Abstract
Gram-positive pathogens, including Staphylococcus aureus cause necrotizing pneumonia. The central feature of S. aureus pneumonia is toxin-induced necroptosis of immune and resident cells, which impedes host defense. However, the role of the NLRC4 in the lung following S. aureus infection remains elusive. Here, we demonstrate that S. aureus activates the NLRC4 to drive necroptosis and IL-18 production, which impaired IL-17A-dependent neutrophil-mediated host susceptibility. In particular, Nlrc4−/− mice exhibit reduced necroptosis, enhanced neutrophil influx into the lungs, decreased bacterial burden, and improved host survival. Loss of NLRC4 signaling in both hematopoietic and non-hematopoietic cells contributes to the host protection against S. aureus pneumonia. Secretion of IL-17A by γδ T cells is essential for neutrophil recruitment into the lungs of Nlrc4−/− mice following infection. Moreover, treatment of wild-type mice with necroptosis inhibitors or genetic ablation of MLKL and IL-18 improves host defense against S. aureus infection, which is associated with increased IL-17A+ γδ T cells and neutrophils. Taken together, these novel findings reveal that S. aureus activates the NLRC4 to dampen IL-17A-dependent neutrophil accumulation through induction of necroptosis and IL-18. Thus, modulating the function of the NLRC4 may be an attractive therapeutic approach for treating S. aureus infections.
Keywords: Pneumonia, Innate Immunity, Neutrophil, Cytokine, Chemokine
INTRODUCTION
Gram-positive pathogens, including Staphylococcus aureus are the major cause of healthcare-associated pneumonia, sepsis, and mortality in post-influenza infection patients 1,2. In particular, the emergence of methicillin-resistant S. aureus (MRSA) as an endemic, dominant strain poses a menacing therapeutic challenge in the United States 3. Unlike other hospital-acquired S. aureus strains, MRSA causes a broad spectrum of necrotizing infections of skin and soft tissues, including pneumonia, and these infections are associated with high morbidity and mortality, even in immunocompetent individuals 3,4 S. aureus pneumonia is characterized by extensive inflammation and localized necrosis leading to loss of alveolar architecture, hemorrhage, and consolidation of lungs 5. Emerging evidence suggests that pathogenesis of S. aureus pneumonia is largely mediated by over-activation of host inflammatory signaling cascades, although the precise mechanisms underlying this remain elusive.
Despite its antibiotic resistance, the success of S. aureus as a highly invasive pathogen is largely attributed to its arsenal of virulence factors, including cytolytic and pore-forming toxins 6. All S. aureus strains produce exotoxins or surface proteins, which have diverse targets in multiple cell types. S. aureus has been shown to activate NLRP3 and NOD2, and to target CD11b and ADAM10 7-10. Unlike Gram-negative pathogen-induced infections, mice deficient in innate immune molecules including ADAM10, NLRP3, NOD2, IFNR-1, and TNFR have improved outcomes in S. aureus (Grampositive) pneumonia 10-14 In general, these gene-deficient mice show enhanced bacterial clearance, attenuated pro-inflammatory cytokine production, and decreased lung injury/pathology contributing to increased survival against pulmonary S. aureus infection. These observations suggest S. aureus may have evolved to utilize host innate immune molecules to dampen immune responses. However, the precise mechanism through which S. aureus manipulates the host inflammatory machinery to favor the pathogen is not clear.
Necroptosis is a recently identified, pro-inflammatory mode of cell death regulated by receptor-interacting serine-threonine kinases (RIP1/3) and executed by mixed lineage kinase domain-like protein (MLKL) 15, 16. Recently, toxin-induced rapid loss of macrophages via necroptosis has been suggested as a major mechanism of lung damage in S. aureus pneumonia 7, 17. Similarly, alveolar macrophages exposed to pore-forming toxins from Serratia marcescens undergo necroptosis, which contributes to the severity of pneumonia through substantial loss of critical immune cells and extensive bystander tissue injury 18. Genetic ablation of Rip3 or treatment with inhibitors of necroptosis protects alveolar macrophages, which is associated with reduced bacterial burden and improved pneumonia outcomes in mice 17, 18. However, the mechanism responsible for the necroptosis-mediated depletion of immune cells that impedes bacterial clearance in S. aureus pneumonia largely remains unknown.
Regarding inflammasomes, NLRC4 belongs to the NLR family of proteins containing an N-terminal CARD domain, a central NACHT domain and a C-terminal leucine-rich repeat domain and is involved in assembly of the inflammasome complex. It was identified as a cytosolic sensor for bacterial flagellin and type III secretion system (T3SS) 19, 20. However, Klebsiella pneumoniae, a non-flagellated bacterium lacking T3SS, and a flagellin-deficient strain of Pseudomonas aeruginosa have been shown to activate the NLRC4, suggesting its activation by other bacterial or endogenous host ligands 21, 22. Nonetheless, the role of NLRC4 in bacterial pneumonia caused by a Gram-positive organism still remains elusive 21, 23. Although recent studies linked NLRP3 and ASC to S. aureus toxin-induced necroptosis, the precise mechanism for this has not been elucidated either 7, 18. Furthermore, Nlrp3−/− mice do not show a completely protective phenotype in S. aureus pneumonia indicating both NLRP3-dependent and independent mechanisms may be important in eliciting lung damage 13. To this end, we investigated whether the activation of the NLRC4 is important in the pathogenesis of S. aureus pneumonia in a mouse model. We found that NLRC4-driven necroptosis and IL-18 suppress IL-17A signaling from γδ T cells leading to attenuated neutrophil recruitment, which is necessary to eradicate S. aureus from the lung. Thus, these findings provide novel insight into how necroptosis and IL-18 dampens host defense in S. aureus pneumonia and suggests the inhibition of NLRC4 as a potential therapeutic approach for control of S. aureus infection in the lungs.
RESULTS
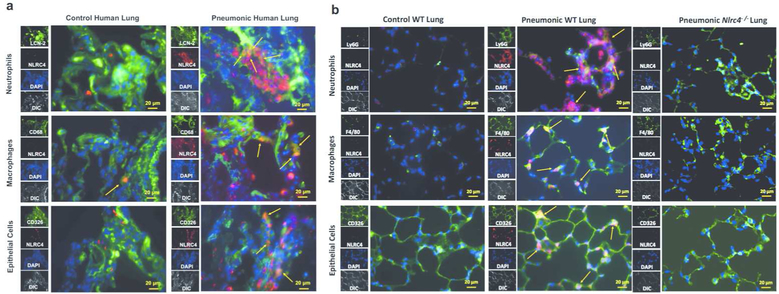
NLRC4 expression is enhanced in human and murine pneumonic lungs.
To determine whether NLRC4 expression is upregulated in human pneumonic lungs, we performed immunofluorescence staining of NLRC4 in lung sections from pneumonic patients and healthy controls. As virulence factors from S. aureus are known to target both myeloid and non-myeloid cells 8, 10, we assessed NLRC4 expression in myeloid cells (neutrophils, macrophages) and non-myeloid cells (epithelial cells) and found increased expression of NLRC4 in Lipocalin-2+ neutrophils, CD68+ macrophages, and CD326+ epithelial cells (Figure 1a). To explore if NLRC4 expression is increased during Gram-positive pneumonia in mouse lungs, we performed immunofluorescence staining in lung sections at 24 hours post- S. aureus infection. We observed enhanced expression of NLRC4 in Ly6G+ neutrophils, F4/80+ macrophages, and CD326+ epithelial cells in infected wild-type (WT) mouse lungs compared to that in control lungs (Figure 1b). Collectively, our results indicate both myeloid and non-myeloid cells show up-regulation of NLRC4 during bacterial pneumonia.
Figure 1. NLRC4 expression is enhanced in human and murine pneumonic lungs.
(a) NLRC4 expression was assessed using immunofluorescence in lung sections from healthy controls and from patients with clinical diagnosis of unspecified bacterial pneumonia. Red staining indicates NLRC4 expression. Neutrophils (Lipocalin-2+), macrophages (CD68+), and epithelial cells (CD326+) were stained green. Nuclei were stained with DAPI (blue). Arrowheads (yellow) indicate co-localization of expression of NLRC4 with specific cell markers. Images are representative of five different sections with similar results. (b) NLRC4 expression in lung sections from control and S. aureus- infected WT and Nlrc4−/− mice at 24 hpi. Red staining indicates NLRC4 expression. Neutrophils (Ly6G+), macrophages (F4/80+), and epithelial cells (CD326+) are stained green. Nuclei were stained with DAPI (blue). Arrowheads (yellow) indicate co-localization of expression of NLRC4 with specific cell markers. Images are representative of five different sections with similar results. Original magnification, 40x. DAPI: 4', 6-diamidino-2-phenylindole, LCN2: lipocalin-2, hpi: hours post infection.
