Abstract
Purpose
Patients with an enlarged prostate and suspicion of prostate cancer pose a diagnostic dilemma. The prostate cancer detection rate of systematic 12-core transrectal ultrasound guided biopsy is between 30% and 40%. For prostates greater than 40 cc this decreases to 30% or less. Magnetic resonance-ultrasound fusion biopsy has shown superior prostate cancer detection rates. We defined the detection rate of magnetic resonance-ultrasound fusion biopsy in men with an enlarged prostate gland.
Materials and Methods
We retrospectively analyzed the records of patients who underwent multiparametric prostate magnetic resonance imaging followed by magnetic resonance-ultrasound fusion biopsy at our institution. Whole prostate volumes were calculated using magnetic resonance imaging reconstructions. Detection rates were analyzed with respect to age, prostate specific antigen and whole prostate volumes. Multivariable logistic regression was used to assess these parameters as independent predictors of prostate cancer detection.
Results
We analyzed 649 patients with a mean ± SD age of 61.8 ± 7.9 years and a median prostate specific antigen of 6.65 ng/ml (IQR 4.35–11.0). Mean whole prostate volume was 58.7 ± 34.3 cc. The overall detection rate of the magnetic resonance-ultrasound fusion platform was 55%. For prostates less than 40 cc the detection rate was 71.1% compared to 57.5%, 46.9%, 46.9% 33.3%, 36.4% and 30.4% for glands 40 to 54.9, 55 to 69.9, 70 to 84.9, 85 to 99.9, 100 to 114.9 and 115 cc or greater, respectively (p <0.0001). Multivariable logistic regression showed a significant inverse association of magnetic resonance imaging volume with prostate cancer detection, controlling for age and prostate specific antigen.
Conclusions
Transrectal ultrasound guided and fusion biopsy cancer detection rates decreased with increasing prostate volume. However, magnetic resonance-ultrasound fusion biopsy had a higher prostate cancer detection rate compared to that of transrectal ultrasound guided biopsy in the literature. Magnetic resonance-ultrasound fusion biopsy represents a promising solution for patients with suspicion of prostate cancer and an enlarged prostate.
Keywords: prostate, prostatic neoplasms, prostatic hyperplasia, magnetic resonance imaging, ultrasonography
According to the American Cancer Society, an estimated 240,000 men were diagnosed with PCa in 2013, resulting in a lifetime risk of 1 of 6 men.1 Most PCa is currently diagnosed by systematic 12-core TRUS guided biopsy.
The systematic TRUS guided approach has evolved since Hodge et al described it in 1989.2 Ten to 14-core extended systematic biopsy is currently considered the standard of care.3 However, it misses many small, nonpalpable and isoechoic (ultrasound invisible) lesions. This is especially relevant given the limitations of PSA screening. Furthermore, the sensitivity and specificity of systematic TRUS guided biopsy decrease as the prostate enlarges, especially for prostates greater than 40 cc.4,5 Several groups reported that the PCa detection rate is less than 30% for prostates greater than 40 cc while it is greater for smaller glands.4,6,7 It is believed that this is related to sampling error since core biopsies are obtained randomly in the prostate gland and lesions are often small, heterogeneous and not uniformly distributed. Also, as prostate volume increases, there is more gland tissue to be responsible for higher PSA and potentially harbor more PCa. However, since the prostate biopsy needle samples a fixed amount of tissue, larger prostates may possibly predispose to a smaller percent of sampling when a standardized number of cores is taken. Thus, in men with BPH PCa may go undiagnosed. These patients commonly undergo multiple biopsy attempts, extended or even saturation biopsies with the resultant increased risk of procedure related complications.8
MP-MRI is increasingly recognized as a method of detecting PCa, especially in men with an enlarged gland.9–12 Although direct in-gantry MR guided biopsy is possible, it is time-consuming, requires special equipment and is cumbersome for the patient. The patient occupies the gantry during the entire procedure, making it costly and impractical. By fusing MRI to a TRUS image it is possible for the urologist to perform MRI guided TRUS biopsies in the office setting. While MR-US fusion biopsies have an increased PCa detection rate compared to TRUS guided biopsies,13 the value of this method in patients with an enlarged prostate gland has not been specifically investigated. We determined the detection rate of MR-US fusion guided biopsies in patients with an enlarged prostate.
