Abstract
Background
A variety of magnetic resonance (MR) lymphographic agents have been proposed for mapping the lymph nodes draining the prostate.
Purpose
To investigate the feasibility of using ferumoxytol (an FDA-approved iron oxide agent) for lymph node mapping of the prostate on imaging (MRI) in a non-human primate (NHP) Macaque model.
Material and Methods
Four NHPs weighing 5–13 kg underwent injection of ferumoxytol after a needle was introduced transrectally under MRI guidance into the prostate using a commercially available intrarectal MRI biopsy guide. Ferumoxytol was administered at dosage in the range of 0.15–0.75 mg Fe/kg in a fixed injection volume of 0.2 mL. T1-weighted MRI was performed at 3 T starting immediately and extending at least 45 min post-injection. Two readers evaluated the images in consensus. The NHPs tolerated the ferumoxytol injections at all doses with no evident side effects.
Results
It was determined that the lowest dose of 0.15 mg Fe/kg produced the best outcome in terms of lymph node visualization and draining nodes were reliably visualized at this dose and volume.
Conclusion
Thus, MRI with intraprostatic injection of ferumoxytol may be considered an effective T1 contrast agent for prospective mapping of lymph nodes draining the prostate and, thus, for attempted sentinel lymph node identification in prostate cancer. Large clinical trials to determine safety and efficacy are needed.
Keywords: Ferumoxytol, lymph node, prostate cancer, iron oxide nanoparticles, magnetic resonance imaging (MRI)
Introduction
Lymph node staging is an important aspect of prostate cancer management. Unfortunately, existing noninvasive imaging such as computed tomography (CT) and magnetic resonance imaging (MRI) are generally considered poor predictors of lymph node metastases as they depend strictly on size criteria that are often misleading. Thus, surgical staging has been the gold standard for nodal assessment in prostate cancer. For patients with suspected localized disease who undergo elective surgery with nomogram determination of possible lymph node involvement, a routine bilateral pelvic lymph node dissection (BPLND) is performed, often sampling the obturator fossa and external iliac chain at the time of prostatectomy to determine the extent of disease. If a higher risk for metastatic disease exists, an extended pelvic lymph node dissection (EPLND) beyond the obturator fossa and external iliac chain is often performed. A recent study showed that patients who underwent EPLND had superior long-term outcomes compared to those who underwent routine BPLND. However, the side-effects and potential complications associated with EPLND including formation of lymphoceles, deep venous thrombosis, and postoperative ileus is more significant (1,2). Thus, more targeted lymph node resections based on the sentinel node theory have been gaining interest, especially in Europe, mirroring a similar successful strategy in breast cancer and melanoma. In theory, macromolecular agents injected into the prostate will drain into the sentinel nodes, or first level of nodal tissues representing the specific drainage pattern of the organ. Thus, Tc-99 m radiolabeled sulfocolloid and other nanoparticulate agents have been proposed along with intraoperative gamma probes to detect the nodes during surgery. Optical probes such as indocyanine green alone or in combination with radiolabeled probes have also been proposed (3). More recently, MRI contrast agents such as gadolinium-based gadofosveset dimeglumine (Ablavar, Lantheus Medical Imaging, N. Billerica, MA, USA), which binds albumin, has shown success (4). The MRI agents have the advantage of providing high resolution images of the nodes without exposing the patient or surgical-pathology team to ionizing radiation. The disadvantage is that they do not provide high fidelity intraoperative guidance. Ideally, the MR lymphographic agent would be safe, FDA-approved, accurate, and provide a means of intraoperative tracking for operative guidance.
While ultrasmall superparamagnetic iron oxides (USPIO) have shown promising results as intravenous lymphographic agents in MRI, interstitial and intratumoral administration of USPIO for MRL of the prostate has not yet been fully explored (5). Ferumoxytol has previously been used as a T1 contrast agent because of its T1 shortening property at low doses (6). Our laboratory has previously demonstrated the utility of the non-human primate (NHP) Macaque model for evaluating lymphatic drainage pathways by MRI (7) and their anatomic similarities to humans. Our goal for this study was to determine the feasibility of intraprostatic injections of ferumoxytol for mapping the nodal drainage patterns of the prostate gland.
