Abstract
Purpose:
For infants with localized rhabdomyosarcoma enrolled on Children’s Oncology Group (COG) ARST0331 and ARST0531, local therapy guidelines were provided but adherence was not mandated given toxicity concerns. We examined adherence to protocol local therapy guidelines, treatment variations, and outcomes for these infants.
Methods:
Children ≤24 months enrolled on ARST0331 and ARST0531 were evaluated. Data were verified through radiologic, surgical, pathologic, and clinical records. Local therapy was assessed for adherence to protocol guidelines, with variations termed individualized local therapy. Subdistribution hazards model assessed local failure (LF); Kaplan-Meier product limit method assessed event-free survival (EFS), and overall survival (OS); log-rank test was used to evaluate prognostic impact.
Results:
124 patients were eligible. Median age was 14 months. Common primary sites were genitourinary (40%), parameningeal (14%), and extremity (11%). Most patients had unresected disease (group III, 64%) and embryonal histology (73%).
58% of patients received radiation therapy (RT) at a median of week 12 (week 1–45). Median radiation dose was 48.6Gy (30.6–54Gy). 43% received individualized local therapy (outside protocol guidelines), typically omission or delay of RT. Delayed primary excision (DPE) was performed in 28% at a median of week 14 (week 7–34). With a median follow-up of 5.6 years, the 5-year LF, EFS, and OS rates were 24%, 68%, and 82%. Local failure was significantly higher in patients receiving individualized local therapy (35%) than in patients who received protocol-specified local therapy (16%; p=0.02). Site of failure was local in 64%, local and distant in 5%, and distant only in 23%. EFS was significantly higher among patients aged 12–24 months, tumors ≤5cm, group I/II disease, and use of protocol-specified therapy.
Conclusions:
Local recurrence was the predominant pattern of failure and more common in those receiving individualized local therapy. De-escalation of effective therapies due to concerns about treatment toxicity should be considered cautiously.
Summary
Infants (≤ 24 months old) enrolled on Children’s Oncology Group ARST0331 and ARST0531 protocols were permitted to undergo individualized local therapy. This retrospective review assesses the treatment approach and outcomes for these infants. While local control, event-free survival, and overall survival did not differ from older children on these protocols, individualized therapy was associated with a lower 5-year event-free survival and double the rate of local failure compared to protocol-specified therapy.
Introduction
Rhabdomyosarcoma (RMS) is the most common childhood sarcoma, and curative therapy includes systemic chemotherapy and local therapy consisting of surgery, radiation therapy (RT), or both (1). Patient age has prognostic importance, with inferior outcomes in adolescents >10 years and infants <1 year (2, 3). Although failure-free survival (FFS) for children with localized RMS has increased over the past 50 years, the youngest children have not experienced the same improvement in outcome. FFS has been significantly worse for infants <1 year of age compared to those aged 1 to 9 years on both North American (4) and European (5) treatment protocols. When controlled for stage and group, age < 1 year remains an independent prognostic factor for inferior outcome (2, 6). These differences in outcome have been attributed to a decrease in therapy for infants due to concerns about treatment toxicity (6, 7).
In recent Children’s Oncology Group (COG) low- and intermediate-risk protocols (ARST0331 and ARST0531, respectively), treating physicians were encouraged to adhere to local therapy protocol guidelines; however, given toxicity concerns, adherence to local therapy guidelines was not mandated for patients ≤24 months of age (8–10). For these young children, individualized local therapy that varied from the protocol guidelines was not considered a protocol deviation. Allowance of local therapy variance could improve the ability to recruit and retain infants on the protocol. We report the outcomes and treatment strategies employed for children aged ≤24 months enrolled on the COG ARST0331 and 0531 protocols.
Materials and Methods
Study Population
Children ≤24 months of age (infants) at the date of enrollment on COG protocols ARST0331 (low-risk) and ARST0531 (intermediate-risk) were eligible for retrospective analysis. These protocols were approved by institutional review boards and informed consent was obtained prior to enrollment. All 126 eligible patients had localized disease. Primary site, disease stage, and group were defined according to COG standard definitions (4). Stage and group were verified by reviewing radiographic, surgical, pathologic, and clinical records, resulting in ineligibility for 2 patients with higher-risk disease.
