Abstract
Background:
Several distinct risk factors for arrhythmia recurrence and mortality following Ventricular Tachycardia (VT) ablation have been described. The effect of concurrent risk factors has not been assessed so far, thus it is not yet possible to estimate these risks for a patient with several comorbidities. Aim of the study was to identify specific risk groups for mortality and VT recurrence using the Survival Tree analysis method.
Methods:
In 1251 patients 16 demographic, clinical and procedure-related variables were evaluated as potential prognostic factors using Survival Tree (ST) analysis employing a recursive partitioning algorithm that searches for relationships among variables. Survival time and time to VT recurrence in groups derived from ST analysis were compared by a log-rank test. A random forest analysis was then run to extract a variable importance index and internally validate the ST models.
Results:
LVEF, ICD/CRT device, previous ablation (Previous Abl) were, in hierarchical order, identified by ST analysis as best predictors of VT recurrence, while LVEF, previous Abl, Electrical Storm (ES) were identified as best predictors of mortality. Three groups with significantly different survival rates were identified. Within the HIGH risk group, 65.0% patients survived and 52.1% were free from VT recurrence; within MEDIUM and LOW risk groups, 84.0% and 97.2% patients survived, 72.4% and 88.4% were free from VT recurrence, respectively.
Conclusions:
Our study is the first to derive and validate a decisional model that provides estimates of VT recurrence and mortality with an effective classification tree. Pre-procedure risk stratification could help optimize peri- and post-procedural care.
Keywords: ventricular tachycardia, catheter ablation, risk assessment, ischemic cardiomyopathy, nonischemic cardiomyopathy, Arrhythmias, Electrophysiology, Catheter Ablation and Implantable Cardioverter-Defibrillator
INTRODUCTION
Radiofrequency catheter ablation has been shown to reduce VT burden and appropriate ICD therapies in patients with structural heart disease1, 2,3. Catheter ablation has become a widespread treatment for post-infarct VTs during the last decade4, 5. Despite several improvements in techniques6–10 and technologies11–14, patients are still exposed to a significant risk of recurrence and mortality after VT ablation. Several studies have attempted to identify predictors of adverse outcomes in single center and multicenter studies. However, a pre-procedural model that estimates patient’s risk of 1-year VT recurrence and mortality has not been developed yet.
The aim of this study was to identify clinical and demographical characteristics allowing to classify patients into subgroups with distinct risks for 1-year VT recurrence and mortality after catheter ablation for VT associated with structural heart disease.
METHODS
Study design
The authors declare that all supporting data and methods are available within the article. The International VT Ablation Center Collaborative Group (IVTCC) consists of 12 international sites that specialize in VT management with a developed protocol for data sharing12. Data concerning 1251 patients undergoing catheter ablation for previous sustained VT in the setting of structural heart disease between 2002 and 2013, with complete data for the variables of interest, were retrospectively collected and analysed. Structural heart disease was defined as ischemic (ICM) and/or nonischemic (NICM) cardiomyopathy with left ventricular ejection fraction (LVEF) <55%, with scar confirmed during electroanatomic mapping (EAM); patients with LVEF>55% were included in cases of RV predominant cardiomyopathy and hypertrophic cardiomyopathy. The diagnosis of ICM was established by prior history of myocardial infarction, or fixed perfusion defect correlated with coronary stenosis or prior coronary artery intervention. Etiologies for NICM included Idiopathic dilated cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy, valvular cardiomyopathy, myocarditis, hypertrophic cardiomyopathy, sarcoidosis, familial, left ventricular non-compaction. Electrical Storm (ES) was defined as ≥ 3 VT/VF episodes within 24 hours. Baseline characteristics of patients are shown in Table 1. The study was approved by the Institutional Review Boards of the respective participating centers.
Table 1:
Baseline characteristics of patients.
| LEVEL | FREQUENCY | PERCENT | |
|---|---|---|---|
| 2 OR MORE AADS | No | 987 | 78.9 |
| Yes | 264 | 21.1 | |
| AF | No | 911 | 72.8 |
| Yes | 340 | 27.2 | |
| AGE GROUPS | <65 years | 648 | 51.8 |
| 65 ≤ years < 80 | 541 | 43.2 | |
| ≥80 years | 62 | 5.00 | |
| CARDIOMYOPATHY | ICM | 601 | 48.0 |
| IDCM | 327 | 26.1 | |
| ARVC | 56 | 4.5 | |
| Myocarditis | 38 | 3.0 | |
| Valvular cardiomyopathy | 28 | 2.2 | |
| Genetic disease | 19 | 1.5 | |
| Sarcoidosis | 16 | 1.3 | |
| HCM | 15 | 1.2 | |
| LVNC | 6 | 0.5 | |
| Uncategorized NICM | 145 | 11.6 | |
| CKD | No | 897 | 71.7 |
| Yes | 354 | 28.3 | |
| DEVICE | No device | 184 | 14.7 |
| ICD | 725 | 58.0 | |
| CRT | 342 | 27.3 | |
| DM | No | 983 | 78.6 |
| Yes | 268 | 21.4 | |
| ES | No storm | 828 | 66.2 |
| VT storm | 423 | 33.8 | |
| GENDER | Male | 1088 | 87.0 |
| Female | 163 | 13.0 | |
| HL | No | 549 | 43.9 |
| Yes | 702 | 56.1 | |
| HTN | No | 565 | 45.2 |
| Yes | 686 | 54.8 | |
| NYHA | 1 or 2 | 823 | 65.8 |
| 3 or 4 | 428 | 34.2 | |
| PREVIOUS ABL | No | 778 | 62.2 |
| Yes | 473 | 37.8 | |
| SHOCKS | No | 538 | 43.0 |
| Yes | 713 | 57.0 | |
Frequencies and percent for categorical variable are reported. 2 or more AADS: use of 2 or more anti arrhythmic drugs; AF: Atrial fibrillation; ICM: ischemic cardiomyopathy; IDCM: Idiopathic dilated cardiomyopathy; ARVC: arrhythmogenic right ventricular cardiomyopathy; HCM: hypertrophic cardiomyopathy; LVNC: left ventricular non-compaction; CKD: chronic kidney disease; ICD: implantable cardioverter defibrillator; CRT: cardiac resynchronization device; DM: diabetes mellitus; ES: electrical storm; HL: hyperlypemia; HTN: hypertension; NYHA: New-York Heart Association classification; Previous Abl: Previous ablation; Shocks: occurrence of ICD shocks before catheter ablation.
Ablation Procedure
Procedural details and ablation strategies have been previously described12. Contemporary approaches for substrate-based ablation guided by electro-anatomic mapping, pace mapping, and when feasible, activation and entrainment mapping, were performed across all centers.
Programmed electrical stimulation (PES) using up to two sites, two drive trains and triple extrastimuli was performed for induction of VT. When a 12-lead ECG of spontaneous VT was available, clinical VT was defined by match in all 12 leads. In absence of 12-lead ECG of the spontaneous VT, clinical VT was defined as the one matching the morphology and cycle length within 30 ms of the ICD stored electrograms from spontaneous VT episodes. The remaining sustained monomorphic VTs induced by PES were defined as nonclinical, but were routinely targeted for ablation. Ablation of areas of late activation or local conduction delay as evidence by split, fractionated or isolated late potentials was performed at the discretion of the treating physician8, 10, 15, 16. After ablation, PES was repeated unless prohibited by hemodynamic instability or procedure duration concerns. Acute procedural success was defined as elimination of all inducible sustained monomorphic VTs. Partial success was defined as elimination of all clinical VTs, but with monomorphic VT still inducible. Failure was defined as persistent inducibility of the clinical VT.
