Abstract
ENaC-mediated sodium reabsorption in the collecting duct (CD) is a critical determinant of urinary sodium excretion. Existing evidence suggest direct stimulatory actions of Angiotensin II (Ang II) on ENaC in the CD, independently of the aldosterone- mineralocorticoid receptor (MR) signaling. Deletion of the major renal AT1 receptor isoform, AT1aR, decreases blood pressure and reduces ENaC abundance despite elevated aldosterone levels. The mechanism of this insufficient compensation is not known. Here, we used patch clamp electrophysiology in freshly isolated split-opened CDs to investigate how AT1aR dysfunction compromises functional ENaC activity and its regulation by dietary salt intake. Ang II had no effect on ENaC activity in CDs from AT1aR −/− mice suggesting no complementary contribution of AT2 receptors. We next found that AT1aR deficient mice had lower ENaC activity when fed with low (<0.01% Na+) and regular (0.32% Na+) but not with high (~2% Na+) salt diet, when compared to the respective values obtained in Wild type (WT) animals. Inhibition of AT1R with losartan in wild-type animals reproduces the effects of genetic ablation of AT1aR on ENaC activity arguing against contribution of developmental factors. Interestingly, manipulation with aldosterone-MR signaling via deoxycosterone acetate (DOCA) and spironolactone had much reduced influence on ENaC activity upon AT1aR deletion. Consistently, AT1aR −/− mice have a markedly diminished MR abundance in cytosol. Overall, we conclude that AT1aR deficiency elicits a complex inhibitory effect on ENaC activity by attenuating ENaC Po and precluding adequate compensation via aldosterone cascade due to decreased MR availability.
Keywords: aldosterone, distal renal tubule, Mas, mineralocorticoid receptors, Na+ reabsorption
1|. INTRODUCTION
Sodium reabsorption by the kidney is central for maintaining sodium balance and setting blood pressure (Crowley & Coffman, 2012). Sodium transport in the distal renal tubule segments, including the collecting duct (CD), plays a major role in matching urinary salt excretion with day-to-day alterations in dietary NaCl intake (Pearce et al., 2015; Staruschenko, 2012). Activity of the epithelial Na+ channel (ENaC) on the apical plasma membrane of principal cells governs electrogenic Na+ influx at these sites (Pearce et al., 2015). Overactivation of ENaC contributes significantly to the pathology of salt- sensitive hypertension rodents and humans (Bhalla & Hallows, 2008; Hummler, 1999; Kakizoe et al., 2009). Likewise, mutations in genes encoding β- and γ-ENaC subunits resulting in gain-of-function of the channel underlie Liddle syndrome associated with high blood pressure (Hansson, Nelson-Williams, et al., 1995; Hansson, Schild, et al. 1995; Shimkets et al., 1994). Deficient ENaC activity leads to renal salt wasting and hypotension, as exemplified in patients with pseudohy- poladosteronism type I (Chang et al., 1996; Schild, 1996).
ENaC is a critical end-effector of the renin-angiotensin- aldosterone system (RAAS). This allows inverse relation between ENaC activity in the distal tubule and dietary salt intake in order to fine-tune systemic Na+ balance largely caused by respective changes in circulating levels of aldosterone and Angiotensin II (Ang II) (Crowley & Coffman, 2012; Zaika, Mamenko, Staruschenko, & Pochynyuk, 2013). Through its binding to the mineralocorticoid receptors (MR) in principal cells, the principal ENaC activator, aldosterone exerts a plethora of stimulatory actions on the channel, including augmented synthesis, trafficking to the apical plasma membrane, etc. (Eaton, Malik, SaxeNa, Al-Khalili, & Yue, 2001; Masilamani, Kim, Mitchell, Wade, & Knepper, 1999; Pacha, Frindt, Antonian, Silver, & Palmer, 1993; Stockand, 2002). In addition to promoting aldosterone secretion from the zoNa glomerulosa of adrenal gland, Ang II is capable of directly increasing ENaC activity non-redundantly to aldosterone (Mamenko, Zaika, Ilatovskaya, Staruschenko, & Pochynyuk, 2012; Peti-Peterdi, Warnock, & Bell, 2002; Sun, Yue, & Wang, 2012). The physiological actions of Ang II are mediated by ATi and AT2 receptors (ATiR and AT2R, respectively). AT1R is a product of two separate genes encoding two subtypes, Namely AT1aR and AT1bR in rodents, with the former being the only isoform expressed in the kidney (Ito et al., 1995). AT1aR activation causes antinatriuresis and vasoconstriction, whereas AT2R antagonize many of the biological effects of AT1aR and cause vasodilation, Natriuresis, and prostaglandin release (Berry, Touyz, Dominiczak, Webb, & Johns, 2001; Kaschina & Unger, 2003). Both receptors are abundantly expressed in the renal tubule, although AT1aR expression predominates (Carey, Wang, & Siragy, 2000; Miyata, Park, Li, & Cowley, 1999; Ozono et al., 1997).
Mice with global AT1aR deletion develop urinary salt wasting and have lower blood pressure at the baseline, which is further exacerbated during the state of dietary salt deficiency (Ito et al., 1995; Oliverio, Best, Smithies, & Coffman, 2000). Interestingly, AT1aR −/− mice have intact regulation of aldosterone secretion during volume depletion, most likely due to the abundant expression of AT1bR isoform in the adrenal gland (Burson, Aguilera, Gross, & Sigmund, 1994). Despite this, AT1aR deficiency has profound effects on ENaC subunit expression. Decreased a-subunit abundance is paralleled by increased β and β subunit expression upon salt restriction, while high salt intake lowers α-subunit levels without any changes to β and γ abundance (Brooks, Allred, Beutler, Coffman, & Knepper, 2002). Moreover, mice with targeted deletion of AT1aR in principal cells only, while having a normal blood pressure independently of salt intake, fail to increase αENaC expression during chronic Ang II infusion (Chen et al., 2016). It is unknown why aldosterone-MR signaling is not potent to correct ENaC expression in the absence of AT1aR. Furthermore, since all three subunits are equally essential to assemble into a functional ENaC, it is hard to predict how discrete and opposite changes in expression of ENaC subunits are translated into changes in ENaC activity during the state of AT1aR deficiency.
