Abstract
Objective:
Quantify tuberculosis (TB) risk attributable to dorm room exposure in addition to classroom exposure.
Methods:
Adolescent school contact investigations were conducted for every reported index TB case, and similar contact investigations were conducted in selected community-control classes from November 2016 to October 2017 in Guangxi, China.
Results:
A total of 6263 contacts of 112 index TB cases and 6130 classmates of 112 controls were investigated. There were 14, 12, and 2 new active TB cases detected among classmates/non-roommates of index cases, classmates/roommates of index cases, and control classmates, respectively. Compared with control contacts, the adjusted relative risk (95% confidence interval (CI)) and population attributable fraction (PAF) for being a classmate/non-roommate of the index case increased the risk of active TB diagnosis to 8.44 (95% CI: 1.31–54.48) and 44.1%. The adjusted RR and PAF for being a classmate/ roommate of the index case was 29.37 (95% CI: 3.80, 227.11) and 41.4%. Being classmates/roommates significantly increased the risk of TB compared to a classmate/non-roommate of the index case (RR = 3.48, 95% CI: 1.64, 7.40).
Conclusion:
The additional risk of TB due to exposure in the dorm room should be taken into account in planning of TB prevention and control in boarding schools.
Keywords: Tuberculosis, adolescent, contact investigation, school
Background
Childhood and adolescent tuberculosis (TB) continues to be a growing concern and problem in countries with a medium or high prevalence of TB. It was estimated that 1.78 million young people (aged 10–24 years) developed tuberculosis, accounting for 17% of all new TB cases globally in 2012 (Snow et al., 2018). The risk of TB increases dramatically during adolescence, and faces challenges in terms of case detection and effective treatment.
Schools are the most common congregate settings reported for community-based outbreaks of TB (Raffalli et al., 1996), particularly in boarding schools, where adolescents are concentrated in relatively overcrowded conditions which predispose each individual to a higher risk of TB transmission (Stein-Zamir et al., 2006). Unlike younger children, adolescents can be effective TB transmitters (Nelson et al., 2004). It has been well documented that classroom exposure increases the risk of active TB by 2–35 times (Anon, 2009; Phillips et al., 2004; Huang et al., 2016; Caley et al., 2010a; Fang et al., 2013a).
China has the third highest number of TB cases worldwide, and in recent years TB outbreaks have frequently occurred in Chinese schools (Huang et al., 2016; Fang et al., 2013b; Wu et al., 2018; Chen et al., 2012). For compulsory education, most Chinese adolescent students are boarding students, sharing a classroom with approximately 50 students and a dorm room with 8–10 roommates. Apart from the classroom, the dorm room can also be locations of close and long-term contacts (Mekonnen and Petros, 2016), where facilitated TB transmission can occur. Several studies have reported that active TB has been detected in dorm roommates of a TB case (Stein-Zamir et al., 2006; Wu et al., 2018; Chen et al., 2012). However, the role of the dorm room in the dynamics of TB transmission has been less well documented. Little is known about the additional risk of TB that can be attributed to dorm room exposure of the index TB case in school. Knowledge on the risk of different types of school contact with active pulmonary TB and TB transmission should enable TB control programs to address the location of TB transmission at schools more properly.
The purpose of this study was to quantify the risk of having active TB between adolescent students who were exposed in classroom and/or dorm room contacts of an index active TB case and adolescents from control classrooms, then evaluate the added risk of TB transmission attributable to dorm room exposure in addition to classroom exposure.
Methods
Study setting
The Guangxi Zhuang Autonomous Region (Guangxi), with a population of 50 million, is one of the highest TB burden provinces in China. According to the Chinese Infectious Diseases Reporting System data (Anon, 2018a), the reported TB incidence was 112 and 55 per 100,000 among the general and adolescent (15–19 years old) populations, respectively in 2016. There were 5 school-related TB outbreaks in 2016 based on the local CDC reports in Guangxi. Our study period was from November 2016 to October 2017.
Study design
We conducted contact investigations for active TB in each classroom where an index active TB patient was identified, and a similar contact investigation was conducted in control classrooms selected from a matched control school in the same county.
