Abstract
Objectives
Apical prostate cancer can be difficult to detect in transrectal ultrasound guided biopsy. Therefore it may be missed before treatment decisions such as HIFU or surgical therapy.
We seek to describe an undescribed “very distal” apical prostate cancer at multiparametric MRI (mpMRI).
Patients and Methods
From January 2011 to December 2012 a total of 210 consecutive patients underwent 3Tesla (mpMRI) with endorectal coil followed by our previously described MRI/ultrasound image fused and directed TRUS biopsies.
Patients also underwent 12 core TRUS sextant biopsies.
The inclusion criteria required at least one distal apical prostate lesion visualized on mpMRI and targeted for biopsy.
Results
A total of 38 men (median age 62 years, median PSA 7.68ng/dl) were identified as having distal apical prostate cancer on mpMRI.
Thirteen patients (34%) had a prior diagnosis of cancer and on active surveillance protocols while 25 (66%) did not. Of those patients 21 (55%) had undergone a median of 2 prior negative biopsies.
Twenty two patients (58%) were positive on biopsy for prostate cancer. On breakdown of patients that were positive, 17 (77%) were positive on TRUS random biopsies and 21 (95%) were positive on MRI targeted biopsies with the majority of patients having multifocal disease.
At the distal apical lesions of interest, 80% were positive on MRI targeted biopsy. In addition 33% of these patients were upgraded based on MRI targeted biopsy at the distal lesion.
Conclusions
Very distal apical prostate cancer can be accurately detected and sampled with mpMRI and subsequent MRI/US fusion biopsy.
This may aid clinicians and patients in decision making for therapeutic modalities.
Keywords: Prostate cancer, MRI, distal apical region, biopsy
INTRODUCTION
Prostate cancer is the second leading cause of cancer in American men, second to non-melanoma skin cancer. An estimated 241,740 men will be diagnosed with prostate cancer in 2012. Prostate cancer is also the second leading cause of cancer related death in American men.1 A significant percentage of men will have prostate cancer in the apex of the prostate, with some studies showing apical prostate cancer increasing in frequency as well.2 This is an area of the prostate that can be difficult to adequately biopsy in standard 10–12 core transrectal US guided prostate schemes. Patients with prior negative biopsies and continued clinical concern for prostate cancer have a high incidence of cancer located at the apex, with rates varying depending on the biopsy approach.3,4 The recognition of significant prostate cancer at the apex is also important in regards to surgical extirpative therapy for prostate cancer, as it is the most common site of positive margins after surgery.5 High intensity focused ultrasound (HIFU) treatment for prostate cancer has gained increasing popularity as a new minimally invasive approach for treatment of localized disease. However a study examining the location of residual disease after HIFU treatment has shown that the apex is the predominant site, possibly related to treatment safety zones established at the sphincter.6 These issues have led many institutions to evaluate the utility of multiparametric MRI in improving localization and detection of prostate cancer. We seek to describe a previously undescribed “very distal” apical prostate cancer on multiparametric MRI and its potential implications for patients and clinicians.
MATERIALS AND METHODS
Study Design & Population
This retrospectively designed, single institution study was approved by the local Institutional Review Board and was compliant with the Health Insurance Portability and Accountability Act of 1996. From January 2011 to December 2012 a total of 210 consecutive patients underwent 3Tesla multi-parametric prostate MRI (mpMRI) with endorectal coil followed by MRI/US fusion biopsy of the prostate, which has been previously described in detail, as well as in subsequent sections of this paper. The inclusion criteria required that at least one distal apical prostate lesion (detailed definition in the upcoming sub-section of materials and methods) was present on mpMRI and sampled by MRI/US fusion biopsy platform.
