeted MRI/US fusion biopsy was 302 days. A nomogram was derived as previously described to predict the likelihood of disqualification from AS upon repeat biopsy (Figure 1). Within the nomogram, patient MRI traits are input into the nomogram, and the output is the probability that upon repeat biopsy (consistent of a standard 12-core biopsy and targeted biopsy of all MR lesions), the individual will demonstrate pathology which makes him unsuitable for continued AS for his PCa. The cohort from which this predictive model was derived consisted of all men who initially were diagnosed with PCa and amenable to active surveillance. All patients underwent MP-MRI with confirmatory biopsy and findings on MRI were correlated to biopsy outcomes. Using this nomogram, a clinician could theoretically decrease the number or frequency of repeat biopsies that are required to follow a patient with low risk PCa while still closely following the patient. If the nomogram scored above the threshold value, then a repeat biopsy was indicated. If it scored below that value, a repeat biopsy could be avoided. We sought to better characterize the optimal nomogram probability below which a repeat biopsy could safely be avoided or delayed.
Figure 1.
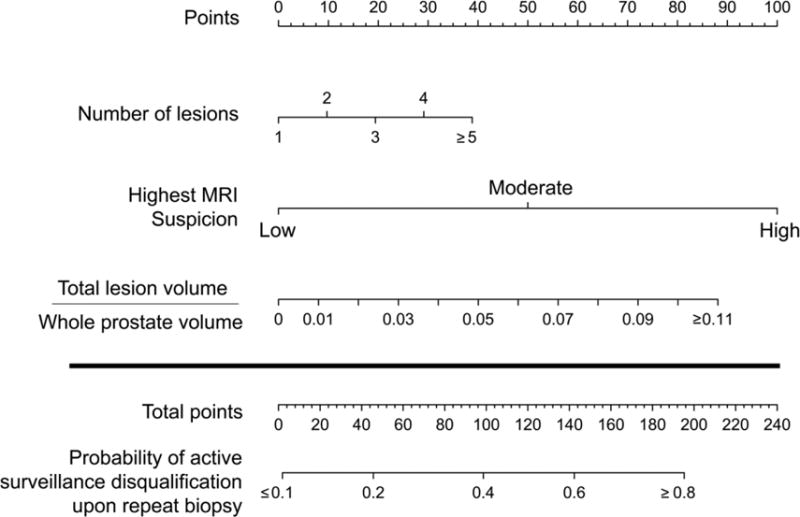
Nomogram to predict probability of disqualification from active surveillance on repeat biopsy utilizing multiparametric-MRI features of number of lesions seen in the prostate, the highest prostate cancer suspicion score of all of the lesions, and the lesion density defined as the total lesion volume over the total prostate volume
Figure 2 demonstrates a waterfall plot of the probabilities of disqualification from AS compared to the actual outcome of AS candidacy based on prostate biopsy. Analysis was performed on the entire cohort to determine how well the nomogram differentiated men who qualified for continued AS versus those who were disqualified on repeat biopsy for AS. We found that this nomogram correlated well with the actual outcome of AS candidacy. For example, 25 patients were within the nomogram probability range of 10–20% predicted rate of disqualification from AS and 3 of these 25 (12%) actually demonstrated progression on repeat biopsy. Similarly, 6 patients were in the nomogram probability range of 60% or higher and 4 out of these 6 (66%) demonstrated repeat progression on repeat biopsy.
Figure 2.
