Abstract
While environmental pollutants have been associated with changes in endocrine health in cetaceans, efforts to link contaminant exposure with hormones have largely been limited to a list of known, targeted contaminants, overlooking minimally characterized or unknown compounds of emerging concern. To address this gap, we analyzed a suite of potential endocrine disrupting halogenated organic compounds (HOCs) in blubber from 16 male short-beaked common dolphins (Delphinus delphis) with known maturity status collected from fishery bycatch in the Southern California Bight. We employed a suspect screening mass spectrometry based method to investigate a wide range of HOCs that were previously observed in cetaceans from the same region. Potential endocrine effects were assessed through the measurement of blubber testosterone. We detected 167 HOCs, including 81 with known anthropogenic sources, 49 of unknown origin, and 37 with known natural sources. The sum of 11 anthropogenic and 4 unknown HOC classes were negatively correlated with blubber testosterone. Evidence suggests that elevated anthropogenic HOC load contributes to impaired testosterone production in mature male D. delphis. The application of this integrative analytical approach to cetacean contaminant analysis allows for inference of the biological consequences of accumulation of HOCs and prioritization of compounds for future environmental toxicology research.
Keywords: Marine mammal, Halogenated organic compounds, Endocrine Disruption, Southern California Bight, Blubber hormones
Graphical Abstract
INTRODUCTION
Many known persistent organic pollutants (POPs), such as organochlorine pesticides, are halogenated organic compounds (HOCs) that are slow to degrade and biomagnify in food webs. Exposure to POPs poses a significant threat to the health of human and wildlife populations.1,2 Due to the difficulty in measuring the large number of known contaminants in the environment and identifying potential unknown compounds, there is a paucity of information on the full extent of HOCs present in the environment and their potential effects3 on wildlife.
Marine mammals are considered environmental sentinels of exposure to and effects of POPs in higher trophic level organisms,4 serving as sensitive, early-warning indicators of contaminant risks to aquatic environments.5 Bioaccumulation and biomagnification of compounds in marine mammals allows for the detection of low-level compounds that are persistent and difficult to measure in lower trophic levels. POP exposure in pinnipeds and cetaceans has been associated with the disruption of endocrine hormones, reproductive failure, decreased immune function, and an increased risk of mortality in primarily observational studies in situ.6–10 However, the majority of studies on HOC exposure in marine mammals focus on measuring a few selected compounds or compound classes, excluding additional known and unexpected contaminants, making it difficult to compare exposure effects to a broad set of HOCs.3,11,12
Recent technological advances have enabled the nontargeted analysis of HOCs in biological samples, enhancing attempts to measure the total load of HOCs in wild individuals. Previous research has identified a broad suite of anthropogenic, natural, and unknown HOCs in fish oil, bird eggs, and dolphin blubber via nontargeted analysis using comprehensive two-dimensional gas chromatography coupled to time-of-flight mass spectrometry (GC×GC/TOF-MS).11–15 Shaul et al.12 identified 327 HOCs in blubber samples from eight bottlenose dolphins (Tursiops truncatus) stranded in the Southern California Bight (SCB), which revealed that 86% of detected compounds are not typically monitored. These included previously unrecognized anthropogenic HOCs, unknown HOCs, and halogenated natural products (HNPs). HNPs are compounds that have physical-chemical properties similar to anthropogenic POPs and are known to bioaccumulate in dolphins.
A high abundance of HOC compounds in mature individuals suggest that HOCs are persistent in the environment and difficult to metabolize, resulting in an increase in HOC body burden over time.16 Given this body burden accumulation and biomagnification, HOC screening paired with biological metrics, including maturity status and hormone analysis can be used to identify potential biological effects of HOC compounds.17 HOCs have been linked to disruption of endocrine hormones that are necessary for development, survival, and reproduction in humans and a range of wildlife species (e.g. birds, amphibians, and terrestrial and marine mammals),2,18,19 including the disruption of testosterone, a hormone associated with male maturity. HOC exposure has also been associated with changes in sexual development and sperm quality in terrestrial mammals.20–22 However, few studies, if any, have investigated the impact of HOCs on the endocrine system of marine mammals especially as they relate to androgen production.
Quantification of hormones in marine mammal blubber specifically allows assessment of reproductive health in free-ranging marine mammal populations.23,24 For most species, biopsies composed of skin and blubber are the only practical way to obtain biological material from wild dolphins in number sufficient for population-level analysis. Testosterone is primarily produced by the testes in males and is lipophilic therefore will partition into lipid-rich tissues, such as marine mammal blubber.25 Previous studies have typically documented relationships between serum hormones and POPs in marine mammals (e.g. Schwacke et al.10) but hormone levels in blubber are less variable than serum providing a better measure of chronic rather than acute changes in endocrine biomarkers.26 Thus, incorporating blubber hormone quantification with comprehensive HOC analysis enables assessment of potential long-term changes in reproductive hormones and provides baseline information for field-based studies on wild marine mammals.
Here we performed contaminant suspect screening on the blubber of mature and immature male short-beaked common dolphins (Delphinus delphis) from the SCB using an established mass spectral library of 268 HOCs generated from a nontargeted analysis of bottlenose dolphins from the same region.12 Marine mammals in the SCB have some of highest levels of dichlorodiphenyltrichloroethane (DDT), polychlorinated diphenyls (PCB), and polybrominated diphenyl ethers (PBDEs) recorded globally due to historical deposition near Los Angeles and proximity to multiple urban areas.12,27–29 D. delphis are likely to bioaccumulate high levels HOCs as they are frequently detected throughout the SCB30 and feed at a high trophic level.31 Blubber testosterone, a measure of reproductive health, was also quantified in each individual. We then paired testosterone measurements with comprehensive HOC screening for the first time to identify ecologically relevant HOCs and evaluate evidence of a potential biological response to HOC exposure and bioaccumulation in wild marine mammals.
MATERIALS AND METHODS
Samples
Sixteen male D. delphis individuals of known maturity (8 immature and 8 mature) were caught incidentally (presumed to be healthy) by fishing gear and selected for HOC and hormone analysis: specimens were collected between 1999 and 2008 and archived at NOAA’s Southwest Fisheries Science Center (Table S1). Sampling from incidental bycatch of non-threatened marine mammals is permitted through the NMFS Marine Mammal Authorization Program under the Marine Mammal Protection Act (16 U.S.C. 1371(a)(5)). All blubber samples were collected, along with basic morphometric measurements, during non-breeding season months (fall and winter) to avoid spurious correlations due to hormone changes from breeding activity during summer. Maturity was determined with reference to testes weight according to criteria defined in Kellar et al.25 A complete cross section of blubber was taken from the dorsal region between 10 minutes and 4 hours after death and stored at −20°C until analysis. Kellar et al25 found no significant relationship between blubber testosterone level and time at −20 °C degrees thus it is assumed testosterone levels in blubber were relatively stable since collection. Two samples were designated as juveniles because they could not be appropriately classified. This was confirmed by obtaining ages determined via tooth histology, available for 14 of the 16 individuals. The two samples were eliminated from all maturity-related analyses because of their intermediate maturity status to focus on individuals within the two defined maturity states. All other immature individuals were presumed to be pre-weaning as all were younger than 16.5 months, the estimate of age-at-weaning for D. delphis in the Eastern Tropical Pacific.32 All mature individuals are within the expected age range for peak reproduction (12–18 years of age) and not likely starting to senesce. According to Westgate and Read33 males in this population reach sexual maturity by 11.9 years of age and can live up to 25+ years.