Protocol Specified Treatment
Radiation was not recommended for embryonal RMS group I disease. However, RT was administered at 36 Gy for alveolar RMS group I disease except in situations where the tumor bed no longer exists following surgery. Clinical group II patients received either 36 Gy for node-negative or 41.4 Gy for node-positive disease. Clinical group III patients received 50.4 Gy. In both studies, gross tumor volume (GTV) was determined by imaging at diagnosis (GTV1) and after induction chemotherapy (GTV2). An additional 1-cm margin, edited for normal anatomic barriers, comprised the clinical target volume (CTV) 1 and CTV2, respectively. An additional ≥0.5 cm margin yielded the planning target volumes (PTV) 1 and PTV2, respectively. A reduction of the RT field to GTV2/CTV2/PTV2 was only permitted in treatment responsive tumors if the total prescribed dose was 50.4 Gy. Major deviations were defined as a >10% deviation between delivered and protocol-specified RT dose, <90% CTV coverage by 95% of the prescribed dose, and exclusion of GTV or CTV from the treatment field. Minor deviations were defined as a 6–10% variance between delivered and protocol-prescribed doses, <100% of the CTV coverage by 95% of the prescribed dose, and CTV/PTV margin less than or significantly greater than protocol specifications.
RT dosing was adjusted, but not omitted, based upon the completeness of delayed primary excision (DPE): 36 Gy for complete R0 resection, 36–41.4 Gy for microscopic residual disease (R1 resection), and 50.4 Gy for gross residual disease (R2 resection). Biopsy only was noted, but not categorized as DPE. DPE was permitted but neither encouraged nor prohibited on ARST0331 and ARST0531. The completeness of both initial resection (group) and DPE (R status) was assessed based on operative, pathologic, and imaging records. From these records it was determined whether resection resulted in the permanent loss of organ function (loss of function) or loss of a structure that was associated with decreased cosmesis or morbidity (resection of vital structures).
All patients received chemotherapy that included vincristine, dactinomycin, and cyclophosphamide with age-specific dosing guidelines (8–10). The timing of local therapy differed between the 2 protocols: week 13 on ARST0331 and week 4 on ARST0531.
Analysis
Infants were assessed for variations to the protocol local therapy guidelines that were required for older children. Treatment of infants with variation that met the protocols’ criteria for major deviation was termed “individualized local therapy,” while those without major variation was termed “protocol-specified.” The original ARST0331 protocol did not recommend adjuvant radiation for patients with group II or III vaginal primaries that had a biopsy proven complete response to chemotherapy or underwent R0 resection through DPE. However, due to a high local recurrence rate with this approach, the protocol was amended in 2009 to require RT in this situation (11). For our analysis, omission of radiation in group II or III patients with vaginal primaries was considered individualized local therapy. Variations that met the protocols’ definitions of minor deviation were not considered individualized local therapy. Chemotherapy modifications were not assessed. The RT treatment records were reviewed at the Imaging and Radiation Oncology Core (IROC) Rhode Island (formerly Quality Assurance Review Center [QARC]) including contours, dose, fields, isodose lines, and dose-volume histograms. DPE within the first 15 weeks from initiation of chemotherapy was considered protocol-specified, but beyond week 15 was considered individualized local therapy. RT initiated more than 3 weeks before or after the protocol-specified timing was considered individualized local therapy, as was RT delivered more than 8 weeks after DPE. These are categorized as delayed radiation in this analysis.
The Chi-square test was used to compare demographic and clinical features between infants and older children. The subdistribution hazards model {Fine, 1999 #32} was used to assess the cumulative incidence of local failure; The Kaplan-Meier product limit method was used to compute the event-free survival (EFS) and overall survival (OS) rates. Local failure was defined as recurrence at the primary site with or without failure elsewhere. The method proposed by Peto-Peto was used to construct the 95% confidence interval (CI). The log-rank test was used for evaluating the prognostic impact of patient characteristics and the impact of protocol deviations.
Results
Patient and disease characteristics
The median age of the 124 eligible patients was 14.0 months (range, 0.07 to 23.4 months). The median follow-up for living patients was 5.6 years (range, 1.2 to 10.9 years). The majority of patients were male (54%), had group III disease (64%), and had embryonal histology (73%). The most common primary sites were genitourinary (GU; 40%), parameningeal (14%), and extremity (11%). Compared to older children, the infants more commonly had an unfavorable primary site (64.5% vs. 54.9%; p=0.048) (Table 1).
