Abstract
Objectives:
To explore the comparative effectiveness of partial nephrectomy (PN), radical nephrectomy (RN), ablative therapies (ablation) and active surveillance (AS) for small renal masses (SRMs; tumor diameter ≤4.0 cm) in the domains of survival, renal function, and quality of life (QOL) using the prospectively-maintained Delayed Intervention and Surveillance for Small Renal Masses (DISSRM) Registry.
Methods:
Estimated glomerular filtration rate (eGFR) was calculated from creatinine values to determine renal function. QOL was measured using the Short Form 12 (SF-12) questionnaire. The Kaplan-Meier method and Cox proportional hazards regression were used for survival analysis. The mixed effects model was used for renal function and QOL analysis.
Results:
Of 638 patients, 231 (36.2%) chose PN, 41 (6.4%) RN, 27 (4.2%) ablation, and 339 (53.1%) AS. CSS at 7 years was 98.8% in PN patients and 100% in all other groups. OS at 7 years was 87.9%, 90.2%, 83.5%, and 66.1% in PN, RN, ablation, and AS patients, respectively. The OS rate was significantly worse in AS than other groups and likely attributable to older age and increased comorbidities. The eGFR was lowest in RN patients but comparable in all other groups. QOL was lowest in AS patients due to lower physical health scores, but mental health scores were similar in all groups.
Conclusions:
With excellent oncologic outcomes in all groups, nephron-sparing approaches like PN and ablation are preferred over RN when intervention is indicated for SRMs. AS is a reasonable option for select patients, given the comparable oncologic and mental health outcomes.
Keywords: Active Surveillance, Renal Surgery, Small Renal Mass, Renal Function, Quality of Life
Introduction
Nephron-sparing strategies have increasingly gained traction for the management of localized renal masses to reduce the risk of chronic kidney disease (CKD) and its associated comorbidities [1, 2]. As such, the American Urological Association (AUA) issued guideline statements in 2017 for the management of localized renal masses suspicious for cancer [3]. The guideline committee reviewed the evidence of four contemporary approaches in the management of clinical stage T1a tumors (diameter ≤4.0 cm): partial nephrectomy (PN), radical nephrectomy (RN), ablative therapies (ablation), and active surveillance (AS). While oncologic outcomes remain the priority of management, additional endpoints such as renal function and quality of life (QOL) were recognized as important considerations.
A 2016 meta-analysis compared the oncologic and functional outcomes of these management strategies in a head-to-head fashion [4]. While the strength of evidence was often low to moderate for direct comparisons of interventional approaches (PN, RN, or ablation), the paucity of data regarding AS rendered analysis of this modality largely insufficient to draw conclusions. As a result, the AUA guidelines committee and a systematic review of research gaps acknowledged a need to prioritize prospective studies comparing the effectiveness of these various approaches in patients with localized renal masses [3, 5].
The Delayed Intervention and Surveillance for Small Renal Masses (DISSRM) Registry is a multi-institutional, prospective trial established in 2009 to evaluate the safety of AS compared to primary intervention for patients with small renal masses (SRMs), defined as clinical stage T1a tumors [6]. While early reports have compared oncologic and functional outcomes between AS and intervention, none have evaluated if differences exist among the three different interventional approaches [6-8]. Thus, the present study aims to evaluate the comparative effectiveness of PN, RN, ablation, and AS on the outcomes of survival, renal function, and QOL in a prospective cohort.
Methods
Study Design and Data Collection
Since January 1, 2009, the multi-institutional DISSRM Registry has prospectively enrolled patients ≥18 years old who choose to undergo AS or intervention for a clinically localized, solid, contrast-enhancing renal mass ≤4.0 cm in diameter on axial imaging. Full study design, selection criteria, and surveillance protocol have been previously described [6, 9]. This study is approved by the Institutional Review Board.
Management options for SRMs included PN, RN, ablation, and AS. Patients choosing AS were monitored with repeat imaging every 6-12 months, and intervention was recommended upon evidence of progression, which was defined as a growth rate >0.5 cm/year or tumor diameter >4 cm. Alternatively, patients could choose to pursue delayed intervention at their own discretion.
The estimated glomerular filtration rate (eGFR) was calculated using the CKD-EPI equation from creatinine values obtained at enrollment, 6 months, and 12 months [10]. The validated Short Form 12 (SF-12) questionnaire was administered to estimate self-reported QOL at enrollment, 6 and 12 months, and every year thereafter [7, 11]. The SF-12 is comprised of a physical component summary (PCS) and mental component summary (MCS).
Statistical Analysis
Baseline patient and tumor characteristics were compared across the four management groups using ANOVA with Bonferroni correction and the χ2 test. Survival outcomes were estimated using the Kaplan-Meier approach and compared using the log-rank test. The Cox proportional hazards regression was used to identify variables associated with survival. The mixed effects model was used for eGFR and QOL analysis, where linear regression was performed for continuous outcomes and logistic regression was performed for binary outcomes. Statistical significance was set at α=0.05, and analyses were performed using Stata version 13.1 (StataCorp, College Station, TX).