METHODS AND MATERIALS
Study Design
We retrospectively reviewed the records of patients in whom prostate volume was calculated by MRI in-house validated software at imaging. Patients underwent MR-US fusion biopsy at the National Cancer Institute (NCI) from January 2009 to December 2012. All patients were enrolled in an institutional review board approved protocol providing after written informed consent. Since the NCI is a referral center, all patients initially underwent screening by standardized MP-MRI, followed by MR-US fusion biopsy for one of certain reasons, including persistent clinical suspicion of prostate cancer despite prior negative biopsies or confirmation of PCa extent when the diagnosis of low grade, low volume disease was not concordant with high PSA or PSA dynamics.
We used fusion platforms that are several research iterations of the current Invivo UroNav™ system. Patients then underwent standard of care 12-core TRUS guided sextant biopsy, including 2 cores (lateral and medial) from each sextant region. No anterior or transitional directed cores were obtained. At the same setting MR-US fusion biopsy was done, which included 2 cores (axial and sagittal) from each suspicious lesion identified on MRI.13
Repeat MR-US fusion biopsies performed at our institution were excluded from analysis since the fusion platform has the capability to store and resample old biopsy sites. Since prior definitive prostate treatments might alter its volume, patients who underwent radical prostatectomy, radiation therapy, brachytherapy, cryotherapy, high intensity focused US or MR guided focal laser ablation were also excluded.
MRI Acquisition, Interpretation and Prostate Volume Measurement
All images were acquired using a 3.0 Tesla Achieva MRI scanner with a 6 or 16-channel SENSE body coil (Philips Healthcare, Cleveland, Ohio) and a BPX-30 endorectal coil (Medrad®).14 Routine precontrast axial T1-weighted imaging, triplanar (axial, coronal and sagittal) T2 with diffusion guided weighted imaging and apparent diffusion coefficient mapping, multivoxel 3-dimensional localized spectroscopy and axial 3-dimensional fast field echo dynamic contrast enhanced MRI sequences were obtained. As previously described,15 all MP-MRIs underwent centralized radiological evaluation by 2 blinded experienced genitourinary radiologists (BT and PLC) with 6 and 13 years of experience with prostate MRI, respectively. In-house validated software was used to calculate WP volumes based on the high resolution T2-weighted MR images. WP volumes were obtained by planimetric calculations by manually contouring each slice.
Patients with at least 1 suspicious lesion of any grade subsequently underwent MR-US fusion biopsy.13 Patients without MR visible lesions were not eligible to participate in our protocol and were referred to their local urologist for standard of care and followup. All pathological specimens were reviewed by a single genitourinary pathologist (MJM) with 25 years of experience.
Statistical Analysis
Data were analyzed with STATA®, version 11 and R, version 2.15.2 (R Foundation for Statistical Computing, Vienna, Austria). Descriptive statistics were used to depict patient characteristics. Univariate logistic regression was done to assess the association between the change in WP volumes in relation to age, PCa detection rates and Gleason score. Categorical variables were analyzed using the Fisher exact test or the chi-square approximation for larger numbers. We performed multivariate logistic regression controlling for age, PSA and WP volumes to assess for independent predictors of PCa. All tests were 2-tailed with significance considered at 0.05.
RESULTS
We identified 796 MR-US fusion biopsy cases between January 2009 and December 2012, of which 147 biopsy sessions were excluded from study because they were repeat MR-US fusion biopsy sessions or patients had undergone definitive prostate treatment that might have altered prostate volume. Thus, 649 patients were available for inclusion and analysis. In the entire cohort median age was 62 years and median PSA was 6.65 ng/ml. Mean WP volume was 58.7 cc (median 49).