Table 1.
Patient characteristics on ARST0331 and ARST0531.
| Age ≤24 months (N=124) | Age >24 months (N=663) | |||
|---|---|---|---|---|
| Characteristic | No. of patients (%) | No. of patients (%) | P (chi-square) | |
| Sex | ||||
| Male | 67 (54.0) | 398 (60.0) | 0.21 | |
| Female | 57 (46.0) | 265 (40.0) | ||
| Histology* | ||||
| Alveolar | 33 (26.6) | 169 (25.5) | 0.79 | |
| Embryonal | 91 (73.4) | 494 (74.5) | ||
| Primary tumor site | ||||
| Favorable | 44 (35.5) | 299 (45.1) | 0.05 | |
| Unfavorable | 80 (64.5) | 364 (54.9) | ||
| Specific site | ||||
| GU°, non-bladder/prostate | 25 (20.2) | 142 (21.4) | Not applicable | |
| Bladder/prostate | 25 (20.2) | 44 (6.6) | ||
| Extremity | 13 (10.5) | 51 (7.7) | ||
| Head and neck | 10 (8.0) | 66 (10.0) | ||
| Intrathoracic | 1 (0.8) | 3 (0.5) | ||
| Orbit | 7 (5.6) | 82 (12.4) | ||
| Other | 4 (3.2) | 20 (3.0) | ||
| Parameningeal | 17 (13.7) | 191 (28.8) | ||
| Perineum/anus | 2 (1.6) | 9 (1.4) | ||
| Retroperitoneum | 9 (7.3) | 37 (5.5) | ||
| Trunk | 11 (8.9) | 18 (2.7) | ||
| Stage | ||||
| 1 | 44 (35.5) | 292 (44.0) | 0.08 | |
| 2 | 33 (26.6) | 125 (18.9) | ||
| 3 | 47 (37.9) | 246 (37.1) | ||
| Group | ||||
| I | 20 (16.1) | 115 (17.4) | 0.94 | |
| II | 25 (20.2) | 128 (19.3) | ||
| III | 79 (63.7) | 420 (63.4) | ||
| Tumor size (cm) | ||||
| ≤5 | 77 (62.1) | 372 (56.1) | 0.24† | |
| >5 | 45 (36.3) | 276 (41.6) | ||
| Unknown | 2 (1.6) | 15 (2.3) | ||
Histology from the institution was used for 16 patients (1 ≤ 24 months; 15 >24 months)
GU = Genitourinary
Unknown excluded
Enrollment of infants was similar between the ARST0331 and ARST0531 protocols (16%; 124/787) and the earlier D9602 (low-risk) and D9803 (intermediate-risk) protocols (15%; 149/1019) in which individualized local therapy was not permitted (12, 13).
Local therapy
Radiation was delivered using intensity-modulated photon RT in 46% of cases (33/72), 3Dconformal photons in 24% (17/72), and proton therapy in 19% (14/72). Brachytherapy (n=3), electrons alone (n=3), and intraoperative RT (n=1) were used infrequently. For one patient, radiation modality was unknown. RT was given at a median of week 12 (range, 1 to 45) for all patients. For those enrolled on ARST0331, RT was given at a median of week 16 (range, 12 to 45), and for ARST0531 patients, the median week of RT was 4 (range, 1 to 43). The median radiation dose was 48.6 Gy (range, 30.6–54 Gy). 19% (24/124) of the infants enrolled on ARST0331 and ARST0531 had omission of indicated RT, including one group I patient and 23 group II or III patients. Local therapy for group II and III infants is described in Figure 1. Of the 17 group I patients on ARST0331, none received radiation, in keeping with the protocol guidelines. On ARST0531, 1 of 3 group I patients with alveolar RMS received radiation. Per protocol guidelines, 2 patients should have received adjuvant radiation, while the third had a testicular primary tumor treated with orchiectomy and chemotherapy and thus no radiation per protocol guidelines.
Figure 1.