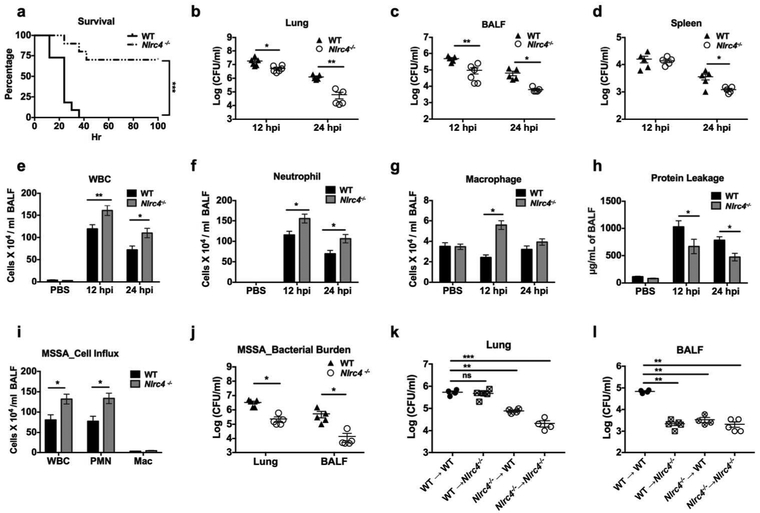
NLRC4 deficiency contributes to host protection against S. aureus pneumonia.
To characterize the importance of NLRC4 in S. aureus pneumonia, WT and Nlrc4−/− mice were infected intratracheally (i.t.) with a lethal inoculum of S. aureus (2×108 CFU/mouse) and survival was monitored up to 100 hours. Compared to WT counterparts, Nlrc4−/− mice showed enhanced survival (Figure 2a). To determine if the enhanced survival in Nlrc4−/− mice was due to increased bacterial clearance, WT and Nlrc4−/− mice were infected i.t. with a sub-lethal inoculum of S. aureus (5×107 CFU/mouse). Compared to WT mice at 12 and 24 hours post infection (hpi), Nlrc4−/− mice showed a diminished bacterial burden in the lung and bronchoalveolar lavage fluid (BALF) along with decreased dissemination in the spleen (Figures 2b-d). We next determined whether the enhanced bacterial clearance was due to augmented neutrophil accumulation in the lungs. Our results revealed that Nlrc4−/− mice had increased numbers of total white blood cells, neutrophil, and macrophage recruited to the lungs (Figures 2e-g). Furthermore, unlike Nlrc4−/− mice, WT mice had increased protein leakage, an indicator of lung permeability (Figure 2h). To assess if the detrimental role of NLRC4 activation is MRSA strain-specific, we infected WT and Nlrc4−/− mice with a methicillin-susceptible S. aureus (MSSA) Newman strain. The protective phenotype seen in Nlrc4−/− mice infected with S. aureus was also seen in pneumonia caused by the MSSA strain (Figures 2i, j).
Figure 2. NLRC4 deficiency confers host resistance against S. aureus pneumonia.
(a) WT (control) and Nlrc4−/− mice were inoculated intratracheally with 2 X 108 CFU of S. aureus and survival was monitored up to 100 hours. A Kaplan Meier plot is used to show survival of mice from each group. (n= 10 mice/group). (b-h) WT and Nlrc4−/− mice were inoculated intratracheally with 5 × 107 CFU of S. aureus or PBS (control). BALF and organs were harvested at the designated time points. Control mice (PBS treated) were sacrificed at 24 hpi. The bacterial burden in the (b) lungs, (c) BALF, and (d) spleen was quantitated at 12 and 24 hpi.. Number of (e) total white blood cells, (f) neutrophils, (g) macrophages, and (h) level of total protein were quantified in BALF at 12 and 24 hpi. (n= 5-6 mice/ pneumonia group, n=3 mice/control group). (i and j) WT and Nlrc4−/− mice were inoculated intratracheally with 5 × 107 CFU of S. aureus Newman strain (MSSA). (i) Cellular influx in BALF and (j) bacterial burden in lungs and BALF were determined at 24 hpi. (k and l) Bone marrow reconstituted mice were inoculated intratracheally with 5 × 107 CFU of S. aureus. The bacterial burden in (k) lung and (l) BALF was quantitated at 24 hpi. Each symbol represents a single mouse (b, c, d, j, k, l). Data from a representative experiment are shown. All experiments were performed three times, but survival and chimera study were performed twice. Statistical significance was determined by log-rank (a), Mann-Whitney (b, c, d, j), unpaired t-test (e-i), one-way ANOVA (followed by Bonferroni’s post hoc comparisons) (k, l). *p<0.05; **p<0.01; ***p<0.001. BALF: bronchoalveolar lavage fluid, CFU: colony forming unit, WBC: white blood cell, nd: non-detectable.
S. aureus targets both myeloid and non-myeloid cells 7, 10, 11. In Figure 1 a-b, we demonstrate increased expression of NLRC4 in both hematopoietic (myeloid cells) and non-hematopoietic (epithelial cells) cells in human and murine pneumonic lungs. Therefore, we next investigated the contribution of hematopoietic versus non-hematopoietic cells to host protection using bone marrow chimeras. In this context, lethally irradiated WT or Nlrc4−/− mice were reconstituted with RBC-lysed bone marrow cells (4×106/mouse) from donor WT or Nlrc4−/− mice. Two months after reconstitution, chimeric mice were infected with S. aureus. Interestingly, the Nlrc4−/− → Nlrc4−/−, Nlrc4−/− → WT but not WT → Nlrc4−/− chimeric groups had decreased bacterial burden in lungs as compared with the WT → WT group (Figures 2k). However, the Nlrc4−/− → Nlrc4−/−, Nlrc4−/− → WT, and WT → Nlrc4−/−groups had reduced bacterial burden in BALF as compared to WT → WT group (Figures 2i). Taken together, our results suggest NLRC4 activation, in both hematopoietic and non-hematopoietic compartments, is detrimental, and their deficiency in mice displays robust protective phenotype in S. aureus pneumonia.
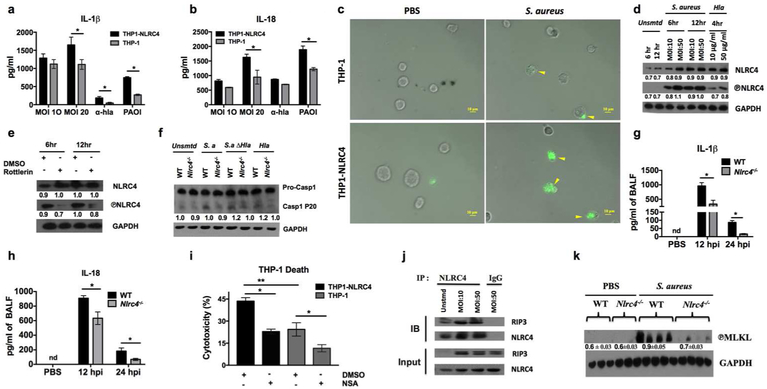
S. aureus activates NLRC4 through PKC-δ to induce IL-1β, IL-18, and necroptosis.
S. aureus α-hemolysin has been shown to activate NLPR3 in human and mouse monocytes 7. Along the same line, to examine the virulence factors associated with NLRC4 activation, we used an isogenic α-hemolysin deficient S. aureus mutant strain, in human and murine cells. Direct evidence of increased NLRC4 expression in humans was obtained using lung lysates from pneumonic patients and comparing them to lysates from healthy individuals (Supplementary Figure 1a). In a similar manner, S. aureus activated NLRC4 in both human monocyte-like (THP-1) and neutrophil-like (HL-60) cells (Supplementary Figure 1a). To further characterize the role of NLRC4 in response to S. aureus infection, we used THP-1 and THP1-NLRC4 cells (stably overexpressing NLRC4). Compared to THP-1 cells, THP1-NLRC4 produced greater amounts of IL-1 β and IL-18 in response to S. aureus (Figure 3a, b). In addition, THP1-NLRC4 cells showed increased caspase-1 activity, as evidenced by their marked staining with the fluorescently labeled inhibitor of caspases (FLICA) reagent (Figure 3c and Supplementary Figure lb). We also observed increased expression and phosphorylation of NLRC4 in a dose- and time-dependent manner with S. aureus and purified α- hemolysin in bone marrow-derived macrophages (BMDMs) from WT mice (Figure 3d and Supplementary Figure 1c). Recently, protein kinase C-δ (PKC-δ) mediated phosphorylation of NLRC4 was shown to be critical for inflammasome activation 24. As anticipated, S. aureus-induced NLRC4 phosphorylation was dependent on PKC-δ in macrophages, as Rottlerin (PKC-δ inhibitor) reduced expression of phospho-NLRC4 (Figure 3e). We also tested whether S. aureus-induced NLRC4 activation leads to caspase-1 processing and IL-1β production, key features of inflammasome activation. To this end, we stimulated BMDMs from WT and Nlrc4−/− mice with S. aureus, its isogenic α- hemolysin deficient mutant, or purified α- hemolysin toxin. As expected, the cleavage of pro-caspase-1 to its active (phospho-20) form was reduced in Nlrc4−/− BMDMs, which was independent of the expression of α- hemolysin by S. aureus (Figure 3f). This observation indicates S. aureus may expresses virulence factors other than α-hemolysin that may serve as activators of NLRC4-dependent caspase-1 processing. In addition, NLRC4 deficiency modestly reduced IL-1 β production in S. aureus-infected BMDMs, so as the pre-treatment with Rottlerin (Supplementary Figure 1d, e). Furthermore, Nlrc4−/− mice had significant reduction in key inflammasome-dependent cytokines, such as IL-1β and IL-18 (Figures 3g, h).