Overall 428 patients (65.9%) had no prior biopsy or negative results on previous biopsies. Of the 649 men 528 (81.4%) underwent 1 or more prior TRUS guided biopsies elsewhere, of whom 149 (45.6%) had a gland of less than 40 cc and 379 (84.6%) had a gland of 40 cc or greater (p <0.0002). Significantly more patients with a gland of 40 cc or greater had prior negative TRUS guided biopsies (239 of 379 or 63.1% vs 68 of 149 or 45.6%, p <0.0002). Of the 307 patients (58%) without a PCa diagnosis MR-US fusion guided biopsy detected PCa in 128 (42%). There were no abnormal findings on digital rectal examination in 544 of the 649 men (83.8%). Abnormal digital rectal examination findings were not used to direct biopsy.
Prostate Volumes in Relation to MR-US Fusion Biopsy Cancer Detection
MR-US fusion biopsy results were positive in 357 of 649 patients, resulting in a 55% cancer detection rate for the entire cohort. Age and prostate volume were significantly associated with the PCa detection rate (table 1). Furthermore, when stratifying the detection rate in relation to different WP volume brackets in 15 cc increments, it was highest for prostates with a volume of less than 40 cc at 71.1% compared to 57.5%, 46.9%, 46.9%, 33.3%, 36.4% and 30.4% for prostates 40 to 54.9, 55 to 69.9, 70 to 84.9, 85 to 99.9, 100 to 114.9 and 115 cc or greater, respectively (p <0.0001). The detection rate in patients with no prior cancer diagnosis was 67.5%, 49.1%, 39.7%, 34.4%, 25.0%, 26.7% and 28.9%, and in those with prior negative biopsies it was 69.1%, 39.2%, 42.4%, 31.0%, 23.3%, 23.1% and 26.5%, respectively (each p <0.0001, fig. 1). Similar results were seen per core on detection rate analysis.
Table 1.
Prostate volume and Gleason score by age
| Age | No. Pts | Mean WP Vol (cc) |
No. Gleason Score
|
|||
|---|---|---|---|---|---|---|
| 6 | 7 | 8 or Greater | Total No. | |||
| Less than 50 | 42 | 58.3 | 11 | 7 | 3 | 21 |
| 50–59 | 208 | 52.1 | 51 | 35 | 19 | 105 |
| 60–69 | 306 | 61.6 | 76 | 51 | 46 | 173 |
| 70 or Greater | 93 | 75.1 | 16 | 17 | 25 | 58 |
|
|
|
|
|
|
||
| Totals | 649 | – | 154 | 110 | 93 | 357 |
| p Value | – | <0.0001 | – | – | – | 0.02 |
Figure 1.
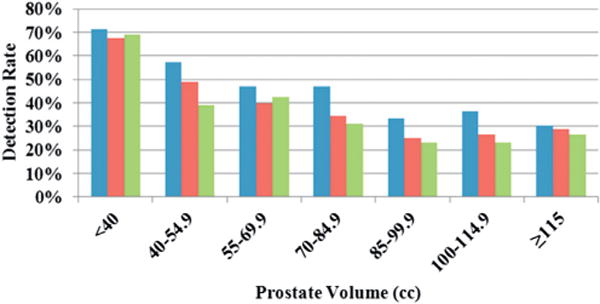
Prostate cancer detection rates overall (blue bars), and for no prior cancer diagnosis (red bars) and prior negative biopsy (green bars) by volume in 15 cc increments.
The median number of prior biopsies was 2 and 96% of patients underwent 1 to 4 prior biopsies. The MR-US fusion biopsy detection rate was 46.6%, 42.7%, 30.9% and 48.1% for 1, 2, 3 and 4 or more prior negative 12-core TRUS biopsies, respectively.