Identifying the school and class of index case
In this study, new cases of adolescents suspected of TB were identified at the local district/county CDC clinics or hospitals and were immediately confirmed and notified to the Guangxi CDC, where a study team led by the author would conduct contact investigation within 15 days of notification. An active pulmonary TB case was defined according to the diagnostic criteria and recommendations of World Health Organization (WHO) (Organization WH, 2002).
Identifying schools and classes without an index case (control schools)
In the same district/county, where an index adolescent TB case was identified, a control school was selected by matching school type (i.e. middle school with middle school, high school with high school, public school with public school). To prevent unbalanced schools, the number of students in a matched school was chosen not to be smaller or larger than that of the index school by 20%. In the control school, a class of the same grade and similar class size as the class size of index TB case was randomly chosen for investigation. The matched control classroom must not have reported a TB case since the class was formed. However, in counties where matched schools were not available due to the small size of the county, a control classroom located the furthest distance from the classroom of the index case was selected within the index school. When TB index cases were identified from different classes in the same county, a control class was selected from the county for each of the index classrooms.
Contact investigation among the dorm roommate of index case
In Guangxi, most middle/high schools are boarding schools, and 8–10 students share the same dorm room with bunk-beds. More than 95% of students sharing the same dorm room usually study in the same class. When a student was a dorm roommate of the index TB case then they were identified and treated as an independent variable. All students from a control class were treated as if they had no index TB cases as a roommate.
Screening survey for active TB
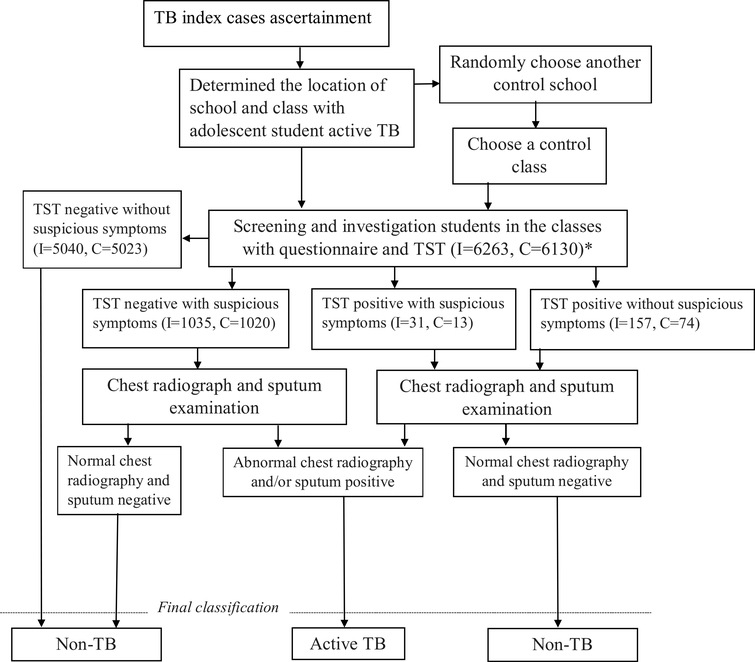
All contacts of the index TB case and all students in the control classes were screened and investigated with parental or guardian consent. Figure 1 summarizes the flow of data collection and the screening process.
Figure 1.
Flow chart indicating screening of subject for active TB.
Note: * I = index case contacts, C = students in control classes.
The investigated procedures included:
Interview questionnaire
Tuberculin skin test (TST)
Chest radiography and sputum examination
Questionnaire
The interview questionnaire contained information on age, gender, ethnicity, school type, birth place, residential status in school, bacillus Calmette – Guérin (BCG) scar (based on direct inspection), suspected symptoms of TB (cough of 2 weeks, fever, hemoptysis, drenching night sweats, and weight loss) and other pertinent medical history. Any subject with a cough >2 weeks or hemoptysis was defined as having suspicious symptoms of TB (Anon, 2017) and underwent chest radiography and sputum examination.
Tuberculin skin testing (TST)
A TST was performed on all students by the Mantoux method using 0.1 ml (2 tuberculin units) of tuberculin RT-23 (Statens Serum Institute, Copenhagen, Denmark) inoculated intradermally into the volar surface of the forearm with a standard tuberculin syringe. The skin test was read 48–72 h after plantation. Induration was read using a ruler or a caliper by trained and well-experienced public health nurses. Students with known immunocompromising conditions and those known to have TB disease at the time of evaluation did not have TST placement. In China, BCG vaccination has been provided to all newborns for more than half a century. Because of the high BCG vaccination rate, Chinese guidelines recommend students with an induration diameter ≥ 15 mm be defined as TST positive (Song et al., 2012; Education NHaFPCMo, 2017).
