Abstract
Cooling is an effective temporary remedy for itch, bringing welcome relief to itchy insect bites, nettle stings, poison ivy, atopic dermatitis, and psoriasis. Menthol, causing a cooling sensation, has similar itch-relieving effects. Palkar et al. demonstrate that TRPM8, a menthol- and cold-activated ion channel, is essential for cooling to relieve itch, suggesting that pharmacologic TRPM8 activation should be explored further as an antipruritic strategy.
The sensation of itch is initiated when specialized primary afferent neurons, the pruriceptors, are excited by pruritic stimuli (Azimi et al., 2016). The cell bodies of these neurons are located within the dorsal root ganglia, inner-vating body skin, and within the trigeminal ganglia, innervating the skin of the head (Azimi et al., 2016; Bautista et al., 2014). Pruriceptors form central connections within the outer laminae of the spinal cord, where itch signals are integrated and relayed to the brain. Histamine, from mast cells and other sources, has long been known as a primary itch stimulus, activating H1 receptors expressed in pruriceptors that couple to phospholipase C and an excitatory ion channel of the transient receptor potential (TRP) family of ion channels, TRPV1. This pathway is inhibited by the antihistamines frequently prescribed for topical or systemic treatment of itch. However, antihistamines are often inadequate for relieving itch, especially in chronic skin conditions, such as chronic allergic contact dermatitis, atopic dermatitis, and psoriasis, suggesting that other, histamine-independent, pruritogenic pathways are involved. In the past few years, substantial progress has been made uncovering the mysteries of histamine-independent itch. This form of itch is often triggered by cytokines produced during inflammation of the skin, such as IL-31, IL-33, or TSLP, via receptors expressed in pruriceptive neurons (Bautista et al., 2014; Liu et al., 2016). The neurotransmitter serotonin has been identified as an additional itch mediator. Advances in neurobiological research uncovered Mas-related G-protein-coupled receptors as specific markers for pruriceptors, and as targets of synthetic pruritogens, such as chloroquine, and of endogenous itch mediators, such as substance P and the skin peptide, Bam8–22 (Azimi et al., 2016; Bautista et al., 2014). TRP ion channels, including TRPV1 and TRPA1, act downstream of these non-histaminergic signals to excite pruriceptors (Bautista et al., 2014; Liu et al., 2013, 2016). Other studies identified central transmitters and circuits essential for the transmission of itch signals within the spinal cord (Bautista et al., 2014). While substantial progress has been made, the diversity and complexity of itch mechanisms has hampered the development of a “onesize fits all” approach to itch therapy (Palkar et al., 2018).
Topical cooling is a frequently used remedy for itch. Cooling the skin by application of ice, gel packs, cool compresses, or cold water can temporarily reduce itch in patients affected by atopic dermatitis, contact urticarial, or psoriasis (Fruhstorfer et al., 1986). In clinical experimental studies, cooling reduced or abolished itch and skin erythema caused by injection of histamine into the skin of healthy volunteers (Bromm et al., 1995; Fruhstorfer et al., 1986).
Menthol, the natural product of peppermint that produces a cooling sensation, also has antipruritic effects. Menthol was shown to alleviate itch in the same conditions in which cooling was effective, including atopic dermatitis and psoriasis, and in experimental studies inducing itch with injections of histamine or hydroxyethyl starch (Bromm et al., 1995). Menthol was also effective in patients with chronic itch after mustard gas injury or in lichen amyloidosis.
Do cooling and menthol engage the same antipruritic mechanism? Cooling can decrease nerve excitability and conduction velocity and slow biochemical mechanisms essential for neurotransmission and neuropeptide release. For example, cooling reduces the activity of TRPV1, the ion channel that excites pruriceptors downstream of H1 histamine receptors. These effects might dampen the responses of pruriceptors to itch-inducing stimuli. However, the topical application of menthol, while making the skin feel cool, does not lower skin temperature and yet diminishes itch sensations (Bromm et al., 1995).