When looking at the different volume categories, the percent of patients diagnosed with high risk (Gleason 8 or greater) PCa remained similar regardless of prostate size (fig. 2). Multivariate logistic regression revealed a significant association of MRI volume with PCa detection, controlling for age and PSA (table 2). Univariate analysis showed that age and PSA were significant predictors of PCa detection. Thus, MR-US fusion technology detected high Gleason score tumors in enlarged prostates.
Figure 2.
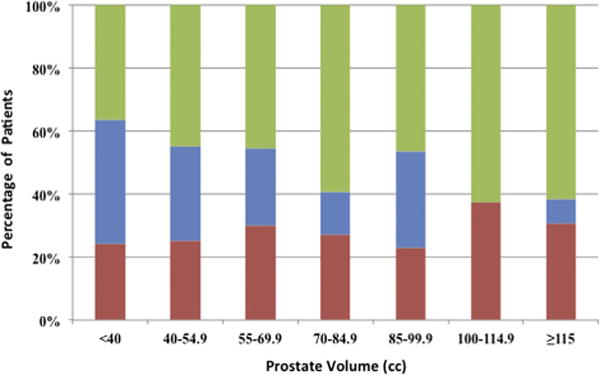
Proportion of Gleason score 6 (green bars), 7 (blue bars) and 8 or greater (red bars) in patients with cancer by prostate volume. Percent of high risk Gleason 8 or greater PCa tended to remain stable as prostate volume increased.
Table 2.
Univariate and multivariate logistic regression of cancer detection predictors
| Univariate
|
Multivariate
|
|||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| PSA/ng/ml | 1.024 (1.007–1.041) | 0.005 | 1.056 (1.031–1.082) | <0.0001 |
| Age/yr | 1.023 (1.002–1.044) | 0.03 | 1.043 (1.019–1.067) | 0.0004 |
| MRI vol/15 cc | 0.776 (0.714–0.843) | <0.0001 | 0.661 (0.595–0.734) | <0.0001 |
DISCUSSION
Since it is situated deep in the pelvis, the anatomical location of the prostate has been a hindrance to uniform, accurate tissue sampling for diagnosis. There are well recognized limitations of TRUS guided biopsy, including under sampling and missing small, isoechoic lesions. Multiple past attempts to improve the TRUS detection rate included applying a systematic sampling approach,2 laterally directed cores to sample the anterior horns of the peripheral zone,17,18 sampling the anterior region of the prostate,19 increasing the number of cores up to 20 and performing transperineal saturation biopsy.20,21 Nevertheless, the PCa detection rate of TRUS guided biopsy is between 30% and 40%.22,23
Specifically in men with clinical suspicion of PCa and an enlarged prostate the yield of TRUS guided biopsy is even lower. This patient subgroup poses a diagnostic challenge since high PSA often prompts urology referral with subsequent biopsies. Additional biopsy specimens or rearrangement of systematic sampling has not solved this dilemma because the amount of tissue sampled is still small compared to the overall prostate. Rather, the answer lies in performing biopsy directed at a lesion identified by imaging. MP-MRI is a useful tool that can aid in identifying, localizing and guiding targeted biopsies of suspicious lesions.14,24,25
Our cohort included a high percent of patients who were previously evaluated by local urologists with extended systematic TRUS guided biopsies. Reported TRUS guided biopsies identified cancer in approximately 41.9% of these patients. Despite this finding MR-US fusion biopsies detected cancer in 55.0% of the cohort. Of the men with prior negative biopsies 42% were found to harbor PCa by fusion technology. Notably, a greater percent of men with a larger prostate (40 cc or greater) had undergone 1 or more previous biopsies with negative results compared to men with a smaller gland (table 1).