Suspect Screening for HOCs and Relative Abundance Quantification
Blubber samples for HOC analysis were prepared following the protocol of Shaul et al.12 Two grams of blubber were extracted with dichloromethane using a pressurized liquid extractor (Dionex ASE 300, Dionex, Sunnyvale, CA, USA). The dichloromethane was evaporated from the sample. One gram of lipid extract was subsampled and spiked with a known amount of internal standard, 13C12-PCB-169, because the final data were corrected by lipid weight rather than blubber weight. Lipid was removed by automatic gel permeation chromatography (GPC) (J2 Scientific, Columbia, MO). The eluent was evaporated to 100 µL in water bath under a N2(g) stream a using a Zymark TurboVap. The extract was spiked with recovery standards (13C12-PCB-189 and 4’-fluoro-2,3,3’,4,5,6-hexabromodiphenyl ether) and concentrated to 100 µL under a N2(g) stream. The final extract of each sample was run on a Pegasus 4D GC×GC/TOF-MS system (LECO, St. Joseph, MI, USA) with the conditions described in Shaul et al.12 Each batch included a procedural blank. Two compounds (a chlorinated benzene and a chlorophosphate) were detected in the procedural blank were discarded from final analysis.
A suspect screening was conducted for HOC analysis. The instrument software (LECO ChromaTOF software, version 4.50.8.0) was used to search for peaks of HOCs at S/N ≥ 50. Each sample produced approximately 6,000 to 10,000 chromatographic features and associated mass spectra, which were manually identified by matching the mass spectra and the GC×GC retention times with the Pacific dolphin library produced by Shaul et al.12 While the Pacific dolphin library contains 327 compounds, we excluded toxaphenes and polychlorinated terphenyls from the suspect screening list prior to analysis due to unclear mass spectra, as well as DDT, dichlorodiphenyldichloroethylene (DDE), and PCBs due to oversaturated peak areas. However, other known DDT-related compounds, including dichlorodiphenyldichloroethane (DDD), and methylsulfonyl-PCBs (PCB metabolites) were included. The resulting suspect screening list included 269 of the 327 compounds from 31 compound classes in the Pacific dolphin library. All compounds were named and classified according to the library produced by Shaul et al.12 Relative abundance was quantified using peak area, using the same ions as in Shaul et al.12 Peak areas for all compounds were corrected relative to the peak area of the internal standard in each sample, and further normalized by each sample’s lipid weight. Isotope-labelled standards are not available for every class such as unknown compounds and HNPs. In addition, we tested multiple internal standards in our previous project12 and found no difference (using isotope labeled PCBs and PBDEs) for comparison of normalized relative abundances. Therefore, the reported values represent a normalized relative abundance of each compound in each sample, i.e. not absolute concentrations, rather a relative concentration of HOCs in each sample in the set.
Hormone analysis
Approximately 100 mg of blubber was homogenized using an Omni BeadRuptor (Omni International, Kennesaw, GA), testosterone was isolated using a biphasic solvent extraction, and extraction efficiency was calculated according to the methods in Kellar et al.34 and Trego et al.35 The final extracts were dried and stored dry at −20° C prior to analysis with a commercially available testosterone enzyme-linked immunosorbent assay (ELISA) kit from Enzo Life Sciences (Farmingdale, NY). Stored samples were reconstituted in 250 µL of phosphate buffered saline (pH 7.5) containing 1% bovine serum albumin prior to analysis on a 96-well ELISA plate. Samples were run in duplicate to account for assay variability. Final hormone concentrations were corrected according to extraction efficiency and blubber weight, and an average blubber hormone concentration (in nanograms per gram) was calculated for each individual.
Data analysis
All data were analyzed using R version 3.3.1.36 Data analyses were broken down into three different steps: characterizing HOC profiles, identifying maturity-related HOC accumulation, and examining evidence of endocrine disruption. For the first step, we ran a principal component analysis (PCA) on compound classes to reduce the dimensionality of the data (167 compounds, 26 classes) and identify co-occurring compound classes accumulating in D. delphis. Next, we used Random Forest37–39 and Mann-Whitney U tests to assess maturity-associated bioaccumulation of compounds classes. Finally, we used linear regression and Random Forest to investigate whether there was evidence of a biological response associated with higher HOC levels. Details on each quantitative analysis are provided in the supplemental information.