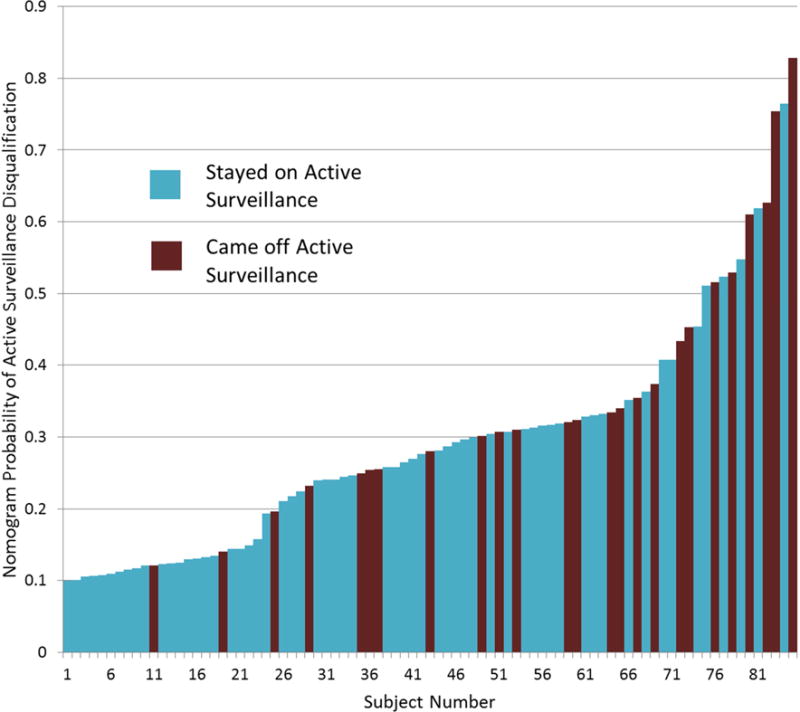
Waterfall plot of all patients with associated nomogram generated probability of disqualification from active surveillance on repeat biopsy color coded by actual biopsy result.
Figure 3a shows the nomogram outperforms any solitary characteristic alone by synthesizing inputs of number of lesions, highest suspicion score, and lesion density. This decision curve analysis quantifies the net benefit of using the nomogram by comparing the true positive and false positive counts (while adjusting for the ratio of expected benefit from the repeat prostate biopsy versus the expected benefit of avoiding a repeat prostate biopsy). It demonstrates that selectively deferring a biopsy using the nomogram was provided a net benefit compared to the strategy of simply performing a biopsy on all patients on AS. Figure 3b demonstrates that the net benefit peaks at the probability cutoff of 32% on the nomogram (above 32% repeat biopsy is indicated, and below 32% continued surveillance is suggested).
Figure 3.
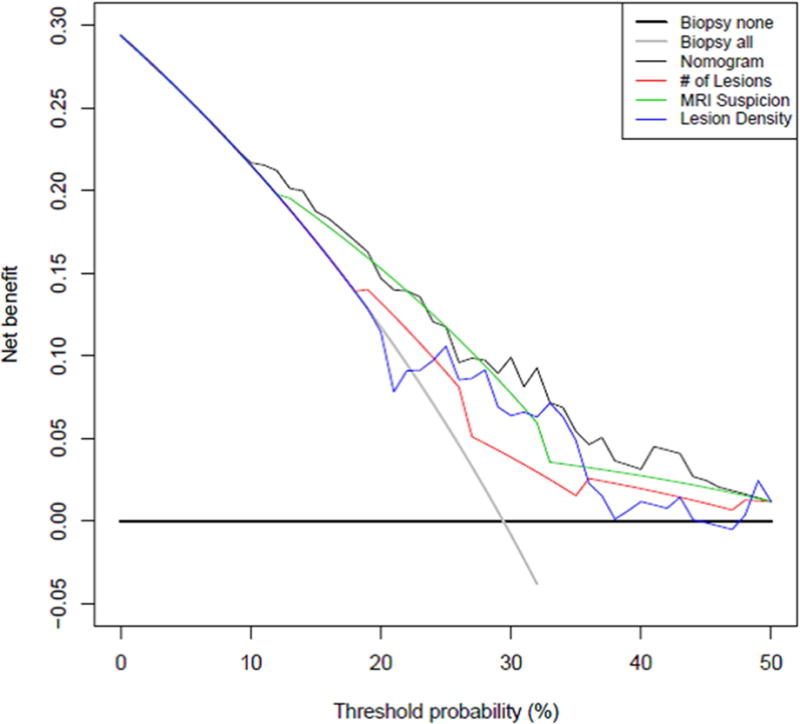
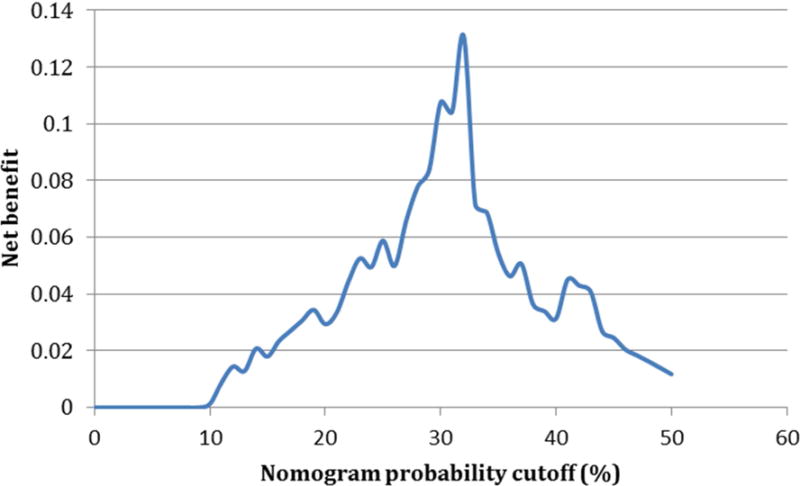
a: Decision curve analysis demonstrating net benefit of utilizing each nomogram component and the nomogram itself for determination of repeat biopsy on active surveillance patients Net benefit in this analysis was defined as the additional correct identification of an individual who is no longer a good candidate for AS. The curves show that the combination of all the factors leads to the most reliable identification of men who are not good candidates for continued AS. b: Additional net