SUMMARY
Salmonella enterica (Se) bacteria cause persistent intracellular infections while stimulating a robust interferon-γ-producing CD4+ T (Th1) cell response. We addressed this paradox of concomitant infection and immunity by tracking fluorescent Se organisms in mice. Se bacteria persisted in nitric oxide synthase (iNOS)-producing resident and recruited macrophages while inducing genes related to protection from nitric oxide (NO). Se-infected cells occupied iNOS+ splenic granulomas that excluded T cells but were surrounded by mononuclear phagocytes producing the chemokines CXCL9 and CXCL10, and Se epitope-specific Th1 cells expressing CXCR3, the receptor for these chemokines. Blockade of CXCR3 inhibited Th1 occupancy of CXCL9/10-dense regions, reduced activation of the Th1 cells, and lead to increased Se growth. Thus, intracellular Se bacteria survive in their hosts by counteracting toxic products of the innate immune response and by residing in T cell-sparse granulomas, away from abundant Th1 cells positioned via CXCR3 in a bordering region that act to limit infection.
Keywords: Th1, CXCR3, phagosomal pathogen, granuloma
Graphical Abstract

eTOC
The pathogen Salmonella enterica (Se) can paradoxically persist in hosts while being controlled by IFN-γ-producing Th1 cells. Goldberg et al. show that the bacteria survive by resisting the effects of toxic host molecules in myeloid cells within granulomas, in areas lacking T cells. Infection is constrained by Th1 cells positioned around the granuloma via the CXCR3-CXCL9/10 chemokine axis.
INTRODUCTION
Salmonella enterica serovar Typhimurium (Se) bacteria cause largely asymptomatic chronic infections in immunocompetent hosts (Monack et al., 2004). These bacteria fall into a large class of microorganisms including Mycobacterium tuberculosis that have evolved to survive within the phagosomes of host phagocytes despite intense pressure from the host’s immune system (Tubo and Jenkins, 2014). Phagosomal infections are controlled by interferon-γ (IFN-γ)-producing CD4+ T cells (Th1 cells) as evidenced by the susceptibility of CD4+ T cell-deficient people to these pathogens (Lee et al., 2011; Prando et al., 2013; Sologuren et al., 2011). CD4+ T cells use αβ T cell receptors (TCRs) to recognize pathogen-derived peptides bound to host major histocompatibility class II (MHCII) molecules on the surface of infected phagocytes (Tubo and Jenkins, 2014). Th1 cells control phagosomal infections by producing IFN-γ, which then binds to the IFN-γ receptors on the nearby-infected phagocytes (Muller et al., 2012). IFN-γ receptor signaling in the phagocytes leads to the expression of anti-microbial effector molecules that generate microbicidal products such as nitric oxide (NO) (MacMicking et al., 1997), which are thought to directly limit microbial replication (Foulds et al., 2006; Hardison et al., 2012; Sharma and Bose, 2001).
For reasons that are not understood, however, this process does not eliminate the microbes from all infected phagocytes. The capacity of CD4+ T cells to control phagosomal pathogens without eliminating them is a fundamental paradox in cellular immunology (Belkaid et al., 2002; Peters et al., 2014; Tubo and Jenkins, 2014). Some evidence indicates that phagosomal pathogens persist by inhibiting innate immunity (Behnsen et al., 2015; Cecilio et al., 2014; Urdahl, 2014) or antigen presentation to T cells (Bayer-Santos et al., 2016; Jackson et al., 2013). It is also possible that the bacteria avoid the immune system by occupying anatomic structures known as granulomas (Pagan and Ramakrishnan, 2018). Early granulomas consist of spherical compact collections of activated macrophages, which over time adopt epithelial cell morphology and form tight junctions eventually developing a fibrotic capsule and central necrotic core. Pathogen peptide:MHCII-specific T cells may not be able to access the inner parts of the granuloma where the bacteria reside (Kauffman et al., 2017), or are poorly activated there by antigen-presenting cells (Bold et al., 2011; Egen et al., 2011; Egen et al., 2008).
The small number of infected phagocytes that are present during the persistent phase of infection has been a barrier to understanding the immune control of phagosomal pathogens. For this reason, knowledge about the type of phagocytes that are infected and their capacity to harbor or kill intracellular bacteria and where this process occurs in the body is limited. We addressed this problem by creating an Se strain expressing a fluorescent protein under the control of the phoN promoter to track the bacteria and the host response. We found that Se infection potently activated the innate immune response of infected cells and stimulated massive proliferation of Se epitope-specific CXCR3+ Th1 cells. Despite this robust innate and adaptive immune response, Se bacteria persisted in resident and recruited macrophages in granulomas. Persistence was associated with Se expression of genes encoding enzymes that break down NO and sparse seeding of granulomas with T cells. These results suggest that intracellular Se bacteria persist by protecting themselves from toxic products of the innate immune response and by residing in niches that exclude the numerous Th1 cells that control the infection.
RESULTS
Generation of a fluorescent Se strain
We produced a fluorescent Se strain to detect infected host cells by histology or flow cytometry. Bumann and colleagues (Barat et al., 2012) showed that the nonspecific acid phosphatase encoded by the phoN gene is highly expressed by intracellular Se organisms in the tissues of chronically infected mice. We used the lambda red recombination system to introduce the dTomato coding sequence after the phoN gene in the Se chromosome (Figure S1A, B). The resulting strain is referred to hereafter as Se-Tomato. Flow cytometric analysis of RAW264.7 macrophages infected in vitro with Se-Tomato bacteria and stained intracellularly with Se-lipopolysaccharide (LPS) antibody revealed cells that contained Se-LPS alone, likely from dead bacteria, or SeLPS and dTomato, while cells infected with wild-type Se only contained Se-LPS signal (Figure S1C). Microscopy revealed that most of the RAW264.7 macrophages that had red fluorescence contained one to three intact Se-Tomato bacteria. Thus, Se-Tomato bacteria generated a strong fluorescent signal in the phagosomes of macrophages.
We then determined whether the Se-Tomato strain could establish a normal persistent infection. Several polymorphic genes encoding innate immune effector molecules expressed primarily by macrophages control natural resistance to Se infection in mice (Dauphinee et al., 2014; Eva et al., 2014; Roy et al., 2007). C57BL/6 (B6) and BALB/c have hypomorphic alleles of these genes and succumb to the infection in less than a week, while 129X1/SvJ (129) and B6 × 129 F1 mice possess functional alleles and develop life-long persistent infections (Loomis et al., 2014; Monack et al., 2004). We therefore used 129 or B6 × 129 F1 animals so that persistent infection could be studied.
129 mice were infected intragastrically with Se-Tomato or wild-type Se bacteria and colony forming units (CFU) were measured after infection to assess the persistence of the two strains. As expected, the wild-type Se bacteria disseminated quickly after inoculation and peaked on day 25 at 105 or 104 organisms in the spleen or mesenteric lymph nodes, respectively. The number of bacteria then fell by day 60 to a stable level of 100–500 organisms in both locations (Figure S1D). Inoculation of Se-Tomato organisms resulted in a similar pattern. Thus, Se-Tomato bacteria were not attenuated and could cause a persistent infection.
Th1 cells control Se-Tomato infection
Work from others indicated that the decline and stable control of Se organisms in resistant hosts is due to the action of CD4+ T cells (Johanns et al., 2010; Weintraub et al., 1997). To determine whether this was the case for the Se-Tomato strain, we tested the effect of Se-Tomato infection on T cell-deficient Tcra−/− and CD4+ T cell-deficient H2dlAb1-Ea strains that we produced by backcrossing the targeted alleles onto the 129 backround. Both mouse strains succumbed to infection with Se-Tomato bacteria on about day 30 (Figure 1A) with 300 times more bacteria in their spleens than wild-type mice (Figure 1B). As reported by others (Johanns et al., 2010; Monack et al., 2004), neutralization of IFN-γ or antibody-mediated depletion of CD4+ T cells but not CD8+ T cells led to a significant increase in Se-Tomato CFU in persistently infected mice (Figure 1C). These results suggest that Th1 cells are the major component of the adaptive immune system required for control of Se-Tomato infection during the persistent phase.
Figure 1. CD4+ T cells control persistent Se-Tomato infection.
(A) Survival of wild-type (n =13), TCRα-deficient (n = 12), or MHCII-deficient, CD4+ T cell-deficient (n = 11) 129 mice infected intragastrically with 108 Se-Tomato bacteria. Survival curves were compared using the Log rank (Mantel-Cox) test to determine significance. (B) Mean CFU (± geometric S.D.) in the spleens of wild-type (n = 7), TCRα-deficient (n = 9), or MHCII-deficient, CD4+ T cell-deficient mice (n = 8), 30 days after intragastric infection with 108 Se-Tomato bacteria. CFU values were log-transformed and columns were compared using one-way ANOVA, with Tukey’s multiple comparisons post-test (*** p ≤ 0.0002) and derived of two independent experiments. (C)Spleen CFU (± geometric S.D.) from 129 mice infected intragastrically with 108 SeTomato bacteria, rested for 60 days, injected intraperitoneally with 0.8 mg of CD4 (n = 15), CD8 (n = 4), IFN-γ (n = 9), or isotype control (n = 25) antibodies twice 3 days apart, before CFU measurement seven days later. Spleen CFU values were log-transformed and analyzed using one-way ANOVA, with Tukey’s multiple comparisons post-test (*** p < 0.001, **** p < 0.0001, n.s. = not significant) to evaluate the significance between groups.
Se-Tomato infection induces massive expansion of protective Se epitope-specific CXCR3+ Th1 cells
An Se peptide:MHCII-specific CD4+ T cell population was tracked to gain a better understanding of how Th1 cells control Se infection. We focused on a population of T cells specific for a peptide (LpdAp) from the Se dihydrolipoamide dehydrogenase protein, which binds to the I-Ab MHCII molecule expressed by 129 mice, and is abundantly displayed on the surfaces of Se-infected dendritic cells (Karunakaran et al., 2017). LpdAp:I-Ab-specific CD4+ T cells were detected by flow cytometry using a cell enrichment method (Moon et al., 2007) based on a fluorochrome-labeled LpdAp:I-Ab tetramer. Uninfected 129 mice contained about 60 LpdAp:I-Ab-specific naïve CD4+ T cells (Figure 2A, 2B). These precursors underwent 6,000-fold proliferation and generated 300,000 and 8,000 effector cells in the spleen and mesenteric lymph nodes, respectively, by day 25 after infection. The population then contracted about 10-fold in the two locations by day 100 and was maintained at this level for the next 250 days (Figure 2B). Subsequent experiments were focused on the spleen because this organ contained most of the Se bacteria and Se epitope-specific T cells.
Figure 2. Expansion and differentiation of Se epitope-specific Th1 cells in Seinfected mice.
