Abstract
Visceral leishmaniasis (VL), a deadly parasitic disease, is a major public health concern globally. Countries affected with VL have signed the London Declaration on Neglected Tropical Diseases and committed to eliminate visceral leishmaniasis as a public health problem by 2020. To achieve and sustain VL elimination, it will become progressively important not to miss any remaining cases in the community who can maintain transmission. This requires accurate identification of symptomatic and asymptomatic carriers using highly sensitive diagnostic tools at primary health service setting. rK39 rapid test (RDT) is the most widely used and is the first choice for decentralized diagnosis of VL in endemic areas, it has good sensitivity and specificity. This test however cannot discriminate between current, subclinical or past infections and is useless for diagnosis of relapses and as prognostic (cure) tests. Importantly, as the goal of VL elimination as a public health problem is approaching, the number of susceptible people to infection will increase. Therefore, correct diagnosis using highly sensitive diagnostic test is crucial to apply appropriate treatment and management of cases. Recent advances in molecular techniques have improved the Leishmania detection and quantification and become increasingly relevant due to possible application to a variety of clinical samples. Most importantly, given current problems in identifying asymptomatic individuals because of poor correlation between the main methods of detection, molecular tests are valuable for VL elimination programs, especially to monitor changes in burden of infection in specific communities. This review provides a comprehensive overview of the available VL diagnostics and discusses the usefulness of molecular methods in the diagnosis, quantification and species differentiation, and its clinical applications.
1. Introduction
Leishmaniasis has been identified as high-priority disease by the World Health Organization [1]. It is caused by protozoa belonging to the genus Leishmania, transmitted by the bite of a 2 to 3 millimeter long insect vector, the phlebotomine sand fly found throughout the world’s inter-tropical and temperate regions [2]. Around 21 species of Leishmania are known to be pathogenic to humans [3]. The disease occurs in three forms: self-healing or chronic cutaneous leishmaniasis (CL), mutilating mucosal or muco-cutaneous leishmaniasis (ML or MCL) and life threatening visceral leishmaniasis (VL). Each form varies in degree of severity, with visceral leishmaniasis (VL) being by far the most devastating with highest mortality.
VL, also known as Kala-azar is the most severe form of leishmaniasis, caused by the obligate intracellular protozoan parasites Leishmania donovani / L. infantum. It is estimated by WHO that worldwide 200-400 thousands new cases of VL occur annually. 90% of VL cases occur in three geographical regions: i) South East Asia: India (especially Bihar), Bangladesh, Nepal; ii) Latin America: mainly North Eastern Brazil; iii) East Africa: Sudan, Ethiopia, Kenya, Uganda and Somalia [4, 5][6, 7]. In the Indian Subcontinent, VL is now being reported in 54 districts in India, 16 upazila in Bangladesh, and 12 districts in Nepal [8]. In Europe, VL is endemic in nine countries and account for less than 2% of the global burden [9]. In Brazil, VL is endemic in 21 out of 26 states and a total of 14,859 cases were reported between 2001-2014 in 25% of Brazilian municipalities [10]. In Sudan, 17 localities in seven states are endemic for VL [11].
The leishmania parasite is transmitted by female Phlebotomine sand flies as a flagellated, metacyclic promastigote, which is phagocytised by host macrophages and then differentiates into the non-flagellated, replicative amastigotes [12]. The commonly affected organs during VL are the bone marrow, liver and the spleen [12]. Thus, clinical symptoms include hepatosplenomegaly, which is characterized by an enlarged abdomen with palpable spleen and liver. Other symptoms include long-term, low-grade fever, muscle wasting, anemia, leukopenia, polyclonal hyper-gammaglobulinemia and weight loss [13, 14]. If left untreated, it has a mortality rate of almost 100%. During an epidemic in the early 1990s in Sudan, there was an estimated 100,000 deaths. Risk of an epidemic still exists in the horn of Africa, at the junction of Eritrea, Ethiopia and Sudan, a highly endemic region where tens of thousands of refugees, returnees and agricultural workers have been resettled. Especially in Sudan, Ethiopia, Kenya, Uganda and Somalia, VL is the cause of much morbidity and mortality, and only a small minority of patients have access to diagnosis and treatment [15]. VL is endemic in several tropical and subtropical regions and has been reported from 56 countries around the world (Figure-1). Importantly, the disease affects mostly poverty stricken people with over 80% of patients living below the poverty threshold (daily income < US$) whose source of income is agriculture and /or animal husbandry [16]. More than 75% live in mud or grass covered houses. These patients are thus completely dependent for diagnosis or treatment upon charity or public health services, which remain grossly deficient in endemic areas [17, 18].
Figure-1.
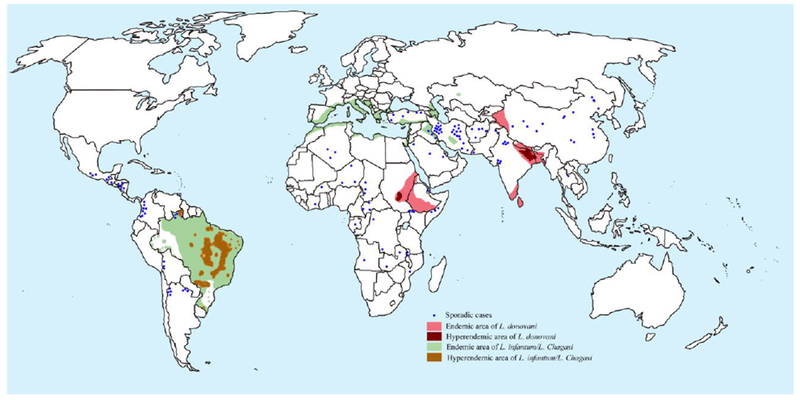
Map of global distribution of visceral leishmaniasis
Importantly, on the Indian Subcontinent, the three countries affected by VL, India, Nepal and Bangladesh, aspired to eliminate visceral leishmaniasis (VL) by 2015 (a deadline later reset to 2020). The aim is to reduce the incidence to less than 1 per 10,000 of population at sub-districts level (i.e. block level in India and Nepal; and upazila level in Bangladesh) through early diagnosis & complete treatment of cases and integrated vector management [19]. Furthermore, as countries move towards elimination goals, number of VL and Post kala-azar dermal leishmaniasis (PKDL) (characterized by skin lesions in which parasites can be identified, in a patient who is otherwise fully recovered from VL) cases will decrease, and a low number of such cases will almost inevitably lead to a decreasing awareness in the communities and in health providers. If cases of VL and PKDL are ignored or missed in such context, a new epidemic phase may start. To avoid such a scenario, there is need for development and validation of an innovative set of tools for VL and PKDL case finding, outbreak management strategy, and surveillance for infection measurement. Thus, there is a direct need for new tools to allow monitoring infections, treatment effectiveness and drug resistance. Molecular detection tools would constitute a more rapid and high throughput alternative to detect parasites. In the present review, we discuss the various molecular methods focusing on the recent developments and its clinical application in leishmania detection, absolute quantification, species differentiation and phylogenetic analysis.
2. Standard diagnostic tools and their limitations
A major challenge in the clinical management of VL is the weakness of health systems at primary care levels in many affected countries with multiple challenges and numerous constraints [8, 20]. Despite multiple techniques for confirming VL cases being available, they are all still far from being ideal. To date, observation of parasites in splenic aspirate is considered the gold standard for VL diagnosis [21]. Though, microscopic examination of spleen aspirates is rapid and cheap approach with high sensitivity and specificity, but is not practical at the PHC level. However, in Kenya, VL policy specifies that all serologically-proven leishmaniasis be confirmed by spleen aspirate, a procedure that can only be performed in referral hospitals [22]. Alternative parasitological diagnostic techniques are the lymph node or bone marrow aspirates (reviewed in [23]), the standard means of diagnosing VL in most countries. Sensitivity of such diagnostic method is highly variable and dependent on sampling procedure and technical skills of physician or personnel’s performing the tests. Although sensitivity of bone marrow is lower than splenic aspiration, the diagnostic potential in combination with serology is adequate for clinical purposes [24, 25]. Again these methods cannot be performed at PHC setting in endemic areas because of requirement of skill persons, high cost and less simplicity. Most importantly, now a day’s, bone marrow/splenic aspirates examination are recommended only when the rK39 RDT test is negative but the suspicion of VL disease is high or in VL patients diagnosed by rK39 who do not respond to first line treatment [23]. Importantly, very few practitioners currently have the skills to perform these dangerous aspirates and very limited numbers of these biopsies are taken in the ISC. Culturing leishmania promastigotes from tissue biopsies/ PBMC/ whole blood is another method of diagnosis but is expensive and requires a sophisticated laboratory [26].
Human VL is associated with high level of plasma antibodies. Although it is useful in diagnosis, but the role of antibodies in VL pathogenesis is not clear [27][28]. A number of non invasive serological tests to detect Leishmania antibodies are now available: Direct Agglutination Test (DAT) extensively validated in endemic areas and recommended by the World Health Organization (WHO) for VL control programs [21, 29]. The requirements of relatively specific material and expertise make it difficult for their use in peripheral health centers. Similarly, the Indirect Immunofluorescent Antibody Test (IFAT) requires an immune-fluorescence microscope which restricts its use to referral hospitals. Much hope is now laid on a rapid immunochromatographic test based on a recombinant 39-amino acid repeat antigen (rK39 dipstick), which despite the variability initially observed among different producers and countries, seems to be the first choice for decentralized diagnosis of VL with a good sensitivity and specificity [30, 31]. However, it shows decreased sensitivity in East Africa when compared to the Indian subcontinent [31, 32]. rK39 dipstick is stable, easy to use and it is a “rapid test” (results available in 10 minutes). The performance of this test in various studies has been comprehensively reviewed recently [23, 24, 31]. In India, Bangladesh and Nepal, the VL elimination initiative has adopted the rK39 RDT as the main tool, but its limitations are sorely felt as it cannot be used to diagnose relapse or to assess response to treatment (test of cure)[23, 33]. Approximately 10-20% of healthy people living in endemic areas are positive with this test [34, 35]. Importantly, despite its limitations, the rK39 RDT has been and still a great asset in the struggle against VL as it allows decentralizing diagnosis and treatment as close as possible to the villages where the patients live. Although rK39 RDT test represents a sound approach in highly endemic areas, it fails in situations of low infection intensity. Furthermore, these antibody detection tests are of limited used in immunocompromised patients (i.e. HIV co-infection)[23]. Antigen detection is required as a means of identifying symptomatic infections in immunocompetent and immunocompromised patients (e.g. diagnosis of primary VL in Sudan where rK39 RDTs lack sensitivity and complex diagnosis of VL relapse) and as an indicator of cure. Latex agglutination test (KATEX) detects of leishmanial antigen in urine and record the results in scoring system that correlated well with the parasite load. However, KATEX is currently not an ideal test as it had a poor sensitivity when it was tested in different centers [32, 36-38].