Figure 3. S. aureus activates NLRC4 through PKC-δ to induce IL-1β, IL-18, and necroptosis.
Levels of (a) IL-1β and (b) IL-18 in the supernatant of THP-1 and THP1-NLRC4 cells incubated with S. aureus (MOI 10 or 20), purified α-hemolysin (25 μg/ml), or Pseudomonas aeruginosa PAO1 (MOI 10) for 8 hours. (c) Active FLICA-caspase-1 (green) was detected in THP-1 and THP1-NLRC4 cells infected with S. aureus (MOI 50) or PBS for 6 hours. Original magnification, 40x. (d) Immunoblot analysis of total NLRC4 and phospho-NLRC4 in cell lysates from WT-BMDMs infected with S. aureus (MOI 10 or 50) for 6 or 12 hours or stimulated with commercially purified α-hemolysin (10 or 50 μg/ml) for 4 hours. (e) Immunoblot analysis of total NLRC4 and phospho-NLRC4 in cell lysates from WT-BMDMs infected with S. aureus (MOI 10) for 6 or 12 hours with or without one-hour pre-treatment with 10 uM Rottlerin (PKC-δ inhibitor). (f) Immunoblot analysis of pro-caspase-1 and cleaved caspase-1 (p-20) in cell lysates from WT and Nlrc4−/−-BMDMs infected with MOI 10 of S. aureus or its isogenic α-hemolysin deficient mutant or stimulated with purified α- hemolysin (50 μg/ml) for 6 hours. (g and h) WT and Nlrc4−/− mice were inoculated with S. aureus (5 X 107 CFU/mouse). Level of (g) IL-1β and (h) IL-18 measured BALF collected at 12 and 24 hpi. (n= 4-6 mice / pneumonia group, n=3 mice/control group). (i) Cytotoxicity in THP-1 and THP1-NLRC4 cells stimulated with S. aureus (MOI 100) for 2 hours with or without 1 hour pre-treatment with 100 uM of necrosulfonamide (NSA). (j) BMDMs from WT mice were left untreated or were incubated with S. aureus (MOI 10 or 50) for 6 hours. Cell lysates were immunoprecipitated with ant-NLRC4 Ab or IgG, and then immunoblotted for RIP3 and NLRC4. Whole cell lysates are shown as the input. (k) Immunoblot analysis of phospho-MLKL in the lung homogenates of WT and Nlrc4−/− mice infected with S. aureus (5 × 107 CFU/mouse). Data from a representative experiment are shown. All experiments were performed three times. In vitro experiments have at least four biological replicates. Numeric values in immunoblot indicate ratio of mean grey scale value of protein of interest to its GAPDH control. Statistical significance was determined by unpaired t-test in all experiment except in cell death assay (i), by one-way ANOVA (followed by Bonferroni’s post hoc comparisons). *p<0.05; **p<0.01. Hla: α-hemolysin, ΔHla: α-hemolysin deficient, MOI: multiplicity of infection, DMSO: dimethyl sulfoxide IP: immunoprecipitation, IB: immunoblotting..
S. aureus has been shown to induce necroptosis in lungs 17 Therefore, in order to assess the role of NLRC4 in context of necroptosis, we performed cytotoxicity assay in THP-1 and BMDM following S. aureus infection. We detected increased cytotoxicity of THP1-NLRC4 and WT-BMDM compared to THP-1 and Nlrc4−/−-BMDMs (Figure 3i and Supplementary Figure 1f), providing direct evidence of the involvement of NLRC4 in S. aureus-induced cell death. In addition, pre-treatment with necrostatin-1 (Nec-1: RIP1 inhibitor) or necrosulfonamide (MLKL inhibitor) protected THP1-NLRC4 and WT-BMDM from S. aureus-induced necroptosis respectively (Figures 3i and Supplementary Figure 1f). Furthermore, we observed increased co-localization of RIP3 and phospho-MLKL in S. aureus-infected WT-BMDMs compared to Nlrc4−/−-BMDMs (Supplementary Figures 1g, h) as well as the co-immunoprecipitation of RIP3 and NLRC4 indicating their potential interaction in the necrosome complex following S. aureus infection (Figure 3j). Given that MLKL pore formation is a critical step in necroptosis, we sought to detect the level of phospho-MLKL in S. aureus-infected lungs. Corroborating our in-vitro results, Nlrc4−/− mice show reduced MLKL phosphorylation in their lungs compared to WT controls (Figure 3k). In a similar manner, WT mice displayed enhanced LDH release and secretion of necroptosis-related alarmins, such as HMGB-1 and IL-1 α, in BALF at 12 and 24 hpi (Supplementary Figures 1i-k). Taken together, our results indicate that S. aureus phosphorylates NLRC4 in PKC-δ-dependent manner and activation of which leads to production of IL-1β, IL-18, and necroptotic cell death.
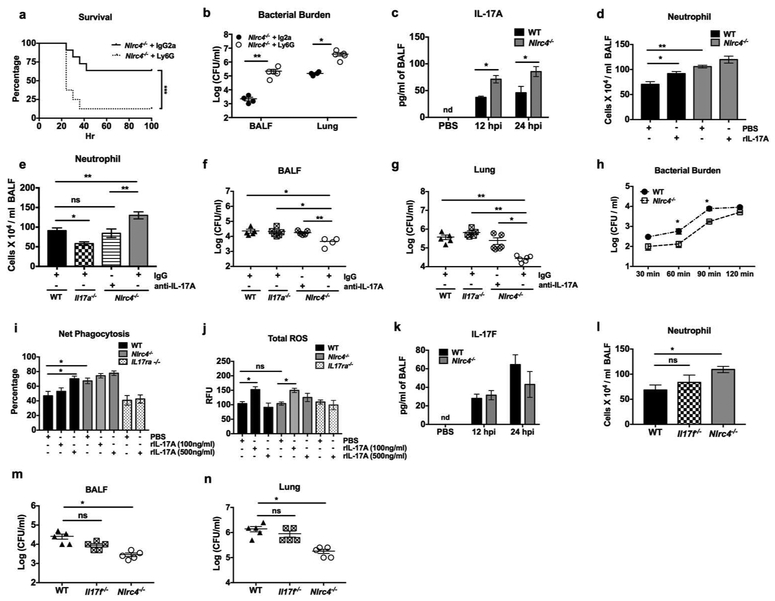
NLRC4 deficiency promotes IL-17A-mediated neutrophil recruitment and bacterial clearance.
Neutrophil recruitment is a critical event for bacterial clearance during S. aureus infection 25. Nlrc4−/− mice displayed enhanced neutrophil recruitment (Figure 2f). Furthermore, neutrophil depletion with anti-Ly6G antibody (IA8) increased the mortality and bacterial burden in Nlrc4−/− mice (Figure 4a, b). However, lower levels of pro-inflammatory cytokines in BALF of Nlrc4−/− mice did not explain the higher neutrophil counts, indicating these cytokines (TNF-α, MCP-1, Il-6) may not be associated with neutrophil recruitment in S. aureus infection (Supplementary Figures S2a-c). It should be noted that IL-17A has been shown to be critical for neutrophil influx and bacterial clearance at the site of S. aureus infection 26. Thus, we measured the level of IL-17A in BALF and found that IL-17A was increased in Nlrc4−/− mice at 12 and 24 hpi (Figure 4c). To further implicate IL-17A-mediated neutrophil recruitment in Nlrc4−/− mice, administration of recombinant IL-17A in WT mice one-hpi increased neutrophil influx to a level comparable to Nlrc4−/− mice (Figure 4d). Further, ablation of Il17a or blockade with anti-IL-17A antibody in Nlrc4−/− mice abrogated neutrophil influx, which was associated with an increased bacterial burden in BALF and lungs (Figures 4e-g). Since IL-17A is not directly involved in neutrophil recruitment, we explored whether enhanced recruitment may be due to increased level of CXC chemokines and neutrophil mobilization through IL-17A/G-CSF axis. Accordingly, Nlrc4−/− mice also showed increased production of CXCL5, but not CXCL1 and CXCL2, at 12 hpi and higher trend of G-CSF at both 12 and 24 hpi (Supplementary Figures S2d-g). To examine if NLRC4 deficiency also alters the phagocytic function of neutrophils, we utilized a phagocytosis assay in bone marrow-derived neutrophils (BMDNs). To our surprise, WT-BMDNs show higher intracellular bacterial survival than Nlrc4−/−- BMDNs following S. aureus infection (Figure 4h). Further, Nlrc4−/− BMDNs were also effective at particle uptake (Figure 4i), suggesting NLRC4 deficiency improved both uptake and clearance in BMDN. Furthermore, the defect in uptake/phagocytosis of WT-BMDN was rescued by pre-treatment with IL-17A, which was associated with increased production of intracellular reactive oxygen species (ROS) (Figures 4i, j). Unlike in BMDNs, NLRC4 was dispensable for the phagocytic function of BMDMs (Supplementary Figure S3a). Moreover, deficiency of IL-17F, another IL-17 homodimeric cytokine complex that binds to the same receptors (IL-17RA or IL-17RC) 27, did not alter neutrophil recruitment or bacterial clearance in S. aureus pneumonia (Figures 4k-n). Collectively, our data indicate that the increased level of IL-17A drives the influx and improves the phagocytic abilities of recruited neutrophil in Nlrc4−/− mice.