Outcomes
Patients were followed up by ICD interrogation at 3, 6 and 12 months. For patients not followed up at an IVTCC center, referring cardiologists were contacted and ICD interrogations reviewed. Remote control follow-ups were carefully checked for VT recurrence. Telephone interviews were also routinely performed with patients or family members. Recurrent VT/VF was defined as documented VT/VF lasting >30 sec, or any appropriate ICD therapy including anti-tachycardia pacing. Study endpoints were VT recurrence following the last ablation procedure, death. Antiarrhythmic therapy after ablation was at the discretion of the treating physician.
Primary efficacy outcome was 1-year all-cause mortality after VT ablation. Secondary outcomes were 1-year incidence of VT recurrence and time to VT recurrence. Clinical outcomes of patients are shown in Table 2. Recurrent VT/VF was defined as documented VT/VF lasting >30 sec, or any appropriate ICD therapy including anti-tachycardia pacing. Data were pooled into a central database for analysis.
Table 2.
Clinical outcomes of patients
| N | LEVEL | FREQUENCY | PERCENT | |
|---|---|---|---|---|
| PES AFTER ABL | 1210 | Clinical VTs inducible | 80 | 6.60 |
| Nonclinical VTs inducible | 236 | 19.5 | ||
| No Inducible VTs | 838 | 69.3 | ||
| Not tested | 56 | 4.60 | ||
| Yes | 713 | 57.0 | ||
| VT RECURRENCE | 1251 | No | 928 | 74.2 |
| Yes | 323 | 25.8 | ||
| DEATH | 1251 | Alive | 1098 | 87.8 |
| Dead | 153 | 12.2 | ||
Frequencies and percent for categorical variable are reported. PES After Abl: Programmed electrical stimulation after ablation.
Statistical analysis
Survival tree (ST) analysis was applied to discover groupings of subjects with homogeneous survival outcome, using information provided by clinical and procedural covariates 17. ST is a machine learning procedure based on binary recursive partitioning of a group of subjects, aiming at the choice of optimal cut-points for binary, ordinal, or continuous covariates, which maximizes split criterion18. The output is a decision tree, consisting of nodes and leaves, with each leaf indicating a class or a predicted outcome value (Figure 1).
Figure 1.
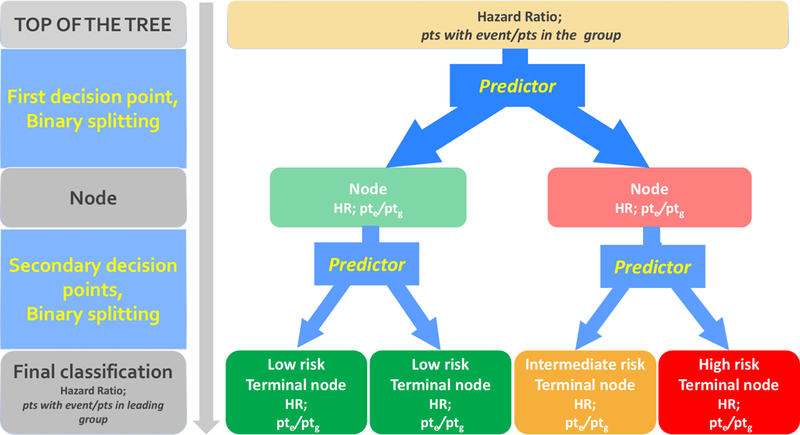
Schema of the decision tree, consisting of nodes and leaves. The root note of the tree (shown on top) comprises all the patients; it splits into daughter nodes; the splitting process continues recursively for each subsequent node. Subsequent splitting allows for the identification of subgroups with homogeneous risk profile. At the top of the tree HR is 1, since the baseline hazard of the entire cohort of the study is used as reference. Each intermediate node and final leaf (lower boxes) indicates a class with a homogeneous risk profile: low-risk patients (HR<0.7) are in green boxes, medium-risk (0.7≤HR≤1.4) in yellow boxes, high-risk (HR>1.4) in red boxes. HR: Hazard Ratio for the patients in the group (leaf). Pte: number of patients with the event in the group. Ptg: total number of patients in the group.
Statistical analysis was performed on a cohort of patients with complete data for all covariates. The root node of the tree (top of the tree), comprises all the observations; it is split into daughter nodes; the splitting process continues recursively for each subsequent node. The following covariates, showing statistical significance in previously published papers12, 19–23, were considered for the preoperative analysis: gender, age, hyperlipidemia (HL), hypertension (HTN), Diabetes Mellitus (DM), atrial fibrillation (AF), chronic kidney disease (CKD), New York Heart Association Functional Classification (NYHA), type of cardiomyopathy (ICM, NICM), left ventricular ejection fraction (LVEF), use of 2 or more AADs, type of cardiac device already implanted before the ablation (none, ICD, CRT), previous ICD shocks, ES, occurrence of a previous VT ablation (Previous Abl). Age was categorised into 3 classes (<65 years, 65–79 and ≥80) based on preliminary ST analyses that included age as continuous variable. At each step of the tree-growing procedure, the algorithm selected the best predictor and the best splitting cut-off according to an exponential model, as described in rpart24. At the end of the process, subjects with similar survival profiles end up in the same terminal node. A variable may be chosen as best predictor several times throughout the procedure.
Patients were classified in 3 risk groups based on Hazard Ratio (HR) for VT recurrence and mortality risk scores of the final leaves of the trees: low ≤ 0.7, medium: 0.8–1.4, high >1.4. Kaplan-Meier estimates of VT recurrence and death in groups derived from each ST analysis were obtained and compared by log-rank test.
Random survival forests (RSF) approach was applied to internally validate the results, by evaluating prediction accuracy, robustness of the results and assessing covariate importance 18, 25, 26. In the present study, 5000 bootstrap samples were drawn from the original data and a survival tree was derived for each bootstrapped sample without applying pruning. In the construction of each tree, at each node of the tree grown on bootstrapped data, the procedure randomly selected a narrowed set of predictors on which to base splitting. Hence, the splitting variable was searched within the reduced set of predictors26. A final prediction was then obtained by combining each individual tree.
Predictive value for each variable in the tree was assessed by evaluating the minimal depth26, that measures the depth of the first split with respect to the root node; the shorter is the split, the more predictive is the variable.
In previous studies, acute VT inducibility following ablation was shown to have an impact on VT recurrence and survival12, 19. To check whether risk stratification early after the ablation using programmed electrical stimulation (PES) could add benefit to the present analysis of procedure-related covariates, we developed additional models including PES in the list of variables analysed by ST and RSF. Statistical analysis for the post procedure model was performed on a cohort of 1210 patients with complete data for all analysed covariates.
We calculated the PAAINESD score27 for the IVTCC cohort and compared its prediction of mortality to the I-VT score using ROC curves. In order to evaluate if the in low/mid/high categories corresponded to an increasing rate pattern of VT recurrence and death, the model was externally validated in a population of consecutive patients with structural heart disease, undergoing VT ablation at San Raffaele Hospital between January 2014 and February 2017; population characteristics are shown in Supplementary Table 1. Analyses were performed using R statistical software (version 3.4.2)28. In particular, the survival29 along with rpart24 and randomForestSRC30 packages were used to implement survival analysis, obtain survival trees, build random forest and derive variable importance respectively. Significance level was considered to be <0.05.