2.1. Molecular diagnosis and detection of infection
Most of the Leishmania species have been sequenced revealing an overall conservation of gene order, chromosome structure and discrete differences in gene content. These recent research advancements helped in development of more appropriate rapid molecular diagnostic devices and platforms [39]. However, despite the technological development, there is huge difference in using a commercially available and standardized molecular diagnostic as opposed to in-house kits. So far, several molecular methods have been developed for detection, identification, quantification and phylogenetic analysis, and these are summarized in Figure-2.
Figure 2.
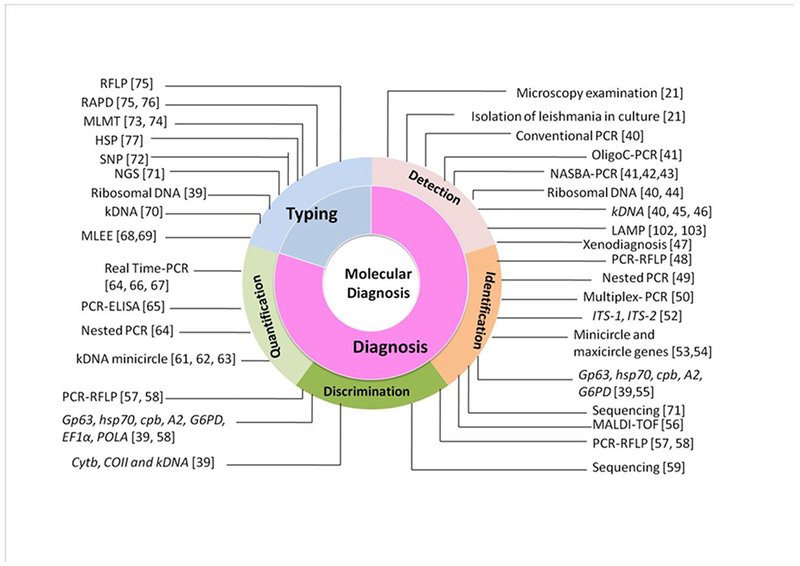
Molecular tools and markers for visceral leishmaniasis diagnosis.
One of the most sensitive and specific methods for diagnosis of clinical VL is the development polymerase chain reaction (PCR) kits that amplifies parasite DNA, and can be visually read without sophisticated equipment [44, 78-80]. Sensitivity of PCR assay mostly depends upon the biological sample used (e.g blood, bone marrow or splenic fluids etc.) and primers to amplify the target sequence (variable or conserved target region) [81, 82]. Most commonly used amplification targets are nuclear DNA such as small subunit ribosomal rRNA (SSU rRNA) gene [45, 83, 84], extra-chomosomal DNA such as repetitive kinetoplastid DNA (kDNA) [45, 46, 85], mini exon genes [86], and ribosomal internal transcribed spacer (ITS) region [52]. A comparative overview of frequently used PCR targets and its sensitivity and specificities in different tissue samples are summarized in Table-1. One of the major limitation of DNA based PCR is the counting dead parasite DNA (as half life of DNA is 24hrs within the body, which is still controversial and not proven); thus, RNA based amplification target is preferred [82, 87]. However, a reliable RNA extraction is difficult at PHC settings. Srivastava et al [44] validated 18S r-RNA based PCR on the blood of largest number of patients and controls, and found sensitivity of 87.8% (95%CI 84.1-89.8) and specificity of 94.6% (95%CI 92.8-96.1). Leishmanial DNA has been detected by PCR in peripheral blood of persons with asymptomatic infection in Brazil and recently this was also documented in India and Nepal [88-90]. To date, several cohort studies conducted in India, Nepal, Bangladesh, Italy, Ethiopia, Sudan and Brazil for detection of asymptomatic L.donovani infection in endemic villages has confirmed the increased capacity of PCR tests to detected infection in healthy individuals [89-94]. PCR assays has been also performed in non invasive samples like buccal swab and urine with sensitivity of 83% and 88% respectively [95, 96]. Molecular diagnosis using PCR is very useful in HIV-VL patients where clinical picture is confusing and, serological as well as immunological tests are not reliable due to low sensitivity [97]. Furthermore, sensitivity and specificity of PCR for detection of low level parasitemia has been shown to be significantly improved by performing nested and semi nested PCR which involves two sets of primers (targeting single gene locus) used in two successive runs. Second set of primers amplify the secondary target within the product of first PCR product [49]. Furthermore, nested PCRs are prone to contamination and not recommend except in accredited laboratories. Sensitivity and specificity of nested PCR using SSU-rRNA in diagnosis of VL is reported as 97% and 100% respectively [98]. Similarly, multiplex PCR involve amplification of different DNA targets at the same time [99]. Although such assays are more sensitive than conventional PCR, their high costs make this test not appropriate in a field setting. Another form of PCR such as OligoC-test [100], PCR-ELISA [101] and Nucleic acid sequence based amplification (NASBA) have been developed and found to be more sensitive than conventional PCR [43]. More recently, rapid and highly specific loop mediated isothermal amplification (LAMP) has emerged as powerful tool for point of care diagnosis and has been validated on VL & PKDL in several countries [102, 103]. One of the advantages of this assay is that the test can be performed without requiring sophisticated equipments, making it a more attractive tool for field based diagnosis. This assay is rapid and cost effective than conventional PCR, but limited in utility due to false positivity. Importantly, PCR-Oligo and LAMP are the only available commercially and this offers huge benefits over in-house kits in terms of reliability, of course this comes at a price. Most recently, recombinase polymerase amplification (RPA) assay (simple and molecular assay as mobile suitcase laboratory) was developed for canine VL [104]. This assay has been tested and proved promising diagnostic method for VL which tremendously decreases the cost associated with testing [105].
Table-1.
Comparison of molecular methodsin detection, differentiation, identification and quantification leishmania species.
| Molecular methods | Capacity to detect leishmania parasites in clinical samples | Levels of leishmania discrimination# | Sample used | Sensitivity (%) | Specificity (%) | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| G | SG | S | ||||||
| PCR methods | Yes | yes | yes | yes | Whole blood | 70-100 | 85-99 | [45, 46, 106] |
| Buccal swab | 79-83.00 | 86-90.56 | [107, 108] | |||||
| Urine | 88.0 -96.8 | 100 | [95] | |||||
| Bone Marrow | 95.30 -97 | 92.60-100 | [109] | |||||
| Buffy coat cells | 80-100 | 63-100 | [110] [111] |
|||||
| Serum | 85- 96 | 100 | [112] | |||||
| Blood spotted filter | 60-98 | 100 | [113] | |||||
| Bone marrow spotted filter | 99-100 | 9.0-87% | [114] | |||||
| PCR-ELISA | Yes | yes | yes | yes | Whole Blood | 83.90 - 100 | 100 | [101] |
| Real time PCR / qPCR | Yes | No | No | No | Whole blood | 90 - 100 | 83.3-100 | [61, 115, 116] |
| Buffy coat cells | 100 | 90 | [117] | |||||
| Oral fluids | 95 | 90-100 | [117] | |||||
| Oligo-C test | Yes | yes | yes | yes | Whole blood | 96.2 | 90.0 | [41, 43] |
| Lymph node | 65-96.8 | 70-100 | [41] | |||||
| Bone marrow | 89-96.9 | 57 -99 | [41] | |||||
| Gene sequencing | Yes | yes | yes | yes | Buufy coat | 69 | 100 | [118] |
| NASBA | Yes | No | No | No | Whole blood | 79.8- 93.3 | 100 | [41, 43, 116] |
| Bone Marrow | 85-99 | 50-98.9 | [41] | |||||
| Lymph node | 64-95 | 70-100 | [41] | |||||
| LAMP | Yes | No | No | No | Whole blood | 83.0 -96.4 | 98.0 -99 | [102, 116, 119, 120] |
| Buffy coat | 90.7-95.0 | 86-100 | [103, 121] | |||||
| PCR-HRM | Yes | yes | yes | yes | Biopsy | NA | NA | [144, 182] |
#Levels of discrimination amenable; G: Genus; SG: Subgeneus; S: species.
Abbreviations
PCR: Polymerase Chain Reaction; ELISA: Enzyme linked immunosorbent assay; IFAT: immunofluorescence antibody test; NASBA: nucleic acid sequence-based amplification; LAMP: Loop-mediated isothermal amplification; qPCR: quantitative PCR; Oligo C test: Oligochromatography PCR test; HRM: High Resolution Melting.
Importantly, in view of mounting drug pressure, PCR diagnostic assays play a key role in monitoring drug efficacy and early reporting of drug resistance which are essential to bring corrective actions in drug policy; this is even more important when the drug arsenal is limited, as in the case of VL [122-124]. The molecular assays are the only standard, rapid and high throughput and easy method to track parasite resistance that can completely replace tedious in vitro susceptibility assays. Furthermore, such tools should be simple as much as possible to be applicable and affordable in the endemic countries. Recently, Srivastava et al identified single nucleotide polymorphism in cysteine proteinase B gene associated with amphotericin-B drug resistance [125].
2.2. Quantification of parasites (Severity of Disease)
As an analytical technique the conventional PCR method has some limitations. By first amplifying the DNA sequence and then analyzing the product, quantification was exceedingly difficult since the PCR give rise to essentially the same amount of product independently of the initial amount of DNA template molecules that were present. Therefore conventional PCR (qualitative analysis) test shows only Leishmania presence or absence without quantification of parasite load. With the highly efficient detection chemistries, sensitive instrumentation, and optimized assays that are available today by Real Time PCR (also known as quantitative Real Time PCR if DNA is the starting genetic material for quantification of parasites), the number of DNA molecules of a particular sequence in a complex sample can be determined with unprecedented accuracy and sensitivity sufficient to detect a single molecule. However, quantitative PCR (qPCR) does not directly measure the number of viable parasites circulating in the blood, but rather the amount of circulating parasite DNA. Therefore, sensitivity of qPCR depends on assay design (primer and target region), chemistry used (SYBER Green or TaqMan), nature of clinical samples (blood, skin, bone marrow or splenic fluids) and the DNA extraction methods (manual vs commercial kits) [126] (Table-2). Using this technique, it was earlier demonstrated that the simultaneous quantitative evaluation of Leishmania DNA and cytokine by Real Time PCR assay allows prediction of the development of disease in asymptomatic infected dogs [127]. Using qPCR, we have shown that the parasite load decreases during treatment in treated VL cases. Amplification of 18S r RNA gene sequence from a small volume of heparinised whole blood by Real Time-PCR revealed a wide range of blood parasitemia in VL patients prior to treatment that in each case began to decline within a few days of the start of their antileishmanial drug therapy [128], and thus can be used as a marker of treatment response as well as measurement of parasitic burden over time. Recently, Hossain et al, evaluated the use of real time PCR and revealed the difference in parasite loads between primary VL and relapse VL [129]. Subsequently on a larger cohort of asymptomatic subjects, we established the threshold parasitemia (>5 L. donovani parasite genomes detected/ml) in blood for clinical symptoms of VL to occur [61]. Later on, in the enlarged cohort of 1,606 healthy individuals of whom 442 were recent sero-converters with DAT and/or rK39, the risk for progression of disease was found much higher in qPCR positives (odds ratio: 14.8, 95% CI 5.1-42.5) (Chakravarty et al unpublished data).