Figure 4. NLRC4 deficiency promotes IL-17A-mediated neutrophil recruitment and bacterial clearance.
(a and b)Nlrc4−/− mice were injected with anti-Ly6G (IA8) Ab intraperitoneally at 24 and 2 hr prior to intratracheal infection with 2 × 108 CFU for survival and 5 × 107 CFU for pneumonia. (a) A Kaplan Meier plot is used to show survival of mice from each group. (n= 10 mice/group). (b) Bacterial burden was determined at 24 hpi. Each symbol represents a single mouse. (c and d) WT and Nlrc4−/− mice were inoculated intratracheally with 5 × 107 CFU of S. aureus or PBS followed by administration of recombinant murine IL-17A or PBS 1 hour later. (c) Level of IL-17A at 12 and 24 hpi and (d) number of neutrophils at 24 hpi in the BALF were enumerated. (e-g) WT, Il-17a−/− and Nlrc4−/− mice were inoculated intratracheally with 5 × 107 CFU of S. aureus or PBS. Nlrc4−/− mice received a single dose of anti-IL17A Ab or IgG at 12 hours prior to infection. (e) Number of neutrophils and (f) bacterial burden in BALF and (g) lungs were determined at 24 hpi. Each symbol represents a single mouse. (n= 4-6 mice/pneumonia group, n=3 mice/control group). (h-j) BMDNs were isolated from WT, Nlrc4−/− and IL-17ra−/− mice and stimulated with S. aureus (MOI 1) in the presence or absence of recombinant murine IL-17A (100 or 500 ng/ml) or PBS. (h) Intracellular killing of neutrophils was determined at indicated time points by assessing the intracellular bacterial burden as described in supplementary methods. (i) Net phagocytosis and (j) total ROS production at 1 hpi was determined as described in supplementary methods. (k-n) Wild type, Il-17f−/−, and Nlrc4−/− mice were inoculated intratracheally with 5 × 107 CFU of S. aureus. BALF and lungs were collected at 12 and 24 hpi. (k) Level of IL-17F, (l) number of neutrophils in BALF, and bacterial burden in (m) BALF and (n) lung were determined at 24 hpi. Each symbol represents a single mouse. (n= 5 mice /pneumonia group, n=3 mice/control group). Data from a representative experiment are shown. All experiments were performed three times. In vitro experiments have at least four biological replicates. Statistical significance was determined by log-rank (a), Man-Whittney (b, h), unpaired t-test (c, k), and one-way ANOVA (followed by Bonferroni’s post hoc comparisons) (d-g, i, j, l-n). *p<0.05; **p<0.01. IgG: immunoglobulin G, ROS: reactive oxygen species.
NLRC4 deficiency augments IL-17A+γδ T cells and neutrophils to regulate neutrophil influx.
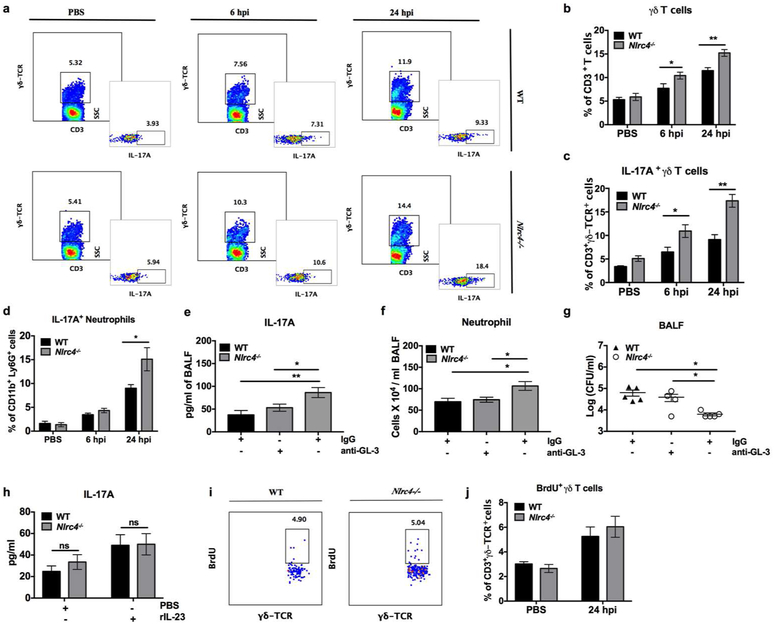
Although IL-17A is known as a signature cytokine for CD4+ T cells (Th17 subsets), emerging evidence has revealed that multiple cell types, such as γδ T cells, NK/NKT cells, neutrophils, innate lymphoid cells, CD8+ T cells, and colonic epithelial cells, also secrete IL-17A 27. To investigate the primary source of IL-17A during the early stage of infection, we utilized intracellular flow cytometry on lung single cell suspensions at 6 and 24 hpi. There was an increase in total and IL-17A producing CD3+γδ-TCR+ cells in the Nlrc4−/− mice at 6 and 24 hpi (Figures 5a-c). While neutrophils (CD11b+ Ly6G+), CD4+ T cells, CD8+ T cells, and NK1.1+ cells also produce IL-17A during S. aureus infection, but NLRC4 deficiency did not alter their number in our model (Supplementary Figures S4a-d). However, IL-17A producing neutrophils were higher at 24 hpi in Nlrc4−/− mice, indicating recruited neutrophils help sustain IL-17A levels and recruit more inflammatory cells (Figure 5d and Supplementary Figure 4a). Human neutrophils activated with S. aureus also produced IL-17A (Supplementary Figure 4e). To further implicate γδ T cells in IL-17A-mediated antimicrobial signaling in Nlrc4−/− mice, we depleted γδ T cells using an anti-γδ-TCR antibody (GL-3). Administration of the GL-3 antibody reduced the level of IL-17A in Nlrc4−/− mice to a level comparable with WT mice receiving isotype-matched IgG control Ab (Figure 5e). Furthermore, depletion of γδ T cells impaired host defense in Nlrc4−/− mice, as there was decreased neutrophil influx and increased bacterial burden in the BALF of these mice at 24 hpi (Figures 5f, g). We next asked if genetic deficiency of NLRC4 alters the production of IL-17A from γδ T cells in addition to their recruitment to the lungs. To this end, we FACS sorted CD3+γδ-TCR+ cells from spleens of naïve mice and stimulated them with S. aureus in the presence or absence of IL-23 in vitro. Surprisingly, the γδ T cells from WT and Nlrc4−/− mice did not show altered production of IL-17A following IL-23 stimulation (Figure 5h). To examine the possibility of increased γδ T cell proliferation in lungs of Nlrc4−/− mice, we injected BrdU intraperitoneally before S. aureus infection and stained for BrdU positive γδ T cells. We found that NLRC4 deficiency did not regulate the proliferation of this cell type in the lungs (Figures 5i, j). Thus, these data indicate increased frequency of γδ T cells from early stage and neutrophil at late stage in Nlrc4−/− mice produce IL-17A that is critical of neutrophil recruitment to the lungs in S. aureus pneumonia.
Figure 5. NLRC4 deficiency augments IL-17A+γδ T cells and neutrophils to regulate neutrophil influx.