Normalized relative peak abundances were natural log transformed prior to all statistical analyses except in Random Forest models, which make no assumption of normality. All nondetects were set to zero and all compounds were summed according to compound class as in Shaul et al.12 The 5 individual peaks of 3 compounds were too saturated to get an accurate estimate of abundance and were excluded from all analyses except for the Random Forests: MBP-Cl7 in two individuals and trans-nonachlor, o,p’-DDD, and p,p’-DDD each in one individual.
RESULTS
Characterizing HOC profiles in Delphinus delphis
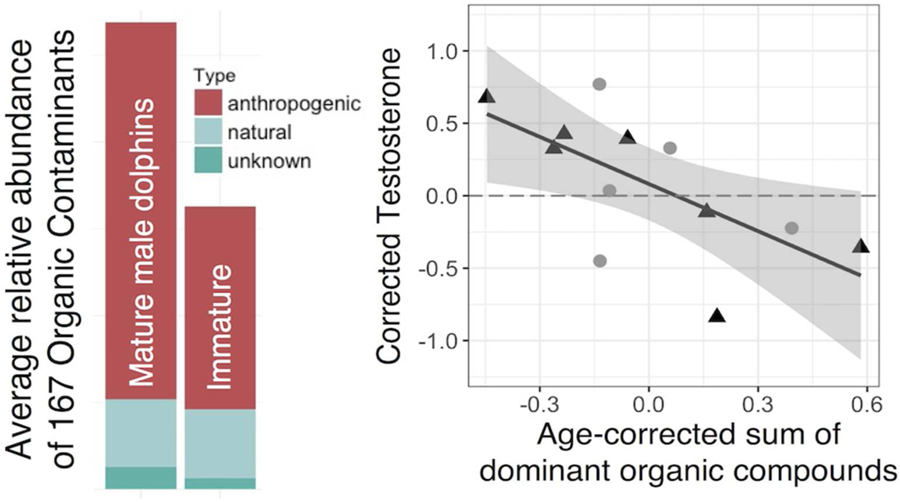
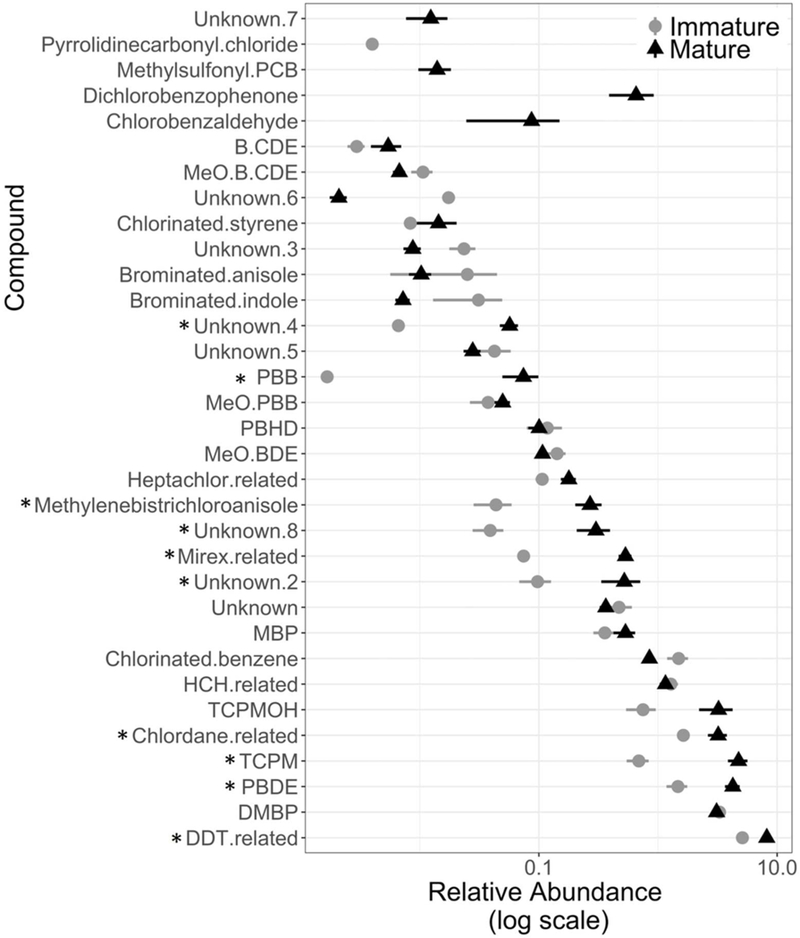
Of the 269 HOCs screened, we confirmed the presence of 167 HOCs from 26 out of 31 screened compound classes (see table S2 for the complete list). Eighty compounds were of anthropogenic origin, 49 were of unknown origin, 37 were from natural compounds, and one was from mixed sources. In terms of abundance, anthropogenic compounds were the predominant type of HOC (61%, Figure 1a). While there were a greater number of unknown compounds detected compared to natural HOCs, unknown compounds represented 8% of total relative HOC abundance compared to 27% for natural HOCs. The compound classes in highest abundance were DDT-related compounds, PBDEs, dimethyl bipyrroles (DMBPs), tris(4-chlorophenyl)methane (TCPM), chlordane-related compounds, and tris(4-chlorophenyl)methanol (TCPMOH; Figure 1b). Of the 167 HOCs detected, only 28 are typically monitored (e.g DDD, PBDEs, and chlordane), with the remaining 139 compounds identified as largely unmonitored (e.g. TCPM, TCPMOH, HNPs, and unknown compounds).
Figure 1:
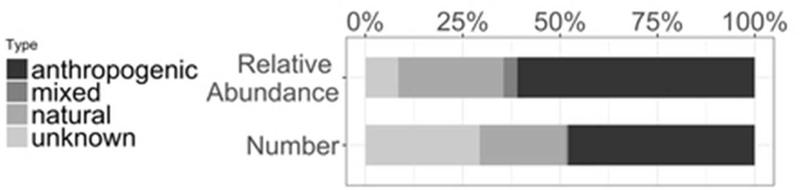
A) The overall number and average relative abundance of halogenated organic compounds (HOCs) classified by origin (gray scale) among all samples.
B) The average relative abundance (in log scale) and standard error of all compound classes by maturity type (black circles and gray triangles represent data from immature and mature animal samples, respectively). The * denotes compound classes with significantly different abundance between mature and immature animals, as determined by randomForest permutation tests.
We identified a group of 15 co-occurring compound classes using PCA. PC1, which accounted for 44% of the total variance in the dataset, represented several known anthropogenic compound classes (here referred to as the “AC group” to distinguish this set of compounds from general anthropogenic compounds, Figure 2), the majority of which are known to bioaccumulate. Several unknown compound classes also loaded strongly with this group, including unknown classes 2, 4, 7, and 8 (numbers as defined in Shaul et al.12). Unknown 2 compounds were recently identified as novel isomers of TCPMOH,28 a metabolite of anthropogenic compound TCPM, (hereafter referred to as unknown 2 (TCPMOHN)). Several HOCs with natural origin, including DMBPs, methyl bipyrroles (MBPs), and methoxy brominated diphenyl ethers (MeO-BDEs), were loosely grouped in the upper right of the biplot along with unknown compound classes 3 and 5 and a few additional anthropogenic compounds. A PCA biplot with the samples plotted based on maturity state is available in the supplementary material (Figure S1).
Figure 2:
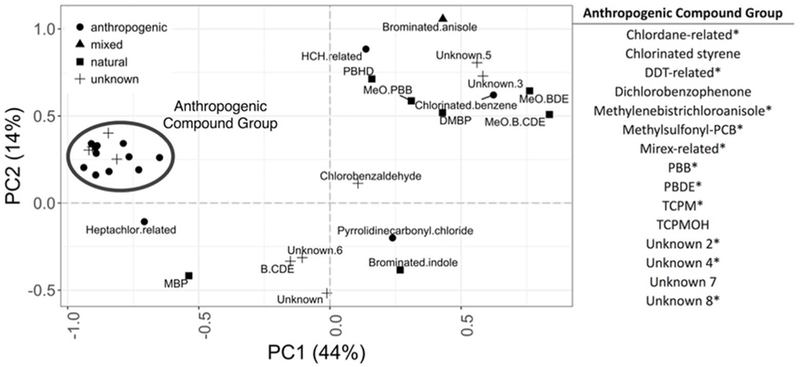
A principal components analysis (PCA) plot of the loadings for all compound classes according to origin (anthropogenic, mixed, natural, or unknown). The drawn circle highlights the anthropogenic compound group and the compound classes contributing to this group are listed on the right. Within the group, compound classes with significant higher relative abundance according to the Random Forest are marked with an asterisk (*).
Identifying maturity-related HOC bioaccumulation
Overall, differences in HOC patterns were apparent between mature and immature individuals (Figure 3). Mature individuals had a significantly higher number of HOCs (104 ± 4.2 vs. 81 ± 4.2, mean ± standard error respectively, p = 0.01, Mann-Whitney U) as well as the total relative abundance compared to immature animals (32.1 ± 3.5 vs. 17.3 ± 1.2 respectively, p = 0.001). The two juvenile individuals of intermediate maturity level had a similar number of compounds as confirmed immature individuals (63 and 76), but differed greatly in total relative abundance (17.9 vs. 88.3).
Figure 3:
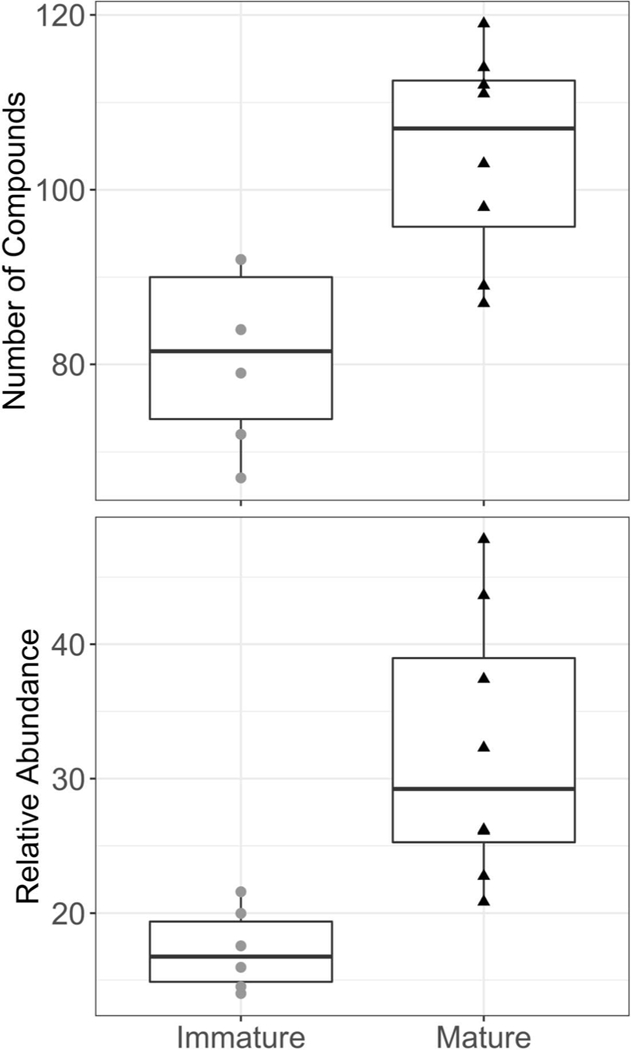
Difference in average total number and relative abundance of compounds detected in confirmed immature and mature individuals. Compound classes that differed between immature and mature individuals were mostly of anthropogenic origin and some of unknown origin. No compound classes of natural origin differed between maturity groups.