benefit at any given nomogram probability cutoff over the strategy of non-selective repeat biopsy on all men utilizing the nomogram to select patient for repeat biopsy. This figure shows that the ability of the nomogram to correctly identify men for AS increases until a certain threshold probability on the nomogram at which point it begins to decrease again.
The analytic performance of this test was defined by characterizing the sensitivity and negative predictive value of all the possible cutoffs on the nomogram which are shown in Figure 4a. Of note, the sensitivity decreases rapidly in the 30–40% cutoff range. Figure 4b shows the number of people who are spared a repeat biopsy as a function of the nomogram cutoff.
Figure 4.
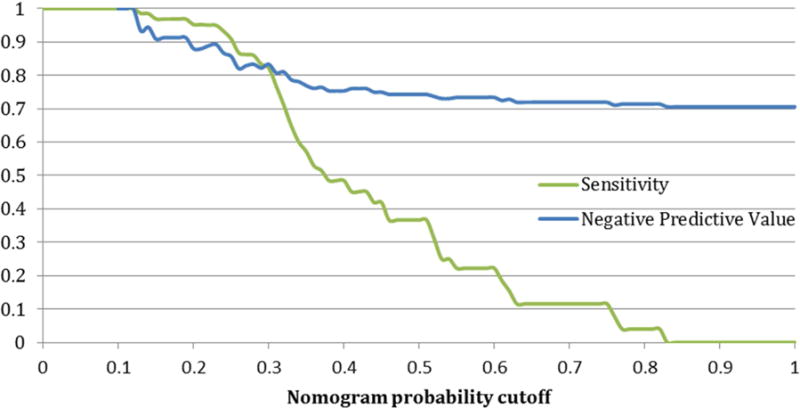
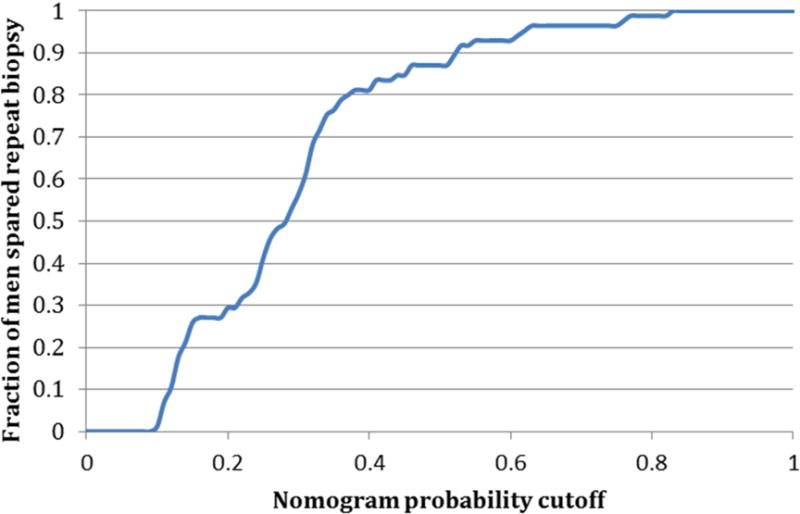
a: Sensitivity and specificity of the nomogram at all possible probability cutpoints for selecting patients correctly for repeat biopsy on active surveillance b: Fraction of patients spared repeat biopsy on active surveillance for each given nomogram cutpoint
Table 1 synthesizes this data for different cut-points on the nomogram below which men would continue AS with no repeat prostate biopsy, and above which they would undergo repeat biopsy to confirm AS candidacy. The cut-point of optimal benefit according to statistical analysis on decision curve analysis was 32%. This correlated with a sensitivity of 71%, a NPV of 81%, and a ratio of 4.3 men spared a biopsy for every one man who incorrectly failed to have a biopsy for disease progression. Theoretically, depending on the individual features