Table-2.
Summary of the comparative analytical sensitivity of real time PCR assay targeting leishmania DNA region
| Target Sequence | Tissue tested | Assay Chemistry | Analytical Sensitivity (parasite/ml or parasite DNA equivalence/reaction) | Reference |
|---|---|---|---|---|
|
kDNA |
Blood | Intercalating dye | 0.07-0.1 | [130] |
| Fluorescent dye | 0.001-0.002 | [131, 132] | ||
| Bone Marrow/Lymph Nodes | Intercalating eye | 0 | [133] | |
| Fluorescent dye | 0.001-0.0125 | [66, 132] | ||
| Skin Biopsy | Intercalating dye | 0.0001-0.5 | [134, 135] | |
| Fluorescent dye | 0.005 | [136] | ||
|
rDNA |
Blood | Intercalating dye | 0.5-10.0 | [137, 138] |
| Fluorescent dye | 1.0 – 6.25 | [139, 140] | ||
| Bone Marrow/Lymph Nodes | Intercalating eye | 0.1 – 10.0 | [138, 141] | |
| Fluorescent dye | 1.0 | [140] | ||
| Skin Biopsy | Intercalating dye | 0.5 – 10.0 | [137, 138] | |
| Fluorescent dye | 1.0 – 1000.0 | [64, 140] | ||
| GPI | Skin biopsy | Fluorescent dye | 165.4 | [142] |
| G6PD | Biopsy | Intercalating dye | 0.005 | [136] |
| HSP70 | Parasite culture | Intercalating dye | 10.0 | [143] |
| cpb | Biopsy | Fluorescent dye | 0.01 | [144] |
Abbreviations
G6PD: Glucose-6-phosphate dehydrogenase; HSP70: Heat-shock protein 70; kDNA: Kinetoplast DNA; rDNA: ribosomal DNA; cpb: cystein protease B; GPI: Glucose phosphate isomerase; qPCR: Quantitative real-time PCR
Elevated levels of IL-10 during active disease is a hallmark of VL, and this overproduction of IL-10 promote parasite replication and disease progression. Verma et al evaluated the parasitic burden measured by qPCR and its association with IL-10 production in VL and found that high qPCR load is strongly correlate with plasma IL-10 levels, making it suitable for biomarker of disease severity[145]. Later on, Wilson group developed several qPCR methods and strategies for leishmania species differentiation and quantification in clinical specimens[63]. Leon et al evaluated the analytical performance of qPCR methods (designed on primers directed to kDNA, HSP70, 18S and ITS-1 targets) and found that 18S marker presented the high sensitivity and specificity [146].
Since, qPCR assay usually provides a measure of parasite load of blood at a given time point, but it remains unclear how this load can be correlated to the load at infection because the parasite load may vary with time, and likely reflect both host parasite interactions as well as initial load. A number of researchers are using PCR-ELISA for early detection and quantification which allows multiple sample testing using whole blood with sensitivity of 87% [101, 147]. However this method is tedious, expensive, and less sensitive than qPCR and tested on limited number of clinical samples [101, 148].
2.3. Species identification
In the Indian subcontinent, visceral leishmaniasis is the disease that mainly occur, however, recent identification of cutaneous leishmaniasis (CL) patients in Rajasthan (caused by L. tropica) and Himachal Pradesh (caused by L.donovani and L.tropica) [149-151], suggest that clinical profile of CL are different in these states. Therefore, species identification assays are useful in such areas for proper management of control program. Importantly, in the Indian subcontinent (mainly India and Bangladesh) as well as in Africa (mainly Sudan), where L. donovani is the causative parasite for VL, a common complication of VL is post kala-azar dermal leishmaniasis (PKDL) [152] which occurs in the months following treatment, in up to 50% of people who have recovered from VL. It is much less common in India, with an incidence of less than 5-10 %, and when it occurs, does so many years after the acute infection [153]. In Africa, PKDL is even more common but there are important intraregional differences; in Sudan it is most common: up to 50-60% of VL cases develop PKDL, usually within 6 months and virtually all cases develop within 12 months (mean 4.5 months). In Ethiopia, Kenya and Uganda PKDL is less common for reasons that are not well understood.
Through whole genome sequencing, Downing et al reported that there are a large amount of chromosome copy number variations between L.donovani strains and between leishmania species on the Indian subcontinent [71]. Therefore, better characterization of parasite strain (i.e species differentiation) is needed to resolve the mystery as to whether the disease is due to reactivation of persistent parasites following clinical cure of VL, or due to re-infection; and also to establish the cause of different forms of PKDL.
Commonly used target genes in leishmania for species identification includes ITS (non coding spacer DNA located between the 18S rRNA and 5.8S rRNA)[52, 154-156], repetitive nuclear DNA sequences[157], cytochrome-b genes[158, 159], mini exon genes[160], G6PD genes [161], cpb genes [162, 163], gp63 genes[164] and hsp70 genes [165, 166]. For example, digestion of ITS-1 PCR product with Hae-III restriction enzyme differentiates most of leishmania species. Since, RFLP pattern dependent on restriction enzyme used, and thus it is suggested to go for sequencing for confirmation. RAPD is another molecular assay where amplification of DNA is performed using arbitrarily short primes without knowing the target sequences. Several studies have been done using RAPD for investigation of genomic diversity [167-170], but its use in leishmaniasis is restricted due to need of specific PCR standardization condition and poor reproducibility [171]. AFLP is more advanced assay for investigation of variations in strains or closely related species [172]. It uses restriction enzyme for genomic DNA digestion followed by selective PCR amplification of restriction fragments. Recently developed more sensitive PCR-fingerprinting techniques include multilocous sequence typing (MLST) which is based on the PCR amplification of multiple unlinked housekeeping genes followed by sequencing [68]. Moreover, a multilocus microsatellite typing (MLMT) approach has been recently developed by which East African strains of L. donovani and Mediterranean strains of L. infantum could be resolved and assigned to genetically isolated populations [57, 74]. Srivastava et al explored the discriminatory power of different molecular assay and markers to detect genetic heterogeneity in clinical isolates of L.donovani from India [70]. Multilocous enzyme electrophoresis (MLEE) is protein based methods which differentiate leishmania parasites to species and subspecies levels based on electrophoretic mobility of enzymes [173]. This method has been known as gold standard for characterization and identification of parasite strains. However, requirement of mass cultivation of parasites, low differentiation power in homology population and developments of more sensitive molecular markers as an alternative methods are the major drawbacks of MLEE [174]. Hernandez et al identified six new world leishmania species through implementation of High Resolution Melting (HRM) genotyping assay which is another robust, highly sensitive and reproducible genotyping technique [175].
2.4. Phylogenetic analysis
The evolutionary pattern among species and taxonomic status of leishmania parasites is essential to understand the divergence among closely related species, designing of reliable diagnostic tools and development of novel control methods. The malaria field is driving much of the relevant technology for this type of work. A major limiting factor to leishmaniasis is a critical lack of expertise throughout endemic areas. So far, many leishmania strains have been typed by MLEE. On the other hand, introduction of numerous molecular typing methodologies with multicopy targets or multigene families have improved the analysis of phylogenetic, taxonomic and genetic studies. These includes DNA targets such as ITS [176], single copy gene for the catalytic polypeptide from DNA polymerase α (polA) [177], cytochrome oxidase II gene[178, 179], cpB genes [180], 7SL RNA [181], and most recently hsp70 subfamily sequence[165]. For example, Zhang et al investigated the phylogenetic relationship using ITS1 and kinetoplast cytochrome oxidase II (COII) gene sequencing and hypothesized that phylogeny of Chinese Leishmania strains is associated with the geographical origin rather than clinical form of disease [73]. Fraga et al analysed the phylogenetic study of 43 leishmania strain from different geographic origins using hsp70 sequence and found that monophylactic genus Leishmania consisted of three distinct subgenera, the L. (Leishmania), L. (Viannia), and L. (Sauroleishmania) [77].
3. Technical challenges and future prospects of molecular based assays
VL affected patients living in endemic areas will not have access to quality care unless efforts are made to integrate existing innovative diagnostic technology into clinical management. For example, in Sudan 3520 VL cases were reported in 2014 and only 62% were diagnosed as confirmed VL. Molecular diagnostics are not only beneficial for the patient, but if done through active case detection (as like sero surveys with k39 RDT) in the villages will also reduce the parasite reservoir in highly endemic areas since humans are the only host reservoir for L. donovani in the Indian subcontinent. Though sensitivity and diagnostic accuracy of molecular assays are reasonable to excellent in laboratory-based evaluations (in reference laboratories), these methods are not currently adapted to a primary health care setting due to the expensive infrastructure and technical expertise required. Overall cost associated with PCR assay is less than US $ 5 (INR 230.0) per sample [44]. Though, this cost is two times greater than rK39 RDT test, it is currently not very clear how such innovative techniques will replace k39 RDT testing and how it can be meaningfully applied within the health system context of VL endemic areas. However, assuming that such raid and highly sensitive molecular tools to assist clinicians working in the frontline of the primary health care setting will help them to better manage their patients presenting with fever related clinical syndromes. Strengthening of early diagnosis and treatment capacities at primary health care settings may provide long-term sustainability of elimination effort through integrated case management as close as possible to the patient’s village. Importantly, emergence and spreading of drug resistance is challenging the VL control program. Therefore, monitoring drug efficacy and early reporting are most essential as drug arsenal is limited. Molecular detection tools would constitute a more rapid and high throughput alternative to detect drug resistant parasites, but requires a standardized way to use them and a structure to implement them in the sentinel sites.