Chest radiography and sputum examination
All students with a positive TST or who had suspected TB symptoms had a chest radiograph performed and sputum examination with 3 sputum specimens (night, morning and a spot sputum). Acid fast smears were prepared, stained (Ziehl-Neelsen), and graded according to WHO recommendations (Organization WH, 2002). Lowenstein-Jensen cultures were evaluated twice within the first week of inoculation and then once per week for 42 days. Further diagnostic procedures for active TB followed the diagnostic criteria by WHO (Organization WH,2002). TST positive students who had a normal chest radiograph result were managed according to the national school TB guideline (Education NHaFPCMo, 2017). Students were recommended to take prophylactic treatment after obtaining parental consent. Those students who did not agree to prophylactic treatment had a chest-X-ray performed at the end of 3, 6 and 12 months after contact investigation.
Statistical analysis
Data was entered into an EpiData (version: 3.1, Odense, Denmark) database. All records were cross-checked with the original datasheets. All analyses were conducted with R (version 3.4.2, Vienna, Austria). Characteristics were compared for the adolescent contacts of the index cases and of the controls, and the impact of exposure to the index case in the same classroom and/or dorm room was evaluated for active TB. With the risk of active TB being binary (TB and non-TB), a relative risk (RR), adjusted relative risk and 95% confidence interval (CI) were analyzed. Survey logistic regression was used in multivariate analysis (Lumley, 2004). Classroom was considered as a cluster or primary sampling units (PSU). We assumed all students had an equal probability to be selected, thus equal weight parameter. The student population was also assumed to be very large, so no finite population correction factors were used. A population attributable fraction (PAF) was estimated with the formula: PAF = p (RR-1)/RR (Rockhill and Weinberg, 1998) (where p is the proportion of new active TB exposures to an adolescent index TB case in the classroom and/or dorm room). The final significance level for regression analyses was set at <0.05.
Results
Index TB case identified
There were 112 adolescents identified with active TB. Among the 112 index cases, 101 cases were from 101 individual schools. The median duration between the onset of symptoms and the date of index TB diagnosis was 11 days (range 0–365), inter-quartile range (1, 36). Four sets of 2 TB cases were from 4 schools and 1 set of 3 TB cases were from 1 school. Altogether, there were 42 middle schools and 64 high schools with TB cases were identified and reported. The counties/districts where these schools were located were distributed relatively uniformly in Guangxi province. (See Figure 2).
Figure 2.
Location of study districts/counties in Guangxi, China.
Control group
A total of 112 matched control classes without any index TB case were selected from 99 schools (55 schools with an index TB case and 44 schools without any index TB cases).
Characteristics of adolescent contacts of the index cases and of the controls
In our study, 34 (30.3%) index TB case classes and 34 (30.3%) control classes had boarding (housing) for all students in the school. The remaining 78 (69.7%) matched sets had a mixture of boarding and ambulatory (non-boarding) students. The mean class size for the index TB case classes was 60.1 ±33.7 (n ± sd) students sharing one classroom, which was not significantly different from the mean control class size of (59.4 ± 33.8).
No classmate of the index cases and no student of the control classes refused to participate. Characteristics of the students in both groups (cases and controls) are summarized in Table 1. Distribution of gender, age, ethnicity, school type, school location and BCG scar were not significantly different between the two groups. The control class students were more likely to be school boarders and had originated from rural areas.
Table 1.