Results
Patient Characteristics
At the time of administrative censoring, 638 patients were enrolled in the DISSRM Registry: 299 patients (46.9%) chose intervention whereas 339 (53.1%) chose AS. Within the intervention group, 231 (36.2% of entire cohort) received PN, 41 (6.4%) received RN, and 27 (4.2%) received ablation. Baseline patient characteristics are detailed in Supplementary Table 1. Notably, PN patients were younger and had fewer comorbidities than all other groups. Furthermore, RN patients were more likely to have high complexity tumors whereas ablation patients had more low complexity tumors, as defined by the RENAL nephrometry score. There were a total of 46 patients (13.6% of AS patients) who underwent delayed intervention: 29 (63.0% of delayed intervention patients) PN, 5 (10.9%) RN, and 12 (26.1%) ablation. Over half of the delayed intervention cases were performed electively, with no indication of clinical progression [Supplementary Figure 1]. Of the patients who demonstrated progression, the vast majority were found based on a growth rate >0.5 cm/year. The pathological outcomes of patients who underwent intervention or biopsy are listed in Supplementary Table 2. The majority of patients who underwent primary or delayed intervention had low-grade (Fuhrman Grade 1-2) clear cell renal cell carcinoma (RCC). Among active surveillance patients who underwent biopsy but did not undergo subsequent intervention, the most common histological finding was oncocytoma.
Survival Outcomes
The median follow-up time was 3.0 years [IQR 1.6-5.0], and 158 patients (24.7%) were followed for ≥5 years. There were 40 deaths (6.3%) in the registry. Two deaths were due to high-grade (Fuhrman Grade 3-4) RCC, and both patients developed metastasis after undergoing PN as primary intervention. Cancer-specific survival (CSS) was 98.8% in the PN group and 100% in all other groups through 7 years of follow-up [Figure 1A]. There was no statistically significant difference in CSS among the four management groups (log-rank P=0.5).
Figure 1.
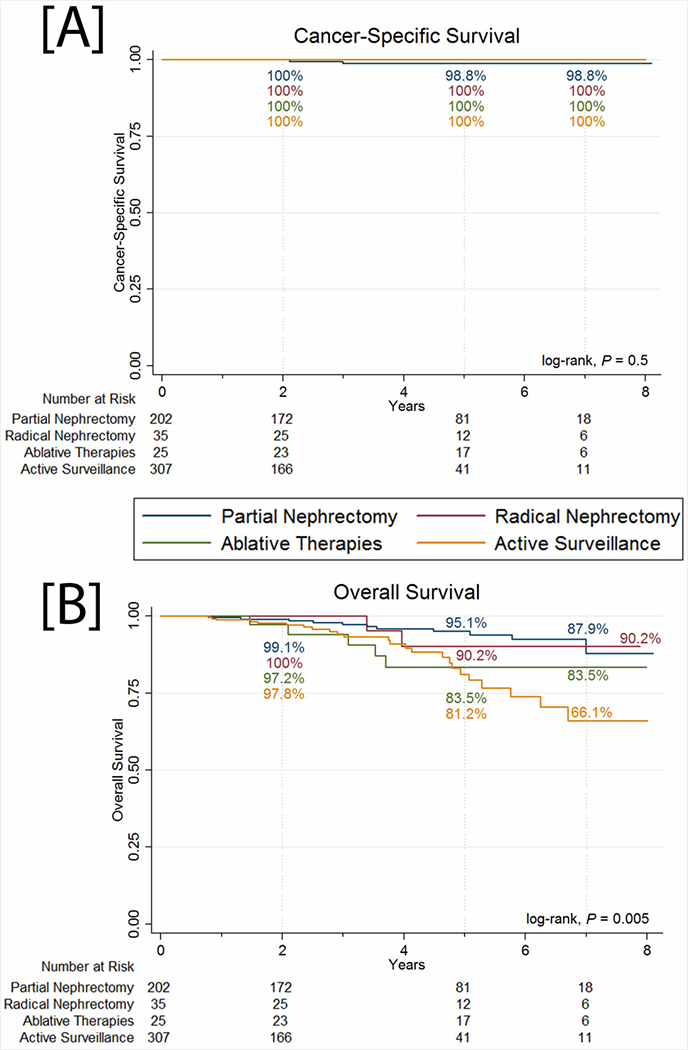
[A] Cancer-specific survival and [B] overall survival of patients in the DISSRM Registry.
Overall survival (OS) was significantly lower in the AS group (66.1% at 7 years) compared to all three intervention groups (log-rank P=0.005) [Figure 1B]. Among the PN, RN, and ablation groups, where OS was 87.9%, 90.2%, and 83.5% at 7 years, respectively, there was no significant difference in OS (log-rank P=0.3). Unadjusted regression of intervention type on the outcome of all-cause mortality demonstrated an increased hazard of death with AS (HR 3.27, 95% CI 1.55-6.92, P=0.002) [Table 1]. However, adjustment with age, sex, and Charlson Comorbidity Index (CCI) demonstrated that AS as a management strategy was not independently associated with worse OS compared to any of the intervention groups (HR 1.66, 95% CI 0.72-3.81, P=0.2). Instead, increased age and CCI were associated with a higher hazard of all-cause mortality.