Local therapy for group II and III infants on ARST0331 and ARST0531 protocols consisted of radiation alone, surgery alone, surgery and radiation, and omission of local therapy
DPE was utilized as a component of local therapy in 28% (29/104) of infants with group II (n=3) and III (n=26) disease at a median of week 14 (range, week 7–34). Most patients had R0 (28%) or R1 (41%) resections, while 14% had R2 resection and 17% had an unknown extent of resection. DPE altered the RT dose in 38% of patients and radiation was omitted after DPE in 41% of patients. In addition, 2 patients did not receive RT following exam-under-anesthesia (n=1) and biopsy-only (n=1) at the time local therapy was due. Table 2 identifies both initial surgeries and DPE that resulted in loss of function or resection of vital structures.
Table 2.
Surgical resections affecting function of vital structures.
| A. LOSS OF FUNCTION | ||
| Site | At diagnosis | At time of local therapy |
| Genitourinary and/or gynecologic | Orchiectomy (n=10) | Hysterectomy, bilateral salpingectomy (n=1) Hysterectomy, cystectomy, vaginectomy (n=1) Cystourethrectomy (n=1) Urethrectomy (n=1) |
| Extremity | Amputation of foot (n=1) | Amputation of leg, below-knee (n=1) |
| B. RESECTION OF VITAL STRUCTURES | ||
| Site | At diagnosis | At time of local therapy |
| Genitourinary and/or gynecologic | Partial cystectomy with or without resection of bladder neck (n=2) Partial vaginectomy (n=1) |
Partial cystectomy (n=1) Partial vaginectomy (n=2)* |
| Extremity | Radial nerve resection (n=1) | |
| Head and Neck | Partial rhinectomy and partial maxillectomy (n=1) Medial maxillectomy (n=1) Infraorbital nerve resection (n=1) |
Parotidectomy, resection of zygomatic arch and mandibular coronoid (n=1) Superficial parotidectomy, resection of mandibular ramus and angle, resection of mandibular branch of trigeminal nerve (n=1) |
| Trunk | Resection of costal cartilage and portion of diaphragm (n=1) Hepatic lobectomy, resection of bile duct, cholecystectomy (n=1) |
|
Presumed (operative data incomplete)
Local therapy was individualized in 43% of infants (53/124), most commonly due to delay or omission of RT (Figure 2). Group III patients (51%) were more likely to receive individualized local therapy compared to group I/II patients (30%; p=0.019). While no major variations were found in target coverage or CTV/PTV expansions, minor variations occurred in 25% of the infants receiving radiation.
Figure 2.
t Types of individualized local therapy for infants treated on ARST0331 and ARST0531. Some patients had multiple factors contributing to individualized local therapy.
Outcomes
Infant 5-year EFS was 68.3% (95% CI: 57.2% to 79.4%) and OS was 81.9% (95% CI: 72.9% to 91.0%). By univariate analysis, age <1 year, primary tumor size >5 cm, and group III disease were associated with inferior EFS (Table 3). Local therapy performed later than protocol specifications did not affect EFS. However, 5-year EFS after individualized local therapy (55.6%) was lower than protocol-specified therapy (77.5%) (p=0.04). Overall survival did not differ between infants treated with individualized local therapy compared to protocol-specified therapy (78.5% vs. 84.3%, p=0.42).
Table 3.
Univariate analysis of event free survival for infants enrolled on ARST0331 and ARST0531.