(a-d) Flow cytometric analysis of the lungs from WT and Nlrc4−/− mice either uninfected or intratracheally infected with 5 × 107 CFU of S. aureus. (a) FACS plots of CD3+ γδ-TCR+ cells (larger) and IL-17A+ CD3+ γδ-TCR+ cells (smaller) at 6 and 24 hpi. Mice receiving PBS were sacrificed at 24-hour time points. (b) The percentage of total γδ T cells, (c) IL-17A producing γδ T cells, and (d) IL-17A producing CD11b+ Ly6G-1+ neutrophils enumerated by flow cytometry in lungs at 6 and 24 hpi. (n= 5 mice / pneumonia group, n= 3 mice / control group). (e-g) WT and Nlrc4−/− mice were inoculated intratracheally with 5 × 107 CFU of S. aureus. Nlrc4−/− mice received an intraperitoneal injection of anti-γδ-TCR ab (GL-3) or IgG at 12 hours prior to infection. (e) Level of IL-17A, (f) number of neutrophils, and (g) bacterial burden in BALF was determined at 24 hpi. (n= 5 mice / group) (h) The CD3+ γδ-TCR+ cells were sorted from the spleen of the uninfected wild-type and Nlrc4−/− mice and incubated with S. aureus (MOI 10) for 18 hours in the presence or absence of IL-23 (40 ng/ml). (h) IL-17A was measured in the supernatant. (i and j) WT and Nlrc4−/− mice were inoculated intratracheally with 5 × 107 CFU of S. aureus or PBS. These mice received a single injection of BrdU (1mg/mouse) intraperitoneally 1-hour prior to infection. (i) FACS plot showing BrdU+ CD3+ γδ-TCR+ cells and (j) their percentage in the lungs of mice 18 hpi. (n= 5 mice /pneumonia group, n=3 mice/control group). Data from a representative experiment are shown. All experiments were performed three times. In vitro experiments have at least four biological replicates. Statistical significance was determined by unpaired t-test (b-d) and one-way ANOVA (followed by Bonferroni’s post hoc comparisons) (e-g) *p<0.05; **p<0.01. BrdU: bromodeoxyuridine.
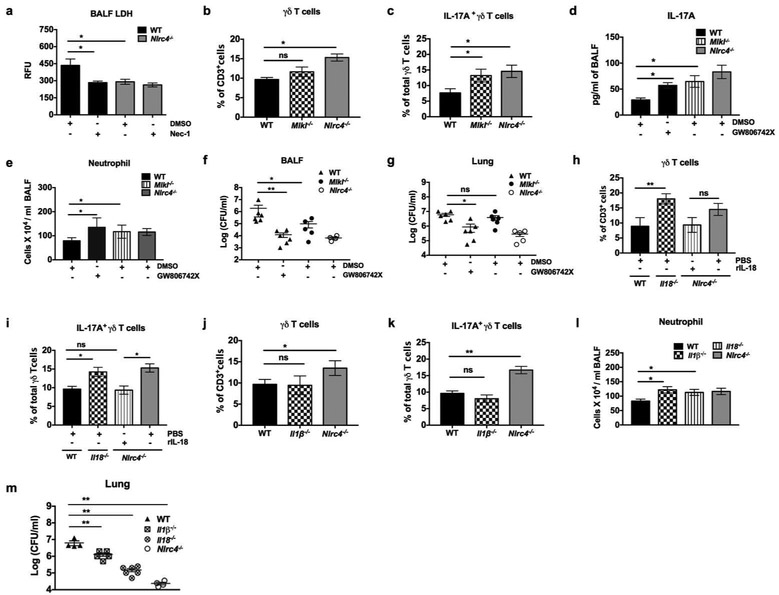
NLRC4-driven necroptosis and IL-18 suppress IL-17A-dependent neutrophil recruitment by limiting γδ T cell expansion.
We showed that S. aureus-induced NLRC4 activation results in the production of IL-1β, IL-18, and necroptosis in macrophages and lungs (Figure 3 and Supplementary Fig 1). Necroptosis-mediated loss of immune cells in WT mice, but not in the Rip3−/− mice, has been shown to worsen the outcome of S. aureus pneumonia 17. Here, we sought to elucidate whether NLRC4-coupled necroptosis suppress IL-17A-mediated antibacterial defense in pneumonia. To confirm necroptosis in vivo, we administered Nec-1 intraperitoneally to block RIP1 and found that it inhibited LDH release in BALF of WT mice at 24 hpi (Figure 6a). To identify if necroptosis alters γδ T cell-derived IL-17A signaling axis, we enumerated percentage of total and IL-17A producing γδ T cells in the lungs of S. aureus infected Mlkl−/− mice. As anticipated, Mlkl−/− mice had a higher percentage of IL-17A+ γδ T cells, but not total γδ T cells, at 24 hpi (Figures 6b, c, and Supplementary Figure S5a). Furthermore, the genetic ablation or blockade of MLKL with GW806742X impaired IL-17A production, neutrophil recruitment and bacterial clearance from BALF and lungs of Nlrc4−/− mice (Figures 6d-g). Previous reports have shown that NLRC4 processed IL-18 is deleterious in P. aeruginosa-induced pneumonia 23, 28. In this regard, mice lacking expression of Il18 had increased total and IL-17A producing γδ T cells compared to WT controls, with numbers comparable to those of Nlrc4−/− mice (Figures 6h, i, and Supplementary Figure 5b). Similarly, administration of recombinant IL-18 in Nlrc4−/− mice decreased both total and IL-17A producing γδ T cells in the lungs at 24 hpi (Figures 6h, i, and Supplementary Figure 5b). However, deficiency of IL-1β, a key NLRC4-dependent cytokine, did not alter the frequency of γδ T cells in S. aureus pneumonia (Figures 6j, k, and Supplementary Figure 5c). Intriguingly, IL-1β−/− mice did augment host defense with enhanced neutrophil recruitment and bacterial clearance, similar to Nlrc4−/− and IL-18−/− (Figures 81, m). Taken together, our results indicate that NLRC4-mediated necroptosis and IL-18 act synergistically, independent of IL-1β, to suppress the recruitment of γδ T cells, which dampens IL-17A-mediated antibacterial defense in S. aureus pneumonia.
Figure 6. NLRC4-driven necroptosis and IL-18 suppress 17A-dependent neutrophil recruitment by limiting γδ T cell expansion.
(a) WT and Nlrc4−/− mice were treated with Necrostatin-1 or DMSO intraperitoneally 12 hours prior to infection with S. aureus (5 ×107 CFU/mouse). RFU of LDH release in BALF was measured at 24 hpi. (n= 5 mice /group). (b-g) WT, Mlkl−/− and Nlrc4−/− mice were infected with S. aureus (5 ×107 CFU/mouse) intratracheally with administration of GW806742X (MLKL inhibitor) or DMSO intraperitoneally 1 hour prior. BALF and lungs were harvested at 24 hpi. (b) The percentage of total γδ T cells and (c) IL-17A producing γδ T cells in the lungs were determined by flow cytometry. (d) IL-17A level, (e) number of neutrophils, and (f) bacterial burden in BALF, and (g) lungs were quantitated. Each symbol represents a single mouse. (n= 4-6 mice /group). (h-m) WT, IL-18−/−, Il-1β−/−, and Nlrc4−/− mice were infected with S. aureus (5 ×107 CFU/mouse) intratracheally. At 24 hpi, the lungs were harvested and processed for flow cytometric analysis. (h) The percentage of total γδ T cells and (i) IL-17A producing γδ T cells in the lungs of WT, IL-18,−/−, and Nlrc4−/− mice receiving recombinant murine IL-18 (1μg/mouse) or PBS 1 hpi were enumerated. (j) The percentage of total γδ T cells and (k) IL-17A producing γδ T cells in the lungs of WT, Il-1β,−/− and Nlrc4−/− mice. (l) The number of neutrophils in BALF and (m) bacterial burden in the lungs at 24 hpi. Each symbol represents a single mouse. (n= 4-6/group). Data from a representative experiment are shown. All experiments were performed three times. Statistical significance was determined by one-way ANOVA (followed by Bonferroni’s post hoc comparisons) in all experiments. *p<0.05; **p<0.01.
Blockade of NLRC4 signaling or necroptosis improves host defense in S. aureus pneumonia.
Like other inflammasomes, activation of NLRC4 results in cleavage of caspase-1 leading to production of IL-1β and IL-18 during S. aureus infection (Figure 3). To determine if deficiency of caspase-1 (downstream of NLRC4) results in a similar outcome in S. aureus pneumonia, mice lacking expression of Casp1/11 or pre-treated with Ac-yvad-cmk (caspase-1 inhibitor) were infected with S. aureus. As compared to WT controls, mice lacking Casp1/11 or WT mice pre-treated with Ac-yvad-cmk demonstrate reduced bacterial burden in the BALF and lungs as well as improved survival (Supplementary Figures S6a-c). To determine if blockade of necrosome components also had a similar effect in S. aureus pneumonia, we infected mice pre-treated with Nec-1 with S. aureus. As anticipated, blockade of RIP1 with Nec-1 enhanced neutrophil recruitment and subsequent bacterial clearance in the BALF, which was associated with improved host survival rates (Supplementary Figures S6d-f). As S. aureus-induced NLRC4 activation was dependent on PKC-δ (Figures 3e), we investigated the role of PKC-δ inhibition in host defense. To this end, mice were pretreated with Rottlerin or DMSO at 12 hours prior to infection. As expected, Rottlerin pre-treated mice show reduced IL-1β production, increased neutrophil influx and bacterial clearance at 24 hpi (Supplementary Figures S6g-i). Collectively, our results indicate that the inhibition of the NLRC4 signaling or necroptosis signaling cascades improves host defense against S. aureus infection.