Materials and Methods
All studies conducted were in accordance with NIH guidelines on animal use in research, and received the approval of the institutional Animal Care and Use Committee (ACUC). Four SPF (McHV-1, SRV, SIV, STLV) rhesus macaques weighing 5–13 kg were housed and cared for in an AAALAC accredited facility. All research was conducted in the presence of a certified veterinarian and their trained staff specializing in the care of NHPs.
Ferumoxytol (FeraHeme, AMAG Pharmaceutical, Cambridge, MA, USA), a USPIO of 20–30 nm in size, is an agent approved by the FDA for iron replacement therapy in patients with chronic renal failure and associated iron-deficiency anemia. It is commercially available at a stock concentration of 30 mg Fe/mL. Contrast dosage was optimized by testing at different dilutions at a fixed injection volume with varying MRI acquisition times. Ferumoxytol was diluted with phosphate buffer saline (Life Technologies, Grand Island, NY, USA). Ferumoxytol is typically a T2* contrast agent, however, when diluted it can also serve to be an effective T1 contrast agent. Therefore various doses of ferumoxytol (in the range of 0.15–0.75 mg Fe/kg body weight) were tested in NHPs. Based on previous experience, 0.2 mL is the optimal volume for injection, as larger doses will often lead to extravasation from the NHP prostate. Therefore, a fixed volume of 0.2 mL was used in each case for the purposes of this experiment.
NHPs were anesthetized with 10 mg/kg ketamine and 25 ug/kg dexmedetomidine intramuscularly, followed by intubation and administration of isoflurane 1–3% and anesthetic monitoring by trained veterinary staff, transferred to the MRI scanner, and kept in the prone position for the duration of the procedure and imaging protocol. All MRI was performed with a 32-channel cardiac coil (SENSE; Philips Medical Systems, Best, The Netherlands) at 3.0 T (Achieva, Philips Medical Systems). An initial baseline 3D T1-weighted (T1W) gradient echo sequence with fat suppression was acquired (Table 1). Next, an FDA-approved, commercially-available device (DynaTRIM, Invivo, Schwerin, Germany), developed for MR-guided prostate biopsies was inserted transrectally into the NHP subjects, and was appropriately positioned and directed for prostate targeting and intra-prostatic injections. A 0.2 mL dose with varying dose of ferumoxytol was administered intraprostatically into each prostate lobe. A 10 cycle massage was applied at the site of injection. Four phases of post-injection T1W MRI were then acquired over 45–75 min. If an additional injection was performed in the contralateral lobe then an interval of at least 30 min was used between the two intraprostatic injections. After image acquisition, all primates were reversed from anesthesia and returned to their housing facility and subsequently evaluated by a veterinarian. Physical examination of the animals as well as a complete blood count (CBC) and serum chemistries revealed no complications associated with the intraprostatic administration of Ferumoxytol.
Table 1.
MRI sequence parameters.
| Sequence parameters | T1 3D GRE with fat suppression |
|---|---|
| FOV (mm) | 300 |
| Acquisition matrix | 600 × 160 |
| TR/TE (ms) | 7.0/3.3 |
| Flip angle (degrees) | 25 |
| Slice thickness (mm), no gaps | 0.35 |
| Voxel size (mm/pixel) | 0.35 × 0.35 × 0.35 |
| Time for acquisition (min:s) | 6:22 |
All MRI analysis was performed by two readers on a commercially-available work station (Extended MR WorkSpace [EWS], Philips Medical Systems). For each imaging session, lymph nodes were evaluated on T1W MR images using a 3-point scoring system (0, no enhancement; 1, hypointense enhancement; 2, mixed enhancement; 3, hyperintense enhancement).
Results
Injection 1: Intra-prostatic injection of 0.75 mg Fe/kg
The first primate (8 kg) was injected with 0.2 mL of undiluted ferumoxytol (30 mg Fe/mL) in the left lobe of the prostate for a dose of 0.75 mg Fe/kg. Serial T1W scans were acquired for 70 min post-injection. Upon evaluation of the first phase, the left internal iliac, left common iliac, and retroperitoneal lymph nodes were seen as hypointense regions (Table 2). By the fourth phase (63-min post-injection), a mixed signal was seen in the draining lymph nodes.
Table 2.