Table 1.
Unadjusted and adjusted Cox proportional hazards regression of variables associated with the outcome of all-cause mortality.
| Unadjusted (Univariable) Analysis | Adjusted (Multivariable) Analysis | |||
|---|---|---|---|---|
| Characteristics | Hazard Ratio [95% CI] | P-value | Hazard Ratio [95% CI] | P-value |
| Management | ||||
| Partial Nephrectomy | Referent | – | Referent | – |
| Radical Nephrectomy | 1.26 [0.28-5.75] | 0.8 | 0.74 [0.16-3.51] | 0.7 |
| Ablative Therapies | 2.45 [0.77-7.82] | 0.1 | 2.24 [0.68-7.44] | 0.2 |
| Active Surveillance | 3.27 [1.55-6.92] | 0.002 | 1.66 [0.72-3.81] | 0.2 |
| Age (per year) | 1.07 [1.04-1.10] | <0.001 | 1.05 [1.02-1.09] | 0.004 |
| Male | 1.38 [0.71-2.69] | 0.3 | 1.38 [0.70-2.72] | 0.4 |
| Charlson Comorbidity Index | ||||
| 0 | Referent | – | Referent | – |
| 1-3 | 3.39 [1.59-7.23] | 0.002 | 2.99 [1.38-6.45] | 0.005 |
| ≥4 | 5.78 [1.78-18.82] | 0.004 | 4.26 [1.28-14.15] | 0.02 |
Renal Function Outcomes
The eGFR was calculated from 762 creatinine values obtained from 381 patients, including 140 PN patients, 38 RN patients, 27 ablation patients, and 176 AS patients [Supplementary Table 3]. Comparisons of the management groups showed that RN patients demonstrate worse absolute renal function than PN and ablation patients at enrollment, 6 months, and 12 months [Figure 2]. RN patients also had lower eGFR than AS patients at 6 and 12 months. Finally, AS patients demonstrated lower eGFR than PN patients at enrollment and 6 months.
Figure 2.
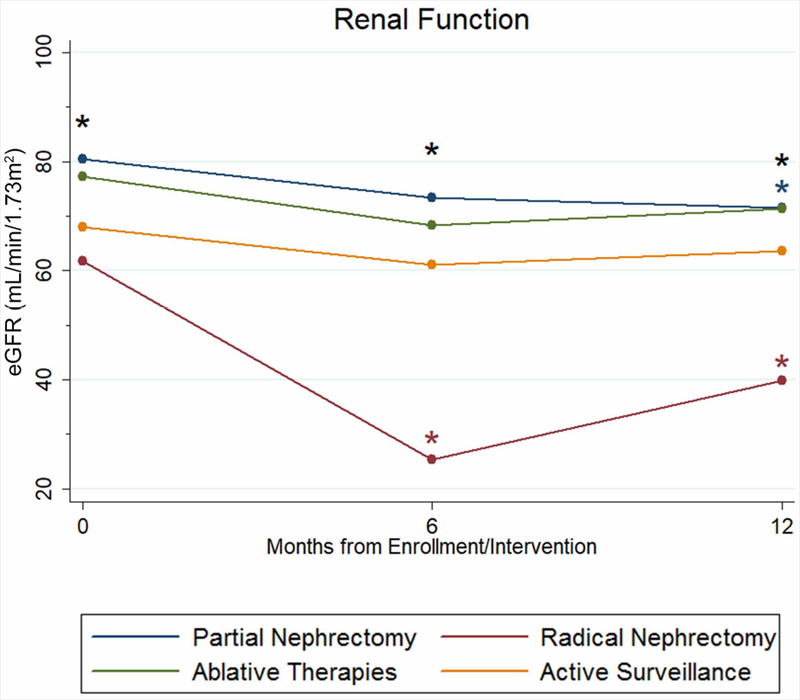
Estimated glomerular filtration rate (eGFR) of patients in the DISSRM Registry. Black stars indicate the presence of a significant difference between two or more groups at that time point. The blue star indicates a significant difference in eGFR for partial nephrectomy patients at that time point when compared to enrollment. Red stars indicate a significant difference in eGFR for radical nephrectomy patients at that time point when compared to enrollment.
Within management groups, renal function also changed significantly over time. PN patients had, on average, a decline of 8.9 mL/min/1.73m2 in eGFR at 12 months when compared to enrollment (P=0.03). RN patients demonstrated a decline in eGFR of 36.5 mL/min/1.73m2 at 6 months and 21.9 mL/min/1.73m2 at 12 months when compared to enrollment (P<0.001 and P=0.05, respectively), but the interval improvement was not statistically significant (14.6 mL/min/1.73m2, P=0.5).