Characteristics of adolescent contacts of the index TB cases and of the controls.
| Exposure variables | Control class (N = 6130) |
Index TB class (N = 6263) |
P-value chi-square test | ||
|---|---|---|---|---|---|
| n | % | N | % | ||
| School type | |||||
| Middle school | 2233 | 36.43 | 2333 | 37.25 | 0.352 |
| High school | 3897 | 63.57 | 3930 | 62.75 | |
| School location | |||||
| Urban district | 1881 | 30.69 | 1891 | 30.19 | 0.33 |
| County | 4249 | 69.31 | 4372 | 69.81 | |
| School residential | |||||
| Ambulatory | 475 | 7.7 | 607 | 9.69 | <0.001 |
| Boarding | 5655 | 92.3 | 5656 | 90.31 | |
| Gender | |||||
| Female | 3142 | 51.26 | 3153 | 50.34 | 0.318 |
| Male | 2988 | 48.74 | 3110 | 49.66 | |
| Age, years | |||||
| ≤15 | 1792 | 29.23 | 1847 | 29.49 | 0.768 |
| >15 | 4338 | 70.77 | 4416 | 70.51 | |
| Ethnicity | |||||
| Others | 2494 | 40.69 | 2456 | 39.21 | 0.098 |
| Han | 3636 | 59.31 | 3807 | 60.79 | |
| Birth place | |||||
| City | 763 | 12.45 | 991 | 15.82 | <0.001 |
| Rural | 5367 | 87.55 | 5272 | 84.18 | |
| BCG scar | |||||
| Present | 4399 | 71.76 | 4403 | 70.3 | 0.076 |
| Absent | 1731 | 28.24 | 1860 | 29.7 | |
New active TB cases detected
In the contact investigation, when the TST induration cut-off value was specified at ≥10 mm, the TST positive rate was 6.39% (400/6263) in the contact group, and 3.28% (201/6130) in the control group. When the TST induration cut-off value was established at ≥15 mm, the TST positive rate was 3.00% (188/ 6263) in the contact group, and 1.42% (87/6130) in the control group.
In those adolescents who were TST positive ( ≥15 mm) contacts of the index TB cases, 26 new active TB patients were detected (6 with culture positive sputum). Eleven (11) different classes/schools were identified by 9, 3, 3, 3, 2, 1, 1, 1, 1, 1, and 1 newly detected TB students. Students who lived in the same dorm room with index case were identified by 5, 3, 2, 1, and 1 new active TB cases. There was no active TB case detected among 17 student contacts who shared the same dorm room with the index TB case but studied in different classrooms. This small group was excluded in the subsequent analysis to reduce the complexity of exposure group. They were not significantly different between the duration of exposure to the index case and the number of new active TB detection in the classroom (P-value = 0.379) or in the dorm room (P-value = 0.108).
The number of contacts of each group and the number of new active TB cases identified are summarized in Table 2. Two new active TB cases were detected among the control group. We did not subdivide the control contacts into subgroups because the number of new TB cases among them was too small (n = 2). The overall relative risk of exposure to an adolescent index TB case for active TB diagnosis was 12.77 (95% CI: 3.2, 111.16).
Table 2.
The association of exposure index case in the classroom and dorm room with new active TB.
| Type of contacts | New active TB |
Crude RR (95% CI) | P-value | |
|---|---|---|---|---|
| No | Yes | |||
| Contacts of control | 6128 | 2 | 1 | |
| Contacts of index case | ||||
| Classmate and dorm roommate | 1117 | 12 | 32.58 (12.08, 87.87) | <0.001 |
| Classmate living in different dorm room | 4497 | 13 | 8.83 (2.34, 33.37) | 0.001 |
| Ambulatory classmate | 606 | 1 | 5.05 (0.01, 4771.59) | 0.141 |
Abbreviation: TB: tuberculosis; RR, relative risk.
The association of exposure to index TB in the classroom and dorm room
Table 2 presents the association between exposure to the index TB case in the classroom and dorm room in detecting././Lenovo/ AppData/Local/youdao/dict/Application/7.5.2.0/resultui/dict/active TB. Being classmates and dorm roommates of the index cases and classmates living in different dorm rooms had a significantly higher risk of TB diagnosis compared to the group who had no contact with index case (contacts of the control) (P ≤ 0.001). The number of ambulatory classmate contacts was too small (n = 606) to detecte a significant increase in TB risk (95% CI: 0.01, 4771.59), thus we merged the latter two groups together to be “classmate/ non-roommate” used in subsequent analysis.