In this study, we undertook direct measurements of ENaC activity with patch clamp in freshly isolated split-opened CDs to investigate how disruption of the stimulatory Ang II input compromises channel function during adaptations to dietary salt intake. We found that direct Ang II actions on single channel ENaC activity depend solely on AT1aR. Deletion of the receptor had a moderate inhibitory effect during regular salt intake and this defect was exacerbated during dietary salt deficiency. Changes in ENaC activity were much reduced in AT1aR −/− mice upon stimulation and inhibition of MR receptors with DOCA and spironolactone, respectively. Furthermore, MR availability was significantly reduced in mutant mice on both low and high salt intake providing a mechanism for insufficient compensation by aldosterone. Overall, our results demonstrate indispensable role of Ang II - AT1aR mechanisms for adequate regulation of ENaC activity in the CD by dietary salt intake.
2 |. MATERIALS AND METHODS
2.1 |. Reagents and animals
All chemicals and materials were from Sigma (St. Louis, MO), VWR (Radnor, PA), and Tocris (Ellisville, MO) unless noted otherwise and were at least of reagent grade. For experiments, male C57BL/6J mice (Charles River Laboratories, Wilmington, MA) and B6.129P2-Agtr1t- m1Unc/j (AT1aR −/−, JAX strain #002682) 6–10 weeks old, were used. Animal use and welfare adhered to the NIH Guide for the Care and Use of Laboratory Animals following protocols reviewed and approved by the Animal Care and Use Committees of the University of Texas Health Science Center at Houston.
2.2 |. Research diets and treatments
To examine effects of salt intake, animals were provided diets containing nominally free (<0.01% Na+, TD.90228), regular (0.32% Na+, 7012), and high (−2% Na+, TD.92034) salt for 1 week. All diets were nutritionally balanced and were purchased from Envigo (Madison, WI). Spironolactone USP (30 mg/kgBW; Amneal Pharmaceutical) and losartan (10 mg/kgBW) were added to drinking water for 7 days. As necessary, mice were injected with Deoxycorticosterone acetate (DOCA) for three consecutive days (2.4 mg/injection/animal) prior to the experimentation similarly to what we have done previously (Mamenko, Zaika, Doris, & Pochynyuk, 2012; Mamenko et al., 2013, 2017).
2.3 |. Tissue isolation
The procedure for isolation of the CDs suitable for electrophysiology followed previously published protocols (Mamenko, Zaika, Doris, et al., 2012; Mamenko et al., 2013; Mironova, Bugay, Pochynyuk, Staruschenko, & Stockand, 2013; Prieto et al., 2017). Briefly, mice were sacrificed by CO2 administration followed by cervical dislocation and kidneys were removed immediately. Kidneys were cut into thin slices (<1mm) with slices placed into ice-cold physiological saline solution (PSS) containing (in mmol/L) 150 NaCl, 5 KCl, 1 CaCl2, 2 MgCl2, 5 glucose and 10 HEPES (pH 7.35). CDs were visually identified by their morphological features (pale color; coarse surface and, in some cases, bifurcations) and were mechanically isolated from kidney slices by micro-dissection using watchmaker forceps under a stereomicroscope. Isolated CDs were attached to 5 × 5 mm cover glasses coated with poly-L-lysine. A cover-glass containing a CD was placed in a perfusion chamber mounted on an inverted Nikon Eclipse Ti microscope and perfused with PSS at room temperature. CDs were split-opened with two sharpened micropipettes, controlled with different micromanipulators, to gain access to the apical membrane. The tubules were used within 2 hr of isolation. For each experimental condition, CDs from at least four different mice were analyzed.
2.4 |. Single channel recordings in isolated collecting ducts
ENaC activity in principal cells was determined in cell-attached patches on the apical membrane made under voltage-clamp conditions (−Vp =− 60 mV) using standard procedures (Mamenko, Zaika, Doris, et al., 2012; Mamenko, Zaika, Ilatovskaya, et al., 2012; Mamenko et al., 2013; Prieto et al., 2017). Current recordings were made in a permanently perfused bath (1.5 ml/min). Recording pipettes had resistances of 8‒10 megaOhms. Typical bath and pipette solutions were (in mmol/L): 150 NaCl, 5 mM KCl, 1 CaCl2, 2 MgCl2, 5 glucose and 10 HEPES (pH 7.35); and 140 LiCl, 2 MgCl2 and 10 HEPES (pH 7.35), respectively. Gap-free single channel current data from gigaOhm seals were acquired and analyzed with Axopatch 200B (Molecular Devices, Sunnyvale, CA) patch clamp amplifier interfaced via a Digidata 1440 (Molecular Devices) to a PC running the pClamp 10.4 suite of software (Molecular Devices). Currents were low-pass filtered at 100 Hz with an eight-pole Bessel filter (Warner Instruments, Hamden, CT). Events were inspected visually prior to acceptance. ENaC activity was analyzed over a span of 60–120 s for each experimental condition. Using previously described analysis (Mamenko, Zaika, Ilatovskaya, et al., 2012), we can reliably (p < 0.05) estimate the maximal number of functional ENaC in a patch using this time span. Channel activity in individual patches, defined as NPo, was calculated using the following equation: NPo = (t1 + 2t2 + … + ntn), where N and Po are the number of ENaC in a patch and the mean open probability of these channels, respectively, and tn is the fractional open time spent at each of the observed current levels. Po was calculated by dividing NPo by the number of active channels within a patch as defined by all-point amplitude histograms. To estimate total ENaC activity (fNPo) under a particular physiological condition, we normalized NPo to the frequency of observing patches with at least one active channel (f = number of patches with active channels/total number of patches). To assess functional ENaC expression for each experimental condition, the mean of number of active channels within a patch was corrected to f. For representation, current traces were corrected for a slow baseline drifts as necessary.