(A)Plots of LpdAp:I-Ab-specific CD4+ T cells from uninfected (left) or Se-infected (right) mice 60 days after infection identified by tetramer-based cell enrichment and flow cytometry. (B)Mean numbers (± S.D.) of LpdAp:I-Ab tetramer+ CD4+ T cells in the indicated organs of 129 mice at the indicated times after Se infection. (C)Representative histograms showing intracellular T-bet staining of CD44low naïve CD4+ T cells and CD44high LpdAp:I-Ab tetramer+ CD4+ T cells from the same Se-infected mouse. (D)Representative contour plots of LpdAp:I-Ab tetramer+ CD4+ T cells from day 60 Se-Tomato infected 129 mice. (E)Mean numbers (± S.E.M., n ≥ 3 mice per group/timepoint) of LpdAp:I-Ab tetramer+ CXCR3+ or CX3CR1+ CD4+ T cells in the spleens of 129 mice at the indicated times after Se infection. Significance was determined on log-transformed values using twoway ANOVA with Sidak’s multiple comparisons post-test (** p < 0.01, *** p < 0.001, **** p < 0.0001). (F)Mean CFU (± geometric S.D.) per spleen from 129 mice that received total (n = 4), CXCR3+ (n = 5), CX3CR1+ (n = 4), or no CD4+ memory T cells (n = 5) from day 60 Se-infected 129 mice, seven days after intravenous infection with 104 Se-Tomato bacteria. Log-transformed values were compared using one-way ANOVA with Tukey’s multiple comparisons post-test (* p < 0.05, *** p < 0.001, **** p < 0.0001, n.s. = not significant) and is derived from three independent experiments.
The phenotype of the LpdAp:I-Ab-specific T cell population yielded a clue about their protective functions. All the LpdAp:I-Ab-specific CD4+ T cells in day 60 Se-infected mice were Th1 cells as evidenced by expression of large amounts of the Th1 lineagedefining transcription factor T-bet (Ravindran et al., 2005) (Figure 2C) and secretion of IFN-γ after peptide stimulation in vivo (data not shown). Analysis of chemokine receptors revealed that 80% of the LpdAp:I-Ab-specific Th1 cells expressed CXCR3, which binds CXCL9, CXCL10, and CXCL11 (Groom and Luster, 2011), while the other 20% expressed CX3CR1, which binds CX3CL1 (fractalkine) (Bottcher et al., 2015) (Figure 2D). A subset of the CXCR3+ cells expressed CD69, a marker of acute TCR signaling or tissue residence (Cibrian and Sanchez-Madrid, 2017). CX3CR1+ cells expressed KLRG1, a marker of terminal differentiation in other systems (Sallin et al., 2017). The CXCR3+ and CX3CR1+ subsets of LpdAp:I-Ab-specific Th1 cells underwent expansion and contraction during the acute phase of infection and stabilization during the persistent phase although the CXCR3+ cells dominated at later times (Figure 2E) suggesting a more prominent role in the control of persistent Se bacteria. Thus, the LpdAp:I-Ab-specific Th1 cell population contains at least two major types of chemokine receptor-expressing cells as observed for Th1 cells and CD8+ T cells during other phagosomal infections (Chu et al., 2016; Sakai et al., 2014).
The two subsets were then tested for their capacity to control Se-Tomato infection. Total, CXCR3+, or CX3CR1+ CD4+ T cells were purified from Se-infected 129 mice and 106 cells from each population were transferred into naïve 129 mice, which were then challenged intravenously with Se bacteria. The intravenous route was used to produce a more consistent infection than the intragastric route. Recipients of the total CD4+ T cell population contained 15-fold fewer bacteria in the spleen seven days after challenge than mice that did not receive T cells (Figure 2F). The CXCR3+ population afforded the same degree of protection to adoptive recipients as the total population, while the CX3CR1+ population provided less protection. These results indicate that CXCR3+ Th1 cells are the major controllers of Se infection.
Th cells are concentrated near granulomas
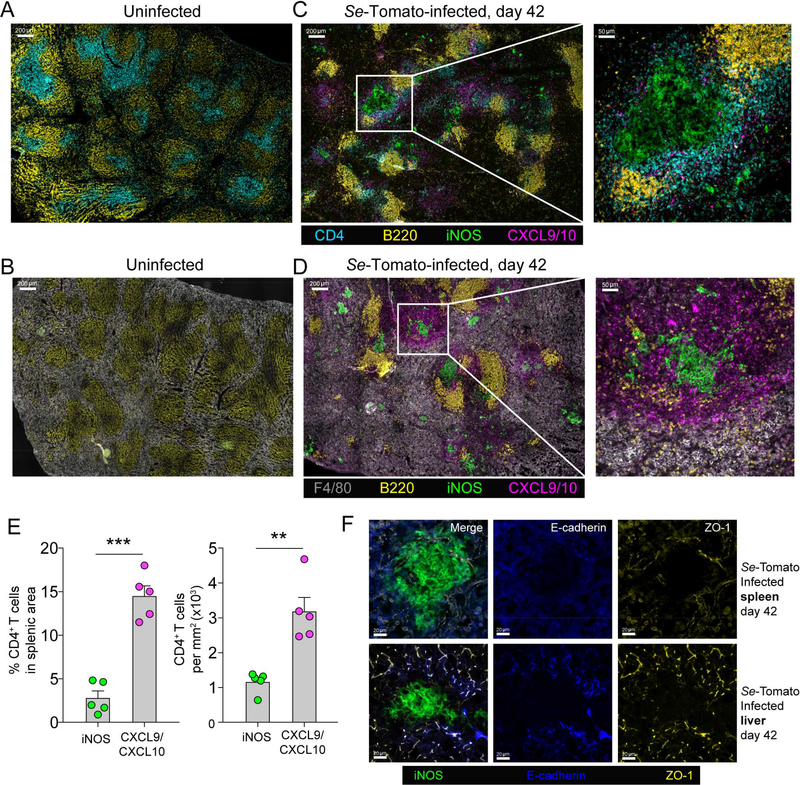
The locations of CD4+ T cells in the Se-infected spleen were then assessed to gain insight into infection control. Thin sections of spleen tissue were stained with fluorochrome-labeled antibodies specific for CD4; CXCL9 and CXCL10; F4/80, a general monocyte/macrophage marker; B220 to highlight B cell follicles; and inducible NO synthase (iNOS), a marker of macrophage activation (Bogdan, 2015). The stained sections were imaged by microscopy. Uninfected mice contained well-organized white pulp cords with B cell-rich follicles surrounding peri-arteriolar sheaths containing CD4+ T cells (Figure 3A). The red pulp areas between the white pulp cords were packed with F4/80+ macrophages (Figure 3B). A few CXCL9/10+ cells were scattered throughout the red pulp regions of the spleens of uninfected mice and iNOS+ cells were rare. As early as 3 days after Se-Tomato infection (data not shown) and thereafter, for example on day 42, the red pulp was expanded and the white pulp cords were compressed (Figure 3C, 3D). Dense clusters of iNOS+ CXCL9/10− F4/80+ cells, usually bordered and often ringed by iNOS− CXCL9/10+ F4/80+ cells formed in the red pulp, often adjacent to white pulp cords. Some CD4+ T cells were still in white pulp cords but many were in the CXCL9/10+ areas bordering the iNOS+ clusters (Figure 3C). Histo-cytometry (Gerner et al., 2012) (Figure S2) was used to quantify the distribution and density of CD4+ T cells throughout the spleen and revealed that the CD4+ T cells were concentrated in the CXCL9/10 regions but were largely excluded from the iNOS+ cores (Figure 3C, 3E).
Figure 3. Se infection stimulates the development of iNOS+ granulomas bordered by CXCL9/10+ mononuclear phagocytes and CD4+ T cells.
(A, B) Spleen sections from an uninfected 129 mouse stained with the indicated antibodies, imaged at 200x magnification, and tiled to cover the entire section. (C, D) Spleen sections from a day 42 Se-Tomato-infected 129 mouse stained with the indicated antibodies, imaged at 200x magnification, and tiled to cover the entire section. Parts of the images in C and D in white boxes are shown enlarged. (E) Mean percentages (± S.E.M.) of total CD4+ T cells in CXCL9/10+ or iNOS+ areas of the spleen images represented in (C), and mean density (± S.E.M.) of CD4+ T cells (per mm2) calculated from dividing the number of CD4+ T cells within each area by the total area of each region. The positions of CD4+ T cells were determined by histo-cytometry and CXCL9/10+ or iNOS+ clusters by a DBSCAN-based algorithm. Images from five individual mice were analyzed. The paired student’s T-test was used to compare the percentage (left) and density (right) of CD4+ T cells within different anatomical locations (** p = 0.007, *** p = 0.0005). (F) Images of spleen (top row) or liver sections (bottom row) from day 42 Se-Tomato infected mice (left column) stained with the indicated antibodies.
The Se-induced clusters of iNOS+ macrophages were suggestive of granulomas. Mycobacterial granulomas are defined by epithelioid macrophages expressing tight junction proteins E-cadherin and ZO-1 (Pagan and Ramakrishnan, 2018). Although this process is less evident in mice than in other species (Guirado and Schlesinger, 2013), these markers have been observed in M. tuberculosis-induced murine granulomas (Cronan et al., 2016). We therefore stained sections from spleen and liver of infected mice, which both had iNOS+ clusters, with antibodies specific for E-cadherin and ZO-1. These proteins were detected at the borders of splenic stromal cells and hepatocytes in Se-infected mice but not in the iNOS+ clusters or CXCL9/10+ borders (Figure 3F). The weak expression of tight junction proteins in the clusters of iNOS+ macrophages indicates that these structures are non-epithelioid granulomas (Pagan and Ramakrishnan, 2018). The composite structures will be referred to as CXCL9/10bordered granulomas.
Se epitope-specific Th cells are concentrated in CXCL9/10+ borders and excluded from granuloma cores
We next determined whether T cells with TCRs specific for Se epitopes were also concentrated in the CXCL9/10 regions as was the case for CD4+ T cells in general. It was first necessary to establish a system for detection of Se epitope-specific T cells since the LpdAp:I-Ab tetramer did not bind to specific T cells in fixed tissue sections (data not shown). An adoptive transfer approach was therefore used for this purpose. CD45.1+ B6 × 129 F1 mice, to be used as T cell donors, were inoculated with Se bacteria or Listeria monocytogenes (Lm) bacteria to induce Th1 cells but specific for different peptide:MHCII complexes (Pepper et al., 2011; Ravindran et al., 2005). Six weeks later, CD4+ T cells were purified from the donor mice and transferred into separate sets of naïve CD45.2+ B6 × 129 F1 recipients, which were then infected with Se-Tomato bacteria. Naïve mice that did not receive CD4+ T cells were also infected. One week after infection, the mice that did not receive T cells or mice that received CD4+ T cells from Lm-infected donors contained about 106 splenic CFU, whereas mice that received CD4+ T cells from Se-Tomato-infected donors contained only 104 (Figure 4A). The 100-fold reduction in CFU observed in recipients of CD4+ T cells from Se-Tomato-infected donors was associated with a 30-fold increase in the number of donor T cells, while the T cells from Lm-infected mice that did not provide protection increased only 3-fold (Figure 4B). The 10-fold greater number of donor-derived T cells in recipients of T cells from Se- than Lm-infected mice indicates that 90 percent of the donor T cells in Se-infected mice on day seven after challenge were specific for Se epitopes. Analysis of spleen sections from CD45.2+ B6 × 129 F1 mice that received CD4+ T cells from Se-Tomato-infected donors revealed that 20 percent of the CD45.1+ donor T cells were in the CXCL9/10+ areas whereas less than five percent were in the iNOS+ areas (Figure 4C, 4D). Thus, Se epitope-specific T cells were concentrated in the CXCL9/10+ cuffs and excluded from the granuloma cores.