Multivariable mixed effects linear regression models evaluated the relative change in eGFR. The impact of adjusting for baseline eGFR is demonstrated by inclusion of this factor in Model 2, which attenuated the drop in eGFR for RN compared to PN patients from −11.82 mL/min/1.73m2 to −4.52 mL/min/1.73m2 [Table 2]. Notably, each additional month of follow-up was associated with an overall decline in eGFR. Model 2 also demonstrated a statistically significant decrease in eGFR for PN patients compared to AS patients. Finally, higher baseline eGFR was associated with higher final eGFR, but the associations with age, race, and comorbidities were no longer significant after adjustment for baseline eGFR.
Table 2.
[A] Multivariable mixed effects linear regression for variables associated with the estimated glomerular filtration rate (eGFR). Change in eGFR is in units of mL/min/1.73m2. [B] Multivariable mixed effects logistic regression for variables associated with an eGFR <45 mL/min/1.73m2 (CKD stage ≥3B). For both regressions, two models were created: Model 1 does not include baseline eGFR and Model 2 includes baseline eGFR.
| [A] Linear Regression | [B] Logistic Regression | |||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |||||
| Characteristics | Change in eGFR [95% CI] | P-value | Change in eGFR [95% CI] | P-value | Odds Ratio [95% CI] | P-value | Odds Ratio [95% CI] | P-value |
| Management | ||||||||
| Partial Nephrectomy | Referent | – | Referent | – | Referent | – | Referent | – |
| Radical Nephrectomy | −11.82 [−19.63-−4.00] | 0.003 | −4.52 [−7.14-−1.89] | 0.001 | 56.48 [7.76-411.18] | <0.001 | 10.92 [3.08-38.77] | <0.001 |
| Ablative Therapies | 1.70 [−4.30-7.70] | 0.6 | −0.25 [−3.28-2.77] | 0.9 | 2.43 [0.30-19.61] | 0.4 | 1.72 [0.39-7.53] | 0.5 |
| Active Surveillance | 2.41 [−1.22-6.03] | 0.2 | 2.30 [0.51-4.09] | 0.01 | 3.68 [0.97-13.96] | 0.06 | 0.97 [0.37-2.51] | >0.9 |
| Baseline eGFR (per mL/min/1.73m2) | – | – | 0.90 [0.87-0.94] | <0.001 | – | – | 0.87 [0.84-0.89] | <0.001 |
| Time (per month) | −0.49 [−0.68-−0.30] | <0.001 | −0.51 [−0.71-−0.32] | <0.001 | 1.16 [1.04-1.20] | 0.002 | 1.10 [1.02-1.18] | 0.01 |
| Age (per year) | −0.80 [−0.98-−0.62] | <0.001 | −0.07 [−0.14-0.004] | 0.06 | 1.07 [1.02-1.13] | 0.01 | 0.99 [0.96-1.03] | 0.8 |
| Male | −0.08 [−4.17-4.01] | >0.9 | 0.45 [−1.19-2.10] | 0.6 | 1.08 [0.35-3.39] | 0.9 | 0.54 [0.26-1.13] | 0.1 |
| African-American | −7.31 [−13.58-−1.03] | 0.02 | −1.25 [−3.56-1.06] | 0.3 | 8.66 [2.25-33.41] | 0.002 | 2.04 [0.95-4.37] | 0.07 |
| Charlson Comorbidity Index | ||||||||
| 0 | Referent | – | Referent | – | Referent | – | Referent | – |
| 1-3 | −9.38 [−13.43-−5.34] | <0.001 | −0.90 [−2.63-0.82] | 0.3 | 19.27 [4.91-75.60] | <0.001 | 2.09 [0.94-4.66] | 0.07 |
| ≥4 | −8.92 [−15.99-−1.85] | 0.01 | −1.39 [−4.31-1.52] | 0.3 | 17.73 [2.33-134.73] | 0.005 | 3.10 [1.07-9.04] | 0.04 |
Multivariable mixed effects logistic regression was performed to determine the odds of clinically significant CKD, defined as an eGFR <45 mL/min/1.73m2 (CKD stage ≥3B). Similar to the outcome for change in eGFR, adjustment for baseline GFR in Model 2 decreased the magnitude of effect but still demonstrated that RN was associated with a higher odds of clinically significant CKD compared to PN (OR 10.92, 95% CI 3.08-38.77, P<0.001). Notably, higher baseline eGFR was associated with lower odds of experiencing clinically significant CKD. AS and PN experienced similar rates of CKD ≥3B after adjustment for baseline eGFR. No patients were initiated on renal replacement therapy during follow-up.
Quality of Life Outcomes
A total of 1957 surveys were collected over a span of 7 years, completed by 218 PN patients, 43 RN patients, 37 ablation patients, and 233 AS patients [Supplementary Table 4]. The median time to questionnaire follow-up was 1 year after enrollment (IQR 0-2), with 232 patients (36.4%) achieving ≥3 years of follow-up.