Results
Risk of VT recurrence
Survival tree analysis (Figure 2A) showed a major impact of LVEF on outcomes; an LVEF of 30% was identified as the best threshold to classify risk of both recurrence and death. Patients with LVEF<30% had a high VT recurrence risk (HR 1.6), compared to patients with LVEF ≥30% (HR 0.7). Among those with LVEF<30%, patients with a previous ablation had the highest risk of VT recurrence after the index procedure (HR 2); among patients with LVEF ≥30%, the absence of an ICD was associated with lower risk (HR 0.38 vs. 0.81). Further risk stratification for VT recurrence in patients with LVEF ≥30% was provided by incorporation of type of Cardiomyopathy (Figure 2A, third decision point): patients with ICM had a lower recurrence risk as compared to those with NICM, both in the group of patients with or without an ICD or CRT (HR: 0.086 vs 0.48, and HR: 0.63 vs 0.98, respectively). Among the 318 patients with NICM, re-analysis of LVEF with a 50% threshold identified narrower risk groups (Figure 2A, fourth decision point): the 94 patients with LVEF≥50% had a lower recurrence rate (15 recurrences, 16%), as compared to those with LVEF<50% (29.0%) (HR:0.59 vs 1.2).
Figure 2.
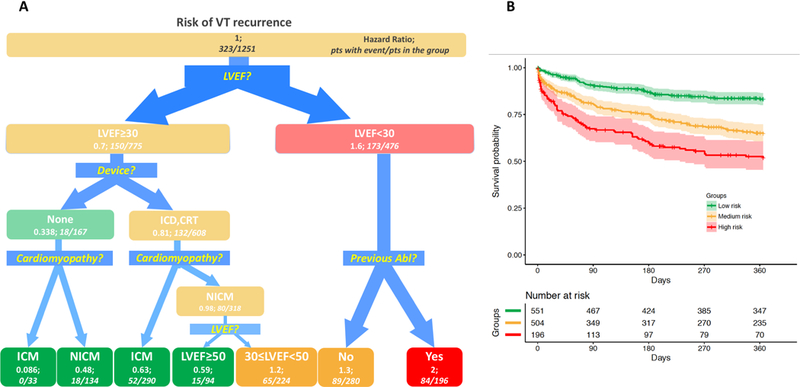
Survival Tree for 1-year VT recurrence and Kaplan-Meyer analysis in derived groups. A. Survival Tree (left panel): The figure follows the schema of the Survival Tree with decisional points, nodes and final classification leafs shown in Figure 1. LVEF appears as the first decision point: patients with LVEF<30% had a higher VT recurrence risk (HR 1.6), as compared to patients with LVEF ≥30% (HR: 0.7). Following split variables are Device, type of cardiomyopathy, Previous Abl. B (Right panel): Kaplan-Meier estimates with 95% confidence intervals of VT recurrence for patients with low (green line), medium (yellow line) and high-risk profile (red line).
Based on the HR, we outlined 3 profiles (Figure 2B): low-risk (HR <0.7, green boxes), medium-risk (HR between 0.7 and 1.4, yellow boxes) and high-risk (HR >1.4, red boxes). Based on the final branches of the ST analysis, patients with LVEF ≥30%, without ICD, and with ICM were assigned to the low-risk group, that experienced a very low-risk of VT recurrence (HR 0.086, no patients with VT recurrences after ablation among 33 in the group). Additionally assigned to the low-risk group were the following: patients with LVEF ≥30%, without ICD, and with NICM (HR 0.48); patients with LVEF ≥30%, with and ICD or CRT, and with ICM (HR 0.63); patients with LVEF ≥50%, with and ICD or CRT, and with NICM (HR 0.59).
In the medium-risk group were assigned patients with LVEF 30%−50%, with an implanted ICD or CRT device and NICM (65 of 224 experienced recurrence, HR 1.2); and patients with LVEF<30% without a previous ablation (89 patients with recurrences among 280 in the group; HR 1.3).
In the high-risk group for VT recurrence were patients with LVEF <30% who had a previous ablation (HR 2). Complete risk stratification for all groups is shown in Figure 2A. The 3 risk groups showed significantly different VT free survivals by log-rank test (Figure 2B) (p<0.001).
Risk of Death
The ST for mortality analysis (Figure 3A) showed that, similar to VT recurrence analysis, a cut-off of 30% for LVEF was the primary classification node for VT patients. Among the lower risk cohort, the occurrence of a previous ablation led to further categorization of 2 low-risk groups: patients without a previously failed VT ablation had the lowest 1-year risk of death (0.96%, HR 0.088), that was significantly better than those with a previous VT ablation (5.2%; HR 0.45).
Figure 3.
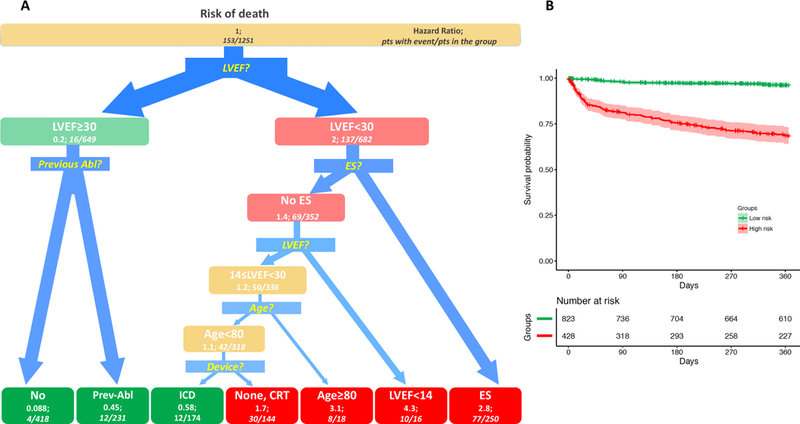
Survival Tree for 1-year death after VT ablation and Kaplan-Meyer analysis in derived groups. A. Survival Tree (left panel): The figure follows the schema of the Survival Tree with decisional points, nodes and final classification leafs shown in Figure 1. LVEF appears as the first decision point: patients with LVEF<30% had a higher death risk (HR 2), as compared to patients with LVEF ≥30% (HR: 0.2). In patients with LVEF≥30% occurrence of a previous ablation (Previous Abl) was identified as further splitting variable. In patients with LVEF<30%, 4 subsequent splitting variable were identified by the procedure, thus allowing for a more precise risk stratification: ES, LVEF, Age, Device. B (Right panel): Kaplan-Meier estimates with 95% confidence intervals of 1-year death for patients with low (green line), medium (yellow line) and high profile (red line).
Patients with LVEF<30% had higher risk of death during the first year after the procedure (HR 2), as compared to those with LVEF≥30 (HR 0.2). In this cohort of patients, the second decisional node was a history of ES: patients with a previous ES were classified in the high-risk group (HR 2.8). In the cohort of patients with LVEF<30% and absence of ES, further classification was provided by presence of LVEF<14%, age and ICD. Noticeably, ST identified patients with LVEF between 14% and 30%, no VT storm, an ICD, and age ˂80 years, as having a low 1-year risk of death (12/174 died, 6.9%; HR 0.58). All other patients with LVEF<30 were classified in the high-risk of death group, with the highest-risk cohort being those with LVEF<14% (HR 4.3). These three groups showed significantly different 1-year survivals by log-rank test (Figure 3B, p<0.001).
An online calculator for the assessment of recurrence and death risk after VT ablation is available at www.vtscore.org.
Risk re-assessment after VT ablation
Results of PES after ablation were previously hypothesised to have an impact on VT recurrence and survival prediction. In order to check whether PES should be considered for accurate risk stratification after the procedure, we developed 2 additional survival models including post-procedure covariates.