Figure 4. Detection and localization of Se epitope-specific T cells using an adoptive transfer system.
(A) CD4+ memory T cells (2.5 × 106) from CD45.1+ B6 × 129 F1 mice infected intravenously with 108 Listeria monocytogenes-ΔactA (Lm) or intragastrically with 108 Se bacteria 30 days earlier were transferred into CD45.2+ B6 × 129 F1 recipients. Five mice received Lm-primed memory cells, five received Se-primed memory cells, and nine did not receive any cells. All recipients were challenged intravenously with 104 Se-Tomato bacteria. Mean splenic CFU (± geometric S.D.) were measured seven days later and analyzed by one-way ANOVA, with Tukey’s multiple comparisons post-test (**** p < 0.0001). The results are derived from two independent experiments. (B)Representative flow cytometry plots of splenic T cells from mice that received Lm-primed (middle), Se-primed (right), or no (left) CD45.1+ CD4+ memory T cells and were challenged with Se-Tomato bacteria seven days earlier. Mean fold expansion (± S.D.) of transferred cells over controls that received T cells but were not infected is shown in the bar graph. Unpaired student’s T-test was used to compare the responses of the two memory T cell populations (**** p < 0.0001). (C) Spleen sections from a B6 × 129 F1 (CD45.2+) mouse that received CD45.1+ memory CD4+ T cells from CD45.1+ Se-infected donors and was challenged intravenously with Se-Tomato bacteria seven days earlier. Sections were stained with the indicated antibodies. Part of the image in the white box is shown enlarged. Scale bars are included on each panel. (D)Quantitative analysis as described in the legend to Figure 3E but applied to images of transferred CD45.1+ CD4+ T cells from eight different recipient mice. (*** p≤ 0.0004).
Se epitope-specific Th cells use CXCR3 to concentrate in CXCL9/10+ borders
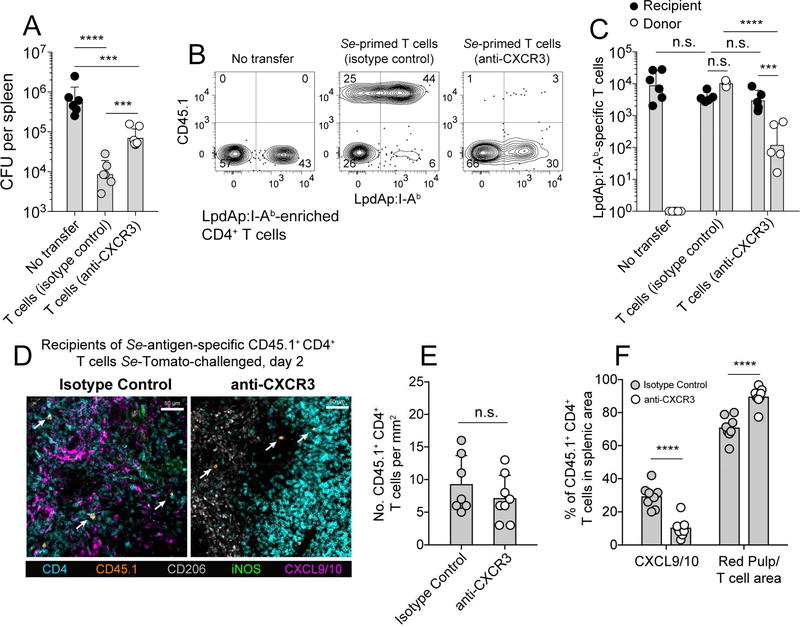
An antibody blocking approach was then used to determine whether CD4+ T cell-concentration in the CXCL9/10+ borders and control of Se infection depended on the function of CXCR3. CD45.1+ CD4+ T cells from day 60 Se-infected mice and containing expanded populations of Se epitope memory/effector T cells including LpdAp:I-Ab-specific cells were transferred into naïve recipients. These recipients and controls that did not receive T cells were challenged with Se-Tomato bacteria and the T cell recipients were treated with an CXCR3 antibody that blocks binding of CXCL10 (Uppaluri et al., 2008), or isotype control antibody every other day for one week. Mice that received CD4+ T cells from day 60 Se-Tomato infected mice and were treated with control antibody had about 70-fold fewer Se-Tomato bacteria than mice that did not receive additional T cells (Figure 5A). In contrast, mice that received T cells and were treated with CXCR3 antibody had 10-fold fewer bacteria than mice that did not receive additional T cells demonstrating that CXCR3 is required for memory/effector T cells to mediate optimal Se control. The fact that CXCR3 blockade did not completely block protection could have been related to residual effects of CXCL9 or other factors.
Figure 5. CXCR3 blockade of Se-specific memory/effector Th1 cells leads to a loss of protection.
(A) Mean CFU (± geometric S.D.) per spleen from 129 mice that received no CD4+ memory T cells or CD45.1+ CD4+ memory/effector T cells from day 60 Se-infected 129 mice and were treated with CXCR3 antibody (n = 6) or normal hamster IgG (n = 6) for seven days after intravenous infection with 104 Se-Tomato bacteria. Log-transformed values were compared using one-way ANOVA with Tukey’s multiple comparisons posttest (*** p ≤ 0.0002, **** p < 0.0001, n.s. = not significant) and are derived from three independent experiments. (B) Representative contour plots from LpdAp:I-Ab tetramer-enriched samples from the spleens of mice from the groups shown in (A). LpdAp:I-Ab tetramer-binding cells of recipient origin are shown in the lower right quadrants and of donor origin in the upper right quadrants. (C)Numbers (± geometric S.D.) of recipient- or donor-derived LpdAp:I-Ab tetramer-binding cells in mice that did not receive donor T cells (left), or received T cells from Se-infected mice and were treated with the indicated antibodies. Significance was determined on log-transformed values using two-way ANOVA with Sidak’s multiple comparisons post-test (*** p = 0.0002, **** p < 0.0001, n.s. = not significant). (D)Spleen sections from B6 × 129 F1 (CD45.2+) mice that received memory/effector CD4+ T cells from CD45.1+Se-infected donors, then isotype control (left) or CXCR3 (right) antibody and intravenous infection with Se-Tomato bacteria before analysis two days later. Sections were stained with the indicated antibodies. White arrows indicate donor-derived T cells. (E) Density of CD45.1+ CD4+ T cells in spleen sections from individual mice of the type shown in (D) determined by manual counting. (F)Mean percentages (± S.E.M.) of CD45.1+ CD4+ T cells (identified by histo-cytometry) in CXCL9/10+ areas (identified by a DBSCAN-based algorithm) or other areas of the spleens of individual mice in the indicated groups. (**** p < 0.0001).
The LpdAp:I-Ab-specific T cells in these mice were analyzed to understand how CXCR3 was involved in Se control. Mice that did not receive T cells from Se-infected mice or received T cells with or without CXCR3 blockade all contained about the same number of LpdAp:I-Ab-specific T cells of recipient origin (Figure 5B, 5C). Thus, CXCR3 antibody did not deplete or mask T cells and did not block the priming of the naïve LpdAp:I-Ab-specific T cells, probably because naïve T cells did not express CXCR3 at the time of initial antigen presentation by dendritic cells in the T cell zones. In contrast, the transferred LpdAp:I-Ab-specific memory/effector T cells, many of which were CXCR3+ at the time of infection (Figure 2D), expanded 30-fold less well in CXCR3 antibody-treated mice than in isotype control-treated mice (Figure 5B, 5C).
The adoptive transfer approach was then used to determine whether CXCR3 antibody treatment blocked the localization of memory/effector Th1 cells in the CXCL9/10+ granuloma borders. CD45.1+ CD4+ T cells from day 60 Se-infected mice were transferred into naïve recipients, which were treated with CXCR3 neutralizing or isotype control antibody and then infected with Se-Tomato bacteria. Spleen sections were analyzed two days after T cell transfer and infection to focus on early migration rather than later proliferation. Indeed, the number of CD45.1+ CD4+ T cells was similar in the spleens of mice that were treated with isotype control or CXCR3 antibody at this time (Figure 5D, 5E) demonstrating that the CXCR3 antibody treatment did not interfere with detection of the transferred cells. About 30 percent of the transferred T cells in mice treated with isotype control antibody were in the CXCL9/10+ cuffs (Figure 5F). In contrast, very few of the transferred T cells were in the CXCL9/10+ cuffs in mice treated with CXCR3 antibody and most were in the red pulp or T cell areas. These results indicate that most memory/effector Th1 cells in Se-infected mice use CXCR3 to position themselves in the CXCL9/10+ granuloma borders.
Se bacteria are concentrated in and around granulomas
The results in Figures 4 and 5 suggest that Se epitope-specific CXCR3+ Th1 cells were concentrated in CXCL9/10+ areas and excluded from the CXCL9/10− iNOS+ granuloma cores. These results raise the possibility that the iNOS+ granuloma cores are safe havens for Se bacteria. We tested this possibility by identifying the locations of the bacteria using immunohistology. High magnification images of spleen sections revealed Se-Tomato bacteria in iNOS+ granuloma cores, CXC9/10+ borders, and the red pulp (Figure 6A). The bacteria in these images were digitally enlarged to allow their visualization in lower magnification views of spleen sections. This procedure showed that most of the rare Se-Tomato bacteria in wild-type mice on day 30 after infection appeared to be in or near the CXCL9/10-bordered granulomas (Figure 6B). Imaris image analysis software and histo-cytometry were used to test this impression objectively. Digital surfaces of iNOS+ cells, CXCL9/10+ cells, and dTomato+ Se-bacteria (Figure 6C, S2, S3) were created and an algorithm was used (Ester et al., 1996) to identify clusters of iNOS+ or CXCL9/10+ cells. The locations of Se-Tomato bacteria were then mapped onto these clusters (Figure 6C, S2I). This analysis revealed that about 60% of the rare Se-Tomato bacteria that were in the spleens of wild-type mice were in the CXCL9/10-bordered granulomas and most of the remaining bacteria were in other areas of the red pulp (Figure 6D). This pattern reflected a remarkable concentration of the bacteria in the CXCL9/10-bordered granulomas since these regions accounted for only 5% of the splenic volume (Figure 6E). Of the Se-Tomato bacteria that were in the CXCL9/10-bordered granulomas, 70% were in the iNOS+ core regions (Figure 6D, 6E). Thus, Se bacteria were concentrated in iNOS+ CXCL9/10− areas that contained very few Se epitope-specific T cells (Figure 4D).
Figure 6. Se bacteria are concentrated in iNOS+ granulomas.