When stratified by increasing WP volume, the PCa detection rate decreased. It was highest for glands greater than 40 cc (71.1%) and lowest for glands 115 cc or greater (30.4%). This pattern was also observed for TRUS guided biopsy since the detection rate was confounded by WP size. Even with a high mean WP ± SD volume of 58.7 ± 34.3 cc the overall PCa detection rate in our series was substantially greater than that of TRUS guided biopsy and comparable to rates in studies using similar fusion technology.13,26,27
The literature is replete with analyses of prostate gland volume and biopsy detection rates (fig. 3). The detection rate of TRUS guided biopsy for prostate glands greater than 40 cc is considerably lower than for glands smaller than 40 cc. A detection rate ranging from 40% in glands less than 34 cc to 24% in glands greater than 64 cc was reported by Ung et al in a cohort of 750 consecutive patients with a median WP volume of 45 cc.4 Yoon et al similarly noted a yield ranging from 30% in glands less than 40 cc to 26.9% for a WP volume of 40 cc or greater in a cohort of 474 patients.7 Even when performing 20-core TRUS guided biopsy, Werahera et al noted a 25.8% PCa detection rate in men with a prostate of greater than 50 cc.6 Our series demonstrated a much higher PCa detection rate of 47.8% for glands 40 cc or greater compared to these historical controls. Importantly, the detection rate of high risk PCa using MR-US fusion did not decrease as a function of prostate volume. In other words, MR-US fusion biopsy is less likely to miss high risk PCa (fig. 2).
Figure 3.
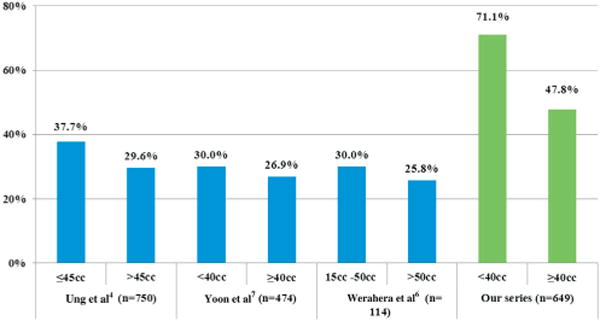
Previous and current PCa detection rates by prostate volume on TRUS guided (blue bars) and MR-US fusion (green bars) biopsy.
These findings emphasize the value of fusion technology to detect and risk stratify cancer on high resolution imaging and accurately target visualized lesions for biopsy. Groups reported that the overall detection rate for MR-US fusion biopsy technology was between 50% and 60%.13,26,27 Others reported a 34% rate in patients with prior negative prostate biopsies.28 We believe that MR-US fusion biopsy is of value for all prostate volumes but especially relevant for enlarged prostates (40 cc or greater) since TRUS guided biopsy in this scenario has an even lower detection rate.
As MR-US fusion technology matures, multiple efforts will be directed toward improving and incorporating it into current treatment guidelines.
Our study has several limitations, including its retrospective nature, heterogeneous population since some patients underwent prior prostate biopsy, and representation of data from a single referral institution. These single institution findings should be further validated in large, prospective, multi-institutional studies. Also, aspects of fusion technology are that are affected by prostate volume and might lead to lower yields. For example, enlarged prostate glands may be more prone to operator dependent deformation during the biopsy procedure, resulting in inaccurate fusion. An endorectal coil used during MP-MRI could also lead to prostate deformation, resulting in inaccurate volume measurements and ultimately fusion. As a precaution, adjustments were routinely made to endorectal coil filling at the beginning of the scan to minimize gland deformation. In addition, fusion technology accuracy per spatial resolution is reported to be approximately 4 to 5 mm, potentially missing lesions 3 mm or less in diameter. However, these small lesions most likely do not represent clinically significant prostate cancer.29,30 Lastly, MP-MRI and MR-US fusion technology requires a high initial investment and technical support. As the technology becomes more readily available, costs may decrease.
CONCLUSIONS
MR-US fusion biopsy represents a promising solution for patients with clinical suspicion of PCa complicated by an enlarged prostate secondary to BPH changes. MR-US fusion guided biopsy shows superior PCa detection compared to extended sextant TRUS guided biopsy. In men with clinical suspicion of PCa and an enlarged prostate due to BPH MR-US fusion biopsy should be considered a first line diagnostic approach.