| Characteristic | No. of patients (%) | 5-year Event Free Survival | P (log-rank) | |
|---|---|---|---|---|
| Age (months) | ||||
| <12 | 52 (42) | 0.58 | 0.04 | |
| ≥12 | 72 (58) | 0.76 | ||
| Site | ||||
| Favorable | 44 (36) | 0.73 | 0.58 | |
| Unfavorable | 80 (65) | 0.66 | ||
| Histology | ||||
| Alveolar | 33 (27) | 0.63 | 0.56 | |
| Embryonal | 91 (73) | 0.70 | ||
| Tumor size (cm) | ||||
| ≤5 | 77 (62) | 0.75 | 0.05* | |
| >5 | 45 (36) | 0.55 | ||
| Unknown | 2 (2) | |||
| Stage | ||||
| 1 | 44 (35) | 0.73 | 0.16 | |
| 2 | 33 (27) | 0.79 | ||
| 3 | 47 (38) | 0.57 | ||
| Group | ||||
| I | 20 (16) | 0.90 | 0.04 | |
| II | 25 (20) | 0.71 | ||
| III | 79 (64) | 0.62 | ||
| Individualized therapy | ||||
| Yes | 53 (43) | 0.56 | 0.04 | |
| No | 71 (57) | 0.77 | ||
| Local therapy | ||||
| None | 21 (17) | 0.46 | ||
| Not delayed | 68 (55) | 0.78 | 0.17† | |
| Delayed | 35 (28) | 0.62 | ||
Unknown excluded
No local therapy excluded
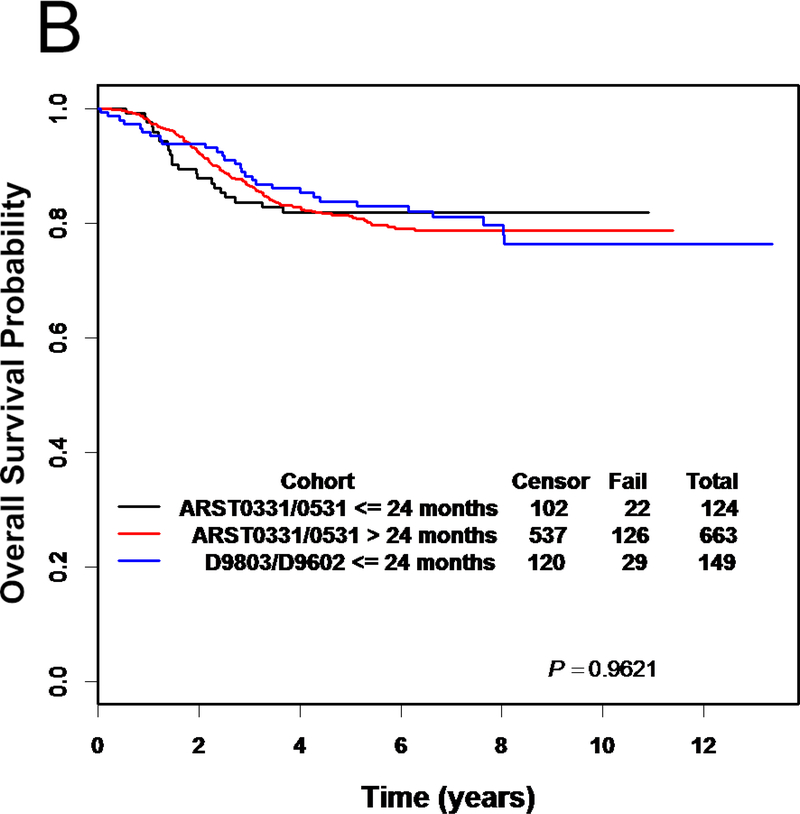
EFS and OS were not significantly different between infants and older children (>24 months) on ARST0331 and ARST0531 or from infants ≤24 months enrolled on the preceding COG RMS trials, D9602 and D9803 (Figure 3) (12, 14). No differences in EFS or OS were identified based on subgroup analysis including risk group and histology.
Figure 3.
(A) Event-free survival and (B) overall survival for infants on ARST0331 and ARST0531, older children on ARST0331 and ARST0531, and infants on D9602 and D9803
Local failure was the predominant pattern of failure (Table 4). The 5-year cumulative incidence of local failure was not significantly different between infants and older children on ARST0331 and ARST0531 (23.6% vs. 17.7%, respectively; p=0.09) (Figure 4). Most failures were local alone (64%) or had a local component (74%). Individualized local therapy was associated with inferior 5-year cumulative incidence of local failure (34.8%; 95% CI: 21.6% to 48.0%) compared to protocol-specified therapy (15.5%; 95% CI: 7.0% to 24.0%; p=0.02).. Delayed local therapy did not significantly impact cumulative incidence of local failure (26.3% vs. non-delayed 16.2%; p=0.29). Of the 24 patients who received individualized local therapy due to omission of RT (with or without DPE), 11 failed locally. Infants recurred within a range of 0.1 to 3.3 years and older children between 0.03 and 5.8 years. The median time to failure cannot be determined as the survival curve did not cross the 0.5 reference line. Distant metastases (n=12) most commonly developed in the brain (n=4), lung (n=3), and bone (n=3; range, 0.3 to 1.6 years). Distant metastases alone comprised the first site of failure in 23% of patients with disease progression.