(A) Spleen sections from day 42 Se-Tomato infected mice stained with the indicated antibodies and shown at high magnification. Se-Tomato bacteria are indicated by white arrows. (B-G) Spleen sections from day 42 Se-infected 129 (B, C) or MHC-deficient, CD4+ T cell-deficient mice (F, G) stained with the indicated antibodies. Se-Tomato bacteria were digitally enlarged using Imaris software to mark their positions in B and F. (C, G) Positional data (centroid) were extracted from the images in B and F and used to construct digital surfaces for each stained cell. Clusters of iNOS+ or CXCL9/10+ cells were generated from these surfaces using a DBSCAN clustering algorithm, and the positions of Se-Tomato bacteria were marked with crosses. (D) The data from rendered surfaces such as those shown in C and G (wild type, n = 6, MHC-deficient, CD4+ T cell-deficient n = 8) were used to calculate the mean percentages (± S.E.M.) of Se-Tomato bacteria in iNOS+ or CXCL9/10 clusters, or other areas of the red pulp. Differences between wild type and CD4+ T cell-deficient mice were tested using two-way ANOVA with Sidak’s multiple comparisons test. (n.s. = not significant). (E) Pie charts displaying the proportion of red pulp containing iNOS+ or CXCL9/10+ regions or the frequency of Se-Tomato bacteria found within each region.
A similar analysis was performed on spleen sections from day 30 Se-Tomatoinfected CD4+ T cell-deficient mice to assess the effect of CD4+ T cells on the location of the bacteria. The spleens of these mice contained many more bacteria and iNOS+ granulomas per unit area and the CXCL9/10+ borders were smaller than those of wildtype mice (Figure 6F, 6G). Despite these differences, the bacteria were distributed in CD4+ T cell-deficient mice in a pattern that was like that observed in wild-type mice (Figure 6D). Most of the bacteria where in the CXCL9/10-bordered granulomas and most of these were in the iNOS+ regions. Therefore, CXCL9/10-bordered granulomas formed in the absence of T cells suggesting that these structures are generated by an innate immune response. The smaller size of the CXCL9/10+ borders, however, indicates that CD4+ T cells play a role in maintaining these structures.
Identification of the cells that comprise the iNOS+ and CXCL9/10+ structures
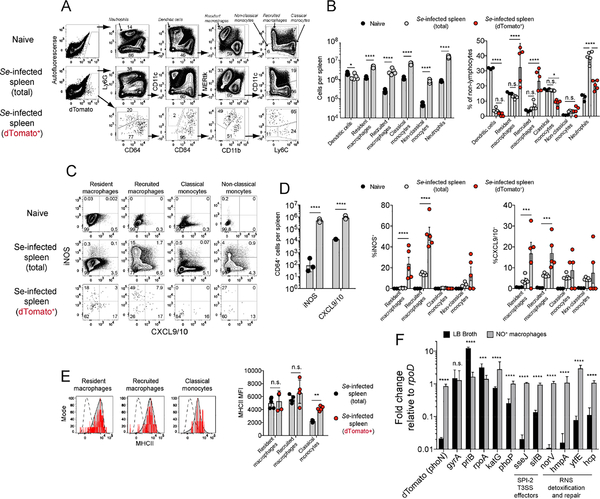
The concentration of Se bacteria in the CXCL9/10-bordered granulomas warranted investigation of the cell types that comprised these structures and harbored the bacteria. Flow cytometry was used for his purpose based on the knowledge that the iNOS+ cells in the granulomas lacked CXCL9/10 and the surrounding CXCL9/10+ cells lacked iNOS (Figure 3C, 3D). We focused on myeloid cells because of abundant evidence in the literature that Se bacteria invade this cell type (Burton et al., 2014; Eisele et al., 2013; Griffin et al., 2011; Nix et al., 2007). Myeloid cells were enriched from spleen samples and stained with an antibody panel designed to resolve prominent myeloid cell populations (Gautier et al., 2012; Jakubzick et al., 2013). The nonlymphocytes from the spleens of uninfected mice contained Ly6G+ neutrophils and a complex population of Ly6G− cells including CD11chigh CD64− dendritic cells and CD64+ monocytes and macrophages (Figure 7A). The CD64+ population contained MERtk+ CD11b− tissue-resident macrophages and CD11b+ cells including CD11c+ recruited macrophages, CD11c− Ly6C+ classical monocytes, and CD11c− Ly6C− non-classical monocytes. These populations were also present in the spleens of Se-infected mice although the numbers of cells of each type except dendritic cells were significantly increased (Figure 7B).
Figure 7. Identification of iNOS+, CXCL9/10+, and Se-infected host cells.
(A) Representative flow cytometry plots of non-lymphocyte-enriched spleen cells from the indicated sources following exclusion of lymphocytes, NK cells, and dead cells. Plots of Se-dTomato+ cells were concatenated from the spleens of five day 42 Se-infected 129 mice (bottom row). (B)Mean number (± geometric S.D.) (left) and mean percentage (± S.E.M.) (right) of the indicated myeloid populations in the spleens of uninfected (n = 3) or day 42 Se-Tomato infected (n = 5) mice. Significance was determined on log-transformed (left) or untransformed (right) values using two-way ANOVA with Sidak’s multiple comparisons post-test (* p ≤ 0.04, **** p < 0.0001, n.s. = not significant). (C)Representative flow cytometry plots showing expression of iNOS and CXCL9/10 by the mononuclear phagocyte populations identified in (A) from the spleens of uninfected or day 42 Se-Tomato-infected mice, or dTomato+ cells from infected mice. (D) Left panel - numbers (± geometric S.D.) of total iNOS+ or CXCL9+ cells from uninfected (n = 3) or day 42 Se-Tomato-infected mice (n = 5), compared with two-way ANOVA and Sidak’s multiple comparisons test (**** p < 0.0001). Middle and right panels - population comparison from the flow cytometry experiment shown in (C). Mean percentages (± S.E.M.) of mononuclear phagocyte populations expressing iNOS (middle) or CXCL9/10 (right) from uninfected (n = 3), total day 42 Se-Tomato-infected (n = 5) or dTomato+ (n = 5) cells from day 42 Se-Tomato-infected 129 mice. Percentages were analyzed using two-way ANOVA and Sidak’s multiple comparisons test (*** p ≤ 0.0004 **** p < 0.0001). (E)Representative flow cytometry histograms of MHCII levels on the indicated mononuclear phagocytes from day 42 Se-Tomato infected 129 mice (gray), neutrophils as negative controls (dashed), or dTomato+ infected cells (red). The bar graph shows geometric mean MHCII mean fluorescence intensity (MFI) (± S.E.M.) levels on total (n = 3) or dTomato+ infected cells (n = 5) from day 42 Se-Tomato infected 129 mice. MFI values were compared using two-way ANOVA and Sidak’s multiple comparisons test (** p = 0.0088, n.s. = not significant). (F) Fold change expression of Se mRNA relative to the housekeeping gene rpoD, between bacteria grown to late-log phase in LB broth (n=6, black bars) and Se bacteria from NO+ macrophages (n=7, gray bars). Data are from two independent experiments, and conditions were compared using two-way ANOVA and Sidak’s multiple comparisons test (*** p = 0.0004, **** p < 0.0001, n.s. = not significant). SPI-2 T3SS = Salmonella pathogenicity island 2 type 3 secretion system; RNS = reactive nitrogen species.
The analysis was then focused on CD64+ cells since preliminary experiments showed that almost all the iNOS+ or CXCL9/10+ cells in infected mice expressed this molecule (data not shown). Very few of the CD64+ cells from uninfected mice expressed iNOS or CXCL9/10 (Figure 7C, 7D). In contrast, cells expressing iNOS or CXCL9/10 were readily detectable in the CD64+ population in Se-Tomato-infected mice and most of the positive cells expressed iNOS or CXCL9/10 but not both as was observed by immunohistology. Five-10% of the cells in each of the four major CD64+ subsets in the spleens of Se-Tomato-infected mice expressed CXCL9/10. Thus, the iNOS− CXCL9/10+ borders in the spleen red pulp were composed of a mixture of monocytes and macrophages. Recruited macrophages were by far the major iNOS+ cell type in Seinfected spleens. Therefore, recruited macrophages must be the major constituents of the iNOS+ granuloma cores.
The Se-infected cells were then identified based on comparison of the auto- and red fluorescence profiles of non-lymphocytes from naïve and Se-infected mice (Figure 7A). The Se-infected cells accounted for about 1 in 1,500 of the total non-lymphocyte population of day 42 Se-Tomato infected animals. The Se-infected population had a lower fraction of neutrophils, dendritic cells, and classical monocytes and a higher fraction of resident and recruited macrophages than the uninfected CD64+ cells in the same mice (Figure 7B). In addition, a higher fraction of the Se-infected resident and recruited macrophages produced iNOS or CXCL9/10 than the comparable uninfected populations (Figure 7C, 7D). The MHCII levels of Se-infected resident and recruited macrophages were the same as those of the comparable populations from uninfected mice and those of classical monocytes were higher (Figure 7E). Thus, the Se-infected population was generally more activated and macrophage-dominated than the uninfected myeloid cell population in the same mice. In addition, Se-infected cells showed no signs of immune evasion at the level of iNOS, CXCL9/10, or MHCII expression.
The finding that Se-infected populations contained more iNOS-producing cells than their uninfected counterparts raised the issue of how the bacteria survive in the presence of NO. We addressed this problem by using the reverse transcriptase polymerase chain reaction (RT-PCR) to assess expression of Se mRNAs involved in bacterial growth and resistance to reactive nitrogen species (Henard and Vazquez-Torres, 2011). RT-PCRs were performed with primers for candidate Se genes on RNA samples from sorted NO-producing CD64+ CD11c+ recruited macrophages containing intracellular Se organisms or Se bacteria cultured in rich broth. The fold change of the PCR value for each gene was calculated relative to a house-keeping gene (rpoD) (Figure 7F). dTomato mRNA was much more highly expressed by intra-macrophage than broth-grown Se bacteria as expected because this transcript was driven from the phoN promoter known to be highly active in intracellular bacteria (Barat et al., 2012). Also, as expected, the house-keeping mRNA, gyrA, was expressed equally by Se bacteria under both conditions. mRNAs involved in DNA replication and growth (priB, rpoA) were expressed to a higher degree in broth-grown than intra-macrophage Se bacteria indicating that the microbes were growing slowly in NO+ macrophages. In contrast, intra-macrophage Se bacteria contained abundant mRNAs required for survival in macrophages. These included a transcriptional regulator of virulence (phoP) (Park and Groisman, 2014), effectors of the Salmonella pathogenicity island 2 type III secretion system (sifB, sseJ) involved in phagolysosome modification (Figueira and Holden, 2012), and a gene for reactive oxygen species detoxification (katG) (Bumann and Cunrath, 2017). Notably, intra-macrophage Se bacteria also contained abundant transcripts encoding proteins (hcp, ytfE, hmpA, norV) involved in sensing and detoxification of reactive nitrogen species (Henard and Vazquez-Torres, 2011). These results suggest that Se bacteria survive in a dormant state in iNOS-producing cells by neutralizing toxic host molecules.