DISCUSSION
The pathogenesis of Gram-positive (S. aureus) pneumonia is mediated by its several virulence factors, such as exotoxins and surface proteins. These virulence factors target different innate signaling molecules in infiltrating leukocytes and resident epithelial cells. Moreover, growing evidence indicates that S. aureus is able to exploit innate signaling pathways, as mice deficient in Adam10, Nlrp3, Nod2, Ifnr-1, and Tnfr expression showed augmented host defense against this pathogen 10-14. Although toxin-induced necroptotic lung damage has been implicated in the pathogenesis of S. aureus pneumonia 17, the precise mechanism underlying the subversion of host immunity is largely unknown. We report that NLRC4 activation leads to necroptotic cell death and IL-18 production, which in turn impedes host defense in S. aureus pneumonia. Furthermore, we demonstrate a novel mechanism by which NLRC4-driven necroptosis and IL-18 production suppress γδ T cell-derived IL-17A-dependent neutrophil recruitment during S. aureus infection. Finally, we show that blockade or genetic deficiency in NLRC4 signaling cascade and necroptosis pathway improve host defense in the context of S. aureus pneumonia.
Pulmonary host defense regulated by the NLRC4 is protective against Gram-negative pathogens, such as K. pneumoniae 21, L. pneumophila 29, 30 and B. pseudomallei 31, but deleterious against P. aeruginosa 23,32. In contrast, our study reports NLRC4 activation is deleterious in the context of Gram-positive pathogens, as Nlrc4−/− mice show improved host defense and survival. In accordance with this, activation of other NOD-like receptors has also been shown to be detrimental during S. aureus infection. For example, Nod2−/− and Nlrp3−/− mice are less susceptible to S. aureus pneumonia 12, 13. Because NLRP3 recognizes a broad range of pathogens, including S. aureus 33, NLRP3 activation was thought to be critical for the pathogenesis of S. aureus pneumonia. However, Nlrp3−/− mice do not have a remarkable phenotype in S. aureus pneumonia, suggesting involvement of an NLRP3-independent host defense mechanism in the response to this pathogen 13. We showed both myeloid and resident cells express NLRC4 during S. aureus pneumonia. Our bone marrow chimeras reveal that NLRC4 expression in both compartments is detrimental with regards to bacterial control. It is important to note that WT → Nlrc4−/− group (hematopoietic) had defective bacterial control in the lungs compared to other chimeric groups. Since neutrophil are a major player of S. aureus clearance and Nlrc4−/− neutrophils are more efficient in controlling bacterial burden, it is possible that NLRC4 expression in myeloid cells of WT → Nlrc4−/− (only hematopoietic expression) group may have dampened phagocytic function of these cells.
Based on data from experiments in THP-1 cells and BMDMs, the NLRC4 was important for caspase-1 activity and IL-1β production in response to S. aureus infection. Our findings also indicate that α-hemolysin induces phosphorylation of the NLRC4, which is a PKC-δ-dependent process like in S. typhimurium-induced NLRC4 activation in macrophages 24. However, α-hemolysin expression in S. aureus was dispensable for caspase-1 processing. In addition, pulmonary infection with K. pneumoniae or P. aeruginosa (non-flagellated strains) has been shown to activate the NLRC4 in vivo 21, 22, indicating that virulence factors other than flagellin can activate NLRC4. In this regard, recent reports related to the involvement of NLRC4 activation in obesity-associated tumors, in progression of melanoma tumors, and in diabetic nephropathy suggest endogenous host proteins may serve as signals for NLRC4 activation 34-36. Therefore, it is possible that unidentified bacterial components of S. aureus or host-derived proteins may act as homologues to known NLRC4-activating bacterial signals.
A number of S. aureus toxins are known to activate necroptosis, a highly pro-inflammatory mode of cell death. S. aureus-derived pore-forming toxins like Hla, Leukocidin AB, and PSMs leading to cytotoxicity has been well characterized 7, 8, 37. Further, necroptosis results in irreparable loss of alveolar macrophages and anti-inflammatory signals, impeding clearance of S. aureus 17. In addition to reduced phosphorylation of MLKL in the lungs of Nlrc4−/− mice, we also found that NLRC4 interacts with RIP3 suggesting a potential role of NLRC4 in necrosome formation. However, it remains unknown if NLRC4 activation also alters the K+ efflux or contributes to membrane destabilization leading to cell lysis. Unlike Nlrp3−/−, Nod2−/−, or Rip3−/− mice infected with S. aureus 12, 13, 17, the central feature of protection in Nlrc4−/− mice is their ability to recruit sufficient numbers of neutrophils to the lungs, which is impaired in WT mice. In Nlrc4−/− mice, this occurs through the increased frequency of γδ T cells, which provide the IL-17A signal to drive neutrophil influx. Previous studies have shown that IL-17A stimulates granulopoesis in bone marrow and regulates expression of CXC chemokines in lungs to recruit neutrophils 38, 39. In our model, it appears that IL-17A may have contributed to neutrophil recruitment via increased CXCL5 and G-CSF production in Nlrc4−/− mice. While numerous immune cells can produce IL-17A at different stages of infection, many studies have highlighted the importance of early IL-17A production by γδ T cells during infection with S. aureus 26, 40, 41 and other extracellular bacteria 39, 42. In addition to γδ T cells, the increased frequency of IL-17A+ neutrophils at 24 hours post- infection may explain that neutrophils are also involved in the recruitment through an autocrine loop. Interestingly, MLKL deficiency or blockade of necroptosis with Nec-1 or MLKL inhibitors improves bacterial clearance, which is also associated with an increased frequency of IL-17A+ γδ T cells and neutrophil recruitment. In addition to necroptosis, NLRC4-induced IL-18 production was found to suppress the γδ T cell-IL-17A-axis, as IL-18 deficiency resulted in improved neutrophil recruitment and clearance of S. aureus. Similar to our results, IL-18 has also been shown to impede host defense in pneumonia and other disease models 23, 28, 43. However, IL-1β, another NLRC4-dependent cytokine, was surprisingly found to be dispensable for this pathway. Thus, these two seemingly disparate NLRC4-dependent mechanisms, the necroptosis and IL-18, work synergistically to impede neutrophil recruitment in S. aureus pneumonia. However, future studies will be required to determine how deficiency of necroptosis and IL-18 leads to the increased frequency of IL-17A producing γδ T cells as early as 6 hpi.
The precise mechanism through which PKC-δ-dependent NLRC4 phosphorylation occurs following Gram-positive (S. aureus) infection remains unclear. It should be noted that a wide range of pathogens or host-derived factors, along with environmental pollutants, can activate NLRP3 33. Along the same lines, it is possible that NLRC4 may recognize host-derived ligands during bacteria-induced inflammation in addition to recognizing pathogens /or their components. For example, since S. aureus - derived pore forming toxins destabilize the membrane of cells, it is possible that endogenous inflammatory ligands are released and subsequently activates NLRC4. However, elucidation of the specific ligands and mechanisms responsible for NLRC4 activation during Gram-positive S. aureus infection of the lung will require future studies.
METHODS
Animals.
Nlrc4−/− mice were generated as described44. The generation of Il-17a−/−, Il-17f−/−, Il-17ra−/−, Casp1−/−/11−/−, Il-1β−/−, IL-18−/− and Mlkl−/− were described previously 22, 27, 45-48. All mouse strains were backcrossed onto the C57BL/6J genetic background at least 10 times. Age and gender-matched WT controls were used. The Institutional Animal Care and Use Committee of the Louisiana State University approved all animal experiments.
Pneumonia model.
The S. aureus USA300 strain, its isogenic α-hemolysin (hla)- deficient, and MSSA Newman strain were used to induce pneumonia, as described previously 21, 49. Bacterial preparation is detailed in supplementary methods. After anesthesia with xylazine/ketamine mix, mice were intratracheally inoculated with S. aureus at a concentration of 5 ×107 CFUs/50μl/mouse for pneumonia or 2 × 108 CFUs/50μl/mouse for survival. Neutrophil depletion was achieved with intraperitoneal injection of 500 μg of anti-Ly6G ab (IA8) (Biolegend) 24 hours and 2 hours prior to infection50. In some experiments, mice were injected intraperitoneally with 300 μg of necrostatin-1 (RIP-1 inhibitor) (Calbiochem), 150 μg of Ac-yvad-cmk (Caspase-1 inhibitor) (Caymen Chemical), 200 μg of murine anti-IL-17A antibody (eBiosccience), 100 μg of anti-γδ-TCR antibody (Biolegend), or 200 μg of Rottlerin (PKC-δ inhibitor) (Calbiochem) 12 hours prior to infection as described with or without slight modifications 17, 51, 52. In other experiments 100 μl of 10 μM of GW806742X (MLKL-inhibitor) (Synkinase) was administered intraperitoneally 1 hour prior to infection18. In additional experiments, mice were treated intratracheally with 1 μg recombinant IL-17A or IL-18 (R&D Systems) 1 hpi21, 26. Control mice received PBS or DMSO, or control rat IgG2a, as appropriate.