Table 4.
Patterns of failure for infants on ARST0331 and ARST0531.
| Location of failure | No. of patients (%) |
|---|---|
| Primary site only | 25 (64) |
| Primary site + regional | 1 (3) |
| Primary site + distant | 2 (5) |
| Primary site + regional + distant | 1 (3) |
| Regional only | 1 (3) |
| Distant only | 9 (23) |
Figure 4.
Cumulative incidence of local failure for infants compared to older children treated on ARST0331 and ARST0531
Progression during therapy occurred more frequently in infants compared to older children (15.3% vs. 5.1%; p<0.0001). Progression on protocol therapy was not associated with protocol-specified vs. individualized local therapy (p=0.57) or delayed vs. on-time local therapy (p=0.80).
Discussion
The guiding principles of local therapy for RMS include disease control while maintaining form, function, and quality of life. These goals may conflict, especially in infants who are in the earliest stages of development. Because of this, varying treatment philosophies have emerged to balance these management principles. Through COG, RT has been employed in initial management to maximize local control, with a primary endpoint of EFS (15). However, the International Society of Paediatric Oncology (SIOP) withholds RT unless there is a lack of response to chemotherapy or recurrent disease, with a primary endpoint of OS (15). One goal of permitting enrollment of infants despite individualized local therapy was to increase infant accrual compared to prior COG RMS studies, like D9602 and D9803, which did not permit individualized local therapy for infants. Interestingly, the percentage of infant accrual was similar on ARST0331 and ARST0531 compared to D9602 and D9803.
For the infants ≤24 months old enrolled on ARST0331 and ARST0531, the 5-year EFS was 68.3% and OS was 81.9%. These outcomes are comparable to published outcomes of infants from previous COG studies. Although most other series define infant as ≤ 12 months, this report includes children ≤ 24 months. We selected ≤ 24 months to correspond to the age cut-off used by ARST0331 and ARST0531 to allow individualized local therapy without classification as a protocol deviation. European series frequently report lower EFS with an approach that minimizes initial RT. Orbach et al (16) reported the outcomes of 102 infants aged ≤12 months. Most survivors (78%) were treated with minimal initial RT. After a complete response to therapy, 34% of patients relapsed, 23% developed metastatic disease, and 69% developed a local recurrence. RT was not recommended for patients of any age in these protocols. Notably, 5 infants were treated with RT and all maintained local control, although definitive conclusions cannot be drawn given the small number. The EFS was 57% and OS was 72% for infants at 5 years. Unlike other COG trials, in this analysis of ARST0331 and ARST0531, no significant difference in local relapse, EFS, or OS occurred for infants compared to older children for the entire cohorts. Ferrari et al (17) described 50 patients aged ≤12 months enrolled on Italian Cooperative Group protocols. Local failure was 54% when RT was omitted due to young age, compared to 28% in those who had RT omitted per protocol. In this study, RT was used only in cases of gross, unresectable disease after induction chemotherapy. At 5 years, the infant EFS was 42.3% and OS was 61.7%, which were lower than older children (OS 67.2%). Historically, 5-year FFS of 57% and OS of 76% were achieved in the infants ≤12 months of age treated on IRS-IV, D9602, and D9803 (7). Infants ≤24 months on ARST0331 and ARST0531 experienced similar EFS and OS as infants ≤24 months enrolled on D9602 and D9803.
While EFS and local control did not vary between infants and older children when the entire cohorts were compared, individualized local therapy within the infant cohort was associated with inferior local control and EFS compared to protocol-specified therapy. EFS, local control, and OS may have been maintained because over 50% of infants were treated with protocol-specified therapy. Similarly, for infants ≤12 months of age treated on IRS-IV, D9602 and D9803, adherence to protocol guidelines was associated with higher FFS and OS (70% and 85%) compared to individualized therapy (FFS 46% and OS 64%, p=0.07 and p=0.03, respectively) (7). Our current study also shows improved EFS and local control with protocol-specified therapy, supporting the use of protocol directed local therapy in infants.