Is it that the sensation of cooling, the mechanism that lets us feel cool temperature, is soothing the itch? Landmark studies by Hansel and Zotterman in the 1950s revealed that cool temperatures excite a small subset of sensory nerves different from the major pain- or itch-transmitting nerves. These cold sensors were also excited by menthol. In 2002, an ion channel was identified in these neurons, TRPM8, which was activated by both cooling and menthol, and was crucial for cooling-induced nerve excitation (McKemy et al., 2002). Studies in TRPM8-deficient mice demonstrated that TRPM8 is essential for cooling-induced behavior, acting as a thermosensor in vivo (Bautista et al., 2007).
In addition to diminishing pruritus, cooling and topical menthol are widely used to alleviate pain. Studies in TRPM8-deficient mice revealed that TRPM8 is the key mediator for menthol- and cooling-induced anal-gesia. In mice and rats, cooling reduced formalin-induced and neuropathic pain via TRPM8. Menthol effectively alleviates pain caused by chemical stimuli, noxious heat, inflammation, and neuropathic pain in a TRPM8-dependent manner (Liu et al., 2013). Eucalyptol, a natural cooling product and TRPM8 agonist, effectively relieved both inflammation and pain in a mouse model of chronic inflammatory pain via TRPM8 (Caceres et al., 2017). TRPM8 activation also attenuated neurogenic inflammation by reducing neuropeptide release in a mouse model of colitis. Cellular ablation of the TRPM8-expressing thermosensors also abolished cooling- or menthol-induced analgesia. However, whether TRPM8 and cold-sensing neurons also contribute to the antipruritic effects of cooling and menthol remained to be elucidated. Complexity was added by the discovery of other menthol-sensitive TRP ion channels, including TRPA1, which contributes to itch signaling in pruriceptors downstream of histamine-independent pathways.
The study by Palkar et al. (2018) sheds light on the role of TRPM8 and TRPM8-expressing sensory neurons in the anti-pruritic effects of cooling and menthol. To test the effects of cooling on itch, the authors first established a mouse model to evaluate the behavioral changes upon pruritogen injection into the hindpaw while the animal is placed on a thermally controlled surface. The authors observed that injection of pruritogens, such as chloroquine, histamine, or the mast cell degranulator 48/80, caused the animals to lick and bite their hindpaw, a behavioral response to itch sensation. Cooling of the surface from 30° C to 20° C or lower essentially abolished chloroquine-, 48/80-, and histamine-induced itch behavior. The antipruritic effect of cooling in mice required continuous cold stimulation. Itch behavior rapidly reappeared when the skin surface was warmed to ambient temperature, an observation consistent with human studies (Fruhstorfer et al., 1986). In TRPM8-deficient mice, the antipruritic effects of mild cooling (20° C) were abolished. However, cooling to lower temperatures in the range where cold can induce pain (17° C and lower) reduced itch behavior in TRPM8-deficient mice, suggesting that TRPM8-independent cold-sensitive mechanisms contribute to thermal sensing at lower temperatures. Furthermore, using a mouse line (Trpm8DTR) in which TRPM8-expressing cells can be ablated by injected diphtheria toxin, Palkar et al. (2018) observed that TRPM8-expressing neurons were essential for the antipruritic effects of both mild cooling and cooling to noxious cold temperature (10° C). Cooling inhibited both histaminergic and non-histaminergic itch in a TRPM8- (and TRPM8+ neuron-) dependent manner.
Palkar et al. (2018) also examined the effects of menthol in their mouse model. Topical application of menthol significantly reduced chloroquine induced itch responses, consistent with a previous study (Kardon et al., 2014). The antipruritic effects of menthol were largely abolished in TRPM8-deficient mice. Of clinical importance, Palkar et al. (2018) demonstrated that cooling reduced chronic itch behavior in a mouse model of dry skin. Taken together, the authors provide strong evidence that TRPM8 is the major cold sensor and menthol target to ameliorate itch in a wide range of skin conditions.