MR Imaging
Patients with clinical concern for prostate cancer, after enrollment in our IRB approved protocol, underwent diagnostic prostate MRI. Studies were performed using a combination of an endorectal coil (BPX-30, Medrad, Pittsburgh, Pennsylvania) tuned to 127.8 MHz and a 16-channel cardiac coil (SENSE, Philips Medical Systems, Best, The Netherlands) on a 3T magnet (Achieva, Philips Medical Systems) without prior bowel preparation. The endorectal coil was inserted using a semi-anesthetic gel (lidocaine) while the patient was in left lateral decubitus position. The balloon surrounding the coil was distended with perfluorocarbon (3 mol/L-Fluorinert, 3M, St. Paul, Minnesota) to a volume of approximately 45 ml to reduce susceptibility artifacts induced by air in the coil’s balloon. The MRI protocol included triplanar T2W turbo spin echo, DW MRI, 3D MR point resolved spectroscopy, axial pre-contrast T1-weighted axial 3D fast field echo DCE MRI sequences, and their detailed sequence parameters were defined in a prior study7.
Prostate Cancer Assessment based on mpMRI
The prostate MRI was evaluated by two radiologists (BT, PLC) with 5 & 13 years, respectively, accumulated experience reading prostate MRI. The radiologists were aware, of course, that the patient underwent the MRI because of clinical concern for prostate cancer, but were blinded to any patient specific details that might have biased their interpretation (e.g. PSA, prior biopsy results, etc.) Each MRI sequence was evaluated for lesion risk. All MRI studies except 3D-MR spectroscopy were analyzed in PACS (Carestream Healthcare). On T2 weighted images the peripheral zone normally has a high signal and is more homogeneous than the transition zone of the prostate. A lesion on T2 weighted images was defined as a “visible” well circumscribed, round-ellipsoid low-signal intensity region within the peripheral prostate gland. Lesions within the transition zone are harder to define as this area is more heterogeneous and has a lower baseline signal intensity. A lesion in the transition zone was defined as a homogenous low signal intensity lesion with irregular borders and without a true capsule. When analyzing prostate cancer with functional MRI imaging using DWI and ADC map sequences to assess the Brownian movement of water through the tissue we also see characteristic changes. Prostate cancers appear hyperintense on DWI images because of reduced diffusion but have a low signal intensity on ADC map sequences compared to the surrounding region.8,9,10 The 3D-MR spectroscopy analysis evaluated choline/citrate (Cho/Cit) ratios within voxels in the biopsy core sites. Voxels were considered abnormal when the (Cho/Cit) ratio was 3 or more standard deviations (SD) above the mean healthy Cho/Cit ratio value (≥0.373), which was defined as 0.13+/−0.081 on the basis of results recorded from 433 healthy voxels from peripheral zone regions with negative biopsy results in 44 additional patients who were referred for prostate MR imaging and who had histologic confirmation.10 DCE MR images were evaluated by direct visual interpretation of raw dynamic enhanced T1W images and the diagnostic criterion for prostate cancer was defined as a focus of early and intense enhancement with rapid wash out compared to the background.10 Each individual sequence was scored as to whether or not the reviewer felt that it represented a cancerous lesion, and then the series were tallied in total to give a final risk for each individual lesion. A lesion was considered “low risk” if it was positive on two or fewer of the sequences, “moderate risk” if it was positive on three of the sequences, and “high risk” if it was positive on all four modalities. The number of suspicious lesions and their individual suspicion level were then compiled so as to plan for our MRI/US fusion platform at a separate biopsy based on this data.
Distal Apical Prostate Cancer Determination
On MRI, we defined the “very distal” apical prostatic zone as “the distal 6mm apical portion of the prostate starting from the distal most visualized part of the prostate in the superior-inferior plane”. That part of the apical prostate gland is believed to be under-sampled due to anatomical challenges. Histological studies have also shown that this portion of the prostate does not normally contain transition zone (TZ), although occasionally as a result of increased intraprostatic pressure TZ may appear to bulge into this area. The bilateral peripheral zones essentially form a ring at the apex, with its thickest portion posteriorly as it continues laterally and anteriorly abutting the anterior fibromuscular stroma.11 This is shown in Figure 1. A recent study by Iremashvili et al evaluated the performance of biopsy cores in their ability to predict disease location on histopathology after radical prostatectomy. This study showed the apical cores to significantly underperform in all measures of diagnostic accuracy compared to other zone of the prostate.12
Figure 1.