of the patient, clinician, and healthcare system, a nomogram threshold could be adjusted such that more men could avoid biopsy for every one man incorrectly biopsied or a higher sensitivity selected. For example, for a ratio of 10, the corresponding nomogram cutoff is 19% and yields 97% sensitivity, 91% NPV, and 27% of patients spared a repeat biopsy. Leave-one-out cross-validation analyses were performed for the various cutoffs to assess the variability within the sensitivities and negative predictive values. There was low variability providing little indication of data overfitting.
Table 1.
Comparison of test characteristics at different cutoffs
| Nomogram Cutoff | Sensitivity | NPV | % of men spared a repeat biopsy | Net benefit on DCA | Number of men spared biopsy for one false negative |
|---|---|---|---|---|---|
| 19% | 97% | 91% | 27% | 0.03 | 10.5 |
| 22% | 95% | 89% | 32% | 0.04 | 8 |
| 25% | 91% | 86% | 41% | 0.06 | 6 |
| 28% | 86% | 83% | 49% | 0.08 | 5 |
| 32% | 71% | 81% | 68% | 0.13 | 4.3 |
| 35% | 57% | 77% | 76% | 0.05 | 3.3 |
DCA=Decision curve analysis
NPV=Negative predictive value
Discussion
Nomogram utilization to guide decision-making offer the benefit of synthesizing multiple pieces of information into a unified analysis. In a literature review of PCa nomograms that aid in the identification of low-grade, low stage organ confined disease, 14 publications were identified that provide such information (Table 2). Despite the improved ability of these nomograms to predict outcomes, physician utilization of nomograms remains low as a decision-making instrument13.
Table 2.