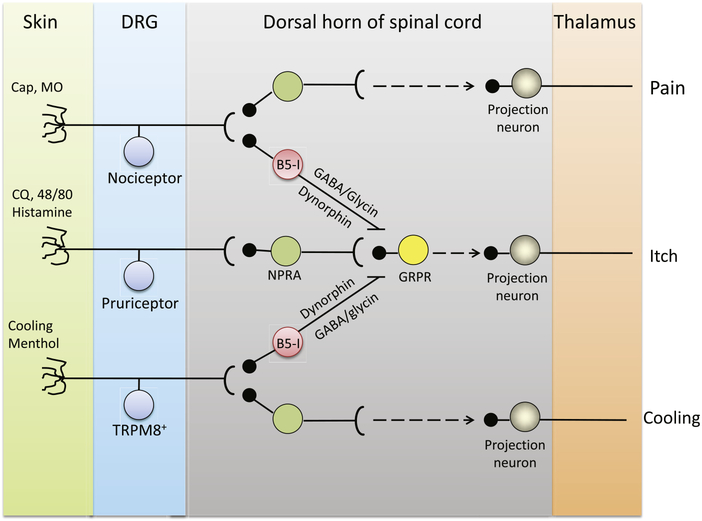
How do TRPM8 and TRPM8-expressing neurons suppress itch when activated by cooling or menthol? TRPM8-expressing cold-sensors are not pruriceptors, so how does excitation of these neurons affect itch signals from pruriceptors? Insight into a potential mechanism came from studies of spinal circuits. B5-I neurons, a type of spinal inhibitory interneuron expressing the transcription factor bHLHb5, have been shown to receive direct synaptic input from menthol-sensitive peripheral neurons (Kardon et al., 2014). B5-I neurons produce dynorphin, which suppresses itch. A crucial experiment demonstrated that menthol’s antipruritic effect was abolished in genetically modified mice that lack B5-I neurons, suggesting that these neurons may inhibit the input from pruriceptors when activated following synaptic transmission from menthol-sensitive peripheral nerves (Kardon et al., 2014). In the study by Palkar et al. (2018), the input from TRPM8-expressing cold receptors is missing, due to either genetic deletion of the TRPM8 gene or cellular ablation of cold receptors, preventing B5-I neurons from inhibiting pruriceptor input when menthol is applied topically (Figure 1). It remains to be investigated whether these inhibitory neurons are also essential for the effects of mild cooling or noxious cold on itch behavior.
Figure 1. Schematic describing cooling and menthol induced antipruritic and analgesic pathways.
Sensory neurons of the dorsal root ganglia (DRG) project primary sensory afferents to the skin, where they detect itch, pain and cooling stimuli. These neurons form synapses with secondary neurons in the dorsal horn of the spinal cord, which connect with other interneurons and/or projection neurons to relay sensory signals to the thalamus. Pruritogens, such as chloroquine, 48/80, or histamine act on corresponding receptors expressed in the skin sensory nerve endings to generate itch signals. The itch signal is transmitted via natriuretic peptide receptor A-gastrin-related peptide receptor (GRPR) pathways in the dorsal horn of spinal cord. Cooling and menthol activate TRPM8 channels expressed in TRPM8+ DRG neurons, which mediate cooling perception. Within the spinal cord, TRPM8+ DRG neurons may form synapses with dorsal horn inhibitory B5-I neurons. B5-I neurons release γ-aminobutyric acid (GABA) and/or glycine, as well as dynorphin, which act on, or downstream of, GRPR neurons to inhibit itch transmission in the dorsal horn.