Summary of experimental results in this study.
| Injection number | Primate weight (kg) | Injected volume (mL/lobe) | Injected dose (Fe mg/kg body weight) | Injection site | Visualized lymph nodes | Visibility window (min post-injection) | Comments |
|---|---|---|---|---|---|---|---|
| 1 | 8 | 0.2 | 0.75 | Left |
|
0–70+ |
|
| 2 | 5 | 0.2 | 0.24 | Left |
|
45–60+ |
|
| 3 | 5 | 0.2 | 0.4 | Left |
|
10–20 |
|
| 4 | 5 | 0.2 | 0.4 | Right |
|
0–50 |
|
| 5 | 13 | 0.2 | 0.15 | Right |
|
0–40+ |
|
Injection 2: Intra-prostatic injection of 0.24 mg Fe/kg
The second primate (5 kg) was injected with 0.2 mL of 20% ferumoxytol diluted with saline (6 mg Fe/mL) in the left lobe of the prostate for a dose of 0.24 mg Fe/kg. Serial T1W scans were acquired for 70 min post-injection. Lymph nodes were not visualized in the first three phases. After a prostatic massage, hyperintensity was seen in the left internal iliac, left common iliac, and retroperitoneal lymph nodes during the fifth phase (post-injection) (Fig. 1a–c).
Fig. 1.
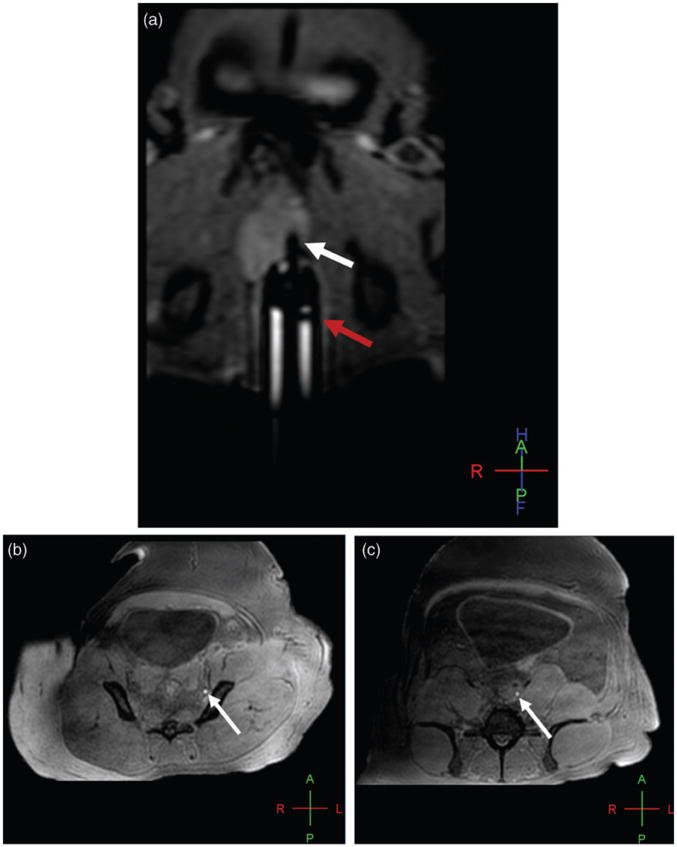
Baseline oblique coronal T1W 3D gradient echo MR image shows the intrarectal probe (red arrow) and intraprostatic needle (white arrow) within the left hemi-prostate prior to the injection (a); axial T1W 3D GRE MR image reveals hyperintense signal enhancement in the left internal iliac (b) and the left common iliac (c) nodal chains (arrows in b and c) 45 min after injection of ferumoxytol at the dose of 0.24 mg Fe/kg.
Injections 3 and 4: Sequential bilateral intra-prostatic injections of 0.4 mg Fe/kg
The third primate (5 kg) was injected with 0.2 mL of 33% ferumoxytol diluted with saline (10 mg Fe/mL) in the left lobe of the prostate for a dose of 0.4 mg Fe/kg. Serial T1W scans were acquired for 76 min post-injection. Upon evaluation of the first phase, the right internal iliac and left internal iliac lymph nodes showed hypointense signal. By the second phase, the same two regions and the left common iliac lymph node had a hypointense signal, but the right common iliac lymph nodes showed hyperintense signal pattern.