Literature review of published nomograms applicable to active surveillance patients
| First Author | Year | Outcome predicted | PSA | PSAD | Clinical stage |
bGS | Variables in nomogram | Age | Biopsy method | MRI | other | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostate volume |
Tumor volume measure |
Benign tissue measure |
|||||||||||
| Partin et al14 | 1997 | Organ confinement, Extracapsular extension, Seminal vesical invasion, Lymph node positivity | x | x | x | ||||||||
| Kattan et al15 | 2003 | Low volume, low grade organ confined disease | x | x | x | x | x | x | |||||
| Nakanishi et al16 | 2007 | Low volume, low grade organ confined disease | x | x | x | ||||||||
| Roemeling et al17 | 2007 | Low volume, low grade organ confined disease | x | x | x | x | x | ||||||
| Kulkarni et al18 | 2007 | Upgrading from bx Gleason 6 to Gleason 7 or greater at RP | x | x | x | x | x | x | x | ||||
| Kattan et al19 | 2008 | Cancer-specific survival for men with clinically localized PCa managed without curative intent | x | x | x | x | x | x | x | ||||
| Shukla-Dave et al20 | 2011 | Low volume, low grade organ confined disease | x | x | x | x | x | ||||||
| Abdollah et al21 | 2011 | 10-yr cancer-specific mortality & other-cause mortality rates with PCa treated with RP or AS | x | x | x | x | |||||||
| O’Brien et al22 | 2011 | Low volume, low grade organ confined disease | x | x | x | x | x | ||||||
| Wong et al23 | 2012 | High grade or high stage Pca | x | x | x | x | |||||||
| Sooriakumar an et al24 | 2012 | Worsening prognosis (either upgrading or upstaging) | x | x | x | ||||||||
| Iremashvili et al25 | 2013 | Progression on repeat biopsy | x | x | x | x | |||||||
| Stamatakis et al6 | 2013 | Progression on repeat biopsy | x | x | x | ||||||||
| Truong et al26 | 2013 | Gleason score upgrading from Gleason 6 | x | x | x | ||||||||
bGS=biopsy Gleason score
PSAD=PSA Density
PCa=Prostate cancer
RP=Radical prostatectomy
AS=Active surveillance
One possible reason for low utilization is that while well designed nomograms may demonstrate good predictive ability, it is often difficult to determine practically how to translate the outcome of the nomogram into a specific patient’s decision process. Patients and their caregivers have to make decision on whether they should proceed with treatment or stay on AS. If a nomogram yields an outcome such as a 30% probability of disease progression, the patient is still left with a difficult decision to make.
We examined the performance of a recently described MRI-based nomogram for PCa AS utilizing easily understood outcome measures. The emphasis was to provide guidance on optimized cutoffs to decide on repeat biopsy of patients on AS based on their MRI findings. We further characterized how the nomogram would influence the number of repeat biopsies avoided. Based on this analysis, a cutoff probability of disqualification from AS in the range of 19–32% on the nomogram resulted in 27–68% of men being monitored only with MRI and without repeat biopsy. Of practical note, the likely range that does not cause too much loss in sensitivity for decreasing the number of biopsies is probably closer to 27–41% as seen in Table 1.
There is great potential benefit in the use of MRI for monitoring AS rather than multiple repeat biopsies. As the process of AS becomes less invasive, greater acceptance amongst patients may follow. Furthermore, with the reliability of MP-MRI to image the entire prostate, it is feasible that patients will feel further reassured that they did not miss any high-grade cancer. The use of MRI for AS monitoring will ultimately be synergistic with better informed repeat prostate biopsies rather than replace them altogether.
One limitation of this study is that the patient population is derived from a population in which many men obtained repeat biopsy for prior negative biopsy in the setting of elevated PSA. Such a cohort may differ from the general population in important ways such as presence of anterior lesions. Another limitation is that application of the nomogram necessitates abnormal findings on the MP-MRI. Men with no lesions on MP-MRI were therefore excluded from this study. Although other studies suggest that lack of any findings on MRI correlate with low-grade PCa if any cancer is present at all, it is unclear if imaging would be effective in following a patient with a baseline normal MP-MRI. One more limitation was that the nomogram was constructed using MRI data only. Future even more powerful nomograms may be possible incorporating all clinical parameters, this was however beyond the scope of this study. Of note, the AS criteria used in this study were amongst the most stringent in practice today. It will be of great interest if MRI performs similarly amongst more lenient criteria. Lastly, a modified AS protocol had to be adapted to incorporate targeted biopsies into the criteria. As the original criteria were based on 12-core biopsies alone, the implications of this change are unclear and will require further studies to better understand. Further development and validation of such clinical decision making aids will be necessary before MRI can be systematically incorporated into AS protocols for patients or applied broadly in consensus recommendations.