2.5 |. Western blotting
Immediately after dissection, kidneys were frozen in liquid nitrogen and stored there for further use. Prior to experimentation the kidneys were homogenized in three volumes of ice-cold hypotonic lysis buffer containing 50 mmol/L Tris, 1% Triton X-100, 5 mmol/L EDTA (pH = 7.4) supplemented with 1mM PMSF and 2 mg/ml protease inhibitor cocktail (Complete mini, Roche Diagnostics, Germany). Homogenates were centrifuged at 1,000g for 15 min at +4C to separate cytosolic fraction. Protein concentration in the Homogenates was determined with a Bradford assay. The samples (25 μg/lane) were separated on 9% polyacrylamide gels at 150 V for 1hr 15 min and transferred to nitrocellulose membranes at 100 V for 1 hr 45 min. The membranes were blocked for 1 hr at RT in 5% nonfat milk in TBS-T (150 mmol/L NaCl, 50mmol/L Tris-HCl pH = 7.4, 0.1% Tween 20). Subsequently the membranes were probed with anti-MR primary rabbit antibodies (1:1000, ab64457, Abcam, Cambridge, UK) followed by peroxidase-conjugated goat anti-rabbit secondary antibodies (1:20000, Bio-Rad, Hercules, CA) for 1 hr at RT. The membranes were re-probed with anti-β-actin (1:5000, ab8227, Abcam) primary rabbit antibodies and peroxidase-conjugated goat anti-rabbit secondary antibodies (1:20000, Bio-Rad). When total MR expression was assessed, MR band intensities were normalized to the intensities of the corresponding β-actin bands.
2.6 |. Statistics
All summarized data are reported as mean ± SEM. All statistical comparisons were made using one-way ANOVA. A p-value of less than 0.05 was considered significant.
3 |. RESULTS
3.1 |. AT1a receptors (AT1aR) are central in regulation of ENaC activity by Angiotensin cascade
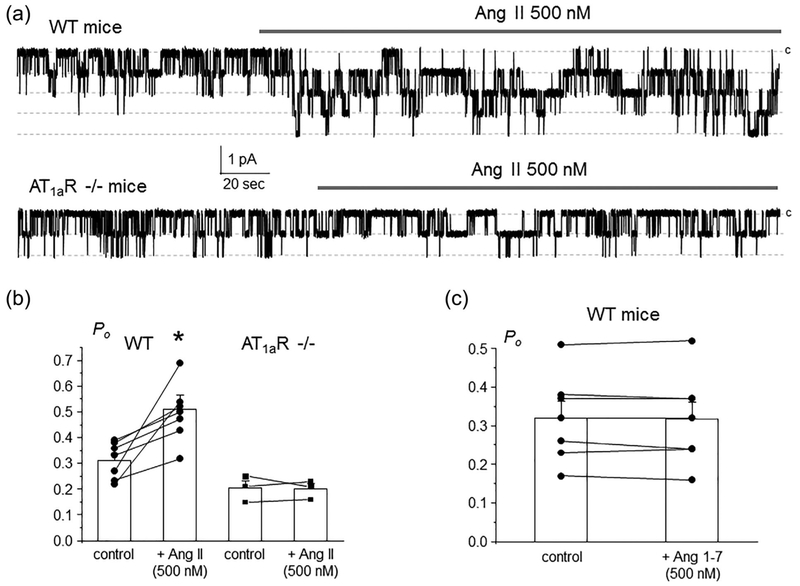
We and others reported previously that Ang II exhibits non-redundant stimulatory actions on ENaC activity beyond those elicited by the classical aldosterone-MR cascade (Mamenko, Zaika, Ilatovskaya, et al., 2012; Mamenko et al., 2013). Consistently, acute application of Ang II (500 nM) increased single channel ENaC activity in freshly isolated split-opened CDs from WT mice (Figure 1a, upper trace). Figure 1b contains the summary graph of stimulatory Ang II actions on ENaC in paired patch clamp experiments. We further took advantage of mice lacking AT1a receptors, as a model of the defective Ang II signaling. Ang II (500 nM) had no measurable effect on single channel ENaC activity in freshly isolated split-opened CDs, as shown on a representative continuous patch clamp experiment in Figure 1a (bottom trace) and the summary graph in Figure 1b. This also argues against a complementary role of AT2R in controlling ENaC function by Ang II cascade. Furthermore, application of Ang 1–7 (500 nM) did not affect ENaC activity in Wild type (WT) mice, as shown in the summary graph in Figure 1c suggesting no contribution of the alternative (vasoprotective) branch of the RAAS and specifically Mas receptors in regulation of ENaC-mediated Na+ reabsorption in the CD. Overall, our results in Figure 1 provide a direct evidence of the exclusive role of AT1aR in conveying stimulatory Ang II signal to ENaC.
FIGURE 1.