4. Conclusion
There is no preventive or therapeutic vaccine for VL and arsenal of antileishmanial drugs are limited, therefore, it is important to identify VL patients likely to relapse after drug treatment, as well as new ways to recognize individuals who have had recent exposure to live parasites. Effective clinical management, chemotherapy and control of transmission depend largely on early and unequivocal diagnosis. Molecular based methods have recently become popular in the field of diagnosis that can detect infection at the low level, pertaining to the goal of VL elimination. So far, several molecular based assays have been developed and evaluated, but PCR based assays are found to be simple, rapid and highly sensitive. The availability of such rapid test to be used to diagnose VL and as a marker of cure at peripheral health centers could have a great impact on the way VL is managed in endemic communities. The test could be an alternative to the current rK39 dipstick test for accurate diagnosis and it could be used to identify treatment failures and relapses.
Key Points:
-
❖
Due to the limited number of currently available anti-leishmanial drugs, effective clinical management, chemotherapy and control of transmission depend largely on early and unequivocal diagnosis. Any patient residing in a VL endemic area, presenting with a history of fever of more than two weeks duration and with no response to antibiotics/antimalarials is to be tested for VL with an rK-39 dipstick test.
-
❖
The rK39 rapid diagnostic test (RDT) in strict clinical criteria is currently used with good results for diagnosis of VL, but this test cannot differentiate between active disease and past VL. Furthermore, a diagnostic algorithm of rK39 RDT for detection of asymptomatic infection has only been validated for high incidence settings in strict combination with clinical criteria (fever for more than two weeks duration plus an enlarged spleen).
-
❖
With the advent of technology, highly specific and sensitive molecular based tools have been developed for detecting infection, diagnosis and species differentiations, these hold considerable further promise for delivering better point-of-care diagnostic tests in elimination and post elimination setting.
-
❖
Molecular based methods play key role in early diagnosis, monitoring of treatment effectiveness and assessment of drug resistance in Leishmania parasites.
Acknowledgments
Funding: This work was supported by the Bill & Melinda Gates Foundation (BMGF), USA (Grant No.OPP 1117011) and, Extramural Program of the National Institute of Allergy and Infectious Disease (NIAID), National Institute of Health (TMRC Grant No. U19AI074321). The funders had no role in design, decision to publish, or preparation of the report.
Footnotes
Compliance with Ethical Standards
Conflict of interest: SS and OPS declare no conflicts of interest.
References
- 1. Research priorities for Chagas disease, human African trypanosomiasis and leishmaniasis World Health Organ Tech Rep Ser 2012 975 v–xii 1–100 Available from: apps.who.int/iris/bitstream/10665/77472/1/WHO_TRS_975_eng.pdf [PubMed] [Google Scholar]
- 2. Tiwary P Singh S Kushwaha AK Rowton E Sacks D Singh OP et al. Establishing, Expanding, and Certifying a Closed Colony of Phlebotomus argentipes (Diptera: Psychodidae) for Xenodiagnostic Studies at the Kala Azar Medical Research Center, Muzaffarpur, Bihar, India J Med Entomol 2017. 54 5 1129–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guerin PJ Olliaro P Sundar S Boelaert M Croft SL Desjeux P et al. Visceral leishmaniasis: current status of control, diagnosis, and treatment, and a proposed research and development agenda Lancet Infect Dis August 2002. 2 8 494–501 [DOI] [PubMed] [Google Scholar]
- 4. Singh OP Hasker E Boelaert M Sundar S Elimination of visceral leishmaniasis on the Indian subcontinent Lancet Infect Dis December 2016. 16 12 e304–e309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le Rutte EA, Coffeng LE, Bontje DM, Hasker EC, Postigo JA, Argaw D. et al. Feasibility of eliminating visceral leishmaniasis from the Indian subcontinent: explorations with a set of deterministic age-structured transmission models. Parasit Vectors. 2016 Jan 19;9:24. doi: 10.1186/s13071-016-1292-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hollingsworth TD, Adams ER, Anderson RM, Atkins K, Bartsch S, Basáñez M-G. et al. Quantitative analyses and modelling to support achievement of the 2020 goals for nine neglected tropical diseases. Parasite & Vectors. 2015;8(1):630. doi: 10.1186/s13071-015-1235-1. 2015. Available from: http://www.parasitesandvectors.com/content/8/1/630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Naghavi M Wang H Lozano R Davis A Liang X Zhou M et al. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: A systematic analysis for the Global Burden of Disease Study 2013 Lancet 2015. 385 9963 117–171 Elsevier Ltd; 2015 Available from: 10.1016/S0140-6736(14)61682-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirve S, Kroeger A, Matlashewski G, Mondal D, Banjara MR, Das P. et al. Towards elimination of visceral leishmaniasis in the Indian subcontinent-Translating research to practice to public health. PLoS Negl Trop Dis. 2017 Oct 12;11(10):e0005889. doi: 10.1371/journal.pntd.0005889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO/Regional Office for Europe. Manual on case management and surveillance of the leishmaniases in the WHO European Region. 2017 cited; Available from: http://www.who.int/leishmaniasis/resources/978-92-89052-51-1/en/
- 10. Reis LLD Balieiro A Fonseca FR Goncalves MJF Changes in the epidemiology of visceral leishmaniasis in Brazil from 2001 to 2014 Rev Soc Bras Med Trop Sep-Oct 2017. 50 5 638–645 [DOI] [PubMed] [Google Scholar]
- 11. Adam GK Ali KM Abdella YH Omar SM Ahmed MA Abdalla TM et al. Trend in cumulative cases and mortality rate among visceral leishmaniasis patients in Eastern Sudan: a 14-year registry, 2002-2015 Int J Infect Dis October 2016. 51 81–84 [DOI] [PubMed] [Google Scholar]
- 12. Stanley A Engwerda C Balancing immunity and pathology in visceral leishmaniasis Immunology and Cell Biology Feb-Mar 2007. 85 2 138–147 [DOI] [PubMed] [Google Scholar]
- 13. Pearson R Sousa A Clinical spectrum of leishmaniasis Clinical Infectious Diseases 1996. 22 1 1–11 [DOI] [PubMed] [Google Scholar]
- 14. Dedet J Pratlong F Cook GC AI Z Leishmaniasis Manson’s Tropical Diseases 2008. 1341–1365 Saunders; London: 22 ed. Avaialbe from: 10.1016/j.trstmh.2009.09.003 [DOI] [Google Scholar]
- 15.Control of the leishmaniases. World Health Organisation Techechnical Report Ser. 2011 Available from: http://apps.who.int/iris/handle/10665/44412.
- 16. Boelaert M Meheus F Sanchez A Singh SP Vanlerberghe V Picado A et al. The poorest of the poor: a poverty appraisal of households affected by visceral leishmaniasis in Bihar, India Trop Med Int Health June 2009. 14 6 639–644 [DOI] [PubMed] [Google Scholar]
- 17. Boelaert M Le Ray D Van Der Stuyft P How better drugs could change kala-azar control. Lessons from a cost-effectiveness analysis Trop Med Int Health November 2002. 7 11 955–959 [DOI] [PubMed] [Google Scholar]
- 18.Singh OP, Singh B, Chakravarty J, Sundar S. Current challenges in treatment options for visceral leishmaniasis in India: a public health perspective. Infect Dis Poverty. 2016 Mar 8;5:19. doi: 10.1186/s40249-016-0112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.London Declaration on Neglected Tropical Diseases. 2012 Available from: http://unitingtocombatntds.org/london-declaration-neglected-tropical-diseases/
- 20. Sundar S Mondal D Rijal S Bhattacharya S Ghalib H Kroeger A et al. Implementation research to support the initiative on the elimination of kala azar from Bangladesh, India and Nepal--the challenges for diagnosis and treatment Trop Med Int Health January 2008. 13 1 2–5 [DOI] [PubMed] [Google Scholar]
- 21. Sundar S Rai M Laboratory diagnosis of visceral leishmaniasis Clin Diagn Lab Immunol September 2002. 9 5 951–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Burki T East African countries struggle with visceral leishmaniasis Lancet 2009. 374 9687 371–372 [DOI] [PubMed] [Google Scholar]
- 23.Singh OP, Sundar S. Developments in Diagnosis of Visceral Leishmaniasis in the Elimination Era. J Parasitol Res. 2015:239469. doi: 10.1155/2015/239469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chappuis F, Rijal S, Soto A, Menten J, Boelaert M. A meta-analysis of the diagnostic performance of the direct agglutination test and rK39 dipstick for visceral leishmaniasis. BMJ. 2006 Oct 7;333(7571):723. doi: 10.1136/bmj.38917.503056.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chappuis F Sundar S Hailu A Ghalib H Rijal S Peeling RW et al. Visceral leishmaniasis: what are the needs for diagnosis, treatment and control? Nat Rev Microbiol November 2007. 5 11 873–82 [DOI] [PubMed] [Google Scholar]
- 26. Maurya R Mehrotra S Prajapati VK Nylen S Sacks D Sundar S Evaluation of blood agar microtiter plates for culturing leishmania parasites to titrate parasite burden in spleen and peripheral blood of patients with visceral leishmaniasis J Clin Microbiol May 2010. 48 5 1932–1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Buxbaum L Scott P Interleukin 10- and Fcγ Receptor-Deficient Mice Resolve Leishmania mexicana Lesions Infection and Immunity 1 April 2005. 73 4 2101–2108 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gidwani K Picado A Ostyn B Singh SP Kumar R Khanal B et al. Persistence of Leishmania donovani antibodies in past visceral leishmaniasis cases in India Clin Vaccine Immunol February 2011. 18 2 346–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jacquet D Boelaert M Seaman J Rijal S Sundar S Menten J et al. Comparative evaluation of freeze-dried and liquid antigens in the direct agglutination test for serodiagnosis of visceral leishmaniasis (ITMA-DAT/VL) Trop Med Int Health December 2006. 11 12 1777–1784 [DOI] [PubMed] [Google Scholar]
- 30. Sundar S Reed SG Singh VP Kumar PC Murray HW Rapid accurate field diagnosis of Indian visceral leishmaniasis Lancet 21 February 1998. 351 9102 563–565 [DOI] [PubMed] [Google Scholar]