After the third phase, a second injection of the same dose was administered in the contralateral side (right lobe). After prostatic massage, the fifth phase in the second injection (42 min post-injection) showed right and left common iliac lymph nodes with hypointense appearance. The right and left internal iliac lymph nodes were of mixed enhancement, and the retroperitoneal lymph nodes had hyper-enhancement (Fig. 2a–c).
Fig. 2.
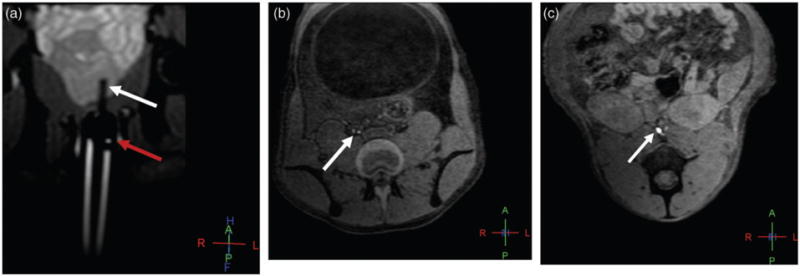
Baseline oblique coronal T1W 3D gradient echo MR image shows the intrarectal probe (red arrow) and intraprostatic needle (white arrow) within the left hemi-prostate prior to the injection (a); axial T1W 3D GRE MR image reveals hyperintense signal enhancement in the right common iliac node (arrow) 20 min after injection of ferumoxytol at the dose of 0.4 mg Fe/kg (b). Additionally, axial T1W 3D GRE MR image demonstrates hyperintense signal enhancement in the retroperitoneal nodes (arrow) 42 min after contralateral (right hemi-prostate) injection of ferumoxytol at the dose of 0.4 mg Fe/kg (c).
Injection 5: Intra-prostatic injection of 0.15 mg Fe/kg
The fourth primate (13 kg) was injected with 0.2 mL of 33% ferumoxytol diluted with saline (10 mg Fe/mL) in the right lobe of the prostate for a dose of 0.15 mg Fe/kg. Serial T1W scans were acquired for 42 min post-injection. After prostatic massage, mixed enhancement was visualized in the common iliac lymph nodes 19 min after injection. However, the retroperitoneal lymph nodes showed hyperintensity. These findings remained consistent until the seventh phase, at which time the image acquisition was concluded.
Discussion
Our study demonstrates the feasibility of using a low volume and low dose ferumoxytol via intra-prostatic injection (0.2 mL of 0.15 mg Fe/kg body weight) for successful lymph node mapping of the prostate on T1W MRI in a NHP model. The appropriate dose was determined by balancing each factor. The appropriate volume of ferumoxytol needed to be limited to 0.2 mL to prevent tissue extravasation into the periprostatic space, which commonly occurs at higher volumes, based upon prior experience (4). Next, determination of the appropriate dose of iron required testing of serial dilutions as described. At higher dose of ferumoxytol, the lymph nodes were subject to signal decrease due to T2 and T2* effects. With time, this excess iron dilutes and begins to shorten the T1 signal. However, based on our observations, this may take several hours to occur, making image acquisition impractical and more variable. Therefore, the doses were tapered so as to result in T1 shortening in the draining nodes within a reasonable image acquisition time (<30 min). A dose of 0.15 mg Fe/kg in 0.2 mL appeared to satisfy both conditions and produced excellent maps of the series of nodes draining the area of the prostate where the intra-prostatic injection of ferumoxytol was performed.
In 2013, a pilot study in patients with breast cancer looked at the ability of SLN biopsy with USPIO to be used as a magnetic tracer (8). In the study, SLNs were identified by USPIO magnetometry in 23/30 (77%), and with blue dye in 24/30 (80%). More recently in 2014, a pilot study in humans has shown the ability of intraoperative magnetometry to identify SLNs during surgery by detecting slight changes in the magnetic field in the draining nodes containing an USPIO (9). It was shown that the detection rate of SLNs in vivo was 90 % and the method yielded 100% sensitivity for metastatic LN. The contrast agent, and the magnetometer (Sienna+ and Sentimag, Endomagnetics, Cambridge, UK) are not yet FDA approved but have recently received investigational device exemption (IDE) status for initiation of further clinical trials. The injected volume for PLND using Sentimag was 10 times larger (2 mL) than that which was used in our MRI study (0.2 mL), however, this likely does not influence the amount of agent taken up and transported to the draining lymph nodes but rather the amount in and around the prostate gland due to volume of tissue extravasation.