AT1aRs are central for regulation of ENaC activity in the CD by Angiotensin II. (a) Representative continuous current traces from cell-attached patches monitoring ENaC activity in the control and during application of 500 nM Ang II (shown with a bar on the top) in a split-opened CD from WT (upper trace) and AT1aR −/− (bottom trace) mice. The patches were held at a test potential of Vh = − Vp = − 60 mV. Inward currents are downward. Dashed lines indicate the respective current state with a c denoting the closed state. (b) Summary graph of ENaC Po changes in response to Ang II from paired patch clamp experiments in WT and AT1aR −/− mice similar to that shown in Figure 1a.*significant increase versus WT control. (c) Summary graph of ENaC Po changes in response to stimulation of Mas receptors with 500 nM Ang 1–7 in split-opened CDs from Wild type mice
3.2 |. Decreased basal ENaC activity and blunted response to dietary salt restriction in AT1aR−/− mice
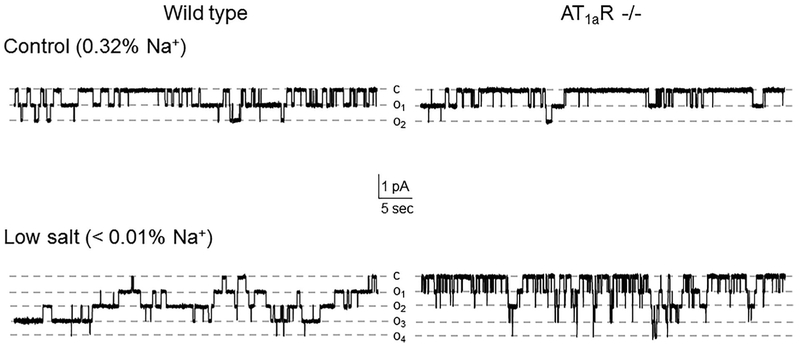
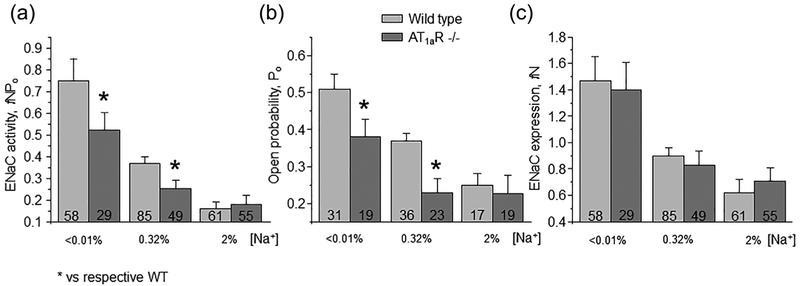
We next investigated how disruption of Ang II signal to ENaC in AT1aR −/− mice affects channel function upon variations in dietary salt intake. As shown in representative current traces in Figure 2 (top panels) and the summary graph in Figure 3a, ENaC activity in split- opened CDs is significantly lower in mutant mice kept on regular (0.32% Na+) salt intake. Detailed analysis of single channel ENaC activity revealed that this difference was due to reduced open probability (Po, Figure 3b), while the average number of active channels per patch (fN) remained similar (Figure 3c).
FIGURE 2.
ENaC activity is reduced in AT1aR −/− mice. Representative current traces of ENaC activity in split-opened CDs isolated from Wild type (left column) and AT1aR −/− (right column) mice kept on control (0.32% Na+, top row) and low (<0.01% Na+, bottom row) salt intake for 1 week. Dashed lines indicate the respective current state with oi indicating the number of simultaneously opened channels; c denotes the closed non-conducting state
FIGURE 3.
AT1aR −/− deletion reduces ENaC salt-sensitivity. Summary graphs of averaged total ENaC activity, fNPo (a); ENaC open probability, Po (b); and number of active channels fN (c) in wild type (light grey) and AT1aR −/− (dark gray) mice kept on low (<0.01% Na+), control (0.32% Na+), and high (~2% Na+) salt diet for one week, respectively. At least four different animals were tested for each condition. *significant decrease versus respective wild type values
We further compared ENaC activity in AT1aR −/− and WT mice subjected to a low (<0.01% Na+) and high (~2% Na+) salt intake for one week. Dietary sodium restriction increased ENaC activity in both animal strains (Figure 2, bottom panel). However, mutant mice had consistently lower total ENaC activity (Figure 3A) and Po (Figure 3b) compared to the respective values in WT mice under these conditions. In contrast, dietary salt loading negated all differences in ENaC activity and open probability between AT1aR −/− and WT mice (Figure 3). Overall, we concluded that disruption of Ang II signaling blunts regulation of ENaC activity in the CD by dietary salt intake due to significantly lower Po changes.
3.3 |. Inhibition of AT1R with losartan decreases ENaC activity similarly to the receptor deletion
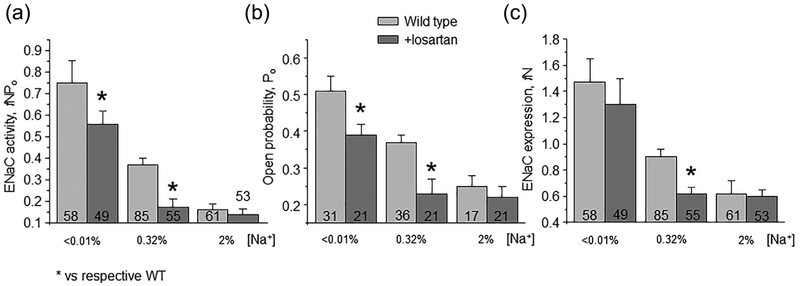
We next compared the effects of AT1aR deletion and receptor antagonism in WT mice on ENaC function to exclude putative compensatory effects due to chronic gene editing. For this, WT animals were fed with low, regular and high salt diets and concomitantly supplied with AT1R blocker losartan (10 mg/kgBW) in drinking water. We similarly observed a reduced ENaC activity in animals kept on regular and low but not high salt intake (Figure 4a). The effect was attributed again to the reduced Po (Figure 4b), the number of functional ENaC channels was moderately lower in mice on the regular salt regimen (Figure 4c). Overall, we concluded the AT1aR deletion and AT1R blockade elicit comparable reduction of ENaC activity suggesting minimal compensation. In addition, Figures 3 and 4 suggest a critical role of Ang II signaling in controlling ENaC activity during regular and low salt intake.
FIGURE 4.