For VT recurrence (Figure 4A), after the first split on LVEF (same as obtained in the pre-procedure tree), PES was a relevant predictor of risk, both in high- and low-risk groups. In particular, patients with clinical VT still inducible and those not tested showed a higher risk of VT recurrence. Other variables detected by the tree were presence of ICD, age and previous ablation.
Figure 4.
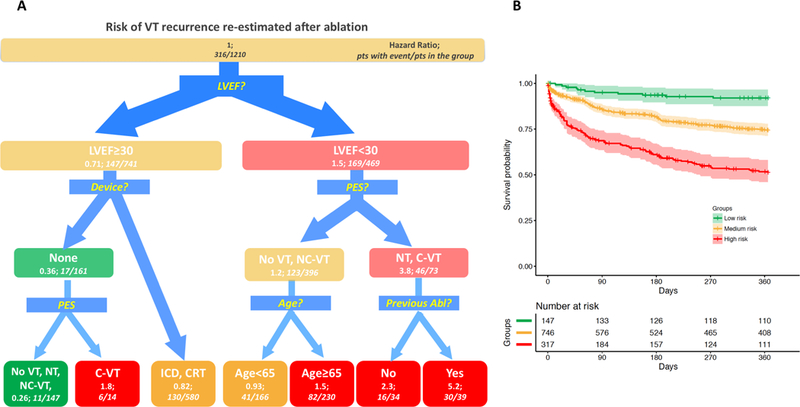
Survival Tree for re-estimation of the VT recurrence risk after the procedure and Kaplan-Meyer analysis in derived groups. A. Survival Tree (left panel): The figure follows the schema of the Survival Tree with decisional points, nodes and final classification leafs shown in Figure 1. LVEF remains as the most predictive variable also for post-operative risk estimation. In patients with LVEF<30%, the result of programmed stimulation after the procedure (PES), Age and occurrence of a previous ablation (Previous Abl) provided further risk stratification. No VT: absence of any VT inducible at programmed stimulation after the ablation; NC-VT: Non clinical ventricular tachycardia inducible after the ablation; C-VT: Clinical ventricular tachycardia inducible after the ablation; NT: not tested. B (Right panel): Kaplan-Meier estimates with 95% confidence intervals of VT recurrence for patients with low (green line), medium (yellow line) and high profile (red line).
For the death analysis (Figure 5A), PES also appeared as a second split but only in the high-risk group (LVEF<30). In this case, patients with no inducible VTs were re-classified as low risk. The remaining splits are similar to those based on pre-procedure variables alone.
Figure 5.
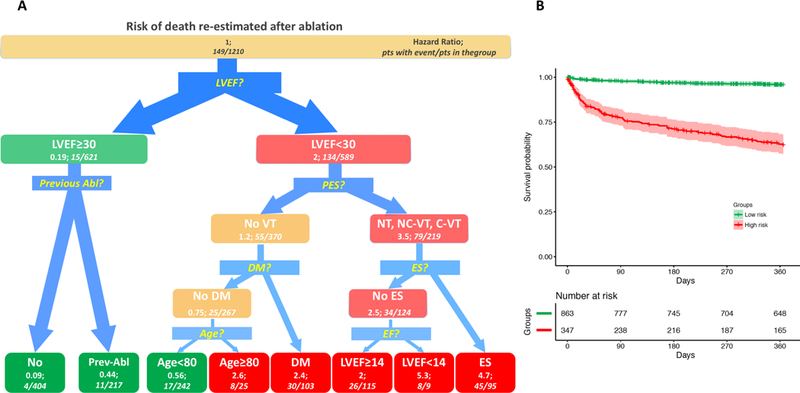
Survival Tree for re-estimation of the mortality risk after the procedure and Kaplan-Meyer analysis in derived groups. A. Survival Tree. Left panel: The figure follows the schema of the Survival Tree with decisional points, nodes and final classification leafs shown in Figure 1. LVEF remains as the most predictive variable also for post-operative risk estimation. In patients with LVEF<30%, the result of programmed stimulation after the procedure (PES), Diabetes Mellitus (DM), ES (Electrical Storm), Age provided further risk stratification. In patients with LVEF≥30% occurrence of a previous ablation (Previous Abl) was identified as further splitting variable. No VT: absence of any VT inducible at programmed stimulation after the ablation; NC-VT: Non clinical ventricular tachycardia inducible after the ablation; C-VT: Clinical ventricular tachycardia inducible after the ablation; NT: not tested. B. Right panel: Kaplan-Meier estimates with 95% confidence intervals of VT recurrence for patients with low (green line), medium (yellow line) and high profile (red line).
Also in the post-procedure variable analysis, Kaplan Meier analysis of the risk groups showed statistically different survivals (all log-rank p<0.001) (Figure 4B and Figure 5B).
Model Validation
In the VT recurrence analysis, LVEF had the highest importance (minimal depth = 0.058), followed by presence of an ICD (minimal depth = 0.117); these variables provided major splits in the survival tree, confirming their importance in discriminating the risk of recurrence among VT patients. The information about minimal depth for each variable is reported in Supplementary Figure 1. In the risk of death analysis, LVEF showed the highest importance (minimal depth = 0.062); other variables showing up in the tree (Figure 3a: ES, previous Abl, age and device) had a high importance (Supplementary Figure 1b). According to RSF, also NYHA had a high importance (minimal depth= 0.118, second in rank among the variables); it did not show up in the trees because NYHA resulted to be a surrogate variable for the primary split. From a practical point of view, in the absence of information on LVEF, which provides the best classification, NYHA may be used for stratification, with NYHA≥3 patients having a worse prognosis than those with NYHA≤2.
When performing internal validation of the post-procedure trees, result of PES showed a high importance in both the recurrence (minimal depth = 0.061) and survival (minimal depth = 0.082), being ranked second just after LVEF (Supplementary Figure 1c and 1d).
The importance of including PES as a predictor is also shown by the increase of accuracy in the prediction of both VT recurrence and mortality; in our sample, 29.2% of patients classified in the high risk group by the pre-procedure risk stratification died, while the mortality increased to 33.7% in the group of patients classified in the high risk group by the post-procedure score (p<0.01) (Table 3). When considering VT recurrence, the prediction improvement was visible in low and mid risk groups: VT recurrence was 15.4% and 30.6% in patients classified in the low and medium risk groups by the pre-procedure score; it decreased to 7.5% and 22.9% respectively, when PES was included in the model (p<0.01).
Table 3.
VT recurrence and death according to risk groups for pre-procedure and post-procedure stratification.
| VT recurrence | ||||||||
| No | Yes | Total | ||||||
| Patients | % | Patients | % | Patients | % | |||
| Pre-procedure risk stratification | LOW | Patients | 466 | 84.6% | 85 | 15.4% | 551 | 44.0% |
| MID | Patients | 350 | 69.4% | 154 | 30.6% | 504 | 40.3% | |
| HIGH | Patients | 112 | 57.1% | 84 | 42.9% | 196 | 15.7% | |
| Total | 928 | 74.2% | 323 | 25.8% | 1251 | 100.0% | ||
| Post-procedure risk stratification | LOW | Patients | 136 | 92.5% | 11 | 7.5% | 147 | 12.1% |
| MID | Patients | 575 | 77.1% | 171 | 22.9% | 746 | 61.7% | |
| HIGH | Patients | 183 | 57.7% | 134 | 42.3% | 317 | 26.2% | |
| Total | 894 | 73.9% | 316 | 26.1% | 1210 | 100.0% | ||
| Death | ||||||||
| No | Yes | Total | ||||||
| Patients | % | Patients | % | Patients | % | |||
| Pre-procedure risk stratification | LOW | Patients | 795 | 96.6% | 28 | 3.4% | 823 | 65.8% |
| HIGH | Patients | 303 | 70.8% | 125 | 29.2% | 428 | 34.2% | |
| Total | 1098 | 87.8% | 153 | 12.2% | 1251 | 100.0% | ||
| Post-procedure risk stratification | LOW | Patients | 831 | 96.3% | 32 | 3.7% | 863 | 71.3% |
| HIGH | Patients | 230 | 66.3% | 117 | 33.7% | 347 | 28.7% | |
| Total | 1061 | 87.7% | 149 | 12.3% | 1210 | 100.0% | ||
Frequencies and percent for categorical variables are reported.