BALF collection, cell counts, and bacterial burden.
BALF and organs were collected as described earlier 21. In brief, mice were sacrificed, the trachea was exposed and cannulated with 20-gauge catheter, and the lungs were flushed four times with 0.8 mL of PBS containing heparin/dextrose. Total and differential cell counts were performed on BALF using light microscopy utilizing Quik-Dip Stain. Lungs/spleen were excised, homogenized in PBS, and plated in serial dilutions on TSA plates to quantitate the bacterial burden in organs after overnight incubation at 37 °C.
Immunofluorescence microscopy.
Immunofluorescence staining for NLRC4 expression on tissue slides was performed as described previously 39. Paraffin-embedded lung sections from healthy or diseased humans (with clinical diagnosis of unspecified bacterial pneumonia) were obtained from Biochain Institute Inc. USA. Following primary antibodies were used: anti-NLRC4 Ab (Cell Signaling), anti-lipocalin-2 Ab for PMNs (R&D systems), anti-CD68 Ab for macrophages (Biolgend) and anti-CD326 Ab for epithelial cells (Biolegend). For mouse slides; we used anti-NLRC4 Ab (EMD Millipore), anti-Ly6G+ Ab for PMNs (Biolegend), anti-CD326 Ab for epithelial cells (Biolegend), and the anti-F4/80 Ab for myeloid cells (Biolegend). Similarly, anti-RIP3 Ab (Cell Signaling) and anti-MLKL (phospho S345) Ab (Abcam) were used to study necroptosis in mouse BMDMs. Alexa conjugated secondary antibodies (Invitrogen) were used.
Flow cytometry.
For flow cytometric analysis, lungs were excised, minced, digested for 90 min at 37°C in collagenase (2 mg/ml) (Worthington) and DNase I (20 U/ml) (Roche) to obtain single cell suspensions. The following surface marker antibodies were purchased from eBioscience: anti-CD11b (clone M1/70), Ly6G (1A8,), F-4/80 (BM8), CD3 (17A2), CD4 (GK1.5), CD8α (53-6.7), γδ-TCR (GL3), and NK1.1 (PK136). For intracellular staining, cells were incubated for 4-5 hours with 3 mg/ml of brefeldin A (eBioscience). The BD Cytofix/Cytoperm Kit was used to permeabilize and fix cells before staining with IL-17A (17B7, eBioscience). Isotype controls were used for compensation. Cells were acquired on a FACs caliber (BD Biosciences). FlowJo 10 (Treestar) was used to analyze data.
Western blotting and immunoprecipitation.
Western blotting for lung homogenates and cell lysates was performed as described in previous publications 21. Antibodies to NLRC4 (EMD Millipore), NLRC4 (phospho S533) (ECM Biosciences), Caspase-1 (Adipogen), RIP3 (Cell Signaling), MLKL (phospho S345) (Abcam), and GAPDH (Cell Signaling) were used. The immunoprecipitation Kit, Dynabeads™ Protein G (Invitrogen), was used to immunoprecipitate RIP3 and NLRC4 according to the manufacturer’s protocol.
Bone marrow chimeras.
Bone marrow transplantation was performed to create Nlrc4−/− chimera mice wherein the Nlrc4 deficiency was confined to either the hematopoietic cells (Nlrc4−/− → WT) or non-hematopoietic tissue (WT → Nlrc4−/− as described in our previous publications 21, 39. In brief, recipient mice were lethally irradiated with a single dose of 1000 rad. Each recipient mouse received a tail vein injection of marrow cells (4 ×106/mouse) and was kept on 0.2% neomycin-sulfate drinking water for 2 weeks. Pneumonia was induced at 2 months after the reconstitution. Four groups were generated (WT → WT, WT → Nlrc4−/−, Nlrc4−/− → WT, and Nlrc4−/− → Nlrc4−/−). Our routine procedure results in at least 75 - 85% reconstitution of blood leukocytes from donor mice, as confirmed by flow cytometry on donor cells expressing GFP.
Statistical analysis.
Data are expressed as means ± standard error of mean (s.e.m.) unless otherwise stated. Prism 7.0a software (GraphPad Software Inc.) was used for statistical analyses. The unpaired t-test, Mann-Whitney U test (non-parametric), or one-way ANOVA (followed by Bonferroni’s post hoc comparisons) were used to analyze differences between groups, as appropriate. Survival curves (Kaplan-Meier plot) were compared using log-rank tests. A P-value of *<0.05, **p<0.01, and ***p<0.001 was considered significant.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by NIH grants F31HL137287 to SP, R01AI113720, R01HL091958 and R21AI133681-01 to SJ, and R01HL133336 to SC. We thank Drs. Juliane Bubeck-Wardenburg (Washington University School of Medicine, St. Louis) for α-hemolysin deficient strains of S. aureus USA300 and Jiahuai Han (Xiamen University, China) for providing Mlkl−/− mice. We thank Marilyn Dietrich for flow cytometry. We also thank the Lung Biology Laboratory members, including Tirumalai Rangasamy, Scott Bergeron, Sangeetha Ravi Kumar, Joseph DeCorte, and John Le for helpful discussion.
REFERENCES
- 1.Chertow DS, Memoli MJ. Bacterial coinfection in influenza: a grand rounds review. JAMA 2013; 309(3): 275–282. [DOI] [PubMed] [Google Scholar]
- 2.David MZ, Daum RS. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev 2010; 23(3): 616–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.King MD, Humphrey BJ, Wang YF, Kourbatova EV, Ray SM, Blumberg HM. Emergence of community-acquired methicillin-resistant Staphylococcus aureus USA 300 clone as the predominant cause of skin and soft-tissue infections. Ann Intern Med 2006; 144(5): 309–317. [DOI] [PubMed] [Google Scholar]
- 4.Francis JS, Doherty MC, Lopatin U, Johnston CP, Sinha G, Ross T et al. Severe community-onset pneumonia in healthy adults caused by methicillin-resistant Staphylococcus aureus carrying the Panton-Valentine leukocidin genes. Clin Infect Dis 2005; 40(1): 100–107. [DOI] [PubMed] [Google Scholar]
- 5.Garnier F, Tristan A, Francois B, Etienne J, Delage-Corre M, Martin C et al. Pneumonia and new methicillin-resistant Staphylococcus aureus clone. Emerg Infect Dis 2006; 12(3): 498–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diep BA, Otto M. The role of virulence determinants in community-associated MRSA pathogenesis. Trends Microbiol 2008; 16(8): 361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Craven RR, Gao X, Allen IC, Gris D, Bubeck Wardenburg J, McElvania-Tekippe E et al. Staphylococcus aureus alpha-hemolysin activates the NLRP3-inflammasome in human and mouse monocytic cells. PLoS One 2009; 4(10): e7446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DuMont AL, Yoong P, Day CJ, Alonzo F 3rd, McDonald WH, Jennings MP et al. Staphylococcus aureus LukAB cytotoxin kills human neutrophils by targeting the CD11b subunit of the integrin Mac-1. Proc Natl Acad Sci U S A 2013; 110(26): 10794–10799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G et al. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem 2003; 278(11): 8869–8872. [DOI] [PubMed] [Google Scholar]
- 10.Inoshima I, Inoshima N, Wilke GA, Powers ME, Frank KM, Wang Y et al. A Staphylococcus aureus pore-forming toxin subverts the activity of ADAM10 to cause lethal infection in mice. Nat Med 2011; 17(10): 1310–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomez MI, Lee A, Reddy B, Muir A, Soong G, Pitt A et al. Staphylococcus aureus protein A induces airway epithelial inflammatory responses by activating TNFR1. Nat Med 2004; 10(8): 842–848. [DOI] [PubMed] [Google Scholar]
- 12.Kapetanovic R, Jouvion G, Fitting C, Parlato M, Blanchet C, Huerre M et al. Contribution of NOD2 to lung inflammation during Staphylococcus aureus-induced pneumonia. Microbes Infect 2010; 12(10): 759–767. [DOI] [PubMed] [Google Scholar]
- 13.Kebaier C, Chamberland RR, Allen IC, Gao X, Broglie PM, Hall JD et al. Staphylococcus aureus alpha-hemolysin mediates virulence in a murine model of severe pneumonia through activation of the NLRP3 inflammasome. J Infect Dis 2012; 205(5): 807–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parker D, Planet PJ, Soong G, Narechania A, Prince A. Induction of type I interferon signaling determines the relative pathogenicity of Staphylococcus aureus strains. PLoS Pathog 2014; 10(2): e1003951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cai Z, Jitkaew S, Zhao J, Chiang HC, Choksi S, Liu J et al. Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat Cell Biol 2014; 16(1): 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaczmarek A, Vandenabeele P, Krysko DV. Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity 2013; 38(2): 209–223. [DOI] [PubMed] [Google Scholar]