Inhibition of AT1R with losartan reduces ENaC activity on low and control salt intake. Summary graphs of averaged total ENaC activity, fNPo (a); ENaC open probability, Po (b); and number of active channels fN (c) in Wild type mice kept on low (<0.01% Na+), control (0.32% Na+), and high (~2% Na+) salt diets for one week in the absence (light gray) and presence (dark gray) of concomitant treatment with AT1R antagonist losartan (10 mg/kgBW) in drinking water, respectively. At least four different animals were tested for each condition. *significant decrease versus respective untreated conditions
3.4 |. AT1aR deletion compromises regulation of ENaC by aldosterone-MR cascade
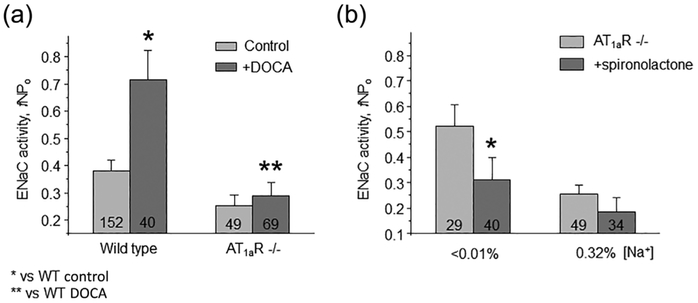
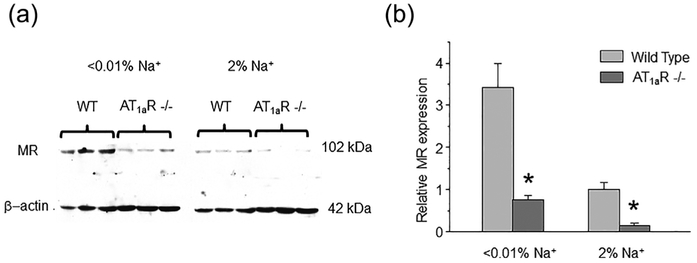
We next tested the effect of maximal activation of MR receptors after repetitive DOCA injections on ENaC function in WT and AT1aR −/− mice. As expected, we detected a marked increase in (fNPo) from 0.38 ± 0.04 to 0.71 ± 0.11 in WT, which was blunted in AT1aR −/− mice: 0.25 ± 0.03 to 0.29 ± 0.04, not statistically significant (Figure 5a). We further probed basal activity of MR-aldosterone axis in AT1aR knockouts with MR antagonist, spironolactone (30 mg/kgBW). As summarized in Figure 5b, spironolactone failed to produce a significant inhibitory effect on ENaC fNPo under regular salt intake decreasing total channel activity from 0.26 ± 0.03 to 0.18 ± 0.05. Impaired response of MR signaling cascade to DOCA despite its low basal activity is indicative of reduced MR abundance. Indeed, Western blot analysis detected a markedly reduced expression of cytosolic MR in AT1aR −/− kidney Homogenates, as compared to the WT mice on both low salt (<0.01% Na+) and high salt (~2% Na+) diets (Figure 6a). At the same time, we observed an upregulation of MR levels upon dietary salt restriction in both strains (Figure 6b). This is consistent with a greater inhibitory effect of spironolactone on single channel ENaC activity in AT1aR −/− mice kept on a low salt diet, where ENaC activity was significantly diminished from 0.52 ± 0.08 to 0.31 ± 0.08 (Figure 5b). Overall, we concluded that AT1aR deletion compromises regulation of ENaC by aldosterone cascade during variations in dietary salt intake at least partially due to reduction in MR availability in the cytosol for stimulation by aldosterone.
FIGURE 5.
AT1aR deletion impairs regulation of ENaC activity by mineralocorticoid receptor (MR) signaling. (a) Summary graph of averaged total ENaC activity, fNPo in Wild type and AT1aR −/− mice in the control and after systemic Deoxycorticosterone acetate (DOCA) injections for 3 consecutive days to maximally stimulate MR. *significant increase versus Wild type control; **significant decrease versus Wild type + DOCA. (b) Summary graph comparing averaged total ENaC activity, fNPo in AT1aR −/− mice kept on low (<0.01% Na+) and control (0.32% Na+) salt intake in the absence (light gray) and presence (dark grey) of concomitant treatment with MR antagonist spironolactone (30 mg/kgBW) in drinking water, respectively. *significant decrease versus AT1aR −/− low salt
FIGURE 6.
Decreased MR availability in AT1aR −/− mice during different salt intake. (a) Representative Western blot from whole kidney lysates (cytosolic fraction) of WT and AT1aR −/− mice (each line reflects individual animal) kept on low (<0.01% Na+) and high (~2% Na+) salt intake for 1 week simultaneously probed with anti-MR and anti-β actin antibodies. (b) Summary graph comparing MR expression normalized to the respective intensity of β-actin in WT and AT1aR −/− mice on the same conditions as described in Figure 6a. *significant decrease versus respective Wild type values
4 |. DISCUSSION
In this study, we employed AT1aR −/− mice as a model deficient of Ang II regulation of ENaC to investigate how this affects regulation of channel activity by dietary salt intake and aldosterone-MR signaling. We report here that AT1aR deletion diminishes ENaC activity at the baseline and blunts upregulation of channel activity by low salt diet. Detailed patch clamp analysis in split-opened CDs revealed that the defect was primarily attributed to altered ENaC gating, Namely decreased open probability, Po. In addition, we found that the lack of AT1aR also interferes with stimulatory input to ENaC from aldosterone-MR cascade.
Using direct measurements of single channel activity, we found that AT1aR ablation precludes regulation of ENaC by Ang II (Figures 1a and 1b). In addition to promoting its anti-Natriuretic actions via AT1R, Ang II is also a potent ligand for AT2R, which generally oppose the effects mediated by AT1R (Berry et al., 2001; Kaschina & Unger, 2003). AT2R deficiency exacerbates Ang II-dependent antinatriuresis (Siragy, Inagami, Ichiki, & Carey, 1999), suggesting AT2R effects of tubular sodium reabsorption. However, this seems to be associated rather with effects in proximal tubule having high levels of AT2R and specifically the sodium hydrogen antiporter 3, NHE-3 and Na+, K+ ATPase (Carey, 2017). We and others showed a dominant role of AT1Rin regulation of sodium reabsorption in the distal tubule, whereas application of AT2R agonist, CGP42112 had no effect on ENaC (Mamenko, Zaika, Ilatovskaya, et al., 2012; Peti-Peterdi et al., 2002; Sun et al., 2012). This is in agreement with the current finding that Ang II has neither stimulatory nor inhibitory effects on ENaC, when AT1aR are deleted (Figure 1). It should be noted though that we used exclusively males in our patch clamp studies. Substantial differences in expression/function of distal tubule sodium transporters between males and females at the baseline as well as in response to salt loading have been reported (Gillis & Sullivan, 2016; Veiras et al., 2017). Specifically, female rats have increased thiazide-sensitive sodium chloride cotransporter (NCC) and ENaC abundance to partially compensate for the diminished volume reabsorption in the proximal tubule (Veiras et al., 2017). While these differences were more pronounced in rats than in mice, further careful examination of sex polymorphism in Ang II-dependent regulation of ENaC is granted. In this regard, AT2R expression and its hypotensive effects are more pronounced in females (Hilliard et al., 2011).