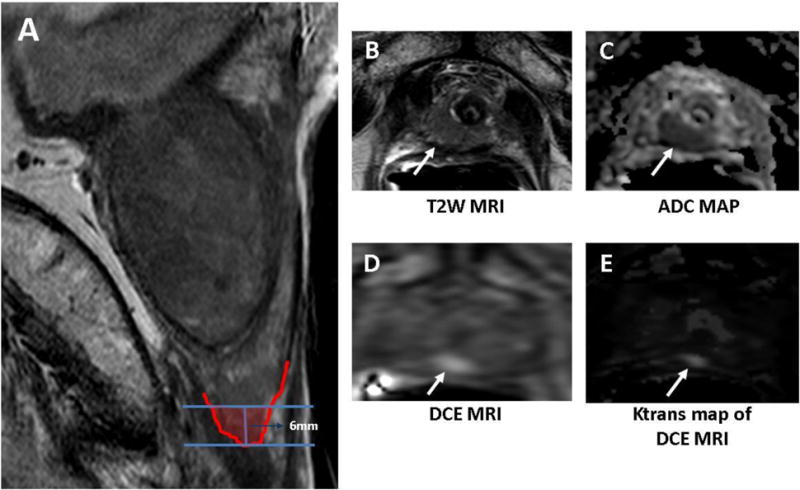
Sagittal T2W MR image shows the distal 6mm apical portion of the prostate corresponding to “distal apical” portion of the prostate (A) in a 72-year old man with a serum PSA of 15.9ng/dL in presence of previous negative prostate biopsies. Axial T2W MRI (A), ADC map of DW MRI (B), raw DCE MRI (C) and Ktrans map derived from DCE MRI (E) demonstrate a right distal apical lesion (arrows). This lesion was sampled with the MRI/US fusion biopsy platform and found to include Gleason 4+4 tumor (90% of core involvement).
Fusion biopsy
The specific details of our MRI/US fusion biopsy platform have been previously described.8 However, all patients enter into the procedure suite having already undergone mpMRI to identify any areas of suspicion within the prostate. Patients received a Fleets® enema as well as antibiotics per AUA guidelines. Patients are placed on the table in the lateral decubitus position, endorectal ultrasound is performed and local anesthesia is provided. Patients first undergo a 12 core standard extended sextant biopsy with medial and lateral samples of the right and left apex, mid gland, and base. In the same setting patients would then undergo MRI/US fusion biopsy. An electromagnetic field (EM) generator (Northern Digital Inc. Ontario, Canada) was placed above the patient. A specifically designed endorectal probe with embedded EM tracking was inserted transrectally. An initial axial sweep of the prostate was performed to create a 3-D image that was then registered to the MRI for fusion, thus allowing MRI defined targets to be superimposed on the ultrasound image.13 Each targeted lesion was then biopsied in both the axial and sagittal plane.
RESULTS
Patient Characteristics
From January 2011 to December 2012 a total of 210 consecutive patients underwent 3Tesla multi-parametric prostate MRI (mpMRI) with endorectal coil followed by MRI/US fusion biopsy of the prostate On retrospective review we identified a total of 38 men with an MRI suspicious lesion at the distal apex. The median age and PSA was 62 years (47 – 73) and 7.68ng/dl (0.33 – 55) respectively. The vast majority of patients had no prior diagnosis of prostate cancer while 12 of 13 patients were on active surveillance w/low volume Gleason 6 disease. One patient was referred for restaging biopsy to determine true disease burden. All patients that had previously undergone outside biopsies had them reread at the NIH as part of our protocol and 2 of the 13 patients had their biopsy re-read as negative or atyipia only. The majority of patients were clinical T1C disease, with 3 patients with T2A disease. The median MRI volume is 47cc for all patients. Patient and staging information are described in table 1.
Table 1.