- 31. Cunningham J Hasker E Das P El Safi S Goto H Mondal D et al. A global comparative evaluation of commercial immunochromatographic rapid diagnostic tests for visceral leishmaniasis Clin Infect Dis 15 November 2012. 55 10 1312–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Boelaert M El-Safi S Hailu A Mukhtar M Rijal S Sundar S et al. Diagnostic tests for kala-azar: a multi-centre study of the freeze-dried DAT, rK39 strip test and KAtex in East Africa and the Indian subcontinent Trans R Soc Trop Med Hyg January 2008. 102 1 32–40 [DOI] [PubMed] [Google Scholar]
- 33. Srividya G Kulshrestha A Singh R Salotra P Diagnosis of visceral leishmaniasis: developments over the last decade Parasitol Res March 2011. 110 3 1065–78 [DOI] [PubMed] [Google Scholar]
- 34. Das VN Siddiqui NA Verma RB Topno RK Singh D Das S et al. Asymptomatic infection of visceral leishmaniasis in hyperendemic areas of Vaishali district, Bihar, India: a challenge to kala-azar elimination programmes Trans R Soc Trop Med Hyg November 2011. 105 11 661–6 [DOI] [PubMed] [Google Scholar]
- 35. Gidwani K Kumar R Rai M Sundar S Longitudinal seroepidemiologic study of visceral leishmaniasis in hyperendemic regions of Bihar, India Am J Trop Med Hyg March 2009. 80 3 345–346 [PubMed] [Google Scholar]
- 36. Rijal S Boelaert M Regmi S Karki BM Jacquet D Singh R et al. Evaluation of a urinary antigen-based latex agglutination test in the diagnosis of kala-azar in eastern Nepal Trop Med Int Health June 2004. 9 6 724–729 [DOI] [PubMed] [Google Scholar]
- 37. Sundar S Agrawal S Pai K Chance M Hommel M Detection of leishmanial antigen in the urine of patients with visceral leishmaniasis by a latex agglutination test Am J Trop Med Hyg August 2005. 73 2 269–271 [PubMed] [Google Scholar]
- 38. Diro E Techane Y Tefera T Assefa Y Kebede T Genetu A et al. Field evaluation of FD-DAT, rK39 dipstick and KATEX (urine latex agglutination) for diagnosis of visceral leishmaniasis in northwest Ethiopia Trans R Soc Trop Med Hyg September 2007. 101 9 908–14 [DOI] [PubMed] [Google Scholar]
- 39. Akhoundi M Downing T Votýpka J Kuhls K Lukeš J Cannet A et al. Leishmania infections: Molecular targets and diagnosis Mol Aspects Med October 2017. 57 1–29 [DOI] [PubMed] [Google Scholar]
- 40. de Ruiter CM van der Veer C Leeflang MM Deborggraeve S Lucas C Adams ER Molecular tools for diagnosis of visceral leishmaniasis: systematic review and meta-analysis of diagnostic test accuracy J Clin Microbiol 2014. 52 9 3147–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saad AA, Ahmed NG, Osman OS, Al-Basheer AA, Hamad A, Deborggraeve S. et al. Diagnostic accuracy of the Leishmania OligoC-TesT and NASBA-Oligochromatography for diagnosis of leishmaniasis in Sudan. PLoS Negl Trop Dis. 2010;4(8):e776. doi: 10.1371/journal.pntd.0000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mugasa CM Deborggraeve S Schoone GJ Laurent T Leeflang MM Ekangu RA et al. Accordance and concordance of PCR and NASBA followed by oligochromatography for the molecular diagnosis of Trypanosoma brucei and Leishmania Trop Med Int Health July 2010. 15 7 800–5 [DOI] [PubMed] [Google Scholar]
- 43.Mugasa CM, Laurent T, Schoone GJ, Basiye FL, Saad AA, El Safi S. et al. Simplified molecular detection of Leishmania parasites in various clinical samples from patients with leishmaniasis. Parasit Vectors. 2010;3(1):13. doi: 10.1186/1756-3305-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Srivastava P, Mehrotra S, Tiwary P, Chakravarty J, Sundar S. Diagnosis of Indian Visceral Leishmaniasis by Nucleic Acid Detection Using PCR. PLoS One. 2011 Apr 29;6(4) doi: 10.1371/journal.pone.0019304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Salotra P Sreenivas G Pogue GP Lee N Nakhasi HL Ramesh V et al. Development of a species-specific PCR assay for detection of Leishmania donovani in clinical samples from patients with kala-azar and post-kala-azar dermal leishmaniasis J Clin Microbiol March 2001. 39 3 849–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Maurya R Singh RK Kumar B Salotra P Rai M Sundar S Evaluation of PCR for diagnosis of Indian kala-azar and assessment of cure J Clin Microbiol July 2005. 43 7 3038–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Molina R, Lopez-Velez R, Gutierrez-Solar B, Jimenez I, Alvar J. Isolation of Leishmania infantum from the blood of a patient with AIDS using sandflies. Trans R Soc Trop Med Hyg. 1992 Sep-Oct;86(5):516. doi: 10.1016/0035-9203(92)90092-q. [DOI] [PubMed] [Google Scholar]
- 48. Dweik A Schonian G Mosleh IM Karanis P Evaluation of PCR-RFLP (based on ITS-1 and HaeIII) for the detection of Leishmania species, using Greek canine isolates and Jordanian clinical material Ann Trop Med Parasitol July 2007. 101 5 399–407 [DOI] [PubMed] [Google Scholar]
- 49. da Silva MA Pedrosa Soares CR Medeiros RA Medeiros Z de Melo FL Optimization of single-tube nested PCR for the diagnosis of visceral leishmaniasis Exp Parasitol June 2013. 134 2 206–10 [DOI] [PubMed] [Google Scholar]
- 50. Noyes HA Reyburn H Bailey JW Smith D A nested-PCR-based schizodeme method for identifying Leishmania kinetoplast minicircle classes directly from clinical samples and its application to the study of the epidemiology of Leishmania tropica in Pakistan J Clin Microbiol October 1998. 36 10 2877–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Harris E Kropp G Belli A Rodriguez B Agabian N Single-step multiplex PCR assay for characterization of New World Leishmania complexes J Clin Microbiol July 1998. 36 7 1989–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schonian G Nasereddin A Dinse N Schweynoch C Schallig HD Presber W et al. PCR diagnosis and characterization of Leishmania in local and imported clinical samples Diagn Microbiol Infect Dis September 2003. 47 1 349–58 [DOI] [PubMed] [Google Scholar]
- 53.Ceccarelli M, Galluzzi L, Diotallevi A, Andreoni F, Fowler H, Petersen C. et al. The use of kDNA minicircle subclass relative abundance to differentiate between Leishmania (L.) infantum and Leishmania (L.) amazonensis. Parasit Vectors. 2017 May 16;10(1):239. doi: 10.1186/s13071-017-2181-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Flegontov PN Strelkova MV Kolesnikov AA The Leishmania major maxicircle divergent region is variable in different isolates and cell types Mol Biochem Parasitol April 2006. 146 2 173–9 [DOI] [PubMed] [Google Scholar]
- 55. Mauricio IL Gaunt MW Stothard JR Miles MA Glycoprotein 63 (gp63) genes show gene conversion and reveal the evolution of Old World Leishmania Int J Parasitol April 2007. 37 5 565–76 [DOI] [PubMed] [Google Scholar]
- 56. Lixia L Jiping L Hongtao J Limin S Bo L Feng W et al. Detection of Leishmania donovani infection using magnetic beads-based serum peptide profiling by MALDI-TOF MS in mice model Parasitol Res March 2012. 110 3 1287–90 [DOI] [PubMed] [Google Scholar]
- 57. Schonian G Kuhls K Mauricio IL Molecular approaches for a better understanding of the epidemiology and population genetics of Leishmania Parasitology April 2010. 138 4 405–25 [DOI] [PubMed] [Google Scholar]
- 58. Montalvo AM Fraga J Monzote L Montano I De Doncker S Dujardin JC et al. Heat-shock protein 70 PCR-RFLP: a universal simple tool for Leishmania species discrimination in the New and Old World Parasitology July 2010. 137 8 1159–68 [DOI] [PubMed] [Google Scholar]
- 59. da Silva LA de Sousa Cdos S da Graca GC Porrozzi R Cupolillo E Sequence analysis and PCR-RFLP profiling of the hsp70 gene as a valuable tool for identifying Leishmania species associated with human leishmaniasis in Brazil Infect Genet Evol January 2010. 10 1 77–83 [DOI] [PubMed] [Google Scholar]
- 60. Cupolillo E Grimaldi G Jr. Momen H Discrimination of Leishmania isolates using a limited set of enzymatic loci Ann Trop Med Parasitol February 1995. 89 1 17–23 [DOI] [PubMed] [Google Scholar]
- 61.Sudarshan M, Singh T, Singh AK, Chourasia A, Singh B, Wilson ME. et al. Quantitative PCR in epidemiology for early detection of visceral leishmaniasis cases in India. PLoS Negl Trop Dis. 2014;8(12):e3366. doi: 10.1371/journal.pntd.0003366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sudarshan M Sundar S Parasite load estimation by qPCR differentiates between asymptomatic and symptomatic infection in Indian visceral leishmaniasis Diagn Microbiol Infect Dis 2014. 80 1 40–2 10.1016/j.diagmicrobio.2014.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Weirather JL Jeronimo SM Gautam S Sundar S Kang M A KM et al. Serial quantitative PCR assay for detection, species discrimination, and quantification of Leishmania spp. in human samples J Clin Microbiol 2011. 49 11 3892–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. van der Meide W Guerra J Schoone G Farenhorst M Coelho L Faber W et al. Comparison between quantitative nucleic acid sequence-based amplification, real-time reverse transcriptase PCR, and real-time PCR for quantification of Leishmania parasites J Clin Microbiol January 2008. 46 1 73–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kobets T Badalova J Grekov I Havelkova H Svobodova M Lipoldova M Leishmania parasite detection and quantification using PCR-ELISA Nat Protoc June 2010. 5 6 1074–80 [DOI] [PubMed] [Google Scholar]
- 66. Mary C Faraut F Lascombe L Dumon H Quantification of Leishmania infantum DNA by a real-time PCR assay with high sensitivity J Clin Microbiol November 2004. 42 11 5249–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Galluzzi L, Ceccarelli M, Diotallevi A, Menotta M, Magnani M. Real-time PCR applications for diagnosis of leishmaniasis. Parasit Vectors. 2018 May 2;11(1):273. doi: 10.1186/s13071-018-2859-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boite MC, Mauricio IL, Miles MA, Cupolillo E. New insights on taxonomy, phylogeny and population genetics of Leishmania (Viannia) parasites based on multilocus sequence analysis. PLoS Negl Trop Dis. 2012;6(11):e1888. doi: 10.1371/journal.pntd.0001888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mauricio IL Yeo M Baghaei M Doto D Pratlong F Zemanova E et al. Towards multilocus sequence typing of the Leishmania donovani complex: resolving genotypes and haplotypes for five polymorphic metabolic enzymes (ASAT, GPI, NH1, NH2, PGD) Int J Parasitol June 2006. 36 7 757–69 [DOI] [PubMed] [Google Scholar]