Recent studies also provided a circumferential evidence of a stronger hypotensive and Natriuretic effect of azilsartan, an AT1R antagonist that was linked to augmented renal ACE2 mRNA and reduced αENaC pointing to an upregulation of the alternative (vasoprotective) branch of the renin-angiotensin-aldosterone system, the ACE2/Ang-(1–7)/Mas cascade in addition to AT1R blockade after antagonist treatment (Iwanami et al., 2014). To our disappointment, we did not observe a measurable inhibitory effect of Ang 1–7 in split- opened CDs (Figure 1C) suggesting no apparent role of Mas receptors in regulation of ENaC activity in the distal tubule at least in mice on a regular salt intake.
We found that deletion of AT1aR blunts salt-sensitivity of ENaC in the CD, explicitly, the dynamic range in which ENaC activity is changed during variations of dietary NaCl from high to low (Figures 2 and 3). ENaC activity was similar between salt loaded Wild type and mutant mice when circulating Ang II levels are low, whereas channel activity was significantly reduced in AT1aR −/− mice during dietary salt restriction (associated with high Ang II levels) indicating lower CD sodium reabsorption and higher urinary sodium excretion in this case. This could potentially contribute to the worsened hypotensive phenotype in these mice on low NaCl diet (Oliverio et al., 2000). On the contrary, Coffman’s group reported recently that mice with targeted deletion of AT1aR in principal cells, while exhibiting a blunted hypertensive response to Ang II infusions, have blood pressure indistinguishable from controls on all tested salt intakes (Chen et al., 2016). Since the employed cre-lox strategy does not guarantee the complete receptor ablation and no positive functional control was provided, it is possible that the residual receptor expression is sufficient to modulate ENaC activity during variations in dietary salt intake. In contrast, we showed no effect of Ang II on ENaC justifying no functional AT1aR in the current mutant model. Alternatively, intact AT1aRin intercalated cells of the CD might play a compensatory role by stimulating electroneutral NaCl reabsorption (Leviel et al., 2010) upon AT1aR deletion in principal cells.
We also report here that the observed defect in ENaC activity in the CD upon deletion of AT1aR is due to decreased channel open probability on regular and low salt intake (Figures 2 and 3). This is consistent with previous observations that Ang II is potent to increase ENaC Po and this effect persists in DOCA- and spironolactone-treated animals, suggesting its aldosterone-independent Nature (Mamenko, Zaika, Ilatovskaya, et al., 2012; Sun et al., 2012). We also previously showed that this Ang II-dependent regulation of ENaC Po may contribute to regulation of channel activity by dietary salt intake. Thus, inhibition of MR signaling with spironolactone precluded salt- dependent regulation of the number of active ENaC measured with patch clamp in split-opened CDs, but failed to affect changes in open probability (Mamenko et al., 2013). In agreement, we observe that the changes in Po are substantially reduced in AT1aR knockouts, while we did not detect significant changes in the average number of active channels per patch between mutant and Wild type mice (Figure 3c). Recall, AT1aR −/− animals retain the ability to increase aldosterone secretion in response to volume depletion most likely due to contribution of unaffected AT1bR isoform in the adrenal gland (Burson et al., 1994). Interestingly, Western blot detection of ENaC in the kidney of AT1aR −/− mice showed reduced abundance of α, but increased abundance of cleaved, most active form of y (Brooks et al., 2002). Since 1:1:1 stoichiometry for α:β:γ is required to assemble into a functional ENaC (Stockand, Staruschenko, Pochynyuk, Booth, & Silverthorn, 2008), this should lead to the overall reduction in the channel numbers with a scarcity being a limiting factor. Since we did not observe differences in the number of active channels between WT and mutant mice, the most likely explanation is increased channel residence on the apical plasma membrane in AT1aR −/− driven by actions of aldosterone. Indeed, we previously observed cumulative inhibitory effects of MR inhibitor spironolactone and AT1R blocker losartan on ENaC activity in the CD (Mamenko et al., 2013). We also found that inhibition of AT1R with losartan recapitulated the major phenotype with regard to decreased ENaC activity in the CD (Figure 4). This argues against major contribution of the developmental factors associated with receptor deficiency. This is consistent with a previous report showing no significant differences in body and kidney weights in AT1aR −/− and Wild type mice suggesting that the receptor is not essential for the normal organogenesis of the kidney (Oliverio et al., 1998).
One of the important results is that AT1aR deficiency cannot be properly compensated by aldosterone-MR cascade. We show that maximal stimulation of MR with DOCA has little stimulatory effect on ENaC activity in AT1aR −/− mice (Figure 5a). This is consistent with reduced availability of the receptors in cytosol, detected by Western blot (Figure 6). While to a much lower extent, dietary salt restriction increases the number of available MR in mutants resulting in the upregulation of ENaC activity. This observation is corroborated by a greater effect of MR inhibitor spironolactone on ENaC activity during this condition (Figure 5b). While the exact mechanism of this MR deficiency requires further investigation beyond the scope of the current manuscript, Ang II was shown to potentiate aldosterone binding to MR in intercalated cells of the CD by removing a specific S843 phosphorylation precluding ligand binding (Shibata et al., 2013). This was proposed to account for discrete adaptive responses to hypokalemia (when only aldosterone is present) and hypovolemia (both aldosterone and Ang II are elevated). Furthermore, inhibition of Ang II pathway with angiotensin converting enzyme (ACE) inhibitor, captopril reduced cardiac fibrosis not only by decreasing Ang II production but also by attenuating the aldosterone-signaling pathway by decreasing the expression of MR receptors (de Resende, Kauser, & Mill, 2006). In summary, our findings support the overarching view that cooperative but yet non-redundant actions of aldosterone and Ang II signaling cascades are necessary to regulate electrolyte transport in principal and intercalated cells of the CD during variations in dietary intake. With respect to ENaC function, deficiency of Ang II signal cannot be properly compensated by aldosterone-MR cascade, as demonstrated in the current study.