Abbreviations and Acronyms
- BPH
benign prostatic hyperplasia
- MP
multiparametric
- MR
magnetic resonance
- MRI
MR imaging
- PCa
prostate cancer
- TRUS
transrectal US
- US
ultrasound
- WP
whole prostate
Footnotes
Study received institutional review board approval.
References
- 1.American Cancer Society. Prostate Cancer. United States of America: 2013. Available at http://www.cancer.org/cancer/prostatecancer/detailedguide/prostate-cancer-key-statistics. Accessed February 27, 2012. [Google Scholar]
- 2.Hodge KK, McNeal JE, Terris MK, et al. Random systematic versus directed ultrasound guided transrectal core biopsies of the prostate. J Urol. 1989;142 doi: 10.1016/s0022-5347(17)38664-0. [DOI] [PubMed] [Google Scholar]
- 3.Heidenreich A, Bellmunt J, Bolla M, et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Uro. 2011;59:61. doi: 10.1016/j.eururo.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 4.Ung JO, San Francisco IF, Regan MM, et al. The relationship of prostate gland volume to extended needle biopsy on prostate cancer detection. J Urol. 2003;169:130. doi: 10.1016/S0022-5347(05)64052-9. [DOI] [PubMed] [Google Scholar]
- 5.Karakiewicz PI, Bazinet M, Aprikian AG, et al. Outcome of sextant biopsy according to gland volume. Urology. 1997;49:55. doi: 10.1016/S0090-4295(96)00360-3. [DOI] [PubMed] [Google Scholar]
- 6.Werahera PN, Sullivan K, Rosa FG, et al. Optimization of prostate cancer diagnosis by increasing the number of core biopsies based on gland volume. Int J Clin Exp Pathol. 2012;5:892. [PMC free article] [PubMed] [Google Scholar]
- 7.Yoon BI, Shin TS, Cho HJ, et al. Is it effective to perform two more prostate biopsies according to prostate-specific antigen level and prostate volume in detecting prostate cancer? Prospective study of 10-core and 12-core prostate biopsy. Urology. 2012;9:491. [PubMed] [Google Scholar]
- 8.de la Taille A, Antiphon P, Salomon L, et al. Prospective evaluation of a 21-sample needle biopsy procedure designed to improve the prostate cancer detection rate. Urology. 2003;61:1181. doi: 10.1016/s0090-4295(03)00108-0. [DOI] [PubMed] [Google Scholar]
- 9.Isebaert S, Van den Bergh L, Haustermans K, et al. Multiparametric MRI for prostate cancer localization in correlation to whole-mount histopa-thology. J Magn Reson Imaging. 2013;37:1392. doi: 10.1002/jmri.23938. [DOI] [PubMed] [Google Scholar]
- 10.Natarajan S, Marks LS, Margolis DJ, et al. Clinical application of a 3D ultrasound-guided prostate biopsy system. Urol Oncol. 2011;29:334. doi: 10.1016/j.urolonc.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh AK, Kruecker J, Xu S, et al. Initial clinical experience with real-time transrectal ultrasonography-magnetic resonance imaging fusion-guided prostate biopsy. BJU Int. 2008;101:841. doi: 10.1111/j.1464-410X.2007.07348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dickinson L, Ahmed HU, Allen C, et al. Magnetic resonance imaging for the detection, localisation, and characterisation of prostate cancer: recommendations from a European consensus meeting. Eur Urol. 2011;59:477. doi: 10.1016/j.eururo.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Pinto PA, Chung PH, Rastinehad AR, et al. Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. J Urol. 2011;186:1281. doi: 10.1016/j.juro.2011.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turkbey B, Mani H, Shah V, et al. Multiparametric 3T prostate magnetic resonance imaging to detect cancer: histopathological correlation using prostatectomy specimens processed in customized magnetic resonance imaging based molds. J Urol. 2011;186:1818. doi: 10.1016/j.juro.