Total SF-12 scores were significantly lower among AS patients only when compared to PN patients at enrollment, 2 years, and 3 years [Figure 3A]. Mixed effects regression demonstrated lower scores in AS patients when compared to PN patients (−3.23 points, 95% CI −5.99–−0.47, P=0.02) after controlling for time, age, gender, CCI, and BMI [Table 3]. Notably, female gender, increased CCI, and increased BMI were associated with lower total SF-12 scores.
Figure 3.
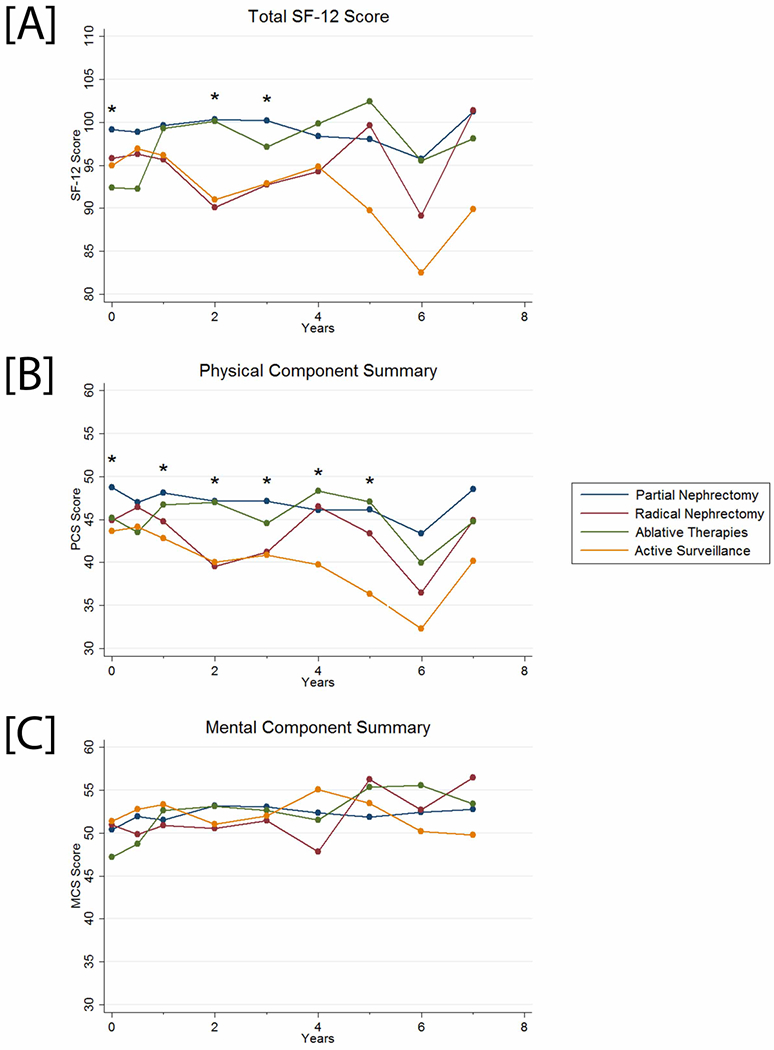
[A] Total SF-12, [B] physical component summary, and [C] mental component summary scores of patients in the DISSRM Registry. Black stars indicate the presence of a significant difference between two or more groups at that time point.
Table 3.
Multivariable mixed effects linear regression for variables associated with [A] total SF-12 scores, [B] physical component summary scores, and [C] mental component summary scores.
| [A] Total SF-12 | [B] Physical Component Summary | [C] Mental Component Summary | ||||
|---|---|---|---|---|---|---|
| Characteristics | Change in Score [95% CI] | P-value | Change in Score [95% CI] | P-value | Change in Score [95% CI] | P-value |
| Management | ||||||
| Partial Nephrectomy | Referent | – | Referent | – | Referent | – |
| Radical Nephrectomy | −3.80 [−8.19-0.60] | 0.09 | −2.61 [−5.63-0.40] | 0.09 | −1.33 [−3.84-1.19] | 0.3 |
| Ablative Therapies | −2.25 [−8.09-3.58] | 0.4 | −1.46 [−5.51-2.58] | 0.5 | −0.90 [−4.27-2.48] | 0.6 |
| Active Surveillance | −3.23 [−5.99-−0.47] | 0.02 | −3.24 [−5.10-−1.37] | 0.001 | −0.03 [−1.60-1.54] | >0.9 |
| Time (per year) | −0.20 [−0.55-0.15] | 0.3 | −0.61 [−0.87-−0.36] | <0.001 | 0.41 [0.20-0.61] | <0.001 |
| Age (per year) | 0.03 [−0.08-0.15] | 0.6 | −0.10 [−0.19-−0.01] | 0.03 | 0.13 [0.07-0.20] | <0.001 |
| Male | 4.36 [1.94-6.78] | <0.001 | 2.82 [1.13-4.52] | 0.001 | 1.51 [0.10-2.91] | 0.04 |
| Charlson Comorbidity Index | ||||||
| 0 | Referent | – | Referent | – | Referent | – |
| 1-3 | −6.39 [−8.86-−3.91] | <0.001 | −4.40 [−6.15-−2.64] | <0.001 | −1.95 [−3.39-−0.51] | 0.01 |
| ≥4 | −6.70 [−12.72-−0.67] | 0.03 | −4.46 [−8.61-−0.32] | 0.04 | −2.40 [−0.16-0.07] | 0.2 |
| BMI (per kg/m2) | −0.35 [−0.54-−0.15] | <0.001 | −0.30 [−0.43-−0.17] | <0.001 | −0.05 [−0.16-0.07] | 0.4 |
PCS scores were significantly lower among AS patients when compared to PN patients at enrollment and annually until year 5 [Figure 3B]. Multivariable mixed effects regression showed lower scores among AS patients compared to PN patients (−3.24 points, 95% CI −5.10–−1.37, P=0.001). In addition to female gender, increased CCI, and increased BMI, each additional year of follow-up and an increased age at enrollment were associated with significantly lower PCS scores.