The current scores, both before and after the procedure (including PES), were associated by ROC analysis to a higher prediction of mortality, as compared PAAINESD score (AUCs 0.82 vs 0.71 and 0.84 vs 0.71, respectively; p<0.001 for both comparisons) (Figure 6). When the model was applied to a different population for external validation, 26.5% of patients classified in the high risk group by the pre-procedure risk stratification died within 1 year, while the mortality increased to 34.3% in the group of patients classified in the high risk group by the post-procedure score (Supplementary Table 2). Among the same population, patients classified in low and high risk groups by the pre-procedure score showed a 1-year VT recurrence of 20.8% and 28.5%; when PES was included in the model, 1-year VT recurrence in low and mid risk groups were 10.5% and 27.2%, respectively.
Figure 6.
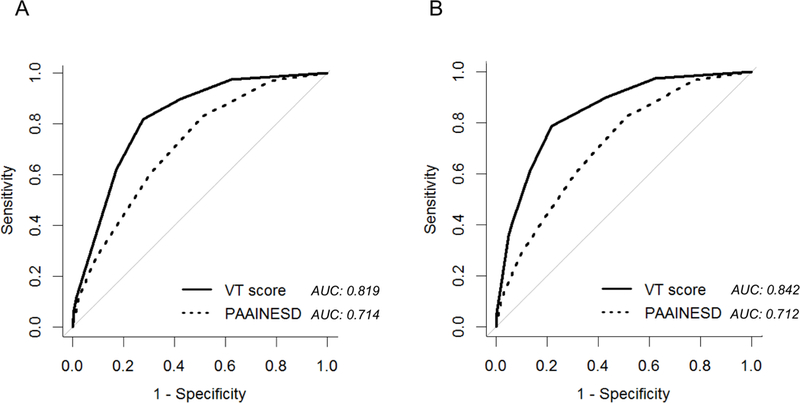
Comparison of mortality prediction between the i-VT score and the PAAINESD score by ROC curves. In the panel A only pre-procedure variables were included in the i-VT score; in panel B, both pre and post procedure variables were included in the i-VT score
DISCUSSION
In this study, we presented the first available prediction model for one-year recurrence and mortality after VT ablation. The model was derived and validated from the largest available and well characterized population of patients undergoing VT ablation in the setting of structural heart disease, treated at 12 international sites. Estimates were obtained from routinely collected clinical and procedural parameters, thus not requiring any additional examinations outside current clinical practice. The broad patient inclusion criteria of the study and the enrolment of consecutive patients following real-world practice allowed the study of a wide spectrum of conditions associated with ventricular tachycardia; thus, the model could be applied to current practice in adult patients with an indication for VT ablation.
In the I-VT score cohort, participants had a 1-year recurrence risk of 25.8% and a 1-year risk of death of 12.2%. The decisional tree identified a gradient of risk across the patients undergoing VT ablation, ranging from negligible to 42.8% for recurrence and from negligible to 62.5% for 1-year mortality; the highest risk groups, thus showed an HR for recurrence and for mortality respectively 23 and 49 times higher than low risk groups. Inclusion of the results of the PES after ablation in the model allowed a more refined risk estimation.
Current clinical guidelines recommend catheter ablation in patients with recurrent ICD shock and consider the procedure after a first episode of sustained VT in patients with an ICD5. However, no further prognostic information is provided to support clinical decision making. Our findings provide a strong step toward identifying patients at risk of death during the first year after VT ablation; they can help physicians to counsel patients before VT ablation procedure and also to target supplementary strategies during or after the procedure, aimed at prevention of death in the highest risk subgroups. In the ST analysis LVEF, Previous Abl, type of cardiac device and type of cardiomyopathy were identified as the best predictors for 1-year VT recurrence; LVEF, ES, age and type of cardiac device were selected as best predictors of 1-year survival.
Significance of left ventricular ejection fraction
LVEF appeared as the most informative variable in predicting both recurrence and death, thus appearing in all the decisional trees as the first branch. LVEF was used as a continuous variable, allowing the investigation of a gradient of risk across a broad range of values, rather than a priori dichotomizing risk; optimal cut-points were derived by the ST procedures. LVEF<30 identified a high-risk population; analysis of additional variables, both in the LVEF<30 and LVEF≥30 groups, allowed a more accurate risk stratification.
Although several markers of sudden cardiac death have been proposed, including signal averaged ECG31, heart rate variability32, baroreflex sensitivity33, QRS prolongation34 and T-wave alternans35, none of those is currently used in clinical practice36. LVEF is the only widely accepted predictor of death in patients with structural heart disease; it has been used for more than a decade in determining eligibility for primary prevention ICD 37, 38 and is still recommended by current clinical guidelines to identify patients who need ICD implant because of high-risk of developing VT/VF39. The reproducible prediction power of LVEF was confirmed by a recent systematic review, that highlighted the inclusion of LVEF in 58% of the 43 published models for death in HF40.
Evaluation of patients with multiple comorbidities
In previous studies advanced heart failure status (NYHA class IV) 21, female gender23, history of a previous VT ablation22 and presentation with ES19 were associated to poor outcomes. The analysis of subgroups of patients sharing one common characteristic provides the opportunity to focus the point of view on the specific field that is analyzed in depth; however, this approach tends to hide the multiple interactions between the various characteristics and comorbidities that constitute the full portrait of each patient. By examining simultaneously all variables and selecting the best splits, the ST analysis may identify subtle differences between patient profiles and maximizing the survival stratification.
About one third of patients are exposed to a one-year risk of death higher than 20% (High-risk group). Comprehensive counseling providing realistic expectations for patients and their families is an important part of the medical mission; this is especially true in situations in which there is greater risk of an unfavorable outcome as in patients with very low LVEF, previously failed ablations, advanced age and ES. The present model helps to identify this particular subset of patients who may require additional interventions to improve procedural efficacy and outcomes. Since all patients in the study underwent catheter ablation, a high-risk score for arrhythmia recurrence or death after the procedure is not a mandatory criterion for denying the treatment. Physician might advise patients at high-risk for recurrence on the possible need for a redo procedure already during the pre-procedure counseling. Patients with a high risk of death may benefit of a proactive institution of therapy, including mechanical hemodynamic support, that is recognized to improve outcomes41, 42. In patients with untreatable VT after a previous ablation and a high-risk for recurrence and death, heart transplant might be considered43. Results of patients risk stratification after the procedure may also be used for individualizing follow-up strategies, thus suggesting more careful monitoring in patients at high-risk for recurrence.
Comparison with existing methods and models for risk prediction
There is an increasing interest in the development of prediction models to justify medical treatments (CHA2DS2-VASc44 and TIMI score45), or to predict outcomes.