DISCUSSION
Our results show that systemic Se infection is associated with the formation of tight clusters of iNOS+ macrophages bordered by a looser collection of CXCL9/10+ monocytes and macrophages in the spleen. The tight clustering of iNOS+ macrophages indicates that these structures are granulomas although the weak expression of E-cadherin or ZO-1 suggests an early stage of epithelialization. The CD11b+ CD11c+ MHCIIhigh iNOS+ Ly6C− phenotype indicates that the main cells that form these granulomas are recruited monocytes that differentiated into macrophages, also known as TNF/iNOS-producing dendritic cells (Shi and Pamer, 2011). Since the iNOS+ granulomas were more numerous in T cell-deficient mice, they were likely initiated by an innate rather than an adaptive immune mechanism. iNOS expression by mononuclear phagocytes is often triggered by synergistic signaling between innate pattern recognition and IFN-γ receptors (Bogdan, 2015; Eisenstein, 2001). Thus, it is conceivable that the iNOS+ granulomas arose in part from monocytes or macrophages that were activated by direct infection or recognition of microbial products. Our finding that macrophages containing bacteria were more likely to produce iNOS than those that did not lends credence to this explanation. It is not clear why Se-induced granulomas do not undergo the macrophage epithelialization process typical of M. tuberculosis-induced granulomas although their location in secondary lymphoid organs rather than the lungs may be a factor. It is also possible that individual Se-induced granulomas turn over relatively quickly and do not persist long enough for the epithelialization process to occur.
Although it has been long appreciated that lymphocytes are restricted to the granuloma border (Guirado and Schlesinger, 2013; Pagan and Ramakrishnan, 2018), the basis this phenomenon was not understood. Our results suggest that CXCR3+ CD4+ T cells are concentrated in these border regions because of local CXCL9/10 production by a mixed population of mononuclear phagocytes that does not express iNOS. IFN-γ is the major known driver of CXCL9 and CXCL10 production in mononuclear phagocytes (Groom and Luster, 2011). It is therefore possible that mononuclear phagocytes surrounding the iNOS+ granulomas are initially stimulated by innate sources of IFN-γ, such as NK cells (Kupz et al., 2013), to produce CXCL9/10, which is later amplified by Se epitope-specific CXCR3+ Th1 cells that are attracted to this area. This possibility is supported by the observation that the CXCL9/10+ cuffs were larger and more organized in wild-type mice than in CD4+ T cell-deficient mice. The fact that blockade of CXCR3 inhibited the localization of Th1 cells to the CXCL9/10+ cuffs, suppressed Th1, proliferation, and lead to increased bacterial growth indicates that such a process is critical for Th1 cell detection and control of infected cells, likely via direct recognition of cognate epitopes.
Our study also sheds light on the capacity of Se bacteria to persist in the face of Th1-based immunity. We found no evidence that Se persistence was related to direct inhibition of T cell activation or Th1 cell formation. Se-infected mononuclear phagocytes expressed the same number or more MHCII molecules than uninfected cells in the same hosts. In addition, the clonal expansion exhibited by Se epitope-specific T cells was 10-fold greater than that reported for epitope-specific T cells in other bacterial infections (Dileepan et al., 2011; Pepper et al., 2010), and the T cells acquired the CXCR3 phenotype associated with control of other phagosomal pathogens (Chu et al., 2016; Sakai et al., 2014). Rather, our results suggest that Se bacteria persist by residing in macrophages in granuloma core regions that are largely inaccessible to CD4+ T cells. This possibility is supported by the observation that most granuloma-associated CD4+ T cells in Mycobacterium tuberculosis-infected rhesus macaques or mice are in outer cuffs not the core regions containing the bacteria (Ernst et al., 2018; Kauffman et al., 2017). The lack of CXCL9/10 production in the cores of the granulomas caused by these pathogens is a plausible explanation for the failure of CXCR3+ Th1 cells to penetrate these areas. Notably, antigen-specific T cells can access the central regions of the granulomas caused by attenuated Mycobacterium bovis-BCG organisms (Egen et al., 2011) suggesting that T cell exclusion from granuloma cores is a property of virulent phagosomal pathogens.
Our data suggest that Se bacteria persist in iNOS+ CD64+ cells by detoxifying NO and other reactive nitrogen species. Intracellular Se bacteria expressed many copies of mRNAs that encode reductases that chemically modify reactive nitrogen species and a protein that repairs reactive nitrogen species-induced damage to iron-sulfur containing proteins. The bacteria may optimize the action of these protective proteins through structural changes to the phagolysosome induced by the Salmonella pathogenicity island 2 type III secretion system (Figueira and Holden, 2012). Even though the bacteria were able to persist in this state, they were probably not thriving as evidenced by repression of genes involved in DNA replication and growth.
Work in the Mycobacterium bovis-BCG murine model and the Mycobacterium marinum zebrafish model (Davis and Ramakrishnan, 2009; Pagan et al., 2015) suggests that a dynamic cycle of bacterial killing and mononuclear phagocyte recruitment also contributes to persistence. Application of this model to Se infection produces a scenario in which classical monocytes are recruited to infected tissues from the circulation and seed the T cell-inaccessible iNOS+ granulomas. The recruited cells are infected by Se bacteria from other cells in the granuloma, turn on iNOS, differentiate into macrophages that ultimately establish residence, and add to the cluster while clearing their infections. Other monocytes become infected as they migrate through the CXCL9/10+ areas, are rapidly detected by a dense network of CXCR3+ Th1 cells and disinfected or killed. This cycle could turn indefinitely if new monocytes seed the granulomas and CD4+ T cell numbers are maintained.
The action of Th1 cells in the CXCL9/10+ borders is an effective mechanism of pathogen control in immunocompetent hosts even though it does not result in sterilizing immunity. Like most humans with phagosomal infections, Se-infected 129 mice show no overt signs of disease unless the CD4+ T cell-dependent control system is compromised. This control, however, can be interrupted in humans with AIDS, malnutrition, or advanced age, resulting in bacterial outgrowth and pathogenesis. New vaccines capable of sterilizing immunity against phagosomal pathogens are therefore desirable. The development of such vaccines may depend on improved understanding of the factors that exclude pathogen-specific Th1 cells from granuloma cores that harbor the pathogen.
STAR Methods
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information or requests for resources and reagents should be made to and will be fulfilled by the corresponding author, Marc K. Jenkins (jenki002@umn.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Experimental Animals.
Mice were housed and bred in a specific-pathogen free facility at the University of Minnesota and supervised by the AAALAC-accredited Department of Research Animal Resources. All experiments were conducted under federal and institutional guidelines and with the approval of the University of Minnesota Institutional Animal Care and Use Committee. Six to 12-week old male and female mice were used for experiments, as we observed no significant differences in male and female littermates in their responses to Se infection.
Cell Lines.
Drosophila S2 cells (ATCC) were used for the production of recombinant IAb monomers for LpdAp:I-Ab tetramers. These cells were propagated in Drosophila S2 media (ThermoFisher) at 30°C.
Bacterial Strains.
Chemically-competent Top10 Escherichia coli bacteria (ThermoFisher) used for molecular cloning and propagation of recombinant DNA were grown overnight in either LB Lennox broth or streaked on LB agar with appropriate antibiotics for selection. Streptomycin-resistant Se serovar Typhimurium strain SL1344 was grown in LB-Lennox broth or streaked on MacConkey Agar containing 100 μg/ml streptomycin.
METHOD DETAILS
Experimental Animals.
129X1/svJ (129) mice were purchased from the Jackson Laboratory. C57BL/6NCr (B6), and B6-Ly5.1/Cr (CD45.1) were purchased from Charles River Labs. B6 mice were bred to 129 mice to produce B6 × 129 F1 mice. 129 mice were bred to CD45.1 mice to generate CD45.1+ B6 × 129 F1 mice. MHCII-deficient B6.129S2-H2dlAb1-Ea/J (Madsen et al., 1999) and TCRα-deficient B6.129S2-TcraTm1mom/J (Mombaerts et al., 1992) mice were obtained from the Jackson Laboratory and backcrossed to 129 mice for five generations before intercrossing F5 heterozygotes to generate 129-H2dlAb1-Ea and 129-TcraTm1mom mice, homozygous for each targeted allele. The genotype of these mice was determined by PCR (Transnetyx) using specific primers to distinguish the wild-type and targeted alleles and the 129 Slc11a1Gly169 and B6 Slc11a1Asp169 alleles (Powell and Frelinger, 2017). All the backcrossed mice used for experiments were homozygous for the 129 Slc11a1Gly169 allele and either the targeted H2dlAb1-Ea or Tcra alleles.
Construction of Se-Tomato.
Se serovar Typhimurium strain SL1344 was used as the parental strain for all experiments. A template plasmid, pJMG01, containing a flippase recognition target (FRT)-flanked kanamycin (KAN) cassette from the pKD4 plasmid was made using a pUC19 backbone by primer-extension PCR and restriction cloning (Figure S1). The Se codon optimized version (Grote et al., 2005) of the fluorescent protein TdTomato with an EM7 promoter and a 43 base pair ribosomal binding site designed for maximal fluorescent protein expression was then inserted upstream of the KAN cassette by InFusion cloning (Tian and Salis, 2015) (Clontech-Takara) to generate plasmid pJMG08. After transformation of SL1344 bacteria with pJMG08 it was noted that one of the tandem-repeats of the TdTomato sequence was frequently deleted and resulted in increased fluorescence intensity. Thus, the template plasmid used for the next step contained only one copy of the dTomato sequence (pJMG09). This template plasmid was used to create a PCR product containing the dTomato-FRT-KAN cassette with 50 base pair arms homologous to a region at the 3’ end of the phoN locus immediately after the stop codon. This PCR product was electroporated into competent Se-SL1344 transformed with the plasmid pWRG730 (Blank et al., 2011) encoding the Lambda Red Recombinase genes exo, bet, and gam under the control of a temperature sensitive promoter which were activated by incubation in a water bath shaker for 15 minutes at 40°C (Datsenko and Wanner, 2000). The b acteria were then plated on LB agar with 50 μg/ml kanamycin to select recombinants. Colonies were then screened by PCR for the dTomato insertion within the phoN locus and the product was sequenced to confirm positional integration. The KAN cassette was removed using the temperaturesensitive pCP20 plasmid encoding the flippase recombinase (FLP) and a chloramphenicol resistance gene. Following FLP-mediated recombination, recombinants were replica plated on LB agar with and without kanamycin at 37°C to cure pCP20, then kanamycin and chloramphenicol sensitive clones were designated Se-Tomato and used for further experiments.
Animal infections and antibody depletion.
SL1344 and Se-Tomato bacteria were cultured overnight in four ml of LB-Lennox broth containing 100 μg/ml of Streptomycin. The next day, these cultures were added to one liter of broth and grown to mid log phase, and single-use aliquots were frozen in 25% glycerol at −80 °C. After thawing, bacterial stocks were diluted in sterile PBS and 108 CFU were delivered using a feeding needle via intestinal gavage after loading the mice with 100 μl of a 5% bicarbonate solution, or 104 CFU were injected intravenously. For cellular depletion experiments, mice infected intragastrically with Se-infected 60 days earlier were injected intraperitoneally with 0.8 mg of purified mouse CD4, CD8a, and IFN-γ antibodies (BioXcell) twice over one week. Control mice were injected with the same amount of rat-isotype control antibodies.