Conclusion
In this study, we analyze the performance of an MP-MRI-based nomogram in order to avoid repeat biopsies on an active surveillance cohort. We found that by varying the cut-point of threshold for biopsy, 27%–68% of biopsies could be safely avoided depending on the tolerance for missing disease that would disqualify the patient for AS. Given the slow growth of most PCa, a relatively higher tolerance for missed disease is justifiable as the lesion is likely to increase in the number of risk features on MP-MRI over time. Elucidation of the performance of the nomogram over the full range of the nomogram generated probabilities allows the patient and caregiver to be better informed decision makers, balancing the risks they are assuming in exchange for the benefit of fewer biopsies. These findings support the further refinement of incorporation of MP-MRI into the AS of patients with low-risk PCa and long-term studies to validate these findings.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. NIH and Philips Healthcare have a cooperative research and development agreement. NIH and Philips share intellectual property in the field. This research was also made possible through the NIH Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from Pfizer Inc, The Leona M. and Harry B. Helmsley Charitable Trust, and the Howard Hughes Medical Institute, as well as other private donors. For a complete list, please visit the Foundation website at http://www.fnih.org/work/programs-development/medical-research-scholars-program.
ABBREVIATIONS
- AS
Active surveillance
- bGS
Biopsy Gleason Score
- DRE
Digital Rectal Exam
- MRI
Magnetic Resonance Imaging
- MR/US
Magnetic Resonance/Ultrasound
- MP-MRI
Multiparametric MRI
- OR
Odds Ratio
- PCa
Prostate Cancer
- PSA
Prostate Specific Antigen
- TRUS
Transrectal Ultrasound
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lu-Yao GL, Albertsen PC, Moore DF, et al. Outcomes of localized prostate cancer following conservative management. JAMA. 2009;302:1202. doi: 10.1001/jama.2009.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson I, Thrasher JB, Aus G, et al. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol. 2007;177:2106. doi: 10.1016/j.juro.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Epstein JI, Feng Z, Trock BJ, et al. Upgrading and downgrading of prostate cancer from biopsy to radical prostatectomy: incidence and predictive factors using the modified Gleason grading system and factoring in tertiary grades. Eur Urol. 2012;61:1019. doi: 10.1016/j.eururo.2012.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siddiqui MM, Rais-Bahrami S, Truong H, et al. Magnetic resonance imaging/ultrasound-fusion biopsy significantly upgrades prostate cancer versus systematic 12-core transrectal ultrasound biopsy. Eur Urol. 2013;64:713. doi: 10.1016/j.eururo.2013.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rais-Bahrami S, Siddiqui MM, Turkbey B, et al. Utility of multiparametric magnetic resonance imaging suspicion levels for detecting prostate cancer. J Urol. 2013;190:1721. doi: 10.1016/j.juro.2013.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walton Diaz A, Hoang AN, Turkbey B, et al. Can magnetic resonance-ultrasound fusion biopsy improve cancer detection in enlarged prostates? J Urol. 2013;190:2020. doi: 10.1016/j.juro.2013.05.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tosoian JJ, Trock BJ, Landis P, et al. Active surveillance program for prostate cancer: an update of the Johns Hopkins experience. J Clin Oncol. 2011;29:2185. doi: 10.1200/JCO.2010.32.8112. [DOI] [PubMed] [Google Scholar]
- 8.Pinto PA, Chung PH, Rastinehad AR, et al. Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. J Urol. 2011;186:1281. doi: 10.1016/j.juro.2011.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turkbey B, Xu S, Kruecker J, et al. Documenting the location of prostate biopsies with image fusion. BJU Int. 2011;107:53. doi: 10.1111/j.1464-410X.2010.09483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turkbey B, Pinto PA, Mani H, et al. Prostate cancer: value of multiparametric MR imaging at 3 T for detection–histopathologic correlation. Radiology. 2010;255:89. doi: 10.1148/radiol.09090475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turkbey B, Mani H, Aras O, et al. Prostate Cancer: Can Multiparametric MR Imaging Help Identify Patients Who Are Candidates for Active Surveillance? Radiology. 