- 17.Kitur K, Parker D, Nieto P, Ahn DS, Cohen TS, Chung S et al. Toxin-induced necroptosis is a major mechanism of Staphylococcus aureus lung damage. PLoS Pathog 2015; 11(4): e1004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzalez-Juarbe N, Gilley RP, Hinojosa CA, Bradley KM, Kamei A, Gao G et al. Pore-Forming Toxins Induce Macrophage Necroptosis during Acute Bacterial Pneumonia. PLoS Pathog 2015; 11(12): e1005337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miao EA, Alpuche-Aranda CM, Dors M, Clark AE, Bader MW, Miller SI et al. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat Immunol 2006; 7(6): 569–575. [DOI] [PubMed] [Google Scholar]
- 20.Miao EA, Mao DP, Yudkovsky N, Bonneau R, Lorang CG, Warren SE et al. Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proc Natl Acad Sci U S A 2010; 107(7): 3076–3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cai S, Batra S, Wakamatsu N, Pacher P, Jeyaseelan S. NLRC4 inflammasome-mediated production of IL-1beta modulates mucosal immunity in the lung against gram-negative bacterial infection. J Immunol 2012; 188(11): 5623–5635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sutterwala FS, Mijares LA, Li L, Ogura Y, Kazmierczak BI, Flavell RA. Immune recognition of Pseudomonas aeruginosa mediated by the IPAF/NLRC4 inflammasome. J Exp Med 2007; 204(13): 3235–3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faure E, Mear JB, Faure K, Normand S, Couturier-Maillard A, Grandjean T et al. Pseudomonas aeruginosa type-3 secretion system dampens host defense by exploiting the NLRC4-coupled inflammasome. Am J Respir Crit Care Med 2014; 189(7): 799–811. [DOI] [PubMed] [Google Scholar]
- 24.Qu Y, Misaghi S, Izrael-Tomasevic A, Newton K, Gilmour LL, Lamkanfi M et al. Phosphorylation of NLRC4 is critical for inflammasome activation. Nature 2012; 490(7421): 539–542. [DOI] [PubMed] [Google Scholar]
- 25.Rigby KM, DeLeo FR. Neutrophils in innate host defense against Staphylococcus aureus infections. Semin Immunopathol 2012; 34(2): 237–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho JS, Pietras EM, Garcia NC, Ramos RI, Farzam DM, Monroe HR et al. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J Clin Invest 2010; 120(5): 1762–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of interleukin-17 family members. Immunity 2011; 34(2): 149–162. [DOI] [PubMed] [Google Scholar]
- 28.Schultz MJ, Knapp S, Florquin S, Pater J, Takeda K, Akira S et al. Interleukin-18 impairs the pulmonary host response to Pseudomonas aeruginosa. Infect Immun 2003; 71(4): 1630–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pereira MS, Morgantetti GF, Massis LM, Horta CV, Hori JI, Zamboni DS. Activation of NLRC4 by flagellated bacteria triggers caspase-1-dependent and -independent responses to restrict Legionella pneumophila replication in macrophages and in vivo. J Immunol 2011; 187(12): 6447–6455. [DOI] [PubMed] [Google Scholar]
- 30.Pereira MS, Marques GG, Dellama JE, Zamboni DS. The Nlrc4 Inflammasome Contributes to Restriction of Pulmonary Infection by Flagellated Legionella spp. that Trigger Pyroptosis. Front Microbiol 2011; 2: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ceballos-Olvera I, Sahoo M, Miller MA, Del Barrio L, Re F. Inflammasome-dependent pyroptosis and IL-18 protect against Burkholderia pseudomallei lung infection while IL-1beta is deleterious. PLoS Pathog 2011; 7(12): e1002452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen TS, Prince AS. Activation of inflammasome signaling mediates pathology of acute P. aeruginosa pneumonia. J Clin Invest 2013; 123(4): 1630–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ravi Kumar S, Paudel S, Ghimire L, Bergeron S, Cai S, Zemans RL et al. Emerging Roles of Inflammasomes in Acute Pneumonia. Am J Respir Crit Care Med 2018; 197(2): 160–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kolb R, Phan L, Borcherding N, Liu Y, Yuan F, Janowski AM et al. Obesity-associated NLRC4 inflammasome activation drives breast cancer progression. Nat Commun 2016; 7: 13007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Janowski AM, Colegio OR, Hornick EE, McNiff JM, Martin MD, Badovinac VP et al. NLRC4 suppresses melanoma tumor progression independently of inflammasome activation. J Clin Invest 2016; 126(10): 3917–3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yuan F, Kolb R, Pandey G, Li W, Sun L, Liu F et al. Involvement of the NLRC4-Inflammasome in Diabetic Nephropathy. PLoS One 2016; 11(10): e0164135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang R, Braughton KR, Kretschmer D, Bach TH, Queck SY, Li M et al. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat Med 2007; 13(12): 1510–1514. [DOI] [PubMed] [Google Scholar]
- 38.Schwarzenberger P, La Russa V, Miller A, Ye P, Huang W, Zieske A et al. IL-17 stimulates granulopoiesis in mice: use of an alternate, novel gene therapy-derived method for in vivo evaluation of cytokines. J Immunol 1998; 161(11): 6383–6389. [PubMed] [Google Scholar]
- 39.Cai S, Batra S, Del Piero F, Jeyaseelan S. NLRP12 modulates host defense through IL-17A-CXCL1 axis. Mucosal Immunol 2016; 9(2): 503–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng P, Liu T, Zhou WY, Zhuang Y, Peng LS, Zhang JY et al. Role of gamma-delta T cells in host response against Staphylococcus aureus-induced pneumonia. BMC Immunol 2012; 13: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maher BM, Mulcahy ME, Murphy AG, Wilk M, O'Keeffe KM, Geoghegan JA et al. Nlrp-3-driven interleukin 17 production by gammadeltaT cells controls infection outcomes during Staphylococcus aureus surgical site infection. Infect Immun 2013; 81(12): 4478–4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dubin PJ, Kolls JK. Th17 cytokines and mucosal immunity. Immunol Rev 2008; 226: 160–171. [DOI] [PubMed] [Google Scholar]
- 43.Dolinay T, Kim YS, Howrylak J, Hunninghake GM, An CH, Fredenburgh L et al. Inflammasome-regulated cytokines are critical mediators of acute lung injury. Am J Respir Crit Care Med 2012; 185(11): 1225–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature 2004; 430(6996): 213–218. [DOI] [PubMed] [Google Scholar]
- 45.Kuida K, Lippke JA, Ku G, Harding MW, Livingston DJ, Su MS et al. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science 1995; 267(5206): 2000–2003. [DOI] [PubMed] [Google Scholar]
- 46.Zheng H, Fletcher D, Kozak W, Jiang M, Hofmann KJ, Conn CA et al. Resistance to fever induction and impaired acute-phase response in interleukin-1 beta-deficient mice. Immunity 1995; 3(1): 9–19. [DOI] [PubMed] [Google Scholar]
- 47.Takeda K, Tsutsui H, Yoshimoto T, Adachi O, Yoshida N, Kishimoto T et al. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity 1998; 8(3): 383–390. [DOI] [PubMed] [Google Scholar]
- 48.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med 2001; 194(4): 519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kulkarni R, Caskey J, Singh SK, Paudel S, Baral P, Schexnayder M et al. Cigarette Smoke Extract-Exposed Methicillin-Resistant Staphylococcus aureus Regulates Leukocyte Function for Pulmonary Persistence. Am J Respir Cell Mol Biol 2016; 55(4): 586–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ocuin LM, Bamboat ZM, Balachandran VP, Cavnar MJ, Obaid H, Plitas G et al. Neutrophil IL-10 suppresses peritoneal inflammatory monocytes during polymicrobial sepsis. J Leukoc Biol 2011; 89(3): 423–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Skelsey ME, Mellon J, Niederkorn JY. Gamma delta T cells are needed for ocular immune privilege and corneal graft survival. J Immunol 2001; 166(7): 4327–4333. [DOI] [PubMed] [Google Scholar]
- 52.Markel G, Bar-Haim E, Zahavy E, Cohen H, Cohen O, Shafferman A et al. The involvement of IL-17A in the murine response to sub-lethal inhalational infection with Francisella tularensis. PLoS One 2010; 5(6): e11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.