The current study has several limitations. The study was conducted in normal NHPs with presumably healthy prostate glands. Thus, MR lymphography with ferumoxytol was not tested in the setting of prostatic adenocarcinoma where identification of cancer-specific lymphatic drainage would be of prime clinical utility. Nevertheless, this is the first report of intraprostatic injection of ferumoxytol for this purpose and can be further developed for injection in cancerous foci, for targeted lymph node mapping via MRI. Also, for the purpose of feasibility and dose ranging the number of animals in the experiment was limited. Further, due to this limitation in the number of NHPs available, tests were unable to be repeated in this study. With regard to the contrast agent, although FDA approved, ferumoxytol is not indicated for SLN mapping by MRI or for interstitial/intraprostatic injection. Therefore, this is currently considered an off-label use of ferumoxytol. Lastly, longer image acquisition was not feasible as the time spent under anesthesia for the NHPs would have been beyond limits of ACUC recommendations; however, it would be interesting to see how long the nodes remain opacified after injection.
In conclusion, this study showed the feasibility of T1W MRI lymph node mapping with ferumoxytol in a non-human primate model. The MR contrast agent, ferumoxytol is FDA approved and therefore, is feasible for rapid clinical translation. It is hoped that intraprostatic injections of ferumoxytol will improve the mapping of sentinel nodes in cases of prostate cancer, thus reducing the need for extended lymph node dissections while still allowing for the benefits of adequate sampling for diagnostic and prognostic purposes.
Acknowledgments
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Footnotes
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Naselli A, Andreatta R, Introini C, et al. Predictors of symptomatic lymphoceles after lymph node excision and radical prostatectomy. Urology. 2010;75:630–635. doi: 10.1016/j.urology.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 2.Liss MA, Palazzi K, Stroup SP, et al. Outcomes and complications of pelvic lymph node dissection during robotic-assisted radical prostatectomy. World J Urol. 2013;31:481–488. doi: 10.1007/s00345-013-1056-9. [DOI] [PubMed] [Google Scholar]
- 3.Manny TB, Patel M, Hemal AK. Fluorescence-enhanced robotic radical prostatectomy using real-time lymphangiography and tissue marking with percutaneous injection of unconjugated indocyanine green: the initial clinical experience in 50 patients. Eur Urol. 2014;65:1162–1168. doi: 10.1016/j.eururo.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 4.Turkbey B, Hoyt HF, Agarwal HK, et al. Magnetic resonance lymph node mapping of the prostate after intra-prostatic injection of Gadofosveset trisodium and albumin: Preliminary results in a canine model. Acad Radiol. 2015;22:646–652. doi: 10.1016/j.acra.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fortuin AS, Smeenk RJ, Meijer HJ, et al. Lymphotropic nanoparticle-enhanced MRI in prostate cancer: Value and therapeutic potential. Curr Urol Rep. 2014;15:389. doi: 10.1007/s11934-013-0389-7. [DOI] [PubMed] [Google Scholar]
- 6.Bashir MR, Bhatti L, Marin D, Nelson RC. Emerging applications for ferumoxytol as a contrast agent in MRI. J Magn Reson Imaging. 2014;41:884–898. doi: 10.1002/jmri.24691. [DOI] [PubMed] [Google Scholar]
- 7.Smedley J, Turkbey B, Bernardo M, et al. Tracking the luminal exposure and lymphatic drainage pathways of intravaginal and intrarectal inocula used in nonhuman primate models of HIV transmission. PLoS One. 2014;9:e92830. doi: 10.1371/journal.pone.0092830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shiozawa M, Lefor AT, Hozumi Y, et al. Sentinel lymph node biopsy in patients with breast cancer using superparamagnetic iron oxide and a magnetometer. Breast Cancer. 2013;20:223–229. doi: 10.1007/s12282-011-0327-9. [DOI] [PubMed] [Google Scholar]
- 9.Winter A, Woenkhaus J, Wawroschek F. A novel method for intraoperative sentinel lymph node detection in prostate cancer patients using superparamagnetic iron oxide nanoparticles and a handheld magnetometer: The initial clinical experience. Ann Surg Oncol. 2014;21:4390–4396. doi: 10.1245/s10434-014-4024-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