The most common RT variations in this study were delayed delivery of RT and omission of indicated RT. CTV and PTV margins were commonly below those specified by protocol, but only to the extent of yielding minor variations according to the protocol guidelines. Results from other series support the importance of RT use and quality. In an analysis of group II RMS patients, 12% recurred locally, and 55% of recurrences had a deviation in RT (41% omission, 37% dose, 20% volume) (18). In group III embryonal RMS patients who achieved gross total resection with DPE, local FFS was 91% with RT compared to 69% without (p=0.14) (19).
There are several limitations of this retrospective review. Given that local failure is the dominant first failure, this report focused solely on the individualization of local therapies. Other studies have found that chemotherapy is also individualized in these young patients (7). Indeed, ARST0331 and ARST0531 included age-specific chemotherapy dosing guidelines given toxicity concerns (20). Another limitation of this study is the lack of detailed toxicity data, affecting a balanced evaluation of the therapeutic ratio. While a primary goal is to preserve form and function, toxicity attributable to local therapy has not been collected for these patients. The surgical toxicity was estimated by assessing the organs resected and categorizing these into loss of function and resection of vital structures. The late RT toxicity for this cohort is not known. Late effects of RT in children from other series are well-documented, but few specifically report on infants, who are at the highest risk of radiation toxicity. One example is Ferrari et al (17) who reported bone and soft tissue hypoplasia in all 6 infant long-term survivors after RT. In addition, we acknowledge that while much of the published data use age < 12 months as a division for age and outcome, this report assesses those aged 24 months and less, in keeping with the protocol stipulation regarding the permissibility of individualized local therapy.
Conclusions
Infants with low- and intermediate-risk RMS treated on the ARST0331 and ARST0531 protocols experienced similar EFS and OS compared to their older counterparts. Local failure predominated as the primary pattern of failure. Individualized local therapy was common in infants and those treated with individualized local therapy had inferior EFS and local control. The use of high-quality local therapy is critical to disease control in this young patient population and adherence to protocol-specified therapy is recommended.
Acknowledgements:
We would like to acknowledge Fran Laurie and her staff at IROC Rhode Island for their assistance during review of the radiation oncology treatment planning records. We would also like to thank Jessica Kirwan and her staff at the University of Florida for manuscript preparation.
Funding: Research reported in this publication was supported by the Children’s Oncology Group and the National Cancer Institute of the National Institutes of Health under award numbers U10CA180899, U10CA180886, U10CA098543, U10CA098413, CA29511, CA18083, and by the Seattle Children’s Foundation, from Kat’s Crew Guild through the Sarcoma Research Fund
Footnotes
Conflict of interest: Julie Bradley: travel grant from Ion Beam Applications for educational program
Disclaimer: The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or Children’s Oncology Group.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Malempati S, Hawkins DS. Rhabdomyosarcoma: review of the Children’s Oncology Group (COG) Soft-Tissue Sarcoma Committee experience and rationale for current COG studies. Pediatr Blood Cancer 2012;59:5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joshi D, Anderson JR, Paidas C, et al. Age is an independent prognostic factor in rhabdomyosarcoma: a report from the Soft Tissue Sarcoma Committee of the Children’s Oncology Group. Pediatr Blood Cancer 2004;42:64–73. [DOI] [PubMed] [Google Scholar]
- 3.Meza JL, Anderson J, Pappo AS, et al. Analysis of prognostic factors in patients with nonmetastatic rhabdomyosarcoma treated on intergroup rhabdomyosarcoma studies III and IV: the Children’s Oncology Group. J Clin Oncol 2006;24:3844–3851. [DOI] [PubMed] [Google Scholar]
- 4.Crist WM, Anderson JR, Meza JL, et al. Intergroup rhabdomyosarcoma study-IV: results for patients with nonmetastatic disease. J Clin Oncol 2001;19:3091–3102. [DOI] [PubMed] [Google Scholar]