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yerram NK, Volkin D, Turkbey B, et al. Low suspicion lesions on multiparametric magnetic resonance imaging predict for the absence of high-risk prostate cancer. BJU Int. 2012;110:E783. doi: 10.1111/j.1464-410X.2012.11646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turkbey B, Huang R, Vourganti S, et al. Age-related changes in prostate zonal volumes as measured by high-resolution magnetic resonance imaging (MRI): a cross-sectional study in over 500 patients. BJU Int. 2012;110:1642. doi: 10.1111/j.1464-410X.2012.11469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stamey T. Making the most out of six systematic sextant biopsies. Urology. 1995;45:2. doi: 10.1016/s0090-4295(95)96168-2. [DOI] [PubMed] [Google Scholar]
- 18.Ochiai A, Babaian RJ. Update on prostate biopsy technique. Curr Opin Urol. 2004;14:157. doi: 10.1097/00042307-200405000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Chon CH, Lai FC, McNeal JE, et al. Use of extended systematic sampling in patients with a prior negative prostate needle biopsy. J Urol. 2002;167:2457. [PubMed] [Google Scholar]
- 20.Zaytoun OM, Moussa AS, Gao T, et al. Office based transrectal saturation biopsy improves prostate cancer detection compared to extended biopsy in the repeat biopsy population. J Urol. 2011;186:850. doi: 10.1016/j.juro.2011.04.069. [DOI] [PubMed] [Google Scholar]
- 21.Pinkstaff DM, Igel TC, Petrou SP, et al. Systematic transperineal ultrasound-guided template biopsy of the prostate: three-year experience. Urology. 2005;65:735. doi: 10.1016/j.urology.2004.10.067. [DOI] [PubMed] [Google Scholar]
- 22.Welch HG, Fisher ES, Gottlieb DJ, et al. Detection of prostate cancer via biopsy in the Medicare-SEER population during the PSA era. J Natl Cancer Inst. 2007;99:1395. doi: 10.1093/jnci/djm119. [DOI] [PubMed] [Google Scholar]
- 23.Eichler K, Hempel S, Wilby J, et al. Diagnostic value of systematic biopsy methods in the investigation of prostate cancer: a systematic review. J Urol. 2006;175:1605. doi: 10.1016/S0022-5347(05)00957-2. [DOI] [PubMed] [Google Scholar]
- 24.McNeal JE. Origin and evolution of benign prostatic enlargement. Invest Urol. 1978;15:340. [PubMed] [Google Scholar]
- 25.McNeal JE. The zonal anatomy of the prostate. Prostate. 1981;2:35. doi: 10.1002/pros.2990020105. [DOI] [PubMed] [Google Scholar]
- 26.Marks L, Young S, Natarajan S. MRI-ultrasound fusion for guidance of targeted prostate biopsy. Curr Opin Urol. 2013;23:43. doi: 10.1097/MOU.0b013e32835ad3ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hadaschik BA, Kuru TH, Tulea C, et al. A novel stereotactic prostate biopsy system integrating pre-interventional magnetic resonance imaging and live ultrasound fusion. J Urol. 2011;186:2214. doi: 10.1016/j.juro.2011.07.102. [DOI] [PubMed] [Google Scholar]
- 28.Sonn GA, Chang E, Natarajan S, et al. Value of targeted prostate biopsy using magnetic resonance-ultrasound fusion in men with prior negative biopsy and elevated prostate-specific antigen. Eur Urol. doi: 10.1016/j.eururo.2013.03.025. Epub ahead of print March 17, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harnden P, Naylor B, Shelley MD, et al. The clinical management of patients with a small volume of prostatic cancer on biopsy: what are the risks of progression? A systematic review and meta-analysis. Cancer. 2008;112:971. doi: 10.1002/cncr.23277. [DOI] [PubMed] [Google Scholar]
- 30.Hoang AN, Volkin D, Yerram NK, et al. Image guidance in the focal treatment of prostate cancer. Curr Opin Urol. 2012;22:328. doi: 10.1097/MOU.0b013e32835482cc. [DOI] [PMC free article] [PubMed] [Google Scholar]