ACKNOWLEDGMENTS
This research was supported by NIH-NIDDK DK095029 (to O. P.), AHA 17GRNT33660488 (to O. P.), AHA 15SDG25550150 (to M. M.), and ASN Ben J. Lipps Research Fellowship (to V. T.). Nabila Boukelmoune (MD Anderson Cancer Center) and Eric Madden (UTHSC at Houston) are recognized for the technical assistance with Western blot.
Funding information
NIH-NIDDK, Grant number: DK095029; American Heart Association, Grant numbers: 15SDG25550150, 17GRNT33660488
REFERENCES
- Berry C, Touyz R, Dominiczak AF, Webb RC, & Johns DG (2001). Angiotensin receptors: Signaling, vascular pathophysiology, and interactions with ceramide. American Journal of Physiology Heart and Circulatory Physiology, 281(6), H2337–H2365. [DOI] [PubMed] [Google Scholar]
- Bhalla V, & Hallows KR (2008). Mechanisms of ENaC regulation and clinical implications. Journal of the American Society of Nephrology: JASN, 19(10), 1845–1854. [DOI] [PubMed] [Google Scholar]
- Brooks HL, Allred AJ, Beutler KT, Coffman TM, & Knepper MA (2002). Targeted proteomic profiling of renal Na+ transporter and channel abundances in Angiotensin II type 1a receptor knockout mice. Hypertension, 39(2 Pt 2), 470–473. [DOI] [PubMed] [Google Scholar]
- Burson JM, Aguilera G, Gross KW, & Sigmund CD (1994). Differential expression of angiotensin receptor 1A and 1B in mouse. The American Journal of Physiology, 267(2 Pt 1), E260–E267. [DOI] [PubMed] [Google Scholar]
- Carey RM (2017). Blood pressure and the renal actions of AT2 receptors. Current Hypertension Reports, 19(3), 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey RM, Wang ZQ, & Siragy HM (2000). Role of the Angiotensin type 2 receptor in the regulation of blood pressure and renal function. Hypertension, 35(1 Pt 2), 155–163. [DOI] [PubMed] [Google Scholar]
- Chang SS, Grunder S, Hanukoglu A, Rosler A, Mathew PM, Hanukoglu I, … Lifton RP (1996). Mutations in subunits of the epithelial sodium channel cause salt wasting with hyperkalaemic acidosis, pseudohypoaldosteronism type 1. Nature Genetics, 12(3), 248–253. [DOI] [PubMed] [Google Scholar]
- Chen D, Stegbauer J, Sparks MA, Kohan D, Griffiths R, Herrera M,… Coffman TM (2016). Impact of angiotensin type 1A receptors in principal cells of the collecting duct on blood pressure and hypertension. Hypertension, 67(6), 1291–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley SD, & Coffman TM (2012). Recent advances involving the renin-angiotensin system. Experimental Cell Research, 318(9), 1049–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Resende MM, Kauser K, & Mill JG (2006). Regulation of cardiac and renal mineralocorticoid receptor expression by captopril following myocardial infarction in rats. Life Sciences, 78(26), 3066–3073. [DOI] [PubMed] [Google Scholar]
- Eaton DC, Malik B, SaxeNa NC, Al-Khalili OK, & Yue G (2001). Mechanisms of aldosterone’s action on epithelial Na+ transport. Journal of Membrane Biology, 184(3), 313–319. [DOI] [PubMed] [Google Scholar]
- Gillis EE, & Sullivan JC (2016). Sex differences in hypertension: Recent advances. Hypertension, 68(6), 1322–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson JH, Nelson-Williams C, Suzuki H, Schild L, Shimkets R, Lu Y, Canessa C,… Lifton RP (1995). Hypertension caused by a truncated epithelial sodium channel gamma subunit: Genetic heterogeneity of Liddle syndrome. Nature Genetics, 11(1), 76–82. [DOI] [PubMed] [Google Scholar]
- Hansson JH, Schild L, Lu Y, Wilson TA, Gautschi I, Shimkets R, … Lifton RP (1995). A de novo missense mutation of the beta subunit of the epithelial sodium channel causes hypertension and Liddle syndrome, identifying a proline-rich segment critical for regulation of channel activity. Proceedings of the National Academy of Sciences of the United States of America, 92(25), 11495–11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard LM, Nematbakhsh M, Kett MM, Teichman E, Sampson AK, Widdop RE,… Denton KM (2011). Gender differences in pressure- Natriuresis and renal autoregulation: Role of the Angiotensin type 2 receptor. Hypertension, 57(2), 275–282. [DOI] [PubMed] [Google Scholar]
- Hummler E (1999). Implication of ENaC in salt-sensitive hypertension. J Steroid Biochem Mol Biol, 69(1–6), 385–390. [DOI] [PubMed] [Google Scholar]
- Ito M, Oliverio MI, Mannon PJ, Best CF, Maeda N, Smithies O, & Coffman TM (1995). Regulation of blood pressure by the type 1A Angiotensin II receptor gene. Proceedings of the National Academy of Sciences of the United States of America, 92(8), 3521–3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwanami J, Mogi M, Tsukuda K, Wang XL, Nakaoka H, Ohshima K, … Horiuchi, M. (2014). Role of angiotensin-converting enzyme 2/angiotensin-(1–7)/Mas axis in the hypotensive effect of azilsartan. Hypertension Research: Official Journal of the Japanese Society of Hypertension, 37(7), 616–620. [DOI] [PubMed] [Google Scholar]
- Kakizoe Y, Kitamura K, Ko T, Wakida N, Maekawa A, Miyoshi T, … Tomita K (2009). Aberrant ENaC activation in Dahl salt-sensitive rats. Journal of Hypertension, 27(8), 1679–1689. [DOI] [PubMed] [Google Scholar]
- Kaschina E, & Unger T (2003). Angiotensin AT1/AT2 receptors: Regulation, signalling and function. Blood Pressure, 12(2), 70–88. [DOI] [PubMed] [Google Scholar]