All compound classes that differed between maturity states were more abundant in mature individuals compared to immature individuals. The Random Forest classification model correctly distinguished between maturity states for all samples based on the relative differences in compound classes abundance with zero out of bag error (i.e. all individuals were correctly classified). TCPM, polybrominated biphenyls (PBB), unknown 4, mirex-related, methylenebistrichloroanisole (MBTCA), unknown 2 (TCPMOHN), PBDE, unknown 8, chlordane-related, and DDT related compounds were significantly more abundant in mature individuals (Figure 1b). Additionally, a few compound classes, chlorbenzylaldehyde, dichlorobenzophenone, methylsulfonyl-PCB (a metabolite of PCB), and unknown 7, were only found in mature individuals, although at relatively low levels, but were not included in the Random Forest classification model because they were detected in fewer than 10 individuals (Figure 1b). Additionally, 14 of the 15 compound classes in the AC group demonstrated evidence of maturity-related bioaccumulation (Figure 2). There was no significant difference in the relative abundance of known natural compound classes between maturity states.
Evidence of endocrine disruption
As expected, blubber testosterone concentrations were significantly higher in mature individuals compared to immature individuals (3.7 ng/g ± 0.6, 1.2 ng/g ± 0.3, p = 0.004). Testosterone negatively correlated with both age-corrected PC1 (p = 0.02, r2 = 0.44, Figure S2) and the age-corrected sum of the AC group (p = 0.02, r2 = 0.42, Figure 4). This trend was stronger when mature individuals were analyzed separately without age-correction (p < 0.01, r2 = 0.86, Figure 4). None of the linear models showed evidence of bias due to age, gonad weight, or maturity.
Figure 4:
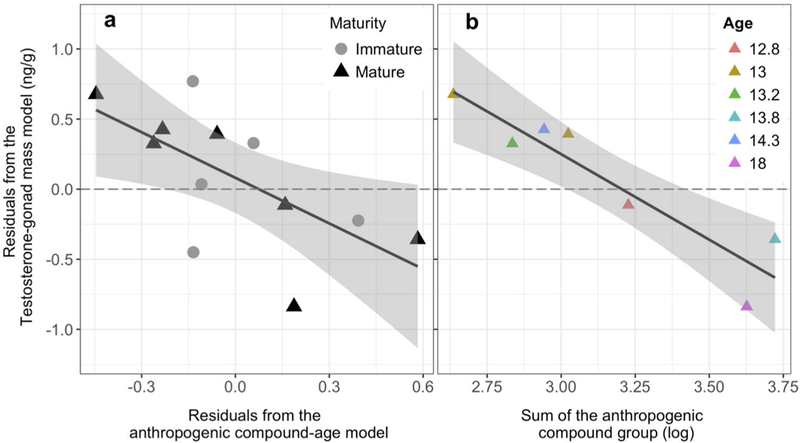
Regressions examining the relationship between the sum of the compound abundances in the anthropogenic contaminant group (AC group) compounds and blubber testosterone for age-corrected data combined (a) and the log-transformed mature-only, non-age-corrected contaminant sum (b). Black circles and triangles represent data from immature (I) and mature (M) animal samples, respectively) and color represents the age of mature individuals. Individuals below the dotted line have less testosterone than expected given the same gonad weight (and age for the age-corrected graph)
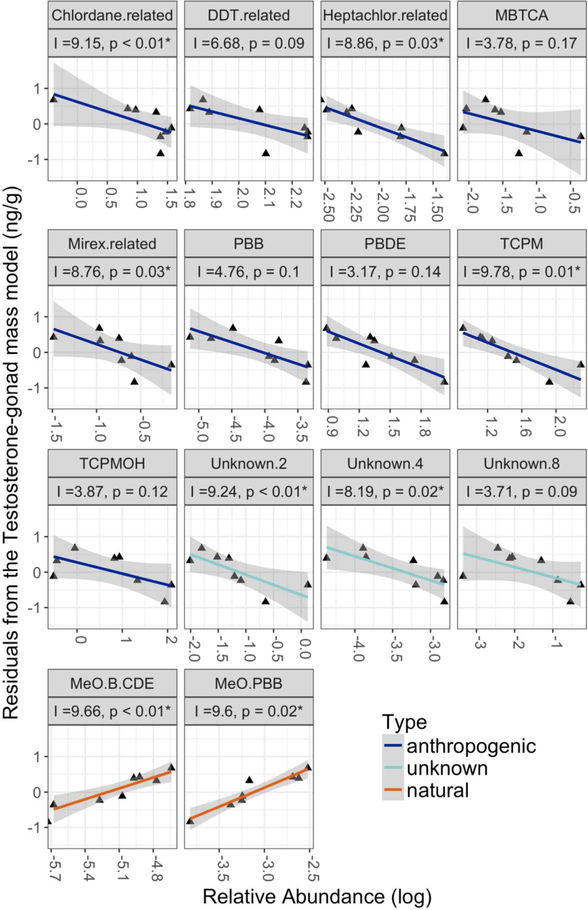
The Random Forest models also independently demonstrated that relative abundance of both compound classes and individual compounds were linked to testosterone. The compound class and individual compound Random Forest models explained 59.02% and 43.74% of the variance in gonad-corrected testosterone, respectively. Of the 23 compound classes tested, four known anthropogenic and two unknown compound classes were negatively related to corrected testosterone in mature individuals and also belonged the AC group: TCPM, chlordane-related, heptachlor-related, mirex-related, unknown 2 (TCPMOHN), and unknown 4. Two natural compound classes, methoxy brominated/chlorinated diphenyl ether (MeO-B/CDE) and methoxy polybrominated biphenyl (MeO-PBB), were positively correlated with corrected testosterone. DDT-related compounds, MBTCA, PBBs, PBDEs, TCPMOH, and Unknown-8 compound classes also had positive variable importance (i.e. increased the predictive power of the model) but the p-values for these classes were above 0.05 (Figure 5, Figure S3).
Figure 5:
The relative abundance of compound classes that increased predictive power of gonad weight adjusted testosterone concentration. Variable importance metrics (I) and p-values (p) are provided for each compound, where p-values below 0.05 were considered significant (*). Color denotes the origin of the compound (anthropogenic, unknown, or natural).