Factors predicting new active TB
Table 3 provides the tabulation between being new active TB and independent variables followed by multivariate analyses. Note that the number of students who shared classrooms only (and not dorm rooms) with the index cases occurred 4.6 (5103/1117) times more often than classmates who also shared dorm rooms with the index cases. Although there was a higher likelihood of only sharing a classroom with the index case, a classmate/non-roommate had an 8.44 (95% CI: 1.31, 54.48) times increased risk (the PAF was 44.08%), and a classmate/roommate had a 29.37 (95% CI: 3.80, 227.11) times increased risk of being diagnosed with new TB (the PAF was 41.40%). Being both a classmate and dorm roommate of the index case had a significantly higher TB diagnosis risk than the classmate/non-roommate contacts (RR = 3.48, 95% CI: 1.64, 7.40).
Table 3.
The independent effects of classroom exposure and/or dorm room exposure to active TB.
| Variables | Active TB [n (%)] |
Adjusted RR | P-value | PAF (%) | |
|---|---|---|---|---|---|
| No | Yes | (95% CI) | |||
| Type of contacts | |||||
| Contacts of control | 6128 (99.97) | 2 (0.03) | 1 | ||
| Contacts of index case | |||||
| Classmate/non-roommate | 5103 (99.7) | 14 (0.3) | 8.44 (1.31, 54.48) | 0.027 | 44.08 |
| Classmate and dorm roommate | 1117 (98.9) | 12 (1.1) | 29.37 (3.80, 227.11) | 0.002 | 41.40 |
| School type | |||||
| Middle school | 4545 (99.5) | 21 (0.5) | 1 | ||
| High school | 7820 (99.9) | 7 (0.1) | 0.19 (0.06, 0.68) | 0.012 | - |
| Gender | |||||
| Female | 6275 (99.7) | 20 (0.3) | 1 | ||
| Male | 6090 (99.9) | 8 (0.1) | 0.44 (0.17, 1.15) | 0.098 | - |
| Age, years | |||||
| ≤15 | 3623 (99.6) | 16 (0.4) | 1 | ||
| >15 | 8742 (99.9) | 12 (0.1) | 0.98 (0.52, 1.84) | 0.947 | - |
| Ethnicity | |||||
| Others | 4930 (99.6) | 20 (0.4) | 1 | ||
| Han | 7435 (99.9) | 8 (0.1) | 0.25 (0.07, 0.88) | 0.033 | - |
| BCG scar | |||||
| Present | 8786 (99.8) | 16(0.2) | 1 | ||
| Absent | 3579 (99.7) | 12(0.3) | 1.68 (0.88, 3.20) | 0.118 | |
| Sputum smear status | |||||
| Control | 6128 (99.97) | 2 (0.03) | - | ||
| Negative | 3887 (99.6) | 15(0.4) | 1 | ||
| Positive | 2350 (99.5) | 11(0.5) | 1.12 (0.26, 4.89) | 0.879 | |
Abbreviation: TB: tuberculosis; RR, relative risk; PAF: population attributable fraction.
Being of a non-Han ethnicity was associated with a greater risk for active TB. Nearly 70% of new TB cases were detected in non-Han student contacts, and they had the same ethnicity with their index case in this study setting. We further analyzed the association of ethnicity of index cases and new TB cases among contacts. Non-Han ethnicity index case had increased odds of detecting new active TB among contacts (OR = 3.42, 95% CI: 1.37, 9.64).
Although middle school was more likely to have students of lower age than high school, overlapping of age groups among students of both types of school allowed us to examine independent effects of each variable. Students of middle schools wereat significantly higher risk of getting active TB than those at high school (OR = 0.19, 95% CI: 0.06, 0.68). However, age did not have independent effect of getting active TB (OR = 0.98, 95% CI: 0.52, 1.84).
Effect of exposure to index TB case by smear status
We further analyzed the effect of exposure to adolescent index TB cases by acid fast bacilli (AFB) smear status. Among the 112 index TB cases, 42 were AFB smear-positive and 70 were AFB smear-negative. Using the control group as the reference, the odds ratios of TST positives after exposure to smear-positive and smear-negative groups were 2.31 (95% CI: 1.67, 3.19) and 2.05 (95% CI: 1.53, 2.76), respectively. These two odds ratios were not significantly different (OR = 1.13, 95% CI: 0.83, 1.53, P-value = 0.45). The corresponding odds of becoming an active TB case were 14.34 (95% CI: 3.18, 64.74) and 11.82 (95% CI: 2.70, 51.72), respectively. These two odds ratios were also not significantly different (OR = 1.21, 95% CI: 0.50, 2.83, P-value = 0.687).