CD4+ T cell isolation and adoptive transfer.
Single cell suspensions were made from spleens and mesenteric lymph nodes of Se-infected 129 or B6 × 129 F1 donors six to eight weeks after infection. CD44high memory/effector CD4+ T cells were enriched by removal of other cells using CD8, CD11b, CD11c, CD19, CD24, B220, CD45RB, CD49b, CD105, or TCRγ/δ (Stem Cell technologies) antibodies to a purity of 80–90% as assessed by CD44high expression. The memory/effector CD4+ T cells (2.5 × 106) were then injected intravenously into recipient mice, which were infected intravenously with Se-Tomato bacteria shortly thereafter. In some cases, memory/effector CD4+ T cells from Se-infected mice were stained with CXCR3 or CX3CR1 antibodies, and CXCR3+ and CX3CR1+ cells were sorted using a BD FACSAria flow cytometer to 99% purity and injected into naïve recipients. For CXCR3 blocking experiments, mice were injected intravenously with memory/effector CD4+ T cells from Se-infected mice, then several days later intravenously with Se-Tomato bacteria. These mice were then injected intraperitoneally with 0.5 mg of anti-mouse CXCR3 or hamster IgG every other day for two or seven days beginning on the day of infection.
Generation of LpdA340–350:I-Ab tetramers.
Sense and anti-sense DNA oligonucleotides (Supplementary Table S1) encoding a signal peptide with an embedded XmaI restriction site, then the LpdAp peptide (HYFDPKVIPSI), and a poly glycine linker with an embedded SpeI site were ordered from Integrated DNA technologies. The primers were designed to expose 5’ and 3’ overhangs complementary to XmaI and SpeI restriction sites present on the digested vector. The sense and antisense oligonucleotides were annealed and ligated into the XmaI/SpeI-digested pRMHa-3 I-Ab beta plasmid (Moon et al., 2007) using T4 DNA ligase (New England Biolabs). One Shot TOP10 Chemically Competent E. coli bacteria (ThermoFisher) were transformed with the ligation mixture and plated out on LB agar containing 100 μg/ml ampicillin. Inserts from transformants were sequenced to confirm that an open reading frame encoding a signal peptide, LpdA peptide, I-Ab beta chain, basic leucine zipper, and 6X histidine tag was present.
Biotinylated LpdA:I-Ab molecules were expressed in Drosophila S2 cells using the DES Drosphila Expression System (ThermoFisher). S2 cells were grown at 28ºC without CO2 and transfected using the Calcium Phosphate Transfection Kit (ThermoFisher) along with the pRMHa-3 I-Ab beta plasmid (encoding the pRMHa-3 I-Ab alpha plasmid encoding a signal peptide, the I-Ab alpha chain, an acidic leucine zipper, and a BirA ligase site (Moon et al., 2007)), an expression plasmid for the E. coli BirA enzyme (Yang et al., 2004), and the pCoBlast plasmid encoding a blasticidin resistance protein. S2 cells were then selected in Express Five serum-free medium designed for insect cell culture containing 25 μg/ml blasticidin (ThermoFisher). After several weeks of growth, LpdAp:I-Ab production was induced by addition of CuSO4 and D-biotin (ThermoFisher). LpdAp:I-Ab monomers were enriched from cell culture supernatant via His-Bind chromatography targeting the 6x His epitope on the C-terminus of the I-Ab beta chain using the Novagen His-Bind Purification Kit (EMD Millipore). The nickel column eluate was then passed over a Pierce Monomeric Avidin UltraLink Resin (ThermoFisher) and eluted with free biotin to purify biotinylated LpdAp:I-Ab molecules according to the manufacturer’s instructions. The biotin was removed from the eluate by four PBS washes through an Amicon Ultra-15 30 kDa concentrating filter (EMD Millipore). The concentration of biotin-labeled LpdAp:I-Ab monomers was determined by absorbance of ultraviolet light at 280 nm. Monomers were combined with streptavidin-phycoerythrin (PE) or streptavidin-allophycocyanin (APC) (Prozyme) in a 4:1 ratio to produce tetramers (Moon et al., 2007).
LpdAp:I-Ab tetramer-based cell enrichment.
Spleen or mesenteric lymph nodes were mashed over nylon mesh in 1–2 ml ice-cold RPMI supplemented with antibiotics, glutamine, and 10% fetal calf serum (complete RPMI) and filtered through nylon mesh into 15 ml polypropylene conical centrifuge tubes. Cells from each mouse were washed in cold sorter buffer (PBS + 2% FBS, 0.1% NaN3), centrifuged, and suspended in sorter buffer plus anti-mouse FcγII/III (CD16/32) blocking antibody to a final volume about twice that of the pellet. LpdAp:I-Ab/streptavidin-PE and/or LpdAp:I-Ab/streptavidin-APC tetramer(s) were then added to a final concentration of 10 nM along with CXCR3 (BV421; BD) and CX3CR1 (PerCP-Cy5.5; Biolegend) antibodies. After an hour incubation in the dark at room temperature, the cells were washed with PBS and centrifuged to a pellet, to which was added 500 μl of sorter buffer and 6.25 μl of Stemcell anti-fluorophore antibody cocktail for 15 minutes in the dark at room temperature followed by 25 μl of Stemcell Easysep Magnetic particles for 15 more minutes. The cells were suspended to a final volume of 2.5 ml in sorter buffer in FACS tubes and placed in an EasySep magnet for five minutes, after which the samples were decanted while still in the magnet. Sorter buffer was added and decanted several times to wash out loosely bound cells. After the final wash, the FACS tube was removed from the magnet and the released cells were washed and centrifuged to a pellet, to which was added 95 μl of Fc receptor blocking solution. The cells were stained with a viability dye (Ghost dye red 780; Tonbo) and CD4 (BUV496; BD), CD90.2 (BUV395; BD), KLRG1 (BV711; BD), CD69 (BUV737; BD), CD45R/B220 (APC-eFluor 780; ThermoFisher), CD11b (APC-eFluor 780; ThermoFisher), CD11c (APC-eFluor 780; ThermoFisher), F4/80 (APC-eFluor 780; ThermoFisher), and NKp46 (APC-eFluor 780; ThermoFisher) antibodies on ice for 30 minutes. Samples were then washed in cold sorter buffer and fixed in 2% paraformaldehyde for 10 minutes at room temperature, followed by additional fixation for 1 hour in Foxp3 Transcription Factor Fixation Buffer (ThermoFisher). The cells were then washed twice in Permeabilization Buffer (Tonbo) before staining overnight at 4°C with anti-mouse T- bet (PE-Cy7; ThermoFisher). Following intracellular staining, the cells were washed once with Permeabilization Buffer and once with PBS, before being suspended in 350 μl of sorter buffer plus 104 123count eBeads counting beads (ThermoFisher) in 50 μl of sorter buffer prior to flow cytometry acquisition and analysis.
Immunohistology.
Tissue pieces were submerged in PLP buffer (Gerner et al., 2012) containing 1% paraformaldehyde overnight, then floated in 30% sucrose in PBS for an additional 24 hours. The tissues were then embedded in OCT, frozen, and stored at −80°C. Seven μm sections were cut on a Leica cryostat and transferred to SuperFrost Plus slides (ThermoFisher) for staining. Sections were permeabilized with tissue staining buffer (PBS with 0.3% Triton-X 100, 0.1 M glycine, 0.1% Cold Fish Skin Gelatin, and 1% BSA) and blocked with tissue staining buffer plus 5% normal rat serum and FcγII/III CD16/32 antibody (2.4G2; BioXcell), before staining with a cocktail of fluorochrome-conjugated E-cadherin (eFluor 660; ThermoFisher), ZO-1 (Alexa Fluor 594; ThermoFisher), CD45R/B220 (Alexa Fluor 594; Biolegend), CD4 (BV480; BD), CD45.1 (BV421; Biolegend), F4/80 (BV480; BD), CD206 (Alexa Fluor 594; Biolegend), iNOS (Alexa Fluor 488; ThermoFisher), and CXCL9 (eFluor 660; ThermoFisher) mouse antibodies, and CXCL10 goat antibody followed by Alexa Fluor 647-conjugated bovine anti-goat IgG (Jackson Immunoresearch). Stained tissue sections were mounted with ProLong anti-fade Diamond medium (ThermoFisher) and cured overnight. Whole section images were acquired on a Leica DM6000B Epi-fluorescence microscope with automated tiling and stitching.
Histo-cytometry.
Images were analyzed using histo-cytometry (Gerner et al., 2012). Imaris imaging software was used to create digital surfaces corresponding to individual host and bacterial cells as described in Figure S2. Imaris batch process extensions designed and modified in house were used to export the mean fluorescence intensity, area, X, Y-position, and sphericity of the digital surfaces as comma separated value (.csv) spreadsheets, which were then imported into FlowJo v10 analysis software for further analysis. A gating strategy was used in FlowJo v10 to remove highly auto-fluorescent non-specific staining as described in Figure S3. The X-Y coordinates of the refined surfaces for CD4+ T cells or Se bacteria were extracted and plotted on images of iNOS+ and CXCL9/10+ clusters created as described below. These plots were used to determine the frequency of CD4+ T cells and dTomato+ Se bacteria in iNOS+ and CXCL9/10+ areas.
Cluster analysis protocol.
iNOS and the CXCL9/10 surfaces were clustered using the Density-Based Spatial Clustering of Applications with Noise (DBSCAN) algorithm, with parameters ε = 30μm and N = 2 (Ester et al., 1996) (Figure S1). Delaunay triangulation was used to convert clusters to polygons by calculating the shape-union of all triangles whose vertices were part of each cluster. Each CD4+ T cell or Se-Tomato+ surface was said to be within a iNOS+ or CXCL9/10 cluster if its centroid was contained in that cluster’s polygon. The proportion of CD4+ T cell or Se-Tomato+ surfaces within either iNOS+ or CXCL9/10+ clusters was calculated. All the cluster analysis was performed in Python (v3.6), using GeoPandas (v0.3.0), Shapely (v1.6.4), SciPy (v1.0.1), NumPy (v1.15.0), scikit-learn (v0.19.1) and Matplotlib (v2.1.1). The code is available upon request at Jupyter Notebooks.