2013 doi: 10.1148/radiol.13121325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turkbey B, Huang R, Vourganti S, et al. Age-related changes in prostate zonal volumes as measured by high-resolution magnetic resonance imaging (MRI): a cross-sectional study in over 500 patients. BJU Int. 2012;110:1642. doi: 10.1111/j.1464-410X.2012.11469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim SP, Karnes RJ, Nguyen PL, et al. Clinical implementation of quality of life instruments and prediction tools for localized prostate cancer: results from a national survey of radiation oncologists and urologists. J Urol. 2013;189:2092. doi: 10.1016/j.juro.2012.11.174. [DOI] [PubMed] [Google Scholar]
- 14.Partin AW, Kattan MW, Subong EN, et al. Combination of prostate-specific antigen, clinical stage, and Gleason score to predict pathological stage of localized prostate cancer. A multi-institutional update. JAMA. 1997;277:1445. [PubMed] [Google Scholar]
- 15.Kattan MW, Eastham JA, Wheeler TM, et al. Counseling men with prostate cancer: a nomogram for predicting the presence of small, moderately differentiated, confined tumors. J Urol. 2003;170:1792. doi: 10.1097/01.ju.0000091806.70171.41. [DOI] [PubMed] [Google Scholar]
- 16.Nakanishi H, Wang X, Ochiai A, et al. A nomogram for predicting low-volume/low-grade prostate cancer: a tool in selecting patients for active surveillance. Cancer. 2007;110:2441. doi: 10.1002/cncr.23055. [DOI] [PubMed] [Google Scholar]
- 17.Roemeling S, Roobol MJ, Kattan MW, et al. Nomogram use for the prediction of indolent prostate cancer: impact on screen-detected populations. Cancer. 2007;110:2218. doi: 10.1002/cncr.23029. [DOI] [PubMed] [Google Scholar]
- 18.Kulkarni GS, Lockwood G, Evans A, et al. Clinical predictors of Gleason score upgrading: implications for patients considering watchful waiting, active surveillance, or brachytherapy. Cancer. 2007;109:2432. doi: 10.1002/cncr.22712. [DOI] [PubMed] [Google Scholar]
- 19.Kattan MW, Cuzick J, Fisher G, et al. Nomogram incorporating PSA level to predict cancer-specific survival for men with clinically localized prostate cancer managed without curative intent. Cancer. 2008;112:69. doi: 10.1002/cncr.23106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shukla-Dave A, Hricak H, Akin O, et al. Preoperative nomograms incorporating magnetic resonance imaging and spectroscopy for prediction of insignificant prostate cancer. BJU Int. 2012;109:1315. doi: 10.1111/j.1464-410X.2011.10612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdollah F, Sun M, Schmitges J, et al. Cancer-specific and other-cause mortality after radical prostatectomy versus observation in patients with prostate cancer: competing-risks analysis of a large North American population-based cohort. Eur Urol. 2011;60:920. doi: 10.1016/j.eururo.2011.06.039. [DOI] [PubMed] [Google Scholar]
- 22.O’Brien BA, Cohen RJ, Ryan A, et al. A new preoperative nomogram to predict minimal prostate cancer: accuracy and error rates compared to other tools to select patients for active surveillance. J Urol. 2011;186:1811. doi: 10.1016/j.juro.2011.06.060. [DOI] [PubMed] [Google Scholar]
- 23.Wong LM, Neal DE, Johnston RB, et al. International multicentre study examining selection criteria for active surveillance in men undergoing radical prostatectomy. Br J Cancer. 2012;107:1467. doi: 10.1038/bjc.2012.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sooriakumaran P, Srivastava A, Christos P, et al. Predictive models for worsening prognosis in potential candidates for active surveillance of presumed low-risk prostate cancer. Int Urol Nephrol. 2012;44:459. doi: 10.1007/s11255-011-0020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iremashvili V, Burdick-Will J, Soloway MS. Improving risk stratification in patients with prostate cancer managed by active surveillance: a nomogram predicting the risk of biopsy progression. BJU Int. 2013;112:39. doi: 10.1111/bju.12112. [DOI] [PubMed] [Google Scholar]
- 26.Truong M, Slezak JA, Lin CP, et al. Development and multi-institutional validation of an upgrading risk tool for Gleason 6 prostate cancer. Cancer. 2013;119:3992. doi: 10.1002/cncr.28303. [DOI] [PMC free article] [PubMed] [Google Scholar]