The individual congener model did not converge (i.e. the significance level of a few compounds fluctuated between model runs) because of the small sample size therefore we also took into account the variable importance. All compounds with a %IncMSE over 5 were consistently significant and variable importance dropped much more rapidly below this threshold (Figure S4). Of the 76 individual compounds tested, sixteen individual compounds were significantly correlated with adjusted testosterone and had a variable importance greater than 5 (Figure S5). Of these, 10 anthropogenic and 2 unknown individual compounds, belonging to the same 6 compound classes identified by the compound class Random Forest, were negatively correlated with testosterone, while four natural compounds were positively correlated.
DISCUSSION
This study identified marine mammal exposure to a region-specific suite of HOCs and evaluated support for a corresponding biological impact, namely endocrine disruption, in a population of small cetaceans in the SCB. The suspect screening analysis successfully detected 167 HOCs in D. delphis on the southern California coast. Among the 167 HOCs, only 28, or 17%, are typically monitored in environmental analyses. We found an additional 139 HOCs that are largely unstudied and unmonitored in wildlife and the environment. A third of these additional HOCs have unknown origins. Several of the compounds we identified exhibit a pattern of bioaccumulation and potential endocrine disruption, including several unknown compound classes and individual compounds. These findings highlight the need for more attention to comprehensive HOC exposure, bioaccumulation, and potential endocrine disruption in higher trophic level organisms.
Characterizing HOC profiles in Delphinus delphis
Anthropogenic compounds represented the largest proportion of HOCs detected in D. delphis within the study area (nearly 50%), suggesting that human activities have significantly influenced the HOCs accumulation in marine mammal populations off of the California coast. Consistent with other studies in the region, we found DDT-related compounds to be the most abundant and prevalent, closely followed by several other legacy contaminant classes that are no longer in use or production in the U.S., including TCPM (a component of DDT’s technical mixture), TCPMOH, PBDEs, and chlordane-related compounds.12,28,40,41 The predominance of these legacy contaminants in marine mammal blubber confirms the long-term persistence of these compounds in the region.
Halogenated natural products (HNPs), which can originate from marine sponges42,43 or bacteria,44 represented 22% (37 of 167) of all compounds detected in this study. The detected proportion of HNPs was similar to the proportion of natural compounds found in coastal T. truncatus in the SCB (24%, from Shaul et al.12). Natural HOCs are of particular interest given the chemical properties shared between anthropogenic and natural compounds.43 Limited evidence suggests that DMBP can activate the aryl hydrocarbon receptor in chickens,45 a pathway commonly associated with toxicity induced by anthropogenic contaminants.
Several unknown compounds or compound classes, such as unknown compound classes 2 (TCPMOHN) and 8, were at similar relative abundance to other known, monitored POPs (e.g. mirex-related compounds). Our PCA indicated that unknown classes 2 (TCPMOHN), 4, 7, and 8 loaded closely with an aggregation of known anthropogenic contaminants, suggesting these unknown compounds may be anthropogenic in origin, either as parent compounds or metabolites. Recent identification of class unknown 2 (TCPMOHN) as novel isomers of TCPMOH by Mackintosh et al.28 support the use of this approach to investigate preliminary accumulation patterns of unknown compounds. This HOC grouping includes mostly known bioaccumulating compounds, suggesting that unknown 2 (TCPMOHN), 4, 7, and 8 may likewise be taken up at a rate faster than lost by catabolism and excretion. Unknown compound classes 3 and 5 loaded with several natural compounds, including DMBPs, MBPs, and MeO-BDEs, providing some evidence that these compounds could be correlated either by compound origin or by similar environmental exposure patterns, though anthropogenic sources cannot be ruled out.
Identifying maturity-related HOC bioaccumulation
By examining the abundance of HOCs in animals with different maturity states, we were able to further characterize the long-term bioaccumulation of several different HOC classes. Overall, mature D. delphis had higher HOC loads compared to immature animals and anthropogenic compounds explained the majority of the bioaccumulation patterns, indicating these compounds pose a long-term bioaccumulation risk. Anthropogenic and unknown compound classes that were more abundant in mature individuals represent compounds that are less likely to be metabolized and thus more likely to bioaccumulate over a lifetime.16 Several compound classes that were higher in mature individuals and are known to bioaccumulate, including TCPM, PBB, PBDE, mirex-related, chlordane-related and DDT-related compounds, were abundant in the individuals sampled.46–49 Furthermore, we observed lesser-known compounds that exhibited the same bioaccumulation pattern, including methylsulfonyl-PCB, MBTCA, chlorbenzylaldehyde, dichlorobenzophenone, and unknown compounds 2 (TCPMOHN), 4, 7, and 8.
Interestingly, there was no difference in relative abundance of natural compound classes between maturity states, though several are thought to bioaccumulate over a lifetime in marine mammal tissues (e.g. DMBPs, MBPs, and MeO-BDEs).47,50 Additionally, several unknown compounds, potentially of anthropogenic or natural origin, were not present in enough individuals to evaluate differences between maturity states. While several of the anthropogenic, natural, and unknown compounds detected here were found at relatively low levels, when combined, both low level and abundant compounds may contribute significantly to the overall HOC load.
Evidence of endocrine disruption
Our results suggest that, beyond characterizing HOC exposure and bioaccumulation in wild marine mammals, there is a continued need to understand how HOC compounds disrupt biological systems. By using an approach that integrates suspect HOC screening with biological markers, we identified a prominent group of bioaccumulating anthropogenic compounds (AC group, Figure 2) that was associated with a decrease in blubber testosterone when corrected for variation in age and gonad weight (Figure 4). Several other studies have also found evidence of reduced testosterone levels in mammals after exposure to several of the HOCs examined here, including DDT-related compounds (e.g. DDE), PBDEs, mirex, heptachlor, and chlordane.19,21,22,51–54 Our analysis found additional supporting evidence that other unstudied compounds that bioaccumulate may also contribute to endocrine disruption, e.g., TCPM, TCPMOH, methlysulfonyl-PCB, and unknown classes unknown 2 (TCPMOHN), 4, 7, and 8. The relationship we observed was strongest in mature individuals, which is consistent with an increase in lifetime accumulation of anthropogenic HOCs contributing to inhibited production of testosterone in male D. delphis.
Within mature individuals, we found additional supporting evidence suggesting that multiple compounds, rather than one dominant compound class within the AC group, are likely contributing to endocrine disruption in D. delphis. Of this dominant group, six compound classes were identified as key predictor variables in the random forest analysis: chlordane-related, heptachlor-related, mirex-related, TCPM, unknown 2 (TCPMOHN), and unknown 4 (Figure 5). In addition, 12 individual congeners from each of these classes were also identified as predictor variables: alpha-chlordane, chlordane-related 14, chlordane-related 9, cis-nonachlor, heptachlor epoxide, mirex, TCPM, TCPM 3, TCPM 4, TCPM 5, unknown 2–3 (TCPMOHN), and unknown 4–3 (Figure S5). While one high abundance compound could possibly be driving the negative trend observed here, the congruence between results from the PCA and the random forest analyses indicates that the negative relationship between HOCs and testosterone is not likely to be explained by variation in one dominant compound class but rather several of the classes within the AC group. There also may be additional compounds or compound classes that are contributing to this relationship that were not included in this analysis, such as PCBs and DDE, due to extreme abundance causing GC peak saturation, both of which have been associated with lower testosterone levels in other mammals.19,20,54,55 This study detected three PCB metabolites (methylsulfonyl PCBs) that covaried with the AC group suggesting PCBs may exhibit a similar pattern of accumulation.