Patient demographics
| N | % | ||
|---|---|---|---|
| Pts. Total | 38 | 100 | |
| Age | Median | 62 | |
| Range | 47 – 73 | ||
| Race | White | 30 | 78.94737 |
| Black | 7 | 18.42105 | |
| Other | 1 | 2.631579 | |
| PSA | Median | 7.68 | |
| Range | 0.33 – 55 | ||
| SD | 9.7 | ||
| Clinical Stage | T1C | 31 | 82 |
| T2A | 3 | 8 | |
| Unknown | 4 | 10 | |
| Prior CaP Dx | 15 | 40 | |
| Positive Bx. | 22 | 58 |
Biopsy Data
Overall cancer detection
Out of the 38 patients 22 (58%) were positive for prostate cancer on the fusion platform when considering disease at any location. Twenty-one (96%) patients were positive on MRI targeted biopsies, 17 (77%) were positive on TRUS random biopsies. Sixteen of the 17 patients (94%) that were positive on random TRUS biopsies were also positive on MRI targeted biopsies. For the 21 patients positive on MRI targeted biopsies, 15 (71%) were positive on random TRUS. Therefore an additional 6 patients (16% of total cohort) were diagnosed with prostate cancer based on MRI targeted biopsies of suspicious lesions. Patient specific staging information and demographic information were not statistically significantly different between patients that were positive and negative on biopsy.
There were 10 patients with 16 sonographic lesions of concern for prostate cancer. Six lesions (37%) in 5 patients were positive at a sonographic lesion. Seventy-nine percent of the ultrasound lesions correspond to an MRI suspicious lesion. There were 2 ultrasound suspicious areas that were positive that did not correlate to an MRI suspicious lesion.
Apical CaP Detection
Of the 38 patients with distal apical lesions on MRI 14 (37%) were positive at the apex. First examining these patients by modality positive, there were 11 patients (73%) positive at the apical lesion on MRI targeted biopsies, whereas 7 patients (47%) were positive on the MRI targeted apical lesion but not positive at the apex on TRUS, 6 (40%) were positive at the apical targeted lesion on both MRI guided fusion biopsy and random TRUS biopsies of the apex and 3 (20%) were positive on random TRUS biopsies of the apex but not positive on MRI targeted biopsy at the apex. More importantly, 5 (33%) of the 15 patients were upgraded based on the distal apical lesion, with 3 of the 5 patients having unifocal disease at the distal apex. All 5 of these patients were upgraded based on the MR targeted distal apical biopsy and 4 out of the 5 were positive only on the MRI target, when comparing the MR targeted with the ultrasound guided 12 core biopsies. Two of the five patients were upgraded to Gleason 4+4 disease.
We examined the effect of MRI volume on apical prostate cancer detection. When we examined MRI volume as a continuous variable, increasing MRI volume was associated with a decrease in apical prostate cancer detection rates, odds ratio of 0.953 (95%CI 0.912 – 0.996). When we examined MRI volume as a categorical variable and split patients into groups of mild prostate enlargement (≤ 40cc), moderate enlargement (> 40cc and ≤ 80cc) and severe enlargement (≥80cc), increasing MRI volume was also associated with decreased apical prostate cancer detection rate (p .036), see table 2.
Table 2.
Differences between Positive & Negative group at distal apical lesion.
| Positive | Negative | P value | ||
|---|---|---|---|---|
| Pts. | 14 | 24 | ||
| Age | 62.2 | 60.5 | 0.45 | |
| Range | 47–72 | 49–73 | ||
| Race | ||||
| White | 8 | 22 | ||
| Black | 5 | 2 | ||
| Other | 1 | 0 | ||
| PSA (mean) | ||||
| Range | 8.77 | 11.56 | 0.4 | |
| MRI Volume | 43.1 | 65.7 | 0.03 | |
| Clinical Stage | 0.54 | |||
| cT1c | 12 | 23 | ||
| cT2a | 2 | 1 | ||
| Prior CaP Dx | 5 | 8 | 1 | |
| MRI Suspicion Level | 0.34 | |||
| Low | 3 | 9 | ||
| Moderate | 9 | 14 | ||
| High | 2 | 1 | ||
A clear correlation was seen between increasing suspicion level on mpMRI, as determined by method described in materials and methods section, and positive biopsy status at the distal apical target lesion. Twenty-three percent (3/13) of patients with low suspicion lesions were positive, 30% (7/23) of patients with moderate suspicion lesions were positive, and 50% (1/2) of high suspicion lesions were positive (Table 3). This is very similar to the trend we have seen with mpMRI imaging and prostate cancer detection overall in our patient population as cited in previous studies.8
Table 3.