- 5.Bisogno G, Compostella A, Ferrari A, et al. Rhabdomyosarcoma in adolescents: a report from the AIEOP Soft Tissue Sarcoma Committee. Cancer 2012;118:821–827. [DOI] [PubMed] [Google Scholar]
- 6.Ragab AH, Heyn R, Tefft M, et al. Infants younger than 1 year of age with rhabdomyosarcoma. Cancer 1986;58:2606–2610. [DOI] [PubMed] [Google Scholar]
- 7.Malempati S, Rodeberg DA, Donaldson SS, et al. Rhabdomyosarcoma in infants younger than 1 year: a report from the Children’s Oncology Group. Cancer 2011;117:3493–3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walterhouse DO, Pappo AS, Meza JL, et al. Reduction of cyclophosphamide dose for patients with subset 2 low-risk rhabdomyosarcoma is associated with an increased risk of recurrence: A report from the Soft Tissue Sarcoma Committee of the Children’s Oncology Group. Cancer 2017;123:2368–2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walterhouse DO, Pappo AS, Meza JL, et al. Shorter-duration therapy using vincristine, dactinomycin, and lower-dose cyclophosphamide with or without radiotherapy for patients with newly diagnosed low-risk rhabdomyosarcoma: a report from the Soft Tissue Sarcoma Committee of the Children’s Oncology Group. J Clin Oncol 2014;32:3547–3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hawkins DS, Anderson JR, Mascarenhas L, et al. Vincristine, dactinomycin, cyclophosphamide (VAC) versus VAC/V plus irinotecan (VI) for intermediate-risk rhabdomyosarcoma (IRRMS): A report from the Children’s Oncology Group Soft Tissue Sarcoma Committee. Journal of Clinical Oncology 2014;32:10004–10004. [Google Scholar]
- 11.Walterhouse DO, Meza JL, Breneman JC, et al. Local control and outcome in children with localized vaginal rhabdomyosarcoma: a report from the Soft Tissue Sarcoma committee of the Children’s Oncology Group. Pediatr Blood Cancer 2011;57:76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raney RB, Walterhouse DO, Meza JL, et al. Results of the Intergroup Rhabdomyosarcoma Study Group D9602 protocol, using vincristine and dactinomycin with or without cyclophosphamide and radiation therapy, for newly diagnosed patients with low-risk embryonal rhabdomyosarcoma: a report from the Soft Tissue Sarcoma Committee of the Children’s Oncology Group. J Clin Oncol 2011;29:1312–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arndt CA, Stoner JA, Hawkins DS, et al. Vincristine, actinomycin, and cyclophosphamide compared with vincristine, actinomycin, and cyclophosphamide alternating with vincristine, topotecan, and cyclophosphamide for intermediate-risk rhabdomyosarcoma: children’s oncology group study D9803. J Clin Oncol 2009;27:5182–5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolden SL, Lyden ER, Arndt CA, et al. Local Control for Intermediate-Risk Rhabdomyosarcoma: Results From D9803 According to Histology, Group, Site, and Size: A Report From the Children’s Oncology Group. Int J Radiat Oncol Biol Phys 2015;93:1071–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donaldson SS, Anderson JR. Rhabdomyosarcoma: many similarities, a few philosophical differences. J Clin Oncol 2005;23:2586–2587. [DOI] [PubMed] [Google Scholar]
- 16.Orbach D, Rey A, Oberlin O, et al. Soft tissue sarcoma or malignant mesenchymal tumors in the first year of life: experience of the International Society of Pediatric Oncology (SIOP) Malignant Mesenchymal Tumor Committee. J Clin Oncol 2005;23:4363–4371. [DOI] [PubMed] [Google Scholar]
- 17.Ferrari A, Casanova M, Bisogno G, et al. Rhabdomyosarcoma in infants younger than one year old: a report from the Italian Cooperative Group. Cancer 2003;97:2597–2604. [DOI] [PubMed] [Google Scholar]
- 18.Million L, Anderson J, Breneman J, et al. Influence of noncompliance with radiation therapy protocol guidelines and operative bed recurrences for children with rhabdomyosarcoma and microscopic residual disease: a report from the Children’s Oncology Group. Int J Radiat Oncol Biol Phys 2011;80:333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cecchetto G, Carretto E, Bisogno G, et al. Complete second look operation and radiotherapy in locally advanced non-alveolar rhabdomyosarcoma in children: A report from the AIEOP soft tissue sarcoma committee. Pediatr Blood Cancer 2008;51:593–597. [DOI] [PubMed] [Google Scholar]
- 20.Arndt C, Hawkins D, Anderson JR, et al. Age is a risk factor for chemotherapy-induced hepatopathy with vincristine, dactinomycin, and cyclophosphamide. J Clin Oncol 2004;22:1894–1901. [DOI] [PubMed] [Google Scholar]