- Leviel F, Hubner CA, Houillier P, Morla L, El Moghrabi S, Brideau G, … Eladari D (2010). The Na+-dependent chloride-bicarbonate exchanger SLC4A8 mediates an electroneutral Na+ reabsorption process in the renal cortical collecting ducts of mice. The Journal of Clinical Investigation, 120(5), 1627–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamenko M, Zaika O, Doris PA, & Pochynyuk O (2012). Salt- dependent inhibition of epithelial Na+ channel-mediated sodium reabsorption in the aldosterone-sensitive distal nephron by bradykinin. Hypertension, 60(5), 1234–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamenko M, Zaika O, Ilatovskaya DV, Staruschenko A, & Pochynyuk O (2012). Angiotensin II increases activity of the epithelial Na+ channel (ENaC) in distal nephron additively to aldosterone. The Journal of Biological Chemistry, 287(1), 660–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamenko M, Zaika O, Prieto MC, Jensen VB, Doris PA, Navar LG, & Pochynyuk O (2013). Chronic Angiotensin II infusion drives extensive aldosterone-independent epithelial Na+ channel activation. Hypertension, 62(6), 1111–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamenko MV, Boukelmoune N,Tomilin VN, Zaika OL, Jensen VB, O’Neil RG, & Pochynyuk OM (2017). The renal TRPV4 channel is essential for adaptation to increased dietary potassium. Kidney International, 91(6), 1398–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masilamani S, Kim GH, Mitchell C, Wade JB, & Knepper MA (1999). Aldosterone-mediated regulation of ENaC alpha, beta, and gamma subunit proteins in rat kidney. The Journal of Clinical Investigation, 104(7), R19–R23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironova E, Bugay V, Pochynyuk O, Staruschenko A, & Stockand JD (2013). Recording ion channels in isolated, split-opened tubules. Methods in Molecular Biology, 998, 341–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata N, Park F, Li XF, & Cowley AW Jr. (1999). Distribution of angiotensin AT1 and AT2 receptor subtypes in the rat kidney. The American Journal of Physiology, 277(3 Pt 2), F437–F446. [DOI] [PubMed] [Google Scholar]
- Oliverio MI, Best CF, Smithies O, & Coffman TM (2000). Regulation of sodium balance and blood pressure by the AT1A receptor for angiotensin II. Hypertension, 35(2), 550–554. [DOI] [PubMed] [Google Scholar]
- Oliverio MI, Madsen K, Best CF, Ito M, Maeda N, Smithies O, & Coffman TM (1998). Renal growth and development in mice lacking AT1A receptors for angiotensin II. The American Journal of Physiology, 274(1 Pt 2), F43–F50. [DOI] [PubMed] [Google Scholar]
- Ozono R, Wang ZQ, Moore AF, Inagami T, Siragy HM, & Carey RM (1997). Expression of the subtype 2 angiotensin (AT2) receptor protein in rat kidney. Hypertension, 30(5), 1238–1246. [DOI] [PubMed] [Google Scholar]
- Pacha J, Frindt G, Antonian L, Silver RB, & Palmer LG (1993). Regulation of Na channels of the rat cortical collecting tubule by aldosterone. The Journal of General Physiology, 102(1), 25–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce D, Soundararajan R, Trimpert C, Kashlan OB, Deen PM, & Kohan DE (2015). Collecting duct principal cell transport processes and their regulation. Clinical Journal of the American Society of Nephrology, 10(1), 135–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peti-Peterdi J, Warnock DG, & Bell PD (2002). Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT1 receptors. Journal of the American Society of Nephrology: JASN, 13(5), 1131–1135. [DOI] [PubMed] [Google Scholar]
- Prieto MC, Reverte V, Mamenko M, Kuczeriska M, Veiras LC, Rosales CB, … Gonzalez AA (2017). Collecting duct prorenin receptor knockout reduces renal function, increases Na+ excretion and mitigates renal responses in ang II induced hypertensive mice. American Journal of Physiology Renal Physiology: Ajprenal, 313(6), F1243–F1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schild L (1996). The ENaC channel as the primary determinant of two human diseases: Liddle syndrome and pseudohypoaldosteronism. Nephrologie, 17(7), 395–400. [PubMed] [Google Scholar]
- Shibata S, Rinehart J, Zhang J, Moeckel G, Castaneda-Bueno M, Stiegler AL, … Lifton RP (2013). Mineralocorticoid receptor phosphorylation regulates ligand binding and renal response to volume depletion and hyperkalemia. Cell Metabolism, 18(5), 660–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimkets RA, Warnock DG, Bositis CM, Nelson-Williams C, Hansson JH, Schambelan M, … Lifton RP (1994). Liddle’s syndrome: Heritable human hypertension caused by mutations in the beta subunit of the epithelial sodium channel. Cell, 79(3), 407–414. [DOI] [PubMed] [Google Scholar]
- Siragy HM, Inagami T, Ichiki T, & Carey RM (1999). Sustained hypersensitivity to angiotensin II and its mechanism in mice lacking the subtype-2 (AT2) angiotensin receptor. Proceedings of the National Academy of Sciences of the United States of America, 96(11), 6506–6510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staruschenko A (2012). Regulation of transport in the connecting tubule and cortical collecting duct. Comprehensive Physiology, 2(2), 1541–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockand JD (2002). New ideas about aldosterone signaling in epithelia. Am J Physiol Renal Physiol, 282(4), F559–F576. [DOI] [PubMed] [Google Scholar]
- Stockand JD, Staruschenko A, Pochynyuk O, Booth RE,&Silverthorn DU (2008). Insight toward epithelial Na+ channel mechanism revealed by the acid-sensing ion channel 1 structure. IUBMB Life, 60(9), 620–628. [DOI] [PubMed] [Google Scholar]
- Sun P, Yue P, & Wang WH (2012). Angiotensin II stimulates epithelial sodium channels in the cortical collecting duct of the rat kidney. American Journal of Physiology Renal Physiology, 302(6), F679–F687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiras LC, Girardi ACC, Curry J, Pei L, Ralph DL, Tran A, … McDonough AA (2017). Sexual dimorphic pattern of renal transporters and electrolyte homeostasis. Journal of the American Society of Nephrology, 28(12), 3504–3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaika O, Mamenko M, Staruschenko A, & Pochynyuk O (2013). Direct activation of ENaC by Angiotensin II: recent advances and new insights. Current Hypertension Reports, 15(1), 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]