- 70. Srivastava P Singh T Sundar S Genetic heterogeneity in clinical isolates of Leishmania donovani from India J Clin Microbiol October 2011. 49 10 3687–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Downing T Imamura H Decuypere S Clark TG Coombs GH Cotton JA et al. Whole genome sequencing of multiple Leishmania donovani clinical isolates provides insights into population structure and mechanisms of drug resistance Genome Res December 2011. 21 12 2143–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Downing T Stark O Vanaerschot M Imamura H Sanders M Decuypere S et al. Genome-wide SNP and microsatellite variation illuminate population-level epidemiology in the Leishmania donovani species complex Infect Genet Evol January 2012. 12 1 149–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang CY, Lu XJ, Du XQ, Jian J, Shu L, Ma Y. Phylogenetic and evolutionary analysis of Chinese Leishmania isolates based on multilocus sequence typing. PLoS One. 2013;8(4):e63124. doi: 10.1371/journal.pone.0063124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kuhls K Keilonat L Ochsenreither S Schaar M Schweynoch C Presber W et al. Multilocus microsatellite typing (MLMT) reveals genetically isolated populations between and within the main endemic regions of visceral leishmaniasis Microbes Infect March 2007. 9 3 334–43 [DOI] [PubMed] [Google Scholar]
- 75. Montalvo AM Monzote L Fraga J Montano I Muskus C Marin M et al. PCR-RFLP and RAPD for typing neotropical Leishmania Biomedica December 2008. 28 4 597–606 [PubMed] [Google Scholar]
- 76. Toledo A Martin-Sanchez J Pesson B Sanchiz-Marin C Morillas-Marquez F Genetic variability within the species Leishmania infantum by RAPD. A lack of correlation with zymodeme structure Mol Biochem Parasitol February 2002. 119 2 257–64 [DOI] [PubMed] [Google Scholar]
- 77. Fraga J Montalvo AM De Doncker S Dujardin JC Van der Auwera G Phylogeny of Leishmania species based on the heat-shock protein 70 gene Infect Genet Evol March 2010. 10 2 238–45 [DOI] [PubMed] [Google Scholar]
- 78. Alam MZ Yasin G Kato H Sakurai T Katakura K PCR-based detection of Leishmania donovani DNA in a Stray dog from a visceral Leishmaniasis endemic focus in Bangladesh J Vet Med Sci 31 January 2012. 75 1 75–8 [DOI] [PubMed] [Google Scholar]
- 79. Blackwell JM Leishmaniasis epidemiology: all down to the DNA Parasitology 1992. 104 Suppl S19–34 [DOI] [PubMed] [Google Scholar]
- 80. Brustoloni YM Lima RB da Cunha RV Dorval ME Oshiro ET de Oliveira AL et al. Sensitivity and specificity of polymerase chain reaction in Giemsa-stained slides for diagnosis of visceral leishmaniasis in children Mem Inst Oswaldo Cruz June 2007. 102 4 497–500 [DOI] [PubMed] [Google Scholar]
- 81. Schallig HD Oskam L Molecular biological applications in the diagnosis and control of leishmaniasis and parasite identification Trop Med Int Health August 2002. 7 8 641–51 [DOI] [PubMed] [Google Scholar]
- 82. Reithinger R Dujardin JC Molecular diagnosis of leishmaniasis: current status and future applications J Clin Microbiol January 2007. 45 1 21–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Srivastava P Dayama A Mehrotra S Sundar S Diagnosis of visceral leishmaniasis Trans R Soc Trop Med Hyg January 2010. 105 1 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Mathis A Deplazes P PCR and in vitro cultivation for detection of Leishmania spp. in diagnostic samples from humans and dogs J Clin Microbiol May 1995. 33 5 1145–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Cortes S Rolao N Ramada J Campino L PCR as a rapid and sensitive tool in the diagnosis of human and canine leishmaniasis using Leishmania donovani s.l.-specific kinetoplastid primers Trans R Soc Trop Med Hyg January 2004. 98 1 12–7 [DOI] [PubMed] [Google Scholar]
- 86. Katakura K Kawazu S Naya T Nagakura K Ito M Aikawa M et al. Diagnosis of kala-azar by nested PCR based on amplification of the Leishmania mini-exon gene J Clin Microbiol August 1998. 36 8 2173–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Prina E Roux E Mattei D Milon G Leishmania DNA is rapidly degraded following parasite death: an analysis by microscopy and real-time PCR Microbes Infect September 2007. 9 11 1307–15 [DOI] [PubMed] [Google Scholar]
- 88. Costa CH Stewart JM Gomes RB Garcez LM Ramos PK Bozza M et al. Asymptomatic human carriers of Leishmania chagasi Am J Trop Med Hyg April 2002. 66 4 334–7 [DOI] [PubMed] [Google Scholar]
- 89. Topno RK Das VN Ranjan A Pandey K Singh D Kumar N et al. Asymptomatic infection with visceral leishmaniasis in a disease-endemic area in bihar, India Am J Trop Med Hyg September 2010. 83 3 502–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Bhattarai NR Van der Auwera G Khanal B De Doncker S Rijal S Das ML et al. PCR and direct agglutination as Leishmania infection markers among healthy Nepalese subjects living in areas endemic for Kala-Azar Trop Med Int Health April 2009. 14 4 404–11 [DOI] [PubMed] [Google Scholar]
- 91. Srivastava P Gidwani K Picado A Van der Auwera G Tiwary P Ostyn B et al. Molecular and serological markers of Leishmania donovani infection in healthy individuals from endemic areas of Bihar, India Tropical Medicine & International Health May 2013. 18 5 548–54 [DOI] [PubMed] [Google Scholar]
- 92. Vallur AC Duthie MS Reinhart C Tutterrow Y Hamano S Bhaskar KR et al. Biomarkers for intracellular pathogens: establishing tools as vaccine and therapeutic endpoints for visceral leishmaniasis Clin Microbiol Infect June 2013. 20 6 O374–83 10.1111/1469-0691.12421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Biglino A Bolla C Concialdi E Trisciuoglio A Romano A Ferroglio E Asymptomatic Leishmania infantum infection in an area of northwestern Italy (Piedmont region) where such infections are traditionally nonendemic J Clin Microbiol January 2009. 48 1 131–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Abbasi I, Aramin S, Hailu A, Shiferaw W, Kassahun A, Belay S. et al. Evaluation of PCR procedures for detecting and quantifying Leishmania donovani DNA in large numbers of dried human blood samples from a visceral leishmaniasis focus in Northern Ethiopia. BMC Infect Dis. 2013 Mar 27;13:153. doi: 10.1186/1471-2334-13-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Fisa R Riera C Lopez-Chejade P Molina I Gallego M Falco V et al. Leishmania infantum DNA detection in urine from patients with visceral leishmaniasis and after treatment control Am J Trop Med Hyg May 2008. 78 5 741–4 [PubMed] [Google Scholar]
- 96. Vaish M Singh OP Chakravarty J Sundar S rK39 antigen for the diagnosis of visceral leishmaniasis by using human saliva Am J Trop Med Hyg April 2012. 86 4 598–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Motazedian M Fakhar M Motazedian MH Hatam G Mikaeili F A urine-based polymerase chain reaction method for the diagnosis of visceral leishmaniasis in immunocompetent patients Diagn Microbiol Infect Dis February 2008. 60 2 151–4 [DOI] [PubMed] [Google Scholar]
- 98. Salam MA Mondal D Kabir M Ekram AR Haque R PCR for diagnosis and assessment of cure in kala-azar patients in Bangladesh Acta Trop January 2010. 113 1 52–5 [DOI] [PubMed] [Google Scholar]
- 99. Jorquera A Gonzalez R Marchan-Marcano E Oviedo M Matos M Multiplex-PCR for detection of natural Leishmania infection in Lutzomyia spp. captured in an endemic region for cutaneous leishmaniasis in state of Sucre, Venezuela Mem Inst Oswaldo Cruz February 2005. 100 1 45–8 [DOI] [PubMed] [Google Scholar]
- 100. Deborggraeve S Laurent T Espinosa D Van der Auwera G Mbuchi M Wasunna M et al. A simplified and standardized polymerase chain reaction format for the diagnosis of leishmaniasis J Infect Dis 15 November 2008. 198 10 1565–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. De Doncker S Hutse V Abdellati S Rijal S Singh Karki BM Decuypere S et al. A new PCR-ELISA for diagnosis of visceral leishmaniasis in blood of HIV-negative subjects Trans R Soc Trop Med Hyg January 2005. 99 1 25–31 [DOI] [PubMed] [Google Scholar]
- 102. Verma S Avishek K Sharma V Negi NS Ramesh V Salotra P Application of loop-mediated isothermal amplification assay for the sensitive and rapid diagnosis of visceral leishmaniasis and post-kala-azar dermal leishmaniasis Diagn Microbiol Infect Dis April 2013. 75 4 390–5 [DOI] [PubMed] [Google Scholar]
- 103.Khan MG, Bhaskar KR, Salam MA, Akther T, Pluschke G, Mondal D. Diagnostic accuracy of loop-mediated isothermal amplification (LAMP) for detection of Leishmania DNA in buffy coat from visceral leishmaniasis patients. Parasit Vectors. 2012;5:280. doi: 10.1186/1756-3305-5-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Castellanos-Gonzalez A Saldarriaga OA Tartaglino L Gacek R Temple E Sparks H et al. A Novel Molecular Test to Diagnose Canine Visceral Leishmaniasis at the Point of Care Am J Trop Med Hyg November 2015. 93 5 970–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mondal D, Ghosh P, Khan MA, Hossain F, Bohlken-Fascher S, Matlashewski G. et al. Mobile suitcase laboratory for rapid detection of Leishmania donovani using recombinase polymerase amplification assay. Parasit Vectors. 2016 May 13;9(1):281. doi: 10.1186/s13071-016-1572-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Srivastava P Gidwani K Picado A Van der Auwera G Tiwary P Ostyn B et al. Molecular and serological markers of Leishmania donovani infection in healthy individuals from endemic areas of Bihar, India Trop Med Int Health May 2013. 18 5 548–54 [DOI] [PubMed] [Google Scholar]