Lesion positivity by MRI Suspicion Level
| MRI Suspicion | N | % |
|---|---|---|
| Low | ||
| Pts. | 12 | |
| Bx Positive | 3 | 25 |
| Moderate | ||
| Pts. | 23 | |
| Bx positive | 9 | 39 |
| High | ||
| Pts. | 3 | |
| Bx Positive | 2 | 66 |
Previous Prostate Biopsy
Of the 38 men identified as having a distal apical lesion on MRI, 31 patients had a previous biopsy. Eighteen patients (47%) had previously undergone negative biopsies with a median of 2 negative biopsies (range 1–4). In this group of patients with prior negative biopsies only, 9 patients (50%) were positive for cancer with all 9 patients positive on MRI targeted lesions and only 6 patients positive on TRUS random biopsies. Nine patients (50%) were negative for cancer on our platform as well. Negative patients were more likely to have had a greater number of prior negative biopsies with a mean of 2.7 biopsies versus 1.9 biopsies in the patients that were positive on our platform, however this did not reach statistically significance (p .20). One patient in this group, that had undergone 4 prior negative biopsies, was positive at the distal apical lesion on targeted biopsy alone with Gleason 8 disease in 90% of the core.
Thirteen patients had a prior cancer diagnosis, with 8 patients positive on our biopsy for prostate cancer, with all except one patient on active surveillance. The solitary exception was being evaluated as a second opinion for true disease burden. Sixty-three percent (5/8) were positive at the apex on the fusion platform with no patient upgraded over prior diagnosis of Gleason 6 disease.
DISCUSSION
The apex of the prostate is an area that has shown to be difficult to biopsy based on current 12 core extended sextant TRUS schemes. False negative biopsies in this area should, theoretically, not be secondary to sampling density as this portion of the prostate is very small. Iremashvill et al reported on a group of 250 men that underwent a 12 core TRUS biopsy of the prostate followed by radical prostatectomy. Histological maps were created of the final prostate specimen and correlated to the prior prostate biopsy. Twenty eight percent of patients were positive at the apex on biopsy and 65.4 % were positive at the apex on final pathologic specimen.14 This poor negative predictive value has led multiple centers to utilize increased biopsy number and approach to target the apical region. Moussa et al prospectively evaluated 181 men with increased risk for prostate cancer and included two additional “extreme” anterior apical biopsies as part of their initial TRUS biopsy scheme. The apical cores achieved the highest cancer detection rate (73%) as well as the highest unique cancer detection rate.15 This approach still is based on the general biopsy of an area and is not targeted for specific lesions. While this is encouraging, one might argue that these very distal lesions might still be missed in these non-targeted schemes, even with an increased proportion of biopsies at the apex. Other authors have utilized saturation biopsy schemes either via transperineal or transrectal approaches. These studies have shown that increasing the sampling density will increase prostate cancer detection rates, with improved rates in the apex specifically.16,17 However many of these modalities require general anesthesia and may have increased morbidity to patients and may detect more clinically insignificant cancers. Additionally, these extended biopsy schemes are not based on pre-biopsy imaging, and therefore still have a significant risk of missing these very distal apical lesions potentially arguing for a targeted approach. In this study, we have demonstrated that directed sampling of the distal apical region can increase the biopsy yield and improve pre-decision staging.