Discussion
In this population-based study, we found that the risk of TB associated with studying in the same classroom and living in the same dorm room as a TB contact was increased by 3.5 times compared to the risk associated with being a classmate/non-roommate, but also had a slightly lower attributable risk than the latter. There was no statistically significant increased risk of TB in school contacts exposed to AFB smear-positive index cases compared to exposure with AFB smear-negative ones.
The possible explanations for the additional risk (3.5-fold increased) of new TB due to exposure in the dorm room may be due to longer duration of exposure with the index TB case. In China, it is a common phenomenon that study time can increase up to 10 h/ day in the classroom in secondary school, particularly under the pressure of a high school or University Entry Exam (Huang et al., 2016). Apart from study time, the dorm roommates also slept about 6–7 h/day in the same dorm room with the index case (Chen et al., 2012). This extra 6–7 h of TB exposure time accounted for this 3.5 fold increase in risk.
The population-attributable fraction represents the effect of newly diagnosed TB in the classroom and dorm room due to exposure of an index TB case. Our data suggest that 44% of adolescent new TB in the community was attributable to classmate/non-roommate exposure of the index case and 41% attributable to classmate and dorm roommate exposure of the index TB case. Whereas the risk of new TB in the latter group was 3.5 times of the former, the attributable fraction was slightly lower. This could be explained by the fact that both classmates and dorm roommates exposure were 4.6 times smaller in number, thus having a limited contribution. Attributable fraction depends on the relative risk, as well as the proportion of the population exposed to the risk factor. For adolescent school TB, the proportion of the population exposed to TB (based on our study) would be reflected by the number of students in the same class (class size) and the number of students in the same dorm room (dorm room size). The Chinese national guidelines suggest an average class size should be 50 students (Anon, 2018b).This is much larger than the average class size of 20.3 students in the United States and approximately 20–35 students generally observed in the United Kingdom (Caley et al., 2010b; Coopersmith, 2009). In the Guangxi Zhuang Autonomous Region, where the economy is underdeveloped, the average number of students per class reached 60 in our study area. If the class size and the dorm room size could be reduced, then we believe the number of students exposed to TB could also be reduced. Control of TB transmission in the classroom and dorm room could appreciably reduce the overall incidence of TB in the region.
In the general population, males are well known to have a higher risk of getting TB than females (Holmes et al., 1998; Borgdorff et al., 2000; Rhines, 2013). The opposite was true in our adolescent population, which was consistent with previous studies (Weber et al., 2000; Bolursaz et al., 2016; Phongsamart et al., 2009). On one hand, this may be explained by difference between lifestyles of the two genders in these two age groups. On the other hand, it may be due to difference context between the open community and the school setting. Further studies are needed to get the answers for risk inequality between genders across social contexts.
Our findings also showed that the non-Han ethnicity was associated with a greater risk for TB diagnosis. One possible explanation for this finding is that those of non-Han ethnicity were likely to come from impoverished mountain counties, which have limited resources for disease prevention and control, and thus had increased risk of getting active TB.
Middle schools were at higher risk of having TB transmission from index cases than high school. This may be due to the fact that at higher level of education, physical closeness among students is likely to decrease (Nickerson and Nagle, 2005), thus reducing the risk of transmission.
Multiple earlier studies have reported that smear-positive patients have been considered more infectious than smear negative patients, but the latter can also contribute to TB transmission (Puryear et al., 2013; Tostmann et al., 2008; Hernandez-Garduno et al., 2004). Although exposure to smear positive cases in our study has an odds ratio of getting new TB above one, we could not find a statistically significant difference between these two exposures. This was due to the relatively small sample size (15 and 11 new TB cases only).
There were several limitations of our study. First, we lacked data on exposure to TB outside the classroom and dorm room setting, such as household exposure to active TB cases in the households. Information on these exposures may lead to better understanding of the linkage between school TB and community TB. Secondly, the effect of boarding on the risk of TB could not be quantified in the current study due to relatively small number of ambulatory students. Thirdly, we provided only chest X-ray screening for all TST positive and symptomatic students, and may have missed TST negative and asymptomatic active TB cases. The number of new active TB detection might be also different if follow-up occurred one or two years after contact investigation.