Flow cytometry analysis of myeloid cells.
pleens and mesenteric lymph node fragments were digested in a serum-free IMDM buffer containing 5 μg/ml Brefeldin-A, 1 mM glutathione, 1 U/ml Heparin Sodium, 0.1 mg/ml DNAse I, 0.8 mg/ml Dispase II, and 0.2 mg/ml Collagenase IV (Worthington), according to a method developed by Turley and colleagues (Fletcher et al., 2011). A portion of the sample was reserved for CFU enumeration. In some cases, myeloid cells were enriched from infected organs by positive selection using a cocktail of magnetically-labeled CD11b, CD11c, and F4/80 (Miltenyi Biotech) antibodies according to the manufacturer’s instructions. After magnetic isolation, the cells were stained with a viability dye (Ghost dye red 780; Tonbo) and fluorochrome-labeled B220, CD90.2, NKp46 (all APC-eF780; ThermoFisher), CD11b (BUV395; BD), CD11c (BUV737; BD), Ly6G (BV510; Biolegend), Siglec F (APC-R700; BD), MERtk (SuperBright 645; ThermoFisher), and Ly6C (PerCP-Cy5.5; ThermoFisher) antibodies. The cells were then fixed with 2% paraformaldehyde, permeabilized with saponin (Tonbo), stained with fluorochrome-labeled iNOS (Alexa Fluor 488; ThermoFisher), CXCL9 (eFluor 660; ThermoFisher) antibodies, and CXCL10 (Unlabeled goat polyclonal; R&D systems) followed by a bovine goat IgG secondary antibody (Alexa Fluor 647; Jackson Immunoresearch) and analyzed on an LSR Fortessa flow cytometer (BD) equipped with a 590/36 bandpass filter on a 561 nm Yellow-Green (YG) laser detector for optimal dTomato detection.
In vivo analysis of Se gene expression in NO-producing macrophages.
Naïve B6 × 129 F1 mice that received 2 × 106 CD44high CD4+ T cells from day 60 Se-Tomato infected mice were subsequently infected intravenously with 105 Se-Tomato bacteria. After five days, splenic mononuclear phagocytes were magnetically enriched as described above and incubated in PBS with the NO indicator 1 μM 4-amino-5-methylamino-2’,7’-difluorescein diacetate (ThermoFisher) (Li et al., 2003) for 10 minutes at 37°C. The cells were then stained with CD64 and CD11c antibodies and 105 infected (dTomato+) or uninfected (dTomato-) NO+ CD64+ CD11c+ macrophages were sorted using a BD FACSAria flow cytometer into RNA protect cell reagent (Qiagen). The sorted cells, and Se-Tomato bacteria grown to log phase in LB-Lennox broth, were pelleted and suspended at room temperature for 30 minutes in 100 μl of 30 mM Tris-HCl 10 mM EDTA pH 8.00 with 0.5% Triton-X 100, 2 mg/ml proteinase K, 10 mg/ml lysozyme (SIGMA), and RNAsin RNAse inhibitor (Promega). RNA was then purified using the RNeasy mini plus kit (Qiagen). cDNA was made from the purified RNA using the SuperScript First Strand Synthesis system (ThermoFisher) with random hexamer primers. One-twenty fifth of the reverse transcriptase reaction was then added to optical grade 96-well PCR plates containing Forget-Me-Not EvaGreen qPCR master mix (Biotium) and individual PCR primers specific for a panel of Se test genes and the rpoD house-keeping gene. Cycle threshold (Ct) values were then obtained using a CFX96 Real-Time PCR detection system (Bio-Rad) and calculated with CFX96 analysis software. Delta Cttest gene values were calculated by subtracting the test gene Ct value for infected NO+ CD64+ CD11c+ macrophages or broth-grown bacteria from the test gene Ct value for uninfected NO+ CD64+ CD11c+ macrophages. Delta CtrpoD values were calculated by subtracting the rpoD Ct value for infected NO+ CD64+ CD11c+ macrophages or broth-grown bacteria from the rpoD Ct value for uninfected NO+ CD64+ CD11c+ macrophages. The fold-change in expression of the test gene over the rpoD house-keeping gene for intracellular or broth-grown bacteria was then calculated according to the formula – fold change = 2(delta Ct test – delta Ct rpoD).
QUANTIFICATION AND STATISTICAL ANALYSIS
Prism software (GraphPad) was used to calculate p values determined by statistical tests indicated within the figure legends. Unless otherwise specified, (n) refers to the number of animals in each experiment. Differences between groups were considered significant for p values ≤ 0.05.
DATA AND SOFTWARE AVAILABILITY
Imaris extensions and tools for histo-cytometry analysis can be found at: https://histocytometry.github.io/Chrysalis/
Source code for the analysis of cell clustering and spatial mapping can be found at: https://github.com/mfgoldberg/Granuloma-Mapping
Supplementary Material
Highlights.
S. enterica (Se) infection stimulates robust expansion of CXCR3+ Th1 cells.
Granulomas bordered by CXCL9/10+ phagocytes form during Se infection.
Se bacteria are in granuloma cores while Th1 cells are excluded to the borders.
Se bacteria produce enzymes that neutralize the effects of nitric oxide.
ACKNOWLEDGMENTS
Supported by grants from the US National Institutes of Health R01 AI039614 and R01 AI103760 to M.K.J., T32 AI83196 to D. I. K., and T32 HL007062 to M. F. G. The authors acknowledge Roman Gerlach (Robert Koch Instituit, Berlin) for the kind provision of plasmids and technical advice on generation of he Se-Tomato stain, Antonio Pagan (Cambridge University, England) for critical advice on the manuscript, Jason Mitchell at the UMN-CFI imaging core facility for technical expertise and training, Jennifer Walter for general technical assistance, and the University of Minnesota University Flow Cytometry Resource for cell sorting and analysis support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Barat S, Willer Y, Rizos K, Claudi B, Maze A, Schemmer AK, Kirchhoff D, Schmidt A, Burton N, and Bumann D (2012). Immunity to intracellular Salmonella depends on surface-associated antigens. PLoS Pathog. 8, e1002966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer-Santos E, Durkin CH, Rigano LA, Kupz A, Alix E, Cerny O, Jennings E, Liu M, Ryan AS, Lapaque N, et al. (2016). The Salmonella effector SteD mediates MARCH8-dependent ubiquitination of MHC II molecules and inhibits T cell activation. Cell Host Microbe 20, 584–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnsen J, Perez-Lopez A, Nuccio SP, and Raffatellu M (2015). Exploiting host immunity: the Salmonella paradigm. Trends Immunol. 36, 112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, and Sacks DL (2002). CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature 420, 502–507. [DOI] [PubMed] [Google Scholar]
- Blank K, Hensel M, and Gerlach RG (2011). Rapid and highly efficient method for scarless mutagenesis within the Salmonella enterica chromosome. PLoS One 6, e15763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan C (2015). Nitric oxide synthase in innate and adaptive immunity: an update. Trends Immunol. 36, 161–178. [DOI] [PubMed] [Google Scholar]
- Bold TD, Banaei N, Wolf AJ, and Ernst JD (2011). Suboptimal activation of antigen-specific CD4+ effector cells enables persistence of M. tuberculosis in vivo. PLoS Pathog. 7, e1002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottcher JP, Beyer M, Meissner F, Abdullah Z, Sander J, Hochst B, Eickhoff S, Rieckmann JC, Russo C, Bauer T, et al. (2015). Functional classification of memory CD8(+) T cells by CX3CR1 expression. Nat. Commun 6, 8306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bumann D, and Cunrath O (2017). Heterogeneity of Salmonella-host interactions in infected host tissues. Curr. Opin. Microbiol 39, 57–63. [DOI] [PubMed] [Google Scholar]
- Burton NA, Schurmann N, Casse O, Steeb AK, Claudi B, Zankl J, Schmidt A, and Bumann D (2014). Disparate impact of oxidative host defenses determines the fate of Salmonella during systemic infection in mice. Cell Host Microbe 15, 72–83. [DOI] [PubMed] [Google Scholar]
- Cecilio P, Perez-Cabezas B, Santarem N, Maciel J, Rodrigues V, and Cordeiro da Silva A (2014). Deception and manipulation: the arms of leishmania, a successful parasite. Front. Immunol 5, 480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu HH, Chan SW, Gosling JP, Blanchard N, Tsitsiklis A, Lythe G, Shastri N, Molina-Paris C, and Robey EA (2016). Continuous effector CD8(+) T cell production in a controlled persistent infection is sustained by a proliferative intermediate population. Immunity 45, 159–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cibrian D, and Sanchez-Madrid F (2017). CD69: from activation marker to metabolic gatekeeper. Eur. J. Immunol 47, 946–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan MR, Beerman RW, Rosenberg AF, Saelens JW, Johnson MG, Oehlers SH, Sisk DM, Jurcic Smith KL, Medvitz NA, Miller SE, et al. (2016). Macrophage epithelial reprogramming underlies Mycobacterial granuloma formation and promotes infection. Immunity 45, 861–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datsenko KA, and Wanner BL (2000). One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U S A 97, 6640–6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauphinee SM, Richer E, Eva MM, McIntosh F, Paquet M, Dangoor D, Burkart C, Zhang DE, Gruenheid S, Gros P, et al. (2014). Contribution of increased ISG15, ISGylation and deregulated type I IFN signaling in Usp18 mutant mice during the course of bacterial infections. Genes Immun. 15, 282–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JM, and Ramakrishnan L (2009). The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell 136, 37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dileepan T, Linehan JL, Moon JJ, Pepper M, Jenkins MK, and Cleary PP (2011). Robust antigen specific th17 T cell response to group A Streptococcus is dependent on IL-6 and intranasal route of infection. PLoS Pathog. 7, e1002252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egen JG, Rothfuchs AG, Feng CG, Horwitz MA, Sher A, and Germain RN (2011). Intravital imaging reveals limited antigen presentation and T cell effector function in mycobacterial granulomas. Immunity 34, 807–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egen JG, Rothfuchs AG, Feng CG, Winter N, Sher A, and Germain RN (2008). Macrophage and T cell dynamics during the development and disintegration of mycobacterial granulomas. Immunity 28, 271–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisele NA, Ruby T, Jacobson A, Manzanillo PS, Cox JS, Lam L, Mukundan L, Chawla A, and Monack DM (2013). Salmonella require the fatty acid regulator PPARdelta for the establishment of a metabolic environment essential for long-term persistence. Cell Host Microbe 14, 171–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein TK (2001). Implications of Salmonella-induced nitric oxide (NO) for host defense and vaccines: NO, an antimicrobial, antitumor, immunosuppressive and immunoregulatory molecule. Microbes Infect. 3, 1223–1231. [DOI] [PubMed] [Google Scholar]
- Ernst JD, Cornelius A, Desvignes L, Tavs J, and Norris BA (2018). Limited antimycobacterial efficacy of epitope peptide administration despite enhanced antigen-specific CD4 T cell activation. J. Infect. Dis [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ester M, Kriegel H-P, Sander J, and Xu X (1996). A density-based algorithm for discovering clusters a density-based algorithm for discovering clusters in large spatial databases with noise. In Proceedings of the Second International Conference on Knowledge Discovery and Data Mining (Portland, Oregon, AAAI Press; ), pp. 226–231. [Google Scholar]
- Eva MM, Yuki KE, Dauphinee SM, Schwartzentruber JA, Pyzik M, Paquet M, Lathrop M, Majewski J, Vidal SM, and Malo D (2014). Altered IFN-gammamediated immunity and transcriptional expression patterns in N-Ethyl-N-nitrosourea-induced STAT4 mutants confer susceptibility to acute typhoid-like disease. J. Immunol 192, 259–270. [DOI] [PubMed] [Google Scholar]