Evidence of endocrine disruption from complex mixtures of anthropogenic contaminants has been found in mammals. A recent study of breeding Arctic foxes found a decrease in testosterone and sperm quality related to an increase in a mixture of HOC concentrations in their fatty tissue after a supervised diet of whale blubber,22 similar to the pattern we detected in common dolphins. Mixtures of other known testosterone disruptors have been shown to alter reproductive development and function in a laboratory setting, even when each compound targets a different part of the testosterone pathway.56–61 There is also limited evidence that PBDE congeners, TCPM, p,p’-DDD, and β-hexachlorocyclohexane (β-HCH) can inhibit sperm quality in humans or rats,62–65 though the mechanism and effect levels are unclear. Thus, exposure to a suite of HOCs that potentially inhibit testosterone production could have important ramifications for the reproductive health and fitness of male marine mammals during the breeding season.
Our analyses indicate that anthropogenic sources, rather than HNPs, are the primary drivers of total HOC load in SCB D. delphis and that these levels are correlated with a measure of endocrine disruption that may pose a reproductive health risk to wild marine mammal populations. As the first study to relate exposure to a wide range of HOCs with an endocrine biomarker in marine mammals, this research highlights the need for continued attention on emerging and unmonitored HOCs in coastal oceans. The relationship between testosterone disruption and long-term accumulation of anthropogenic and unknown compounds supports continued monitoring of legacy HOCs, early detection of compounds of emerging concern, and a greater understanding of endocrine disruption in long-lived mammals.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank NOAAs marine mammal observer program for sample acquisition, Keith Maruya for providing laboratory space and Wayne Lao & David Tsukada on ASE training at SCCWRP, Nellie Shaul, and Susan Mackintosh for providing standard operating procedures and technical expertise for sample preparation, Kayo Watanabe for instrument operation, Kerri Danil and Susan Chivers for providing life history and age data, Camryn Allen and Krista Catelani for contributions to the operation of NOAA’s Marine Wildlife Endocrine Laboratory, and Megan Jennings, Corey Clatterbuck, Tracy Grimes, Hsiang Ling Chen, Alexander Gaos, and Dovi Kacev for providing helpful edits to the manuscript.
Funding Sources
This research was funded in part by the following entities and organizations: National Marine Fisheries Service, California State University Counsel on Ocean Affairs, Science, & Technology (COAST-GDP-2014–001), Southern California Society of Environmental Toxicology and Chemistry, and the National Science Foundation (OCE-1313747) and National Institute of Environmental Health Sciences (P01-ES021921) through the Oceans and Human Health Program
Footnotes
ASSOCIATED CONTENT
Supporting Information. Supplementary information is available free of charge on the ACS Publications website:
A full list of detected compounds, individual sample information, and supplemental tables and figures (PDF)
REFERENCES
- (1).Annamalai J; Namasivayam V Endocrine disrupting chemicals in the atmosphere: Their effects on humans and wildlife. Environ. Int 2015, 76, 78–97. [DOI] [PubMed] [Google Scholar]
- (2).Colborn T; vom Saal FS; Soto AM Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ. Health Perspect 1993, 101 (5), 378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Daughton CG Non-regulated water contaminants: emerging research. Environ. Impact Assess. Rev 2004, 24 (7–8), 711–732. [Google Scholar]
- (4).Van der Schalie WH; Gardner HS Jr; Bantle JA; De Rosa CT; Finch RA; Reif JS; Reuter RH; Backer LC; Burger J; Folmar LC; et al. Animals as sentinels of human health hazards of environmental chemicals. Environ. Health Perspect 1999, 107 (4), 309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Ross PS Marine Mammals as Sentinels in Ecological Risk. Hum. Ecol. Risk Assess 2000, 6 (1), 29–46. [Google Scholar]
- (6).Ross P; De Swart R; Addison R; Van Loveren H; Vos J; Osterhaus A Contaminant-induced immunotoxicity in harbour seals: wildlife at risk? Toxicology 1996, 112 (2), 157–169. [DOI] [PubMed] [Google Scholar]
- (7).Hall AJ; Kalantzi OI; Thomas GO Polybrominated diphenyl ethers (PBDEs) in grey seals during their first year of life—are they thyroid hormone endocrine disrupters? Environ. Pollut 2003, 126 (1), 29–37. [DOI] [PubMed] [Google Scholar]
- (8).Ylitalo GM; Stein JE; Hom T; Johnson LL; Tilbury KL; Hall AJ; Rowles T; Greig D; Lowenstine LJ; Gulland FMD The role of organochlorines in cancer-associated mortality in California sea lions (Zalophus californianus). Mar. Pollut. Bull 2005, 50 (1), 30–39. [DOI] [PubMed] [Google Scholar]
- (9).Murphy S; Pierce GJ; Law RJ; Bersuder P; Jepson PD; Learmonth JA; Addink M; Dabin W; Santos MB; Deaville R; et al. Assessing the effect of persistent organic pollutants on reproductive activity in common dolphins and harbour porpoises. J. Northwest Atl. Fish. Sci 2010, 42, 153–173. [Google Scholar]
- (10).Schwacke LH; Zolman ES; Balmer BC; De Guise S; George RC; Hoguet J; Hohn AA; Kucklick JR; Lamb S; Levin M; et al. Anaemia, hypothyroidism and immune suppression associated with polychlorinated biphenyl exposure in bottlenose dolphins (Tursiops truncatus). Proc. R. Soc. B Biol. Sci 2012, 279 (1726), 48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Hoh E; Dodder NG; Lehotay SJ; Pangallo KC; Reddy CM; Maruya KA Nontargeted Comprehensive Two-Dimensional Gas Chromatography/Time-of-Flight Mass Spectrometry Method and Software for Inventorying Persistent and Bioaccumulative Contaminants in Marine Environments. Environ. Sci. Technol 2012, 46 (15), 8001–8008. [DOI] [PubMed] [Google Scholar]
- (12).Shaul NJ; Dodder NG; Aluwihare LI; Mackintosh SA; Maruya KA; Chivers SJ; Danil K; Weller DW; Hoh E Nontargeted Biomonitoring of Halogenated Organic Compounds in Two Ecotypes of Bottlenose Dolphins (Tursiops truncatus) from the Southern California Bight. Environ. Sci. Technol 2015, 49 (3), 1328–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Alonso MB; Maruya KA; Dodder NG; Lailson-Brito J; Azevedo A; SantosNeto E; Torres JPM; Malm O; Hoh E Nontargeted Screening of Halogenated Organic Compounds in Bottlenose Dolphins (Tursiops truncatus) from Rio de Janeiro, Brazil. Environ. Sci. Technol 2017, 51 (3), 1176–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Hoh E; Lehotay SJ; Pangallo KC; Mastovska K; Ngo HL; Reddy CM; Vetter W Simultaneous Quantitation of Multiple Classes of Organohalogen Compounds in Fish Oils with Direct Sample Introduction Comprehensive Two-Dimensional Gas Chromatography and Time-of-Flight Mass Spectrometry. J. Agric. Food Chem 2009, 57 (7), 2653–2660. [DOI] [PubMed] [Google Scholar]
- (15).Millow CJ; Mackintosh SA; Lewison RL; Dodder NG; Hoh E Identifying Bioaccumulative Halogenated Organic Compounds Using a Nontargeted Analytical Approach: Seabirds as Sentinels. PLOS ONE 2015, 10 (5), e0127205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Yordy JE; Wells RS; Balmer BC; Schwacke LH; Rowles TK; Kucklick JR Life history as a source of variation for persistent organic pollutant (POP) patterns in a community of common bottlenose dolphins (Tursiops truncatus) resident to Sarasota Bay, FL. Sci. Total Environ 2010, 408 (9), 2163–2172. [DOI] [PubMed] [Google Scholar]
- (17).Crisp TM; Clegg ED; Cooper RL; Wood WP; Anderson DG; Baetcke KP; Hoffmann JL; Morrow MS; Rodier DJ; Schaeffer JE; et al. Environmental endocrine disruption: an effects assessment and analysis. Environ. Health Perspect 1998, 106 (Suppl 1), 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Kabir ER; Rahman MS; Rahman I A review on endocrine disruptors and their possible impacts on human health. Environ. Toxicol. Pharmacol 2015, 40 (1), 241–258. [DOI] [PubMed] [Google Scholar]
- (19).Pelch KE; Beeman J; Niebruegge B; Winkeler S; Nagel S Endocrine-disrupting chemicals (EDCs) in mammals. Horm. Reprod Vertebr 2011, 329–371.