MCS scores were comparable across all groups at each point of follow-up [Figure 3C]. On multivariable mixed effects regression, MCS scores were higher with each additional year of follow-up, with greater age at enrollment, and for males. Notably, there were no significant differences in MCS scores among any of the management groups.
An analysis of the delayed intervention patients found that there were no significant differences in total SF-12, PCS, or MCS scores following intervention after a period of active surveillance. However, it should be noted that this cohort is limited in size and the number of data points both before and after intervention are highly variable among patients.
Discussion
The present study directly compares the four major management options for SRMs in the domains of survival, renal function, and QOL in a prospective, contemporaneous registry. To summarize, CSS was excellent and comparable in all groups, but OS was lower in AS patients and likely attributable to older age and increased comorbidities. Renal function was lowest in RN patients but comparable in all other groups. QOL was lowest in AS patients due to lower physical health scores, but mental health scores were similar in all groups. These data address a number of the research gaps previously identified by demonstrating outcomes in a prospective manner, thereby strengthening the rationale behind recent AUA guidelines which were based on retrospective studies [3, 5].
In this cohort of patients with clinical stage T1a tumors, CSS was similar regardless of management. Likewise, data from a randomized controlled trial and recent meta-analysis found no significant difference in oncologic outcomes for patients treated with PN versus RN [4, 12]. In conjunction, these results demonstrate that CSS is determined by stage; therefore, all SRMs would be expected to have similar rates of CSS. Furthermore, longer follow-up from this registry confirms the continued safety of AS compared to various options for intervention in carefully selected patients – namely those who are older and have increased comorbidities.[6] These factors likely explain the lower OS rate among AS patients and suggest that OS is driven primarily by comorbidity status, as previously demonstrated [4, 13, 14].
Given the excellence of oncologic outcomes, the focus has increasingly shifted to preservation of renal function as a secondary goal. Indeed, the AUA guidelines recommend PN over RN when intervention is indicated to mitigate the risk of CKD progression [3]. A recent meta-analysis suggested RN patients exhibit the lowest eGFR when compared to PN, ablation, and AS patients, but the evidence was wrought with retrospective data reporting results at non-standardized time points [5, 15]. Nevertheless, the report found that when compared to PN patients, RN patients demonstrated a lower final eGFR (−10.5 mL/min/1.73m2, 95% CI −9.8–−11.2, P<0.001) and a higher risk of CKD stage ≥3 (RR 2.56, 95% CI 1.97-3.32, P<0.001). The only randomized controlled trial to date evaluating CKD outcomes with overall survival found that there were no differences between patients undergoing RN versus PN, despite significantly worse renal function in the RN group [12]. Although no explanation could be given at the time, recent studies from the Cleveland Clinic have shown that patients with CKD induced by surgery behave more like patients without CKD than those with medically-induced CKD, with the latter group demonstrating worse survival outcomes [16, 17]. Under this paradigm, in which surgically-induced CKD and medically-induced CKD are categorically different pathologies, RN patients would be expected to demonstrate similar survival outcomes when compared to PN patients in spite of the significantly worse renal function. Like the randomized controlled trial, the present study demonstrates this effect and potentially strengthens the observations from the Cleveland Clinic.
In the meta-analysis, no differences in renal function were found between PN and ablation patients, but data was insufficient to compare either intervention against the AS group [15]. Results from the present study corroborate these retrospective findings in a prospective cohort but also provide insight into direct comparisons of renal function to AS, which was previously found not to preserve eGFR any better than PN or ablation [8, 18]. Importantly, our results demonstrate that patients in AS, despite having lower baseline eGFR, have similar or improved renal functional outcomes compared to PN and ablation patients when controlling for patient characteristics and time. Potential explanations point to recent evidence suggesting that baseline eGFR may exert an effect on final eGFR [16, 17, 19, 20]. Intuitively, patients with higher baseline eGFR may be thought to be protected from renal functional decline, and those with compromised renal function may stand to benefit the most from nephron-sparing approaches. Therefore, we included two models for both the linear and logistic regressions to reflect this new paradigm, where Model 2 included baseline eGFR as an independent variable. Adjustment for baseline eGFR reduced the variability in the effect measure sufficiently to show that AS was associated with better preservation of absolute eGFR compared to PN. At the same time, adjustment in the logistic model demonstrated that it was the lower baseline eGFR in the AS group and a general decrease in eGFR over time for all patients that made it appear as though AS patients had a greater rate of CKD stage ≥3B in Model 1, whereas similar rates were observed for both AS and PN in Model 2.