Survival trees are powerful and effective tools useful to identify homogeneous groups of patients with different risk profiles. With respect to standard Cox regression models, they provide several advantages: they automatically select variables which allow to best discriminate among groups, identify optimal cut-offs for the examined variables and uncover interactions among variables without the need to directly specify them in the model.
Widespread application of risk stratification models in routine clinical practice is usually inhibited by the feeling that they are time consuming or providing low value information46. The Seattle score47, 48 included 14 continuous and 10 categorical values, thus making it impractical for computation by hand. In 43 previously published models for prediction of death in heart failure, the median number of final predictors was 9 (range: 3 to 317)40. Identification of only the informative variables and improvement in techniques for easier visualization of the risk profile have been acknowledged to favor the clinical application of the tools 40. The current decisional tree requires only the 6 variables selected among 15 analyzed, based on variable importance; variables not improving the classification were discarded by the procedure.
The previously developed PAAINESD score27 can be used for the evaluation of 30-days mortality after VT ablation. The current I-VT score confirms that LVEF, age, ES, type of cardiomyopathy and DM, already present in the PAAINESD score are important predictors of poor outcome; as compared with the PAAINESD score, I-VT score provides a longer timeframe (1-year) estimation and a more accurate prediction of mortality. When applied to an external population, the I-VT score proved to be effective in predicting VT recurrence and mortality; similarly to the results obtained in the training sample, patients categorized as high risk had an higher rate of recurrence and death then low/mid risk groups.
Limitations
This study was a retrospective analysis of data from high volume, tertiary-referral ablation hospitals. As such, it is possible that a referral bias and the wide lifespan of the study may limit the generalizability of our results beyond centres with extensive experience in VT ablation. Antiarrhythmic drug therapy and ICD therapies programming were left to the discretion of the treating physicians and could influence outcomes. In particular, VT recurrence may have been underreported in patients without an ICD; on the other side, inflated recurrence rates in patients with ICD implants might be due enhanced detection and rapid treatment by ICD also for VTs that could otherwise terminate spontaneously. However, the sample size larger than previously published studies is a major strength. Future studies should test prospectively the present risk model.
Conclusions
The present study is the first to derive and validate a risk model that provides estimates of VT recurrence and mortality with an effective classification tree. Pre-operative risk stratification could help counseling for patients and their families and also planning supplementary care before and after the procedure.
An online calculator for the assessment of recurrence and death risk after VT ablation is available at www.vtscore.org.
Supplementary Material
What is known
Radiofrequency catheter ablation has been shown to reduce VT burden and appropriate ICD therapies in patients with structural heart disease.
Patients are still exposed to a significant risk of recurrence and mortality after VT ablation.
What the Study Adds
The present study is the first to derive and validate a risk model that provides estimates of VT recurrence and mortality with an effective classification tree.
The decisional tree identified Left Ventricular Ejection Fraction as the most informative variable in predicting both recurrence and death
The model helps to identify subset of patients with several comorbidities who may require additional interventions to improve procedural efficacy and outcomes.
Pre-operative risk stratification could help counseling for patients and their families and also planning supplementary care before and after the procedure.
Footnotes
Disclosures: Luigi Di Biase is a consultant to Biosense Webster, St. Jude Medical, and Stereotaxis; Usha Tedrow has received honoraria from Medtronic, Boston Scientific, and St. Jude Medical; and research grants from Biosense Webster and St. Jude Medical; T. Jared Bunch is a consultant to Boston Scientific; J. David Burkhardt is a consultant to Biosense Webster; William G. Stevenson is the recipient of a patent for needle ablation consigned to Brigham and Women’s Hospital; Kalyanam Shivkumar was supported by National Heart, Lung, and Blood Institute Grant R01HL084261; Paolo Della Bella is consultant for St. Jude Medical and has received honoraria for lectures from Biosense Webster, St. Jude Medical and Biotronik.
REFERENCES:
- 1.Reddy VY, Reynolds MR, Neuzil P, Richardson AW, Taborsky M, Jongnarangsin K, Kralovec S, Sediva L, Ruskin JN and Josephson ME. Prophylactic catheter ablation for the prevention of defibrillator therapy. N Engl J Med. 2007;357:2657–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuck KH, Schaumann A, Eckardt L, Willems S, Ventura R, Delacretaz E, Pitschner HF, Kautzner J, Schumacher B and Hansen PS. Catheter ablation of stable ventricular tachycardia before defibrillator implantation in patients with coronary heart disease (VTACH): a multicentre randomised controlled trial. Lancet. 2010;375:31–40. [DOI] [PubMed] [Google Scholar]
- 3.Sapp JL, Wells GA, Parkash R, Stevenson WG, Blier L, Sarrazin JF, Thibault B, Rivard L, Gula L, Leong-Sit P, Essebag V, Nery PB, Tung SK, Raymond JM, Sterns LD, Veenhuyzen GD, Healey JS, Redfearn D, Roux JF and Tang AS. Ventricular Tachycardia Ablation versus Escalation of Antiarrhythmic Drugs. N Engl J Med. 2016;375:111–21. [DOI] [PubMed] [Google Scholar]
- 4.Palaniswamy C, Kolte D, Harikrishnan P, Khera S, Aronow WS, Mujib M, Mellana WM, Eugenio P, Lessner S, Ferrick A, Fonarow GC, Ahmed A, Cooper HA, Frishman WH, Panza JA and Iwai S. Catheter ablation of postinfarction ventricular tachycardia: ten-year trends in utilization, in-hospital complications, and in-hospital mortality in the United States. Heart Rhythm. 2014;11:2056–63. [DOI] [PubMed] [Google Scholar]
- 5.Priori SG, Blomstrom-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, Kirchhof P, Kjeldsen K, Kuck KH, Hernandez-Madrid A, Nikolaou N, Norekval TM, Spaulding C and Van Veldhuisen DJ. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2015;36:2793–867. [DOI] [PubMed] [Google Scholar]
- 6.Berruezo A, Fernandez-Armenta J, Andreu D, Penela D, Herczku C, Evertz R, Cipolletta L, Acosta J, Borras R, Arbelo E, Tolosana JM, Brugada J and Mont L. Scar dechanneling: new method for scar-related left ventricular tachycardia substrate ablation. Circ Arrhythm Electrophysiol. 2015;8:326–36. [DOI] [PubMed] [Google Scholar]
- 7.Di Biase L, Santangeli P, Burkhardt DJ, Bai R, Mohanty P, Carbucicchio C, Dello Russo A, Casella M, Mohanty S, Pump A, Hongo R, Beheiry S, Pelargonio G, Santarelli P, Zucchetti M, Horton R, Sanchez JE, Elayi CS, Lakkireddy D, Tondo C and Natale A. Endo-epicardial homogenization of the scar versus limited substrate ablation for the treatment of electrical storms in patients with ischemic cardiomyopathy. J Am Coll Cardiol. 2012;60:132–41. [DOI] [PubMed] [Google Scholar]