MRI has been shown in to improve prostate cancer detection rates. Pinto et al reported their outcomes on 101 patients with low, moderate, or high suspicion lesions on mpMRI that were subsequently targeted via MRI/US fusion biopsy platform. The prostate cancer detection rates were 27, 66, and 89% respectively.8 Sciarra et al performed a prospective trial in 180 patients with prior negative biopsy and persistent PSA elevation. Patients were randomized to either MRI targeted biopsy followed by random or random TRUS guided biopsy. Prostate cancer detection in the MRI targeted group was 45.5% versus 24.4% in the random group.18 Although our cohort was small, we have depicted a similar positive correlation between mpMRI lesion suspicion score and tumor detection rates for the distal apical target lesions.
The majority of our patients in this small cohort had previously undergone biopsies with 18 that had prior negative biopsies. The true standard of care for these patients has yet to be established but many authors have shown increased prostate cancer detection with saturation biopsies (transperineal or transrectal). Since our current platform involves an extended sextant 12 core TRUS biopsy with laterally directed cores as well as the additional MRI targeted biopsies of any lesion concerning for cancer, we in essence produce a “targeted saturation type” biopsy. In this group of patients with prior negative biopsies the median number of cores taken per patient was 18 (range 13 – 27). Further studies will be needed to determine if saturation biopsy routines are equivalent in detection for these distal apical lesions in comparison to a more targeted approach.
Positive margin rates after radical prostatectomy vary widely from 2 – 59% and are dependent upon many variables such as PSA, clinical stage, and surgical modality.5,19 The apex is consistently reported as the most common location with one modern series showing rates as high as 52%.5 Recent publications have demonstrated that even in those patients with otherwise localized disease that positive surgical margins can negatively impact disease specific outcomes.20,21 Multiparametric-MRI has been shown to have significant value in its ability to correlate prostate disease burden with final histopathologic outcomes. Turkbey et al showed that MRI was able to accurately stage 80% of patients when correlated to whole mount sectioned prostate specimens.7 To the authors knowledge there have been no studies assessing MRI’s ability to localize disease and therefore decrease positive margin status after prostatectomy. However Karavitakis et al evaluated 95 consecutive patients utilizing whole mount sections of radical prostatectomy specimens. Among the thirty-one patients (33%) with a positive margin, 19 patients (61%) had multifocal disease. The index lesion (mean size 5.4cc) was the site of the positive margin in 30% (9/31) while the index lesion and satellite lesion were both sites of the positive margin in 19% (6/31) of patients. The mean size of the satellite lesion in these patients was 1.06cc. Thus, 50% of positive margins involved the index lesion. This supports the role of mpMRI in accurately stratifying these patients as it has been shown to adequately evaluate lesions of >5mm7.22 Pre-operative knowledge of these very distal apical lesions might also impact patient counseling in regards to nerve preservation and continence, especially early in the recovery period. Many authors have also recognized that a better appreciation of apical dissection may be warranted in patients undergoing surgical extirpation. Tewari et al recently described a novel technique for apical dissection to improve visualization and decrease positive surgical margin rates, with a reduction in apical margin rates from 4.4 to 1.4%.23
The location of disease is an important consideration in regards to HIFU treatment for prostate cancer. The apex is difficult to treat because of its close proximity to the striated sphincter causing several groups to begin their HIFU 6mm above the apex of the gland. This creates a theoretical 6mm “safety margin” to protect the sphincter.6 Boutier et al evaluated the largest series of patients that were treated with HIFU with systemic biopsies 3–6 months after treatment. The apex was positive in 60% of patients with positive biopsy post-treatment. This led the authors to conclude that patients with very distal apical lesions might not be good candidates for HIFU therapy.6 With this in mind, patients should have extensive counseling prior to treatment that the location of their lesion could have significant impact on treatment outcomes and an mpMRI might help predict that risk.