To date, only four studies have attempted QOL comparisons in patients undergoing PN versus RN [21-24]. Results generally demonstrated that patients undergoing PN scored higher on physical functioning than patients undergoing RN. Although our findings were not significant, we demonstrate a similar trend. On the other hand, AS patients scored significantly lower than PN patients in overall QOL, which was primarily driven by the physical health component. Even after adjustment for other factors, this difference remains and is present at enrollment, underscoring the favorable physical health status among PN patients. Importantly, this study demonstrates that mental health scores improved with each year of follow-up, independent of management type. While the trend was suggested in a prior report, it was not statistically significant, and the present study provides greater follow-up with more granular comparisons among the different types of intervention [7].
Limitations of this study include the selection bias associated with the non-randomized design of the registry. Patient selection was intentionally designed to optimize outcomes for AS patients in order to represent comparative effectiveness in clinical practice. Furthermore, renal mass biopsy has not been a requirement to enroll in the registry and may therefore introduce heterogeneity in the patient population with respect to the presence of benign lesions. While this reflects the current standard of practice, it is notable that survival may be overestimated [25-27]. However, with no significant difference in biopsy rate between intervention and AS groups, any overestimation that may exist should be distributed equally and randomly. Nevertheless, results from the present study serve to demonstrate effectiveness and reflect practice patterns, in which shared decision-making is encouraged, as opposed to efficacy in an artificially-controlled setting. Finally, it should be noted that missing eGFR and QOL data may introduce additional selection bias as well.
Conclusions
Data from this study prospectively address research gaps identified by the AUA. With excellent oncologic outcomes in all groups, it is reasonable to consider AS for SRMs, especially in older patients with multiple comorbidities. However, if intervention is indicated, nephron-sparing approaches like PN or ablation are favored over RN due to improved renal functional outcomes. Finally, AS patients exhibit lower QOL scores, likely due to worse health at baseline. Notably, mental health is comparable in all groups and improves with time. These results confirm previous comparisons between PN and RN while concurrently describing the comparative effectiveness of two relatively new options, ablation and AS, for patients enrolled in the same time frame.
Supplementary Material
Acknowledgments
This work was supported, in part, by the National Institutes of Health and National Center for Advancing Translational Sciences under Award Number TL1TR001078 to Ridwan Alam. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest
None.
References
- 1.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–305. [DOI] [PubMed] [Google Scholar]
- 2.Huang WC, Levey AS, Serio AM, Snyder M, Vickers AJ, Raj GV, et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol. 2006;7(9):735–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell S, Uzzo RG, Allaf ME, Bass EB, Cadeddu JA, Chang A, et al. Renal Mass and Localized Renal Cancer: AUA Guideline. J Urol. 2017;198(3):520–9. [DOI] [PubMed] [Google Scholar]
- 4.Pierorazio PM, Johnson MH, Patel HD, Sozio SM, Sharma R, Iyoha E, et al. Management of Renal Masses and Localized Renal Cancer: Systematic Review and Meta-Analysis. J Urol. 2016;196(4):989–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel HD, Iyoha E, Pierorazio PM, Sozio SM, Johnson MH, Sharma R, et al. A Systematic Review of Research Gaps in the Evaluation and Management of Localized Renal Masses. Urology. 2016;98:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pierorazio PM, Johnson MH, Ball MW, Gorin MA, Trock BJ, Chang P, et al. Five-year analysis of a multi-institutional prospective clinical trial of delayed intervention and surveillance for small renal masses: the DISSRM registry. Eur Urol. 2015;68(3):408–15. [DOI] [PubMed] [Google Scholar]
- 7.Patel HD, Riffon MF, Joice GA, Johnson MH, Chang P, Wagner AA, et al. A Prospective, Comparative Study of Quality of Life among Patients with Small Renal Masses Choosing Active Surveillance and Primary Intervention. J Urol. 2016;196(5):1356–62. [DOI] [PubMed] [Google Scholar]