- 8.Jais P, Maury P, Khairy P, Sacher F, Nault I, Komatsu Y, Hocini M, Forclaz A, Jadidi AS, Weerasooryia R, Shah A, Derval N, Cochet H, Knecht S, Miyazaki S, Linton N, Rivard L, Wright M, Wilton SB, Scherr D, Pascale P, Roten L, Pederson M, Bordachar P, Laurent F, Kim SJ, Ritter P, Clementy J and Haissaguerre M. Elimination of local abnormal ventricular activities: a new end point for substrate modification in patients with scar-related ventricular tachycardia. Circulation. 2012;125:2184–96. [DOI] [PubMed] [Google Scholar]
- 9.Tzou WS, Frankel DS, Hegeman T, Supple GE, Garcia FC, Santangeli P, Katz DF, Sauer WH and Marchlinski FE. Core isolation of critical arrhythmia elements for treatment of multiple scar-based ventricular tachycardias. Circ Arrhythm Electrophysiol. 2015;8:353–61. [DOI] [PubMed] [Google Scholar]
- 10.Vergara P, Trevisi N, Ricco A, Petracca F, Baratto F, Cireddu M, Bisceglia C, Maccabelli G and Della Bella P. Late potentials abolition as an additional technique for reduction of arrhythmia recurrence in scar related ventricular tachycardia ablation. J Cardiovasc Electrophysiol. 2012;23:621–7. [DOI] [PubMed] [Google Scholar]
- 11.Stevenson WG, Wilber DJ, Natale A, Jackman WM, Marchlinski FE, Talbert T, Gonzalez MD, Worley SJ, Daoud EG, Hwang C, Schuger C, Bump TE, Jazayeri M, Tomassoni GF, Kopelman HA, Soejima K and Nakagawa H. Irrigated radiofrequency catheter ablation guided by electroanatomic mapping for recurrent ventricular tachycardia after myocardial infarction: the multicenter thermocool ventricular tachycardia ablation trial. Circulation. 2008;118:2773–82. [DOI] [PubMed] [Google Scholar]
- 12.Tung R, Vaseghi M, Frankel DS, Vergara P, Di Biase L, Nagashima K, Yu R, Vangala S, Tseng CH, Choi EK, Khurshid S, Patel M, Mathuria N, Nakahara S, Tzou WS, Sauer WH, Vakil K, Tedrow U, Burkhardt JD, Tholakanahalli VN, Saliaris A, Dickfeld T, Weiss JP, Bunch TJ, Reddy M, Kanmanthareddy A, Callans DJ, Lakkireddy D, Natale A, Marchlinski F, Stevenson WG, Della Bella P and Shivkumar K. Freedom from recurrent ventricular tachycardia after catheter ablation is associated with improved survival in patients with structural heart disease: An International VT Ablation Center Collaborative Group study. Heart Rhythm. 2015;12:1997–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sosa E, Scanavacca M, D’Avila A, Piccioni J, Sanchez O, Velarde JL, Silva M and Reolao B. Endocardial and epicardial ablation guided by nonsurgical transthoracic epicardial mapping to treat recurrent ventricular tachycardia. J Cardiovasc Electrophysiol. 1998;9:229–39. [DOI] [PubMed] [Google Scholar]
- 14.Della Bella P, Brugada J, Zeppenfeld K, Merino J, Neuzil P, Maury P, Maccabelli G, Vergara P, Baratto F, Berruezo A and Wijnmaalen AP. Epicardial ablation for ventricular tachycardia: a European multicenter study. Circ Arrhythm Electrophysiol. 2011;4:653–9. [DOI] [PubMed] [Google Scholar]
- 15.Arenal A, Glez-Torrecilla E, Ortiz M, Villacastin J, Fdez-Portales J, Sousa E, del Castillo S, Perez de Isla L, Jimenez J and Almendral J. Ablation of electrograms with an isolated, delayed component as treatment of unmappable monomorphic ventricular tachycardias in patients with structural heart disease. J Am Coll Cardiol. 2003;41:81–92. [DOI] [PubMed] [Google Scholar]
- 16.Bogun F, Good E, Reich S, Elmouchi D, Igic P, Lemola K, Tschopp D, Jongnarangsin K, Oral H, Chugh A, Pelosi F and Morady F. Isolated potentials during sinus rhythm and pace-mapping within scars as guides for ablation of post-infarction ventricular tachycardia. J Am Coll Cardiol. 2006;47:2013–9. [DOI] [PubMed] [Google Scholar]
- 17.Breiman L, Friedman JH, Olshen RA and Stone CI. Classification and regression trees. Belmont, California: Wadsworth; 1984. [Google Scholar]
- 18.Zhou Y and McArdle JJ. Rationale and Applications of Survival Tree and Survival Ensemble Methods. Psychometrika. 2015;80:811–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vergara P, Tung R, Vaseghi M, Brombin C, Frankel D, Di Biase L, Nagashima K, Tedrow U, Tzou WS, Sauer WH, Mathuria N, Nakahara S, Vakil K, Tholakanahalli V, Bunch TJ, Weiss JP, Dickfeld T, Vunnam R, Lakireddy D, Burkhardt JD, Correra A, Santangeli P, Callans D, Natale A, Marchlinski F, Stevenson WG, Shivkumar K and Della Bella P. Successful ventricular tachycardia ablation in patients with electrical storm reduces recurrences and improves survival. Heart Rhythm. 2018;15:48–55. [DOI] [PubMed] [Google Scholar]
- 20.Vakil K, Garcia S, Tung R, Vaseghi M, Tedrow U, Della Bella P, Frankel DS, Vergara P, Di Biase L, Nagashima K, Nakahara S, Tzou WS, Burkhardt JD, Dickfeld T, Weiss JP, Bunch J, Callans D, Lakkireddy D, Natale A, Sauer WH, Stevenson WG, Marchlinski F, Shivkumar K and Tholakanahalli VN. Ventricular Tachycardia Ablation in the Elderly: An International Ventricular Tachycardia Center Collaborative Group Analysis. Circ Arrhythm Electrophysiol. 2017;10: e005332. [DOI] [PubMed] [Google Scholar]
- 21.Tzou WS, Tung R, Frankel DS, Vaseghi M, Bunch TJ, Di Biase L, Tholakanahalli VN, Lakkireddy D, Dickfeld T, Saliaris A, Weiss JP, Mathuria N, Tedrow U, Afzal MR, Vergara P, Nagashima K, Patel M, Nakahara S, Vakil K, Burkhardt JD, Tseng CH, Natale A, Shivkumar K, Callans DJ, Stevenson WG, Della Bella P, Marchlinski FE and Sauer WH. Ventricular Tachycardia Ablation in Severe Heart Failure: An International Ventricular Tachycardia Ablation Center Collaboration Analysis. Circ Arrhythm Electrophysiol. 2017;10:e004494. [DOI] [PubMed] [Google Scholar]
- 22.Tzou WS, Tung R, Frankel DS, Di Biase L, Santangeli P, Vaseghi M, Bunch TJ, Weiss JP, Tholakanahalli VN, Lakkireddy D, Vunnam R, Dickfeld T, Mathuria N, Tedrow U, Vergara P, Vakil K, Nakahara S, Burkhardt JD, Stevenson WG, Callans DJ, Della Bella P, Natale A, Shivkumar K, Marchlinski FE and Sauer WH. Outcomes after repeat ablation of ventricular tachycardia in structural heart disease: An analysis from the International VT Ablation Center Collaborative Group. Heart Rhythm. 2017;14:991–997. [DOI] [PubMed] [Google Scholar]
- 23.Frankel DS, Tung R, Santangeli P, Tzou WS, Vaseghi M, Di Biase L, Nagashima K, Tedrow U, Bunch TJ, Tholakanahalli VN, Dendi R, Reddy M, Lakkireddy D, Dickfeld T, Weiss JP, Mathuria N, Vergara P, Patel M, Nakahara S, Vakil K, Sauer WH, Callans DJ, Natale A, Stevenson WG, Della Bella P, Shivkumar K and Marchlinski FE. Sex and Catheter Ablation for Ventricular Tachycardia: An International Ventricular Tachycardia Ablation Center Collaborative Group Study. JAMA Cardiol. 2016. [DOI] [PubMed] [Google Scholar]
- 24.Therneau T, Atkinson B and Ripley B. rpart: Recursive Partitioning and Regression Trees. R package version 4.1–13. https://CRAN.R-project.org/package=rpart. 2018. [Google Scholar]
- 25.Breiman L Bagging Predictors. Machine Learning. 1996;24:123–140. [Google Scholar]
- 26.Ishwaran H, Kogalur UB, Blackstone EH and Lauer MS. Random survival forests. The Annals of Applied Statistics. 2008;2:841–860. [Google Scholar]
- 27.Santangeli P, Frankel DS, Tung R, Vaseghi M, Sauer WH, Tzou WS, Mathuria N, Nakahara S, Dickfeldt TM, Lakkireddy D, Bunch TJ, Di Biase L, Natale A, Tholakanahalli V, Tedrow UB, Kumar S, Stevenson WG, Della Bella P, Shivkumar K, Marchlinski FE, Callans DJ and International VTACCG. Early Mortality After Catheter Ablation of Ventricular Tachycardia in Patients With Structural Heart Disease. J Am Coll Cardiol. 2017;69:2105–2115. [DOI] [PubMed] [Google Scholar]
- 28.R: a language and environment for statistical computing [computer program]. Version 3.4.2. Vienna, Austria: R Foundation for Statistical Computing; 2017.