- (20).Kelce WR; Stone CR; Laws SC; Gray LE Persistent DDT metabolite p,p’-DDE is a potent androgen receptor antagonist. Nature 1995, 375, 581–585. [DOI] [PubMed] [Google Scholar]
- (21).Lilienthal H; Hack A; Roth-Härer A; Grande SW; Talsness CE Effects of Developmental Exposure to 2,2′,4,4′,5-Pentabromodiphenyl Ether (PBDE-99) on Sex Steroids, Sexual Development, and Sexually Dimorphic Behavior in Rats. Environ. Health Perspect 2006, 114 (2), 194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Sonne C; Torjesen PA; Fuglei E; Muir DCG; Jenssen BM; Jørgensen E; Dietz R; Ahlstrøm Ø Exposure to persistent organic pollutants reduces testosterone concentrations and affects sperm viability and morphology during the mating peak-period in a controlled experiment on farmed Arctic foxes (Vulpes lagopus). Environ. Sci. Technol 2017, 51 (8), 4673–4680. [DOI] [PubMed] [Google Scholar]
- (23).Kellar NM; Trego ML; Chivers SJ; Archer FI Pregnancy patterns of pantropical spotted dolphins (Stenella attenuata) in the eastern tropical Pacific determined from hormonal analysis of blubber biopsies and correlations with the purse-seine tuna fishery. Mar. Biol 2013, 160 (12), 3113–3124. [Google Scholar]
- (24).Kellar NM; Trego ML; Chivers SJ; Archer FI; Perryman WL From progesterone in biopsies to estimates of pregnancy rates: Large scale reproductive patterns of two sympatric species of common dolphin, Delphinus spp. off California, USA and Baja, Mexico. Bull. South. Calif. Acad. Sci 2014, 113 (2), 58–80. [Google Scholar]
- (25).Kellar NM; Trego ML; Marks CI; Chivers SJ; Danil K; Archer FI Blubber testosterone: A potential marker of male reproductive status in short-beaked common dolphins. Mar. Mammal Sci 2009, 25 (3), 507–522. [Google Scholar]
- (26).Kellar N; Keliher J; Trego M; Catelani K; Hanns C; George J; Rosa C Variation of bowhead whale progesterone concentrations across demographic groups and sample matrices. Endanger. Species Res 2013, 22 (1), 61–72. [Google Scholar]
- (27).Blasius ME; Goodmanlowe GD Contaminants still high in top-level carnivores in the Southern California Bight: Levels of DDT and PCBs in resident and transient pinnipeds. Mar. Pollut. Bull 2008, 56 (12), 1973–1982. [DOI] [PubMed] [Google Scholar]
- (28).Mackintosh SA; Dodder NG; Shaul NJ; Aluwihare LI; Maruya KA; Chivers SJ; Danil K; Weller DW; Hoh E Newly Identified DDT-Related Compounds Accumulating in Southern California Bottlenose Dolphins. Environ. Sci. Technol 2016, 50 (22), 12129–12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Meng X-Z; Blasius ME; Gossett RW; Maruya KA Polybrominated diphenyl ethers in pinnipeds stranded along the southern California coast. Environ. Pollut 2009, 157 (10), 2731–2736. [DOI] [PubMed] [Google Scholar]
- (30).Becker EA; Forney KA; Thayre BJ; Debich AJ; Campbell GS; Whitaker K; Douglas AB; Gilles A; Hoopes R; Hildebrand JA Habitat-Based Density Models for Three Cetacean Species off Southern California Illustrate Pronounced Seasonal Differences. Front. Mar. Sci 2017, 4.
- (31).Pauly D; Trites AW; Capuli E; Christensen V Diet composition and trophic levels of marine mammals. ICES J. Mar. Sci 1998, 55 (3), 467–481. [Google Scholar]
- (32).Danil K; Chivers SJ Growth and reproduction of female short-beaked common dolphins, Delphinus delphis, in the eastern tropical Pacific. Can. J. Zool 2007, No. 85, 108–121. [Google Scholar]
- (33).Westgate AJ; Read AJ Reproduction in short-beaked common dolphins (Delphinus delphis) from the western North Atlantic. Marine Biology 2007, 150 (5), 1011–1024. [Google Scholar]
- (34).Kellar NM; Trego ML; Marks CM; Dizon A Determining pregnancy from blubber in three species of delphinids. Mar. Mammal Sci 2006, 22 (1), 1–16. [Google Scholar]
- (35).Trego ML; Kellar NM; Danil K Validation of Blubber Progesterone Concentrations for Pregnancy Determination in Three Dolphin Species and a Porpoise. PLoS ONE 2013, 8 (7), e69709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).R Core Team. R: A language and environment for statistical computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- (37).Breiman L Random Forests. Mach. Learn 2001, 45 (1), 5–32. [Google Scholar]
- (38).Liaw A; Wiener M Classification and regression by randomForest. R News 2002, 2 (3), 18–22. [Google Scholar]
- (39).Archer FI rfPermute: Estimate permutation p-values for Random Forest importance metrics; 2016.