While examining dose-response effects of menthol, Palkar et al. (2018) observed that a high dose of menthol applied topically to the mouse cheek produced robust wiping behavior, which is an indication of a pain response. This response was only partially diminished in TRPM8-deficient mice, suggesting that menthol interacts with other targets to induce pain or irritation. As mentioned, TRPA1, involved in both pain and itch signaling, is activated by menthol, and additional targets of menthol have been described. Thus, menthol, depending on the patient and condition, may cause side effects that may preclude its use due to lack of selectivity. Indeed, some atopic dermatitis patients in clinical studies complained about stinging and burning sensations upon menthol application, resulting in cessation of treatment. Therefore, the use of more specific and potent TRPM8 agonists needs to be explored. A wide range of menthol analogs were developed by the consumer products industry as cooling agents, with some showing high selectivity toward TRPM8 versus TRPA1. One such compound, WS-12, was shown to act as a TRPM8-dependent analgesic (Liu et al., 2013). A recent randomized, double-blind clinical pilot trial demonstrated the utility of this approach, showing that topical application of a combination of two selective TRPM8 agonists ameliorated severe pruritus in dry skin patients, with no adverse events observed (Stander et al., 2017). The results by Palkar et al. (2018) and the promising clinical pilot data suggest that selective TRPM8 activation is a promising strategy for relieving the burden of chronic itch.
Clinical Implications.
Cooling and menthol effectively suppress itch behavior in mice, similar to humans.
TRPM8, an ion channel in cold-sensing peripheral neurons, is essential for cooling and menthol to diminish itch.
Utilization of selective and potent TRPM8 activators as antipruritics may be better tolerated by patients than menthol.
ACKNOWLEDGMENTS
We acknowledge the financial support from Zhejiang Provincial Natural Science Funds for Distinguished Young Scholars (LR17H270001) and National Science Foundation of China (81603676) to BL, and from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (R21AR070554) to SEJ.
Footnotes
CONFLICT OF INTEREST
Sven-Eric Jordt serves on the Scientific Advisory Board of Hydra Biosciences LLC (Cambridge, MA), a biopharmaceutical company developing TRP ion channel inhibitors for the treatment of pain and inflammation. Boyi Liu states no conflict of interest.
REFERENCES
- Azimi E, Xia J, Lerner EA. Peripheral mechanisms of itch. Curr Probl Dermatol 2016;50:18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, et al. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 2007;448:204–8. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Wilson SR, Hoon MA. Why we scratch an itch: the molecules, cells and circuits of itch. Nat Neurosci 2014;17:175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromm B, Scharein E, Darsow U, Ring J. Effects of menthol and cold on histamine-induced itch and skin reactions in man. Neurosci Lett 1995;187:157–60. [DOI] [PubMed] [Google Scholar]
- Caceres AI, Liu B, Jabba SV, Achanta S, Morris JB, Jordt SE. Transient receptor potential cation channel subfamily M member 8 channels mediate the anti-inflammatory effects of eucalyptol. Br J Pharmacol 2017;174:867–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruhstorfer H, Hermanns M, Latzke L. The effects of thermal stimulation on clinical and experimental itch. Pain 1986;24:259–69. [DOI] [PubMed] [Google Scholar]
- Kardon AP, Polgar E, Hachisuka J, Snyder LM, Cameron D, Savage S, et al. Dynorphin acts as a neuromodulator to inhibit itch in the dorsal horn of the spinal cord. Neuron 2014;82: 573–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Fan L, Balakrishna S, Sui A, Morris JB, Jordt SE. TRPM8 is the principal mediator of menthol-induced analgesia of acute and inflammatory pain. Pain 2013;154:2169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Tai Y, Achanta S, Kaelberer MM, Caceres AI, Shao X, et al. IL-33/ST2 signaling excites sensory neurons and mediates itch response in a mouse model of poison ivy contact allergy. Proc Natl Acad Sci U S A 2016;113:E7572–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 2002;416:52–8. [DOI] [PubMed] [Google Scholar]
- Palkar R, Ongun S, Catich E, Li N, Borad N, Sarkisian A, et al. Cooling relief of acute and chronic itch requires TRPM8 channels and neurons. J Invest Dermatol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stander S, Augustin M, Roggenkamp D, Blome C, Heitkemper T, Worthmann AC, et al. Novel TRPM8 agonist cooling compound against chronic itch: results from a randomized, double-blind, controlled, pilot study in dry skin. J Eur Acad Dermatol Venereol 2017;31:1064–8. [DOI] [PubMed] [Google Scholar]