- 29.Therneau TM. A Package for Survival Analysis in S %U https://CRAN.R-project.org/package=survival. 2015. [Google Scholar]
- 30.Ishwaran H and Kogalur UB. Random Forests for Survival, Regression, and Classification (RF-SRC): manual %U https://cran.r-project.org/package=randomForestSRC; 2017. [Google Scholar]
- 31.Huikuri HV, Tapanainen JM, Lindgren K, Raatikainen P, Makikallio TH, Juhani Airaksinen KE and Myerburg RJ. Prediction of sudden cardiac death after myocardial infarction in the beta-blocking era. J Am Coll Cardiol. 2003;42:652–8. [DOI] [PubMed] [Google Scholar]
- 32.Camm AJ, Pratt CM, Schwartz PJ, Al-Khalidi HR, Spyt MJ, Holroyde MJ, Karam R, Sonnenblick EH, Brum JM and AzimiLide post Infarct surVival Evaluation I. Mortality in patients after a recent myocardial infarction: a randomized, placebo-controlled trial of azimilide using heart rate variability for risk stratification. Circulation. 2004;109:990–6. [DOI] [PubMed] [Google Scholar]
- 33.Grimm W, Christ M, Bach J, Muller HH and Maisch B. Noninvasive arrhythmia risk stratification in idiopathic dilated cardiomyopathy: results of the Marburg Cardiomyopathy Study. Circulation. 2003;108:2883–91. [DOI] [PubMed] [Google Scholar]
- 34.Zimetbaum PJ, Buxton AE, Batsford W, Fisher JD, Hafley GE, Lee KL, O’Toole MF, Page RL, Reynolds M and Josephson ME. Electrocardiographic predictors of arrhythmic death and total mortality in the multicenter unsustained tachycardia trial. Circulation. 2004;110:766–9. [DOI] [PubMed] [Google Scholar]
- 35.Ikeda T, Sakata T, Takami M, Kondo N, Tezuka N, Nakae T, Noro M, Enjoji Y, Abe R, Sugi K and Yamaguchi T. Combined assessment of T-wave alternans and late potentials used to predict arrhythmic events after myocardial infarction. A prospective study. J Am Coll Cardiol. 2000;35:722–30. [DOI] [PubMed] [Google Scholar]
- 36.Stein KM. Noninvasive risk stratification for sudden death: signal-averaged electrocardiography, nonsustained ventricular tachycardia, heart rate variability, baroreflex sensitivity, and QRS duration. Prog Cardiovasc Dis. 2008;51:106–17. [DOI] [PubMed] [Google Scholar]
- 37.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW and Andrews ML. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–83. [DOI] [PubMed] [Google Scholar]
- 38.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp-Channing N, Davidson-Ray LD, Fraulo ES, Fishbein DP, Luceri RM and Ip JH. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. /source>. 2005;352:225–37. [DOI] [PubMed] [Google Scholar]
- 39.Epstein AE, DiMarco JP, Ellenbogen KA, Estes NAM, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW and Sweeney MO. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities. Journal of the American College of Cardiology. 2008;51:e1–e62.18498951 [Google Scholar]
- 40.Rahimi K, Bennett D, Conrad N, Williams TM, Basu J, Dwight J, Woodward M, Patel A, McMurray J and MacMahon S. Risk prediction in patients with heart failure: a systematic review and analysis. JACC Heart Fail. 2014;2:440–6. [DOI] [PubMed] [Google Scholar]
- 41.Santangeli P, Muser D, Zado ES, Magnani S, Khetpal S, Hutchinson MD, Supple G, Frankel DS, Garcia FC, Bala R, Riley MP, Lin D, Rame JE, Schaller R, Dixit S, Marchlinski FE and Callans DJ. Acute hemodynamic decompensation during catheter ablation of scar-related ventricular tachycardia: incidence, predictors, and impact on mortality. Circ Arrhythm Electrophysiol. 2015;8:68–75. [DOI] [PubMed] [Google Scholar]
- 42.Baratto F, Pappalardo F, Oloriz T, Bisceglia C, Vergara P, Silberbauer J, Albanese N, Cireddu M, D’Angelo G, Di Prima AL, Monaco F, Paglino G, Radinovic A, Regazzoli D, Silvetti S, Trevisi N, Zangrillo A and Della Bella P. Extracorporeal Membrane Oxygenation for Hemodynamic Support of Ventricular Tachycardia Ablation. Circ Arrhythm Electrophysiol. 2016;9:e004492. [DOI] [PubMed] [Google Scholar]
- 43.Gilljam T, Haugaa KH, Jensen HK, Svensson A, Bundgaard H, Hansen J, Dellgren G, Gustafsson F, Eiskjaer H, Andreassen AK, Sjogren J, Edvardsen T, Holst AG, Svendsen JH and Platonov PG. Heart transplantation in arrhythmogenic right ventricular cardiomyopathy - Experience from the Nordic ARVC Registry. Int J Cardiol. 2017;250:201–206. [DOI] [PubMed] [Google Scholar]
- 44.Lip GY, Nieuwlaat R, Pisters R, Lane DA and Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–72. [DOI] [PubMed] [Google Scholar]
- 45.Antman EM, Cohen M, Bernink PJ, McCabe CH, Horacek T, Papuchis G, Mautner B, Corbalan R, Radley D and Braunwald E. The TIMI risk score for unstable angina/non-ST elevation MI: A method for prognostication and therapeutic decision making. JAMA. 2000;284:835–42. [DOI] [PubMed] [Google Scholar]
- 46.Eichler K, Zoller M, Tschudi P and Steurer J. Barriers to apply cardiovascular prediction rules in primary care: a postal survey. BMC Fam Pract. 2007;8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, Anand I, Maggioni A, Burton P, Sullivan MD, Pitt B, Poole-Wilson PA, Mann DL and Packer M. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation. 2006;113:1424–33. [DOI] [PubMed] [Google Scholar]
- 48.Vakil KP, Roukoz H, Tung R, Levy WC, Anand IS, Shivkumar K, Rector TS, Vaseghi M and Tholakanahalli V. Mortality prediction using a modified Seattle Heart Failure Model may improve patient selection for ventricular tachycardia ablation. Am Heart J. 2015;170:1099–104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