Conclusion
Our findings showed that several newly identified TB cases were detected in the same classrooms and dorm rooms of the index TB cases. The additional risk of TB transmission due to exposure in the dorm room should be taken into account in planning of TB prevention and control programs in boarding schools. A reduction in the number of students sharing the same classroom and those sharing the dorm room is highly recommended so as to decrease the prevalence of school TB outbreak in Guangxi.
Acknowledgements
Drs. Alan Geater and Angkana Chaiprasert are acknowledged for their help in the study design. We thank all the counties/districts doctors, school administrators, teachers and school nurses help in conducting survey. We also thank all students who participated in this study.
Funding
The study was supported by the Guangxi Center for Disease Prevention and Control and the National Natural Science Foundation of China (Grant number 81560549). This study is a part of the first author’s thesis to fulfil the requirement of PhD degree in Epidemiology, Prince of Songkla University. The training program for TB/MDR-TB research is supported by the Fogarty International Center, Grant number D43TW009522.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
The study protocol and informed consent forms were reviewed and approved by the Research Ethics Committee of Prince of Songkla University (No. 59–247-18–5) and Ethical Review Committee of Guangxi (No. GX IRB2016–0049). Before enrollment, for students less than 18 years of age consent from the parents/ guardians was requested and received. Older students used their own judgment in giving consent.
References
- Anon. A school- and community-based outbreak of Mycobacterium tuberculosis in Northern Italy, 1992–3. Epidemiol Infect 2009;113(1):83–93. [PMC free article] [PubMed] [Google Scholar]
- Anon. Diagnosis for pulmonary tuberculosis. 2017. [Google Scholar]
- Anon. Chinese Infectious Diseases Reporting System. 2018. Available from: http://10.249.1.170/.
- Anon, https://baike.baidu.com/item/StandardizationSchool.
- Bolursaz MR, Lotfian F, Aghahosseini F, Hassanzad M, Ghafaripoor H, Khalilzadeh S, et al. Characteristics of tuberculosis among children and adolescents at a referral TB’s Hospital, 2006–2011. J Compr Pediatr 2016;7(3). [Google Scholar]
- Borgdorff M, Nagelkerke N, Dye C, Nunn P. Gender and tuberculosis: a comparison of prevalence surveys with notification data to explore sex differences in case detection. Int J Tuberc Lung Dis 2000;4(2):123–32. [PubMed] [Google Scholar]
- Caley M, Fowler T, Welch S, Wood A. Risk of developing tuberculosis from a school contact: retrospective cohort study, United Kingdom, 2009. Euro Surveill 2010a;15(11). [PubMed] [Google Scholar]
- Caley M, Fowler T, Welch S, Wood A. Risk of developing tuberculosis from a school contact: retrospective cohort study, United Kingdom, 2009. Eurosurveillance 2010b;15(11):19510. [PubMed] [Google Scholar]
- Chen W, Xia Y, Li X, Zhou L, Li C, Wan K, et al. A tuberculosis outbreak among senior high school students in China in 2011. J Int Med Res 2012;40(5):1830–9. [DOI] [PubMed] [Google Scholar]
- Coopersmith J Characteristics of public, private, and bureau of Indian education elementary and secondary school teachers in the United States: results from the 2007–08 schools and staffing survey. First look. NCES 2009–324. National Center for Education Statistics; 2009. [Google Scholar]
- Education NHaFPCMo. Regulations on prevention and control of tuberculosis in schools. 2017.