- 8.Danzig MR, Ghandour RA, Chang P, Wagner AA, Pierorazio PM, Allaf ME, et al. Active Surveillance is Superior to Radical Nephrectomy and Equivalent to Partial Nephrectomy for Preserving Renal Function in Patients with Small Renal Masses: Results from the DISSRM Registry. J Urol. 2015;194(4):903–9. [DOI] [PubMed] [Google Scholar]
- 9.Uzosike AC, Patel HD, Alam R, Schwen ZR, Gupta M, Gorin MA, et al. Growth Kinetics of Small Renal Masses on Active Surveillance: Variability and Results from the DISSRM Registry. J Urol. 2017. [DOI] [PubMed] [Google Scholar]
- 10.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gandek B, Ware JE, Aaronson NK, Apolone G, Bjorner JB, Brazier JE, et al. Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: results from the IQOLA Project. International Quality of Life Assessment. J Clin Epidemiol 1998;51(11):1171–8. [DOI] [PubMed] [Google Scholar]
- 12.Van Poppel H, Da Pozzo L, Albrecht W, Matveev V, Bono A, Borkowski A, et al. A prospective, randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol. 2011;59(4):543–52. [DOI] [PubMed] [Google Scholar]
- 13.Patel HD, Kates M, Pierorazio PM, Allaf ME. Balancing cardiovascular (CV) and cancer death among patients with small renal masses: modification by CV risk. BJU Int. 2015;115(1):58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kutikov A, Egleston BL, Canter D, Smaldone MC, Wong YN, Uzzo RG. Competing risks of death in patients with localized renal cell carcinoma: a comorbidity based model. J Urol. 2012;188(6):2077–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel HD, Pierorazio PM, Johnson MH, Sharma R, Iyoha E, Allaf ME, et al. Renal Functional Outcomes after Surgery, Ablation, and Active Surveillance of Localized Renal Tumors: A Systematic Review and Meta-Analysis. Clin J Am Soc Nephrol. 2017;12(7):1057–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demirjian S, Lane BR, Derweesh IH, Takagi T, Fergany A, Campbell SC. Chronic kidney disease due to surgical removal of nephrons: relative rates of progression and survival. J Urol. 2014;192(4):1057–62. [DOI] [PubMed] [Google Scholar]
- 17.Lane BR, Demirjian S, Derweesh IH, Takagi T, Zhang Z, Velet L, et al. Survival and Functional Stability in Chronic Kidney Disease Due to Surgical Removal of Nephrons: Importance of the New Baseline Glomerular Filtration Rate. Eur Urol 2015;68(6):996–1003. [DOI] [PubMed] [Google Scholar]
- 18.Castaneda CV, Danzig MR, Finkelstein JB, RoyChoudhury A, Wagner AA, Chang P, et al. The natural history of renal functional decline in patients undergoing surveillance in the DISSRM registry. Urol Oncol. 2015;33(4):166 e17–20. [DOI] [PubMed] [Google Scholar]
- 19.Woldu SL, Weinberg AC, Korets R, Ghandour R, Danzig MR, RoyChoudhury A, et al. Who really benefits from nephron-sparing surgery? Urology. 2014;84(4):860–7. [DOI] [PubMed] [Google Scholar]
- 20.Danzig MR, Chang P, Wagner AA, Allaf ME, McKiernan JM, Pierorazio PM. Active Surveillance for Small Renal Masses: A Review of the Aims and Preliminary Results of the DISSRM Registry. Curr Urol Rep 2016;17(1):4. [DOI] [PubMed] [Google Scholar]
- 21.Arnold ML, Thiel DD, Diehl N, Wu KJ, Ames S, Parker AS. Comparison of baseline quality of life measures between renal cell carcinoma patients undergoing partial versus radical nephrectomy. BMC Urol. 2013;13:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Azawi NH, Tesfalem H, Dahl C, Lund L. Do the different types of renal surgery impact the quality of life in the postoperative period? Int Urol Nephrol 2015;47(2):263–9. [DOI] [PubMed] [Google Scholar]
- 23.Poulakis V, Witzsch U, de Vries R, Moeckel M, Becht E. Quality of life after surgery for localized renal cell carcinoma: comparison between radical nephrectomy and nephron-sparing surgery. Urology. 2003;62(5):814–20. [DOI] [PubMed] [Google Scholar]
- 24.Shinohara N, Harabayashi T, Sato S, Hioka T, Tsuchiya K, Koyanagi T. Impact of nephron-sparing surgery on quality of life in patients with localized renal cell carcinoma. Eur Urol. 2001;39(1):114–9. [DOI] [PubMed] [Google Scholar]
- 25.Haifler M, Kutikov A. Current Role of Renal Biopsy in Urologic Practice. Urol Clin North Am 2017;44(2):203–11. [DOI] [PubMed] [Google Scholar]
- 26.Patel HD, Johnson MH, Pierorazio PM, Sozio SM, Sharma R, Iyoha E, et al. Diagnostic Accuracy and Risks of Biopsy in the Diagnosis of a Renal Mass Suspicious for Localized Renal Cell Carcinoma: Systematic Review of the Literature. J Urol. 2016;195(5):1340–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel HD, Druskin SC, Rowe SP, Pierorazio PM, Gorin MA, Allaf ME. Surgical histopathology for suspected oncocytoma on renal mass biopsy: a systematic review and meta-analysis. BJU Int. 2017;119(5):661–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