- 107. Vaish M Mehrotra S Chakravarty J Sundar S Noninvasive molecular diagnosis of human visceral leishmaniasis J Clin Microbiol May 2011. 49 5 2003–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ferreira Sde A, Almeida GG, Silva Sde O, Vogas GP, Fujiwara RT, de Andrade AS. et al. Nasal, oral and ear swabs for canine visceral leishmaniasis diagnosis: new practical approaches for detection of Leishmania infantum DNA. PLoS Negl Trop Dis. 2013;7(4):e2150. doi: 10.1371/journal.pntd.0002150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. de Ruiter CM van der Veer C Leeflang MMG Deborggraeve S Lucas C Adams ER Molecular Tools for Diagnosis of Visceral Leishmaniasis: Systematic Review and Meta-Analysis of Diagnostic Test Accuracy Journal of Clinical Microbiology September 2014. 52 9 3147–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Adhya S Chatterjee M Hassan MQ Mukherjee S Sen S Detection of Leishmania in the blood of early kala-azar patients with the aid of the polymerase chain reaction Trans R Soc Trop Med Hyg Nov-Dec 1995. 89 6 622–4 [DOI] [PubMed] [Google Scholar]
- 111. Disch J Maciel FC de Oliveira MC Orsini M Rabello A Detection of circulating Leishmania chagasi DNA for the non-invasive diagnosis of human infection Trans R Soc Trop Med Hyg Jul-Aug 2003. 97 4 391–5 [DOI] [PubMed] [Google Scholar]
- 112. Wu Z Bao Y Ding Y Yu M Lu L Zhang Y An experimental study on application of PCR in detection of kala-azar Southeast Asian J Trop Med Public Health March 1997. 28 1 169–72 [PubMed] [Google Scholar]
- 113. Osman OF Oskam L Zijlstra EE Kroon NC Schoone GJ Khalil ET et al. Evaluation of PCR for diagnosis of visceral leishmaniasis J Clin Microbiol October 1997. 35 10 2454–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Alam MZ Shamsuzzaman AK Kuhls K Schonian G PCR diagnosis of visceral leishmaniasis in an endemic region, Mymensingh district, Bangladesh Trop Med Int Health May 2009. 14 5 499–503 [DOI] [PubMed] [Google Scholar]
- 115. Sudarshan M Singh T Chakravarty J Sundar S A Correlative Study of Splenic Parasite Score and Peripheral Blood Parasite Load Estimation by Quantitative PCR in Visceral Leishmaniasis J Clin Microbiol December 2015. 53 12 3905–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.de Paiva-Cavalcanti M, de Morais RC, Pessoa-E-Silva R, Trajano-Silva LA, Gonçalves-de-Albuquerque SC, Tavares Dde H. et al. Leishmaniases diagnosis: an update on the use of immunological and molecular tools. Cell Biosci. 2015 Jun 17;5:31. doi: 10.1186/s13578-015-0021-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Galai Y Chabchoub N Ben-Abid M Ben-Abda I Ben-Alaya-Bouafif N Amri F et al. Diagnosis of mediterranean visceral leishmaniasis by detection of leishmania antibodies and leishmania DNA in oral fluid samples collected using an Oracol device J Clin Microbiol September 2011. 49 9 3150–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Hu XS Yang WT Lu HG Yan HP Cheng JP Ma Y et al. Sequencing a specific kinetoplast DNA fragment of Leishmania donovani for polymerase chain reaction amplification in diagnosis of leishmaniasis in bone marrow and blood samples J Parasitol August 2000. 86 4 822–6 [DOI] [PubMed] [Google Scholar]
- 119. Adams ER Schoone GJ Ageed AF Safi SE Schallig HD Development of a reverse transcriptase loop-mediated isothermal amplification (LAMP) assay for the sensitive detection of Leishmania parasites in clinical samples Am J Trop Med Hyg April 2010. 82 4 591–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Takagi H Itoh M Islam MZ Razzaque A Ekram AR Hashighuchi Y et al. Sensitive, specific, and rapid detection of Leishmania donovani DNA by loop-mediated isothermal amplification Am J Trop Med Hyg October 2009. 81 4 578–82 [DOI] [PubMed] [Google Scholar]
- 121.Adams ER, Schoone G, Versteeg I, Gomez MA, Diro E, Mori Y. et al. Development and evaluation of a novel LAMP assay for the diagnosis of Cutaneous and Visceral Leishmaniasis. J Clin Microbiol. 2018 doi: 10.1128/JCM.00386-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Hendrickx S Guerin PJ Caljon G Croft SL Maes L Evaluating drug resistance in visceral leishmaniasis: the challenges Parasitology 21 November 2016. 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Singh OP, Singh B, Chakravarty J, Sundar S. Current challenges in treatment options for visceral leishmaniasis in India: a public health perspective. Infect Dis Poverty. 2016 Mar 8;5:19. doi: 10.1186/s40249-016-0112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Freitas-Junior LH Chatelain E Kim HA Siqueira-Neto JL Visceral leishmaniasis treatment: What do we have, what do we need and how to deliver it? Int J Parasitol Drugs Drug Resist December 2012. 2 11–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Srivastava P Prajapati VK Rai M Sundar S Unusual case of resistance to amphotericin B in visceral leishmaniasis in a region in India where leishmaniasis is not endemic J Clin Microbiol August 2011. 49 8 3088–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Gomes CM Cesetti MV de Paula NA Vernal S Gupta G Sampaio RN et al. Field Validation of SYBR Green- and TaqMan-Based Real-Time PCR Using Biopsy and Swab Samples To Diagnose American Tegumentary Leishmaniasis in an Area Where Leishmania (Viannia) braziliensis Is Endemic J Clin Microbiol 2017. 55 2 526–534 10.1128/JCM.01954-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Manna L Reale S Viola E Vitale F Foglia Manzillo V Pavone LM et al. Leishmania DNA load and cytokine expression levels in asymptomatic naturally infected dogs Vet Parasitol 20 December 2006. 142 3-4 271–80 [DOI] [PubMed] [Google Scholar]
- 128. Sudarshan M Weirather JL Wilson ME Sundar S Study of parasite kinetics with antileishmanial drugs using real-time quantitative PCR in Indian visceral leishmaniasis J Antimicrob Chemother August 2011. 66 8 1751–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hossain F, Ghosh P, Khan MAA, Duthie MS, Vallur AC, Picone A. et al. Real-time PCR in detection and quantitation of Leishmania donovani for the diagnosis of Visceral Leishmaniasis patients and the monitoring of their response to treatment. PLoS One. 2017;12(9):e0185606. doi: 10.1371/journal.pone.0185606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. de Paiva Cavalcanti M Felinto de Brito ME de Souza WV de Miranda Gomes Y Abath FG The development of a real-time PCR assay for the quantification of Leishmania infantum DNA in canine blood Vet J November 2009. 182 2 356–8 [DOI] [PubMed] [Google Scholar]
- 131. Dantas-Torres F da Silva Sales KG Gomes da Silva L Otranto D Figueredo LA Leishmania-FAST15: A rapid, sensitive and low-cost real-time PCR assay for the detection of Leishmania infantum and Leishmania braziliensis kinetoplast DNA in canine blood samples Mol Cell Probes 2017. 31 65–69 10.1016/j.mcp.2016.08.006 [DOI] [PubMed] [Google Scholar]
- 132. Molina I Fisa R Riera C Falco V Elizalde A Salvador F et al. Ultrasensitive Real-Time PCR for the Clinical Management of Visceral Leishmaniasis in HIV-Infected Patients Am J Trop Med Hyg July 2013. 89 1 105–10 10.4269/ajtmh.12-0527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Silva RC Richini-Pereira VB Kikuti M Marson PM Langoni H Detection of Leishmania (L.) infantum in stray dogs by molecular techniques with sensitive species-specific primers Vet Q December 2017. 37 1 23–30 Epub 2016 Nov 9 [DOI] [PubMed] [Google Scholar]
- 134.Ribeiro-Romão RP, Saavedra AF, Da-Cruz AM, Pinto EF, Moreira OC, Ribeiro-Romão RP1, Saavedra AF1, Da-Cruz AM1, Pinto EF1, Moreira OC2. Development of real-time PCR assays for evaluation of immune response and parasite load in golden hamster (Mesocricetus auratus) infected by Leishmania (Viannia) braziliensis. Parasit Vectors. 2016 Jun 27;9(1):361. doi: 10.1186/s13071-016-1647-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Suárez M, Valencia BM, Jara M, Alba M, Boggild AK, Dujardin JC. et al. Quantification of Leishmania (Viannia) Kinetoplast DNA in Ulcers of Cutaneous Leishmaniasis Reveals Inter-site and Inter-sampling Variability in Parasite Load. PLoS Negl Trop Dis. 2015 Jul 23;9(7):e0003936. doi: 10.1371/journal.pntd.0003936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Jara M Adaui V Valencia BM Martinez D Alba M Castrillon C et al. Real-time PCR assay for detection and quantification of leishmania (viannia) organisms in skin and mucosal lesions: exploratory study of parasite load and clinical parameters J Clin Microbiol June 2013. 51 6 1826–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Talmi-Frank D, Nasereddin A, Schnur LF, Schonian G, Toz SO, Jaffe CL. et al. Detection and identification of old world Leishmania by high resolution melt analysis. PLoS Negl Trop Dis. 2010;4(1):e581. doi: 10.1371/journal.pntd.0000581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. de Almeida ME Koru O Steurer F Herwaldt BL da Silva AJ Detection and Differentiation of Leishmania spp. in Clinical Specimens by Use of a SYBR Green-Based Real-Time PCR Assay J Clin Microbiol 28 December 2016. 55 1 281–290 10.1128/JCM.01764-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Bossolasco S Gaiera G Olchini D Gulletta M Martello L Bestetti A et al. Real-time PCR assay for clinical management of human immunodeficiency virus-infected patients with visceral leishmaniasis J Clin Microbiol November 2003. 41 11 5080–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Schulz A Mellenthin K Schonian G Fleischer B Drosten C Detection, differentiation, and quantitation of pathogenic leishmania organisms by a fluorescence resonance energy transfer-based real-time PCR assay J Clin Microbiol April 2003. 41 4 1529–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Miro G Oliva G Cruz I Canavate C Mortarino M Vischer C et al. Multicentric, controlled clinical study to evaluate effectiveness and safety of miltefosine and allopurinol for canine leishmaniosis Vet Dermatol October 2009. 20 5-6 397–404 [DOI] [PubMed] [Google Scholar]
- 142. Wortmann G Hochberg L Houng HH Sweeney C Zapor M Aronson N et al. Rapid identification of Leishmania complexes by a real-time PCR assay Am J Trop Med Hyg December 2005. 73 6 999–1004 [PubMed] [Google Scholar]