Our study is not without limitations. First, our patient population was relatively small and is referral based, however this “very distal apical lesion” is a novel concept brought into our practice and our team is expanding its experience on these newly described lesions. Second, we used our fusion platform to validate our results since radical prostatectomy was not performed in all patients. Criticisms of studies conducted using conventional TRUS biopsy are deserved since they are well known to be inaccurate. However, unlike conventional “blind” TRUS biopsies, these biopsies were guided by the fusion platform which allows direct sampling of each MRI positive distal apical lesions and assures that the lesion identified by MRI was the lesion evaluated histologically. This method of correlation has been previously validated and has been successfully utilized.24
In conclusion, the distal apical portion of the prostate is generally undersampled. Our results indicate that distal apical prostate can harbor cancer lesions that can be accurately detected and sampled with mpMRI and subsequent MRI/US fusion biopsy approach. This may aid clinicians and patients in decision making for therapeutic modalities and could improve prediction of positive margin rates for certain treatments.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Ishii J, Ohori M, Scardino P, Tsuboi T, Slawin K, Wheeler T. Significance of the craniocaudal distribution of cancer in radical prostatectomy specimens. Int J Urol. 2007;14:817. doi: 10.1111/j.1442-2042.2007.01836.x. [DOI] [PubMed] [Google Scholar]
- 3.Dimmen M, Vlatkovic L, Hole KH, Nesland JM, Brennhovd B, Axcrona K. Transperineal prostate biopsy detects significant cancer in patients with elevated prostate-specific antigen (PSA) levels and previous negative transrectal biopsies. BJU Int. 2011 doi: 10.1111/j.1464-410X.2011.10759.x. [DOI] [PubMed] [Google Scholar]
- 4.Lee MC, Moussa AS, Zaytoun O, Yu C, Jones JS. Using a Saturation Biopsy Scheme Increases Cancer Detection During Repeat Biopsy in Men With High-grade Prostatic Intra-epithelial Neoplasia. Urology. 2011;78:1115. doi: 10.1016/j.urology.2011.04.067. [DOI] [PubMed] [Google Scholar]
- 5.Smith JA, Jr, Chan RC, Chang SS, Herrell SD, Clark PE, Baumgartner R, et al. A comparison of the incidence and location of positive surgical margins in robotic assisted laparoscopic radical prostatectomy and open retropubic radical prostatectomy. J Urol. 2007;178:2385. doi: 10.1016/j.juro.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 6.Boutier R, Girouin N, Cheikh AB, Belot A, Rabilloud M, Gelet A, et al. Location of residual cancer after transrectal high-intensity focused ultrasound ablation for clinically localized prostate cancer. BJU Int. 2011;108:1776. doi: 10.1111/j.1464-410X.2011.10251.x. [DOI] [PubMed] [Google Scholar]
- 7.Turkbey B, Mani H, Shah V, Rastinehad AR, Bernardo M, Pohida T, et al. Multiparametric 3T Prostate Magnetic Resonance Imaging to Detect Cancer: Histopathological Correlation Using Prostatectomy Specimens Processed in Customized Magnetic Resonance Imaging Based Molds. J Urol. 2011;186:1818. doi: 10.1016/j.juro.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinto PA, Chung PH, Rastinehad AR, Baccala AA, Jr, Kruecker J, Benjamin CJ, et al. Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. J Urol. 2011;186:1281. doi: 10.1016/j.juro.2011.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turkbey B, Bernardo M, Merino MJ, Wood BJ, Pinto PA, Choyke PL. MRI of localized prostate cancer: coming of age in the PSA era. Diagn Interv Radiol. 2011 doi: 10.4261/1305-3825.DIR.4478-11.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turkbey B, Pinto PA, Mani H, Bernardo M, Pang Y, McKinney YL, et al. Prostate cancer: value of multiparametric MR imaging at 3 T for detection–histopathologic correlation. Radiology. 2010;255:89. doi: 10.1148/radiol.09090475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fine SW, Reuter VE. Anatomy of the prostate revisited: implications for prostate biopsy and zonal origins of prostate cancer. Histopathology. 2012;60:142. doi: 10.1111/j.1365-2559.2011.04004.x. [DOI] [PubMed] [Google Scholar]