- (40).Byard JL; Paulsen SC; Tjeerdema RS; Chiavelli D DDT, Chlordane, Toxaphene and PCB Residues in Newport Bay and Watershed: Assessment of Hazard to Wildlife and Human Health. In Reviews of Environmental Contamination and Toxicology Volume 235; Whitacre DM., Ed.; Springer International Publishing: Cham, 2015; Vol. 235, pp 49–168. [DOI] [PubMed] [Google Scholar]
- (41).Dodder NG; Maruya KA; Lauenstein GG; Ramirez J; Ritter KJ; Schiff KC Distribution and sources of polybrominated diphenyl ethers in the Southern California Bight. Environ. Toxicol. Chem 2012, 31 (10), 2239–2245. [DOI] [PubMed] [Google Scholar]
- (42).Agarwal V; Blanton JM; Podell S; Taton A; Schorn MA; Busch J; Lin Z; Schmidt EW; Jensen PR; Paul VJ; et al. Metagenomic discovery of polybrominated diphenyl ether biosynthesis by marine sponges. Nat. Chem. Biol 2017, 13 (5), 537–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Vetter W; Janussen D Halogenated Natural Products in Five Species of Antarctic Sponges: Compounds with POP-like Properties? Environ. Sci. Technol 2005, 39 (11), 3889–3895. [DOI] [PubMed] [Google Scholar]
- (44).Agarwal V; Li J; Rahman I; Borgen M; Aluwihare LI; Biggs JS; Paul VJ; Moore BS Complexity of Naturally Produced Polybrominated Diphenyl Ethers Revealed via Mass Spectrometry. Environ. Sci. Technol 2015, 49 (3), 1339–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Tittlemier SA; Kennedy SW; Hahn ME; Reddy CM; Norstrom RJ Naturally produced halogenated dimethyl bipyrroles bind to the aryl hydrocarbon receptor and induce cytochrome P4501A and porphyrin accumulation in chicken embryo hepatocytes. Environ. Toxicol. Chem 2003, 22 (7), 1622–1631. [PubMed] [Google Scholar]
- (46).Bachman MJ; Keller JM; West KL; Jensen BA Persistent organic pollutant concentrations in blubber of 16 species of cetaceans stranded in the Pacific Islands from 1997 through 2011. Sci. Total Environ 2014, 488–489, 115–123. [DOI] [PubMed]
- (47).Barón E; Giménez J; Verborgh P; Gauffier P; De Stephanis R; Eljarrat E; Barceló D Bioaccumulation and biomagnification of classical flame retardants, related halogenated natural compounds and alternative flame retardants in three delphinids from Southern European waters. Environ. Pollut 2015, 203, 107–115. [DOI] [PubMed] [Google Scholar]
- (48).Lebeuf M; Bernt KE; Trottier S; Noël M; Hammill MO; Measures L Tris (4chlorophenyl) methane and tris (4-chlorophenyl) methanol in marine mammals from the Estuary and Gulf of St. Lawrence. Environ. Pollut 2001, 111 (1), 29–43. [DOI] [PubMed] [Google Scholar]
- (49).Letcher RJ; Gebbink WA; Sonne C; Born EW; McKinney MA; Dietz R Bioaccumulation and biotransformation of brominated and chlorinated contaminants and their metabolites in ringed seals (Pusa hispida) and polar bears (Ursus maritimus) from East Greenland. Environ. Int 2009, 35 (8), 1118–1124. [DOI] [PubMed] [Google Scholar]
- (50).Pangallo KC; Reddy CM Distribution Patterns Suggest Biomagnification of Halogenated 1′-Methyl-1,2′-Bipyrroles (MBPs). Environ. Sci. Technol 2009, 43 (1), 122–127. [DOI] [PubMed] [Google Scholar]
- (51).Gregoraszczuk EŁ; Rak A; Kawalec K; Ropstad E Steroid secretion following exposure of ovarian follicular cells to single congeners and defined mixture of polybrominateddibenzoethers (PBDEs), p,p′-DDT and its metabolite p,p′-DDE. Toxicol. Lett 2008, 178 (2), 103–109. [DOI] [PubMed] [Google Scholar]
- (52).Meeker JD; Johnson PI; Camann D; Hauser R Polybrominated diphenyl ether (PBDE) concentrations in house dust are related to hormone levels in men. Sci. Total Environ 2009, 407 (10), 3425–3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Oskam IC; Ropstad E; Dahl E; Lie E; Derocher AE; Wiig ystein; Larsen S.; Wiger R.; Skaare JU. Organochlorines Affect the Major Androgenic Hormone, Testosterone, in Male Polar Bears (Ursus Maritimus) at Svalbard. J. Toxicol. Environ. Health A 2003, 66 (22), 2119–2139. [DOI] [PubMed] [Google Scholar]
- (54).Subramanian AN; Tanabe S; Tatsukawa R; Saito S; Miyazaki N Reduction in the testosterone levels by PCBs and DDE in Dall’s porpoises of northwestern North Pacific. Mar. Pollut. Bull 1987, 18 (12), 643–646. [Google Scholar]
- (55).Hallanger IG; Jørgensen EH; Fuglei E; Ahlstrøm Ø; Muir DCG; Jenssen BM Dietary Contaminant Exposure Affects Plasma Testosterone, but not Thyroid Hormones, Vitamin A, and Vitamin E, in Male Juvenile Arctic Foxes (Vulpes lagopus). J. Toxicol. Environ. Health A 2012, 75 (21), 1298–1313. [DOI] [PubMed] [Google Scholar]
- (56).Andric SA; Kostic TS; Stojilkovic SS; Kovacevic RZ Inhibition of rat testicular androgenesis by a polychlorinated biphenyl mixture Aroclor 1248. Biol. Reprod 2000, 62 (6), 1882–1888. [DOI] [PubMed] [Google Scholar]
- (57).Birkhøj M; Nellemann C; Jarfelt K; Jacobsen H; Andersen HR; Dalgaard M; Vinggaard AM The combined antiandrogenic effects of five commonly used pesticides. Toxicol. Appl. Pharmacol 2004, 201 (1), 10–20. [DOI] [PubMed] [Google Scholar]
- (58).Earl Gray L; Wilson VS; Stoker T; Lambright C; Furr J; Noriega N; Howdeshell K; Ankley GT; Guillette L Adverse effects of environmental antiandrogens and androgens on reproductive development in mammals. Int. J. Androl 2006, 29 (1), 96–104. [DOI] [PubMed] [Google Scholar]
- (59).Gray LE; Ostby J; Furr J; Wolf CJ; Lambright C; Parks L; Veeramachaneni DN; Wilson V; Price M; Hotchkiss A; et al. Effects of environmental antiandrogens on reproductive development in experimental animals. Eur. Soc. Hum. Reprod. Embryol 2001, 7 (3), 248–264. [DOI] [PubMed] [Google Scholar]
- (60).Hotchkiss AK; Parks-Saldutti LG; Ostby JS; Lambright C; Furr J; Vandenbergh JG; Gray LE A Mixture of the “Antiandrogens” Linuron and Butyl Benzyl Phthalate Alters Sexual Differentiation of the Male Rat in a Cumulative Fashion1. Biol. Reprod 2004, 71 (6), 1852–1861. [DOI] [PubMed] [Google Scholar]
- (61).Reddy ML; Reif JS; Bachand A; Ridgway SH Opportunities for using Navy marine mammals to explore associations between organochlorine contaminants and unfavorable effects on reproduction. Sci. Total Environ 2001, 274 (1), 171–182. [DOI] [PubMed] [Google Scholar]
- (62).Foster WG; Desaulniers D; Leingartner K; Wade MG; Poon R; Chu I Reproductive effects of tris (4-chlorophenyl) methanol in the rat. Chemosphere 1999, 39 (5), 709–724. [DOI] [PubMed] [Google Scholar]
- (63).Mumford SL; Kim S; Chen Z; Gore-Langton RE; Boyd Barr D; Buck Louis GM Persistent organic pollutants and semen quality: The LIFE Study. Chemosphere 2015, 135, 427–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Pant N; Pant AB; Chaturvedi PK; Shukla M; Mathur N; Gupta YK; Saxena DK Semen quality of environmentally exposed human population: the toxicological consequence. Environ. Sci. Pollut. Res 2013, 20 (11), 8274–8281. [DOI] [PubMed] [Google Scholar]
- (65).Pflieger-Bruss S; Heitkamp S; Hagemann S; Körner W; Köhn F-M; Müller C; Schill W-B Influence of tris (4-chlorophenyl) methanol, non-ortho PCB 77 and γ-hexachlorocyclohexane on human sperm function in vitro. Andrologia 2006, 38 (2), 39–47. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.