- 143.León CM, Muñoz M, Hernández C, Ayala MS, Flórez C, Teherán A. et al. Analytical Performance of Four Polymerase Chain Reaction (PCR) and Real Time PCR (qPCR) Assays for the Detection of Six Leishmania Species DNA in Colombia. Front Microbiol. 2017 Oct 4;8:1907. doi: 10.3389/fmicb.2017.01907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zampieri RA, Laranjeira-Silva MF, Muxel SM, Stocco de Lima AC, Shaw JJ, Floeter-Winter LM. High Resolution Melting Analysis Targeting hsp70 as a Fast and Efficient Method for the Discrimination of Leishmania Species. PLoS Negl Trop Dis. 2016 Feb 29;10(2):e0004485. doi: 10.1371/journal.pntd.0004485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Verma S, Kumar R, Katara GK, Singh LC, Negi NS, Ramesh V. et al. Quantification of parasite load in clinical samples of leishmaniasis patients: IL-10 level correlates with parasite load in visceral leishmaniasis. PLoS One. 2010;5(4):e10107. doi: 10.1371/journal.pone.0010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Leon CM, Munoz M, Hernandez C, Ayala MS, Florez C, Teheran A. et al. Analytical Performance of Four Polymerase Chain Reaction (PCR) and Real Time PCR (qPCR) Assays for the Detection of Six Leishmania Species DNA in Colombia. Front Microbiol. 2017;8:1907. doi: 10.3389/fmicb.2017.01907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Medeiros FA, Gomes LI, Oliveira E, de Souza CS, Mourao MV, Cota GF. et al. Development and Validation of a PCR-ELISA for the Diagnosis of Symptomatic and Asymptomatic Infection by Leishmania (Leishmania) infantum. J Trop Med. 2017;2017:7364854. doi: 10.1155/2017/7364854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sue MJ, Yeap SK, Omar AR, Tan SW. Application of PCR-ELISA in Molecular Diagnosis. Biomed Res Int. 2014;2014:653014. doi: 10.1155/2014/653014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Aara N Khandelwal K Bumb RA Mehta RD Ghiya BC Jakhar R et al. Clinco-Epidemiologic Study of Cutaneous Leishmaniasis in Bikaner, Rajasthan, India Am J Trop Med Hyg July 2013. 89 1 111–5 10.4269/ajtmh.12-0558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Khandelwal K Bumb RA Mehta RD Kaushal H Lezama-Davila C Salotra P et al. A patient presenting with diffuse cutaneous leishmaniasis (DCL) as a first indicator of HIV infection in India Am J Trop Med Hyg July 2011. 85 1 64–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Sharma NL Mahajan VK Kanga A Sood A Katoch VM Mauricio I et al. Localized cutaneous leishmaniasis due to Leishmania donovani and Leishmania tropica: preliminary findings of the study of 161 new cases from a new endemic focus in himachal pradesh, India Am J Trop Med Hyg June 2005. 72 6 819–24 [PubMed] [Google Scholar]
- 152. Zijlstra EE Musa AM Khalil EA el-Hassan IM el-Hassan AM Post-kala-azar dermal leishmaniasis Lancet Infect Dis February 2003. 3 2 87–98 [DOI] [PubMed] [Google Scholar]
- 153. Rahman KM Islam S Rahman MW Kenah E Ghalib CM Zahid MM et al. Increasing incidence of post-kala-azar dermal leishmaniasis in a population-based study in Bangladesh Clin Infect Dis 1 January 2009. 50 1 73–6 [DOI] [PubMed] [Google Scholar]
- 154. Cupolillo E Grimaldi Junior G Momen H Beverley SM Intergenic region typing (IRT): a rapid molecular approach to the characterization and evolution of Leishmania Mol Biochem Parasitol July 1995. 73 1-2 145–55 [DOI] [PubMed] [Google Scholar]
- 155. Mauricio IL Stothard JR Miles MA Leishmania donovani complex: genotyping with the ribosomal internal transcribed spacer and the mini-exon Parasitology March 2004. 128 Pt 3 263–7 [DOI] [PubMed] [Google Scholar]
- 156. Chargui N Haouas N Jaouadi K Gorcii M Pratlong F Dedet JP et al. Usefulness of a PCR-based method in the detection and species identification of Leishmania from clinical samples Pathol Biol (Paris) December 2012. 60 6 e75–9 [DOI] [PubMed] [Google Scholar]
- 157. Piarroux R Azaiez R Lossi AM Reynier P Muscatelli F Gambarelli F et al. Isolation and characterization of a repetitive DNA sequence from Leishmania infantum: development of a visceral leishmaniasis polymerase chain reaction Am J Trop Med Hyg September 1993. 49 3 364–9 [DOI] [PubMed] [Google Scholar]
- 158. Luyo-Acero GE Uezato H Oshiro M Takei K Kariya K Katakura K et al. Sequence variation of the cytochrome b gene of various human infecting members of the genus Leishmania and their phylogeny Parasitology May 2004. 128 Pt 5 483–91 [DOI] [PubMed] [Google Scholar]
- 159.Yang BB, Chen DL, Chen JP, Liao L, Hu XS, Xu JN. Analysis of kinetoplast cytochrome b gene of 16 Leishmania isolates from different foci of China: different species of Leishmania in China and their phylogenetic inference. Parasit Vectors. 2013;6:32. doi: 10.1186/1756-3305-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Marfurt J Nasereddin A Niederwieser I Jaffe CL Beck HP Felger I Identification and differentiation of Leishmania species in clinical samples by PCR amplification of the miniexon sequence and subsequent restriction fragment length polymorphism analysis J Clin Microbiol July 2003. 41 7 3147–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Castilho TM Shaw JJ Floeter-Winter LM New PCR assay using glucose-6-phosphate dehydrogenase for identification of Leishmania species J Clin Microbiol February 2003. 41 2 540–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Hide M Banuls AL Polymorphisms of cpb multicopy genes in the Leishmania (Leishmania) donovani complex Trans R Soc Trop Med Hyg February 2008. 102 2 105–6 [DOI] [PubMed] [Google Scholar]
- 163. Hide M Banuls AL Species-specific PCR assay for L. infantum/L. donovani discrimination Acta Trop December 2006. 100 3 241–5 [DOI] [PubMed] [Google Scholar]
- 164. Victoir K Banuls AL Arevalo J Llanos-Cuentas A Hamers R Noel S et al. The gp63 gene locus, a target for genetic characterization of Leishmania belonging to subgenus Viannia Parasitology July 1998. 117 Pt 1 1–13 [PubMed] [Google Scholar]
- 165. Fraga J Montalvo AM Van der Auwera G Maes I Dujardin JC Requena JM Evolution and species discrimination according to the Leishmania heat-shock protein 20 gene Infect Genet Evol 28 May 2013. 18C 229–37 [DOI] [PubMed] [Google Scholar]
- 166. Garcia L Kindt A Bermudez H Llanos-Cuentas A De Doncker S Arevalo J et al. Culture-independent species typing of neotropical Leishmania for clinical validation of a PCR-based assay targeting heat shock protein 70 genes J Clin Microbiol May 2004. 42 5 2294–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Hamad SH Khalil EA Musa AM Ibrahim ME Younis BM Elfaki ME et al. Leishmania donovani: genetic diversity of isolates from Sudan characterized by PCR-based RAPD Exp Parasitol August 2010. 125 4 389–93 [DOI] [PubMed] [Google Scholar]
- 168. Mauricio IL Howard MK Stothard JR Miles MA Genomic diversity in the Leishmania donovani complex Parasitology September 1999. 119 Pt 3 237–46 [DOI] [PubMed] [Google Scholar]
- 169. Martinez E Alonso V Quispe A Thomas MC Alonso R Pinero JE et al. RAPD method useful for distinguishing Leishmania species: design of specific primers for L. braziliensis Parasitology December 2003. 127 Pt 6 513–7 [DOI] [PubMed] [Google Scholar]
- 170. Khanra S Bandopadhyay SK Chakraborty P Datta S Mondal D Chatterjee M et al. Characterization of the recent clinical isolates of Indian Kala-azar patients by RAPD-PCR method J Parasit Dis October 2012. 35 2 116–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Eisenberger CL Jaffe CL Leishmania: identification of Old World species using a permissively primed intergenic polymorphic-polymerase chain reaction Exp Parasitol January 1999. 91 1 70–7 [DOI] [PubMed] [Google Scholar]
- 172. Kumar A Boggula VR Misra P Sundar S Shasany AK Dube A Amplified fragment length polymorphism (AFLP) analysis is useful for distinguishing Leishmania species of visceral and cutaneous forms Acta Trop February 2010. 113 2 202–6 [DOI] [PubMed] [Google Scholar]
- 173. Rioux JA Lanotte G Serres E Pratlong F Bastien P Perieres J Taxonomy of Leishmania. Use of isoenzymes. Suggestions for a new classification Ann Parasitol Hum Comp 1990. 65 3 111–25 [DOI] [PubMed] [Google Scholar]
- 174. Banuls AL Hide M Prugnolle F Leishmania and the leishmaniases: a parasite genetic update and advances in taxonomy, epidemiology and pathogenicity in humans Adv Parasitol 2007. 64 1–109 [DOI] [PubMed] [Google Scholar]
- 175.Hernandez C, Alvarez C, Gonzalez C, Ayala MS, Leon CM, Ramirez JD. Identification of six New World Leishmania species through the implementation of a High-Resolution Melting (HRM) genotyping assay. Parasit Vectors. 2014 Nov 14;7:501. doi: 10.1186/s13071-014-0501-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Berzunza-Cruz M Cabrera N Crippa-Rossi M Sosa Cabrera T Perez-Montfort R Becker I Polymorphism analysis of the internal transcribed spacer and small subunit of ribosomal RNA genes of Leishmania mexicana Parasitol Res October 2002. 88 10 918–25 [DOI] [PubMed] [Google Scholar]
- 177. Croan DG Morrison DA Ellis JT Evolution of the genus Leishmania revealed by comparison of DNA and RNA polymerase gene sequences Mol Biochem Parasitol November 1997. 89 2 149–59 [DOI] [PubMed] [Google Scholar]
- 178. Ibrahim ME Barker DC The origin and evolution of the Leishmania donovani complex as inferred from a mitochondrial cytochrome oxidase II gene sequence Infect Genet Evol July 2001. 1 1 61–8 [DOI] [PubMed] [Google Scholar]
- 179. Asato Y Oshiro M Myint CK Yamamoto Y Kato H Marco JD et al. Phylogenic analysis of the genus Leishmania by cytochrome b gene sequencing Exp Parasitol April 2009. 121 4 352–61 [DOI] [PubMed] [Google Scholar]
- 180. Hide M Bras-Goncalves R Banuls AL Specific cpb copies within the Leishmania donovani complex: evolutionary interpretations and potential clinical implications in humans Parasitology March 2007. 134 Pt 3 379–89 [DOI] [PubMed] [Google Scholar]
- 181. Zelazny AM Fedorko DP Li L Neva FA Fischer SH Evaluation of 7SL RNA gene sequences for the identification of Leishmania spp Am J Trop Med Hyg April 2005. 72 4 415–20 [PubMed] [Google Scholar]
- 182.Pita-Pereira D, Lins R, Oliveira MP, Lima RB, Pereira BA, Moreira OC, Brazil RP, Britto C. SYBR Green-based Real-Time PCR targeting kinetoplast DNA can be used to discriminate between the main etiologic agents of Brazilian cutaneous and visceral leishmaniases. Parasit Vectors. 2012;5:15. doi: 10.1186/1756-3305-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]