- 12.Iremashvili V, Pelaez L, Jorda M, Manoharan M, Arianayagam M, Rosenberg DL, et al. Prostate sampling by 12-core biopsy: comparison of the biopsy results with tumor location in prostatectomy specimens. Urology. 2012;79:37. doi: 10.1016/j.urology.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 13.Pinto PA, Chung PH, Rastinehad AR, Baccala AA, Jr, Kruecker J, Benjamin CJ, et al. Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. J Urol. 2011;186:1281. doi: 10.1016/j.juro.2011.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iremashvili V, Pelaez L, Jorda M, Manoharan M, Arianayagam M, Rosenberg DL, et al. Prostate sampling by 12-core biopsy: comparison of the biopsy results with tumor location in prostatectomy specimens. Urology. 2012;79:37. doi: 10.1016/j.urology.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 15.Moussa AS, Meshref A, Schoenfield L, Masoud A, Abdel-Rahman S, Li J, et al. Importance of additional “extreme” anterior apical needle biopsies in the initial detection of prostate cancer. Urology. 2010;75:1034. doi: 10.1016/j.urology.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 16.Pal RP, Elmussareh M, Chanawani M, Khan MA. The role of a standardized 36 core template-assisted transperineal prostate biopsy technique in patients with previously negative transrectal ultrasonography-guided prostate biopsies. BJU Int. 2011 doi: 10.1111/j.1464-410X.2011.10355.x. [DOI] [PubMed] [Google Scholar]
- 17.Taira AV, Merrick GS, Galbreath RW, Andreini H, Taubenslag W, Curtis R, et al. Performance of transperineal template-guided mapping biopsy in detecting prostate cancer in the initial and repeat biopsy setting. Prostate Cancer Prostatic Dis. 2010;13:71. doi: 10.1038/pcan.2009.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sciarra A, Panebianco V, Ciccariello M, Salciccia S, Cattarino S, Lisi D, et al. Value of magnetic resonance spectroscopy imaging and dynamic contrast-enhanced imaging for detecting prostate cancer foci in men with prior negative biopsy. Clin Cancer Res. 2010;16:1875. doi: 10.1158/1078-0432.CCR-09-2195. [DOI] [PubMed] [Google Scholar]
- 19.Ficarra V, Cavalleri S, Novara G, Aragona M, Artibani W. Evidence from robot-assisted laparoscopic radical prostatectomy: a systematic review. Eur Urol. 2007;51:45. doi: 10.1016/j.eururo.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 20.Psutka SP, Feldman AS, Rodin D, Olumi AF, Wu CL, McDougal WS. Men with organ-confined prostate cancer and positive surgical margins develop biochemical failure at a similar rate to men with extracapsular extension. Urology. 2011;78:121. doi: 10.1016/j.urology.2010.10.036. [DOI] [PubMed] [Google Scholar]
- 21.Preston MA, Carriere M, Raju G, Morash C, Doucette S, Gerridzen RG, et al. The prognostic significance of capsular incision into tumor during radical prostatectomy. Eur Urol. 2011;59:613. doi: 10.1016/j.eururo.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 22.Karavitakis M, Ahmed HU, Abel PD, Hazell S, Winkler MH. Margin status after laparoscopic radical prostatectomy and the index lesion: implications for preoperative evaluation of tumor focality in prostate cancer. J Endourol. 2011 doi: 10.1089/end.2011.0345. [DOI] [PubMed] [Google Scholar]
- 23.Tewari AK, Srivastava A, Mudaliar K, Tan GY, Grover S, El DY, et al. Anatomical retro-apical technique of synchronous (posterior and anterior) urethral transection: a novel approach for ameliorating apical margin positivity during robotic radical prostatectomy. BJU Int. 2010;106:1364. doi: 10.1111/j.1464-410X.2010.09318.x. [DOI] [PubMed] [Google Scholar]
- 24.Turkbey B, Xu S, Kruecker J, Locklin J, Pang Y, Shah V, et al. Documenting the location of systematic transrectal ultrasound-guided prostate biopsies: correlation with multi-parametric MRI. Cancer Imaging. 2011;11:31. doi: 10.1102/1470-7330.2011.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]