- Figueira R, and Holden DW (2012). Functions of the Salmonella pathogenicity island 2 (SPI-2) type III secretion system effectors. Microbiology 158, 1147–1161. [DOI] [PubMed] [Google Scholar]
- Fletcher A, Malhotra D, Acton S, Lukacs-Kornek V, Bellemare-Pelletier A, Curry M, Armant M, and Turley S (2011). Reproducible isolation of lymph node stromal cells reveals site-dependent differences in fibroblastic reticular cells. Front. Immunol 2, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds KE, Wu CY, and Seder RA (2006). Th1 memory: implications for vaccine development. Immunol. Rev 211, 58–66. [DOI] [PubMed] [Google Scholar]
- Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, Helft J, Chow A, Elpek KG, Gordonov S, et al. (2012). Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat. Immunol 13, 1118–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerner MY, Kastenmuller W, Ifrim I, Kabat J, and Germain RN (2012). Histocytometry: a method for highly multiplex quantitative tissue imaging analysis applied to dendritic cell subset microanatomy in lymph nodes. Immunity 37, 364–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin AJ, Li LX, Voedisch S, Pabst O, and McSorley SJ (2011). Dissemination of persistent intestinal bacteria via the mesenteric lymph nodes causes typhoid relapse. Infect. Immun 79, 1479–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groom JR, and Luster AD (2011). CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunol Cell Biol 89, 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote A, Hiller K, Scheer M, Munch R, Nortemann B, Hempel DC, and Jahn D (2005). JCat: a novel tool to adapt codon usage of a target gene to its potential expression host. Nucleic Acids Res. 33, W526–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guirado E, and Schlesinger LS (2013). Modeling the Mycobacterium tuberculosis Granuloma - the Critical Battlefield in Host Immunity and Disease. Front. Immunol 4, 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardison SE, Herrera G, Young ML, Hole CR, Wozniak KL, and Wormley FL Jr. (2012). Protective immunity against pulmonary cryptococcosis is associated with STAT1-mediated classical macrophage activation. J. Immunol 189, 4060–4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henard CA, and Vazquez-Torres A (2011). Nitric oxide and salmonella pathogenesis. Front. Microbiol 2, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson NP, Kang YH, Lapaque N, Janssen H, Trowsdale J, and Kelly AP (2013). Salmonella polarises peptide-MHC-II presentation towards an unconventional Type B CD4+ T-cell response. Eur. J. Immunol 43, 897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubzick C, Gautier EL, Gibbings SL, Sojka DK, Schlitzer A, Johnson TE, Ivanov S, Duan Q, Bala S, Condon T, et al. (2013). Minimal differentiation of classical monocytes as they survey steady-state tissues and transport antigen to lymph nodes. Immunity 39, 599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanns TM, Ertelt JM, Rowe JH, and Way SS (2010). Regulatory T cell suppressive potency dictates the balance between bacterial proliferation and clearance during persistent Salmonella infection. PLoS Pathog. 6, e1001043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunakaran KP, Yu H, Jiang X, Chan Q, Goldberg MF, Jenkins MK, Foster LJ, and Brunham RC (2017). Identification of MHC-Bound Peptides from Dendritic Cells Infected with Salmonella enterica Strain SL1344: Implications for a Nontyphoidal Salmonella Vaccine. J. Proteome Res 16, 298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman KD, Sallin MA, Sakai S, Kamenyeva O, Kabat J, Weiner D, Sutphin M, Schimel D, Via L, Barry CE 3rd, et al. (2017). Defective positioning in granulomas but not lung-homing limits CD4 T-cell interactions with Mycobacterium tuberculosis-infected macrophages in rhesus macaques. Mucosal Immunol. 11, 462–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupz A, Scott TA, Belz GT, Andrews DM, Greyer M, Lew AM, Brooks AG, Smyth MJ, Curtiss R 3rd, Bedoui S, and Strugnell RA (2013). Contribution of Thy1+ NK cells to protective IFN-gamma production during Salmonella typhimurium infections. Proc. Natl. Acad. Sci. U S A 110, 2252–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WI, Huang JL, Yeh KW, Jaing TH, Lin TY, Huang YC, and Chiu CH (2011). Immune defects in active mycobacterial diseases in patients with primary immunodeficiency diseases (PIDs). J. Formos. Med. Assoc 110, 750–758. [DOI] [PubMed] [Google Scholar]
- Li N, Sul JY, and Haydon PG (2003). A calcium-induced calcium influx factor, nitric oxide, modulates the refilling of calcium stores in astrocytes. J. Neurosci 23, 10302–10310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis WP, Johnson ML, Brasfield A, Blanc MP, Yi J, Miller SI, Cookson BT, and Hajjar AM (2014). Temporal and anatomical host resistance to chronic Salmonella infection is quantitatively dictated by Nramp1 and influenced by host genetic background. PLoS One 9, e111763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMicking JD, North RJ, LaCourse R, Mudgett JS, Shah SK, and Nathan CF (1997). Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc. Natl. Acad. Sci. U S A 94, 5243–5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen L, Labrecque N, Engberg J, Dierich A, Svejgaard A, Benoist C, Mathis D, and Fugger L (1999). Mice lacking all conventional MHC class II genes. Proc. Natl. Acad. Sci. U S A 96, 10338–10343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, and Papaioannou VE (1992). RAG-1-deficient mice have no mature B and T lymphocytes. Cell 68, 869–877. [DOI] [PubMed] [Google Scholar]
- Monack DM, Bouley DM, and Falkow S (2004). Salmonella typhimurium persists within macrophages in the mesenteric lymph nodes of chronically infected Nramp1+/+ mice and can be reactivated by IFNgamma neutralization. J. Exp. Med 199, 231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, and Jenkins MK (2007). Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity 27, 203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller AJ, Filipe-Santos O, Eberl G, Aebischer T, Spath GF, and Bousso P (2012). CD4+ T cells rely on a cytokine gradient to control intracellular pathogens beyond sites of antigen presentation. Immunity 37, 147–157. [DOI] [PubMed] [Google Scholar]
- Nix RN, Altschuler SE, Henson PM, and Detweiler CS (2007). Hemophagocytic macrophages harbor Salmonella enterica during persistent infection. PLoS Pathog. 3, e193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagan AJ, and Ramakrishnan L (2018). The formation and function of granulomas. Annu. Rev. Immunol 36, 639–665. [DOI] [PubMed] [Google Scholar]
- Pagan AJ, Yang CT, Cameron J, Swaim LE, Ellett F, Lieschke GJ, and Ramakrishnan L (2015). Myeloid growth factors promote resistance to Mycobacterial infection by curtailing granuloma necrosis through macrophage replenishment. Cell Host Microbe 18, 15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, and Groisman EA (2014). Signal-specific temporal response by the Salmonella PhoP/PhoQ regulatory system. Mol. Microbiol 91, 135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper M, Linehan JL, Pagan AJ, Zell T, Dileepan T, Cleary PP, and Jenkins MK (2010). Different routes of bacterial infection induce long-lived TH1 memory cells and short-lived TH17 cells. Nat. Immunol 11, 83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper M, Pagan AJ, Igyarto BZ, Taylor JJ, and Jenkins MK (2011). Opposing signals from the Bcl6 transcription factor and the interleukin-2 receptor generate T helper 1 central and effector memory cells. Immunity 35, 583–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters NC, Pagan AJ, Lawyer PG, Hand TW, Henrique Roma E, Stamper LW, Romano A, and Sacks DL (2014). Chronic parasitic infection maintains high frequencies of short-lived Ly6C+CD4+ effector T cells that are required for protection against re-infection. PLoS Pathog. 10, e1004538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell DA, and Frelinger JA (2017). Efficacy of resistance to Francisella imparted by ITY/NRAMP/SLC11A1 depends on route of infection. Front. Immunol 8, 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prando C, Samarina A, Bustamante J, Boisson-Dupuis S, Cobat A, Picard C, AlSum Z, Al-Jumaah S, Al-Hajjar S, Frayha H, et al. (2013). Inherited IL-12p40 deficiency: genetic, immunologic, and clinical features of 49 patients from 30 kindreds. Medicine (Baltimore) 92, 109–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindran R, Foley J, Stoklasek T, Glimcher LH, and McSorley SJ (2005). Expression of T-bet by CD4 T cells is essential for resistance to Salmonella infection. J. Immunol 175, 4603–4610. [DOI] [PubMed] [Google Scholar]
- Roy MF, Riendeau N, Bedard C, Helie P, Min-Oo G, Turcotte K, Gros P, Canonne-Hergaux F, and Malo D (2007). Pyruvate kinase deficiency confers susceptibility to Salmonella typhimurium infection in mice. J. Exp. Med 204, 29492961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai S, Kauffman KD, Schenkel JM, McBerry CC, Mayer-Barber KD, Masopust D, and Barber DL (2014). Cutting edge: control of Mycobacterium tuberculosis infection by a subset of lung parenchyma-homing CD4 T cells. J. Immunol 192, 2965–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallin MA, Sakai S, Kauffman KD, Young HA, Zhu J, and Barber DL (2017). Th1 differentiation drives the accumulation of intravascular, non-protective CD4 T cells during Tuberculosis. Cell Rep. 18, 3091–3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, and Bose M (2001). Role of cytokines in immune response to pulmonary tuberculosis. Asian Pac. J. Allergy Immunol 19, 213–219. [PubMed] [Google Scholar]
- Shi C, and Pamer EG (2011). Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol 11, 762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sologuren I, Boisson-Dupuis S, Pestano J, Vincent QB, Fernandez-Perez L, Chapgier A, Cardenes M, Feinberg J, Garcia-Laorden MI, Picard C, et al. (2011). Partial recessive IFN-gammaR1 deficiency: genetic, immunological and clinical features of 14 patients from 11 kindreds. Hum. Mol. Genet 20, 1509–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian T, and Salis HM (2015). A predictive biophysical model of translational coupling to coordinate and control protein expression in bacterial operons. Nucleic Acids Res. 43, 7137–7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubo NJ, and Jenkins MK (2014). CD4+ T Cells: guardians of the phagosome. Clin. Microbiol. Rev 27, 200–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uppaluri R, Sheehan KC, Wang L, Bui JD, Brotman JJ, Lu B, Gerard C, Hancock WW, and Schreiber RD (2008). Prolongation of cardiac and islet allograft survival by a blocking hamster anti-mouse CXCR3 monoclonal antibody. Transplantation 86, 137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urdahl KB (2014). Understanding and overcoming the barriers to T cell-mediated immunity against tuberculosis. Semin. Immunol 26, 578–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub BC, Eckmann L, Okamoto S, Hense M, Hedrick SM, and Fierer J (1997). Role of alphabeta and gammadelta T cells in the host response to Salmonella infection as demonstrated in T-cell-receptor-deficient mice of defined Ity genotypes. Infect. Immun 65, 2306–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Jaramillo A, Shi R, Kwok WW, and Mohanakumar T (2004). In vivo biotinylation of the major histocompatibility complex (MHC) class II/peptide complex by coexpression of BirA enzyme for the generation of MHC class II/tetramers. Hum. Immunol 65, 692–699. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.