- Fang Y, Zhang L, Tu C, Ye D, Fontaine R, Ma H, et al. Outbreak of pulmonary tuberculosis in a Chinese high school, 2009–2010. J Epidemiol 2013a;23 (4):307–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, Zhang L, Tu C, Ye D, Fontaine R, Ma H, et al. Outbreak of pulmonary tuberculosis in a Chinese high school, 2009–2010. J Epidemiol 2013b;23 (4):307–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Garduno E, Cook V, Kunimoto D, Elwood R, Black W, FitzGerald J. Transmission of tuberculosis from smear negative patients: a molecular epidemiology study. Thorax 2004;59(4):286–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes CB, Hausler H, Nunn P. A review of sex differences in the epidemiology of tuberculosis. Int J Tuberc Lung Dis 1998;2(2):96–104. [PubMed] [Google Scholar]
- Huang Y, Zhong J, Wu Q, Liu Z, Pan A, Zhu L, et al. Investigation of a large school-based outbreak of tuberculosis infection in Eastern China. Pediatr Pol 2016;91 (6):541–6. [Google Scholar]
- Lumley T Analysis of complex survey samples. J Stat Softw 2004;9(1):1–19. [Google Scholar]
- Mekonnen A, Petros B. Burden of tuberculosis among students in two Ethiopian universities. Ethiop Med J 2016;54(4):189–96. [PMC free article] [PubMed] [Google Scholar]
- Nelson LJ, Schneider E, Wells CD, Moore M. Epidemiology of childhood tuberculosis in the United States, 1993–2001: the need for continued vigilance. Pediatrics 2004;114(2):333–41. [DOI] [PubMed] [Google Scholar]
- Nickerson AB, Nagle RJ. Parent and peer attachment in late childhood and early adolescence. J Early Adolesc 2005;25(2):223–49. [Google Scholar]
- Organization WH. An expanded DOTS framework for effective control Tuberculosis. Geneva: WHO; 2002. [PubMed] [Google Scholar]
- Phillips L, Carlile J, Smith D. Epidemiology of a tuberculosis outbreak in a rural Missouri high school. Pediatrics 2004;113(6):e514–9. [DOI] [PubMed] [Google Scholar]
- Phongsamart W, Kitai I, Gardam M, Wang J, Khan K. A population-based study of tuberculosis in children and adolescents in Ontario. Pediatr Infect Dis J 2009;28 (5):416–9. [DOI] [PubMed] [Google Scholar]
- Puryear S, Seropola G, Ho-Foster A, Arscott-Mills T, Mazhani L, Firth J, et al. Yield of contact tracing from pediatric tuberculosis index cases in Gaborone, Botswana. Int J Tuberc Lung Dis 2013;17(8):1049–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffalli J, Sepkowitz KA, Armstrong D. Community-based outbreaks of tuberculosis. Arch Intern Med 1996;156(10):1053–60. [PubMed] [Google Scholar]
- Rhines AS. The role of sex differences in the prevalence and transmission of tuberculosis. Tuberculosis 2013;93(1):104–7. [DOI] [PubMed] [Google Scholar]
- Rockhill BNB, Weinberg C. Use and misuse of population attributable fractions. Am J Public Health 1998;88(1):15–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow KJ, Sismanidis C, Denholm J, Sawyer SM, Graham SM. The incidence of tuberculosis among adolescents and young adults: a global estimate. Eur Respir J 2018;51(2)1702352. [DOI] [PubMed] [Google Scholar]
- Song Q, Guo H, Zhong H, Liu Z, Chen X, Wang C, et al. Evaluation of a new interferon- gamma release assay and comparison to tuberculin skin test during a tuberculosis outbreak. Int J Infect Dis 2012;16(7):e522–6. [DOI] [PubMed] [Google Scholar]
- Stein-Zamir C, Volovik I, Rishpon S, Atamna A, Lavy A, Weiler-Ravell D. Tuberculosis outbreak among students in a boarding school. Eur Respir J 2006;28(5):986–91. [DOI] [PubMed] [Google Scholar]
- Tostmann A, Kik SV, Kalisvaart NA, Sebek MM, Verver S, Boeree MJ, et al. Tuberculosis transmission by patients with smear-negative pulmonary tuberculosis in a large cohort in the Netherlands. Clin Infect Dis 2008;47 (9):1135–42. [DOI] [PubMed] [Google Scholar]
- Weber H, Beyers N, Gie R, Schaaf H, Fish T, Donald P. The clinical and radiological features of tuberculosis in adolescents. Ann Trop Paediatr 2000;20(1):5–10. [DOI] [PubMed] [Google Scholar]
- Wu X, Pang Y, Song Y, Dong W, Zhang T, Wen S, et al. Implications of a school outbreak of multidrug-resistant tuberculosis in Northern China. Epidemiol Infect 2018;146(5):584–8. [DOI] [PMC free article] [PubMed] [Google Scholar]