Abstract
PURPOSE:
The goals of this study were to evaluate geographic and racial/ethnic variation in breast cancer mortality, and evaluate whether observed geographic differences are explained by county-level characteristics.
METHODS:
We analyzed data on breast cancer deaths among women in 3108 contiguous United States (US) counties from years 2000 through 2015. We applied novel geospatial methods and identified hot spot counties based on breast cancer mortality rates. We assessed differences in county-level characteristics between hot spot and other counties using Wilcoxon rank-sum test and Spearman correlation, and stratified all analysis by race/ethnicity.
RESULTS:
Among all women, 80 of 3108 (2.57%) contiguous US counties were deemed hot spots for breast cancer mortality with the majority located in the southern region of the US (72.50%, p value <0.001). In race/ethnicity-specific analyses, 119 (3.83%) hot spot counties were identified for NH-Black women, with the majority being located in southern states (98.32%, p value <0.001). Among Hispanic women, there were 83 (2.67%) hot spot counties and the majority was located in the southwest region of the US (southern = 61.45%, western = 33.73%, p value < 0.001). We did not observe definitive geographic patterns in breast cancer mortality for NH-White women. Hot spot counties were more likely to have residents with lower education, lower household income, higher unemployment rates, higher uninsured population, and higher proportion indicating cost as a barrier to medical care.
CONCLUSIONS:
We observed geographic and racial/ethnic disparities in breast cancer mortality: NH-Black and Hispanic breast cancer deaths were more concentrated in southern, lower SES counties.
Keywords: Breast Cancer, Health Disparities, Socioeconomic Factors, Geospatial Analysis
INTRODUCTION
Breast cancer is a leading cause of death among women in the United States (US), responsible for more than 41,000 annual deaths [1]. The overall survival for breast cancer has increased over the past few decades with an estimated 90.8% five-year relative survival rate among all US women, a 16.0% absolute increase since 1977 [2]. However, racial differences in breast cancer mortality persist as Non-Hispanic (NH)-Black (71% increased risk) and Hispanic (14% increased risk) women have poorer five-year cause-specific survival when compared with their NH-White counterparts [1, 2]. Though research suggests that many of these women with lower survival rates are more likely to have more aggressive forms of breast cancer, there is also evidence that suggest socioeconomic status is an integral factor in decreased survival rates [3].
NH-Black and Hispanic women have up to a 50% increased risk for late-stage breast cancer diagnoses [4, 5], and experience higher breast cancer mortality when compared with NH-Whites [6-11]. An increased risk of late stage breast cancer diagnosis is associated with residing in highly segregated areas and decreased access to mammography.[12]. In addition, Haas et al. (2008) reported that the mediating role of racial segregation on the association between Black race and adequate breast cancer care was responsible for nearly 10% of the total effect on adequate breast cancer care [13]. Prior research also suggests that Black and Hispanic women living in poor and/or racially segregated communities are more likely to be diagnosed with late-stage breast cancer and when compared with their NH-White counterparts [4-10, 12-18]. In addition, a recent analysis of data from Surveillance, Epidemiology, and End Results (SEER), showed that when compared with NH-White women, and after controlling for breast cancer hormone-receptor subtype, area-level socioeconomic status, and healthcare access, NH-Blacks and Hispanics had a 39% and 5% increased risk of breast cancer mortality, respectively [6]. These studies suggest that social and built environmental risk factors such as availability of quality cancer screening and treatment resources may lead to clustering of breast cancer mortality in specific regions, and partly explain racial disparities in mortality rates among US women.
While prior studies have reported on the influence of race and geographic residence on breast cancer outcomes in the US [19-27], these studies are limited in scope due to limited generalizability to the entire US population (i.e., few studies among the entire contiguous US), lack of granularity (i.e., many studies have been state-level analyses), and most have lacked data on specific county-level characteristics as potential explanations for observed geographic patterns. Here, we will evaluate geographic variations in breast cancer mortality among US women by race/ethnic group, and evaluate the contribution of county-level characteristics important for breast cancer outcomes. Together, these data will add a nuanced view to our understanding of breast cancer mortality in the US, and highlight areas where targeted cancer prevention strategies are needed.
MATERIALS AND METHODS
Study Design and Data Source
This was a cross-sectional study of breast cancer mortality among women between 2000 through 2015 in counties within the contiguous US. County-level breast cancer mortality rates were obtained from the Center for Disease Control and Prevention (CDC) underlying causes of death file [28], while county-level characteristics were obtained from the 2014 County Health Rankings (CHR) and American Community Survey (ACS) [29, 30]. This study was considered exempt by the Institutional Review Boards of the University of Alabama at Birmingham and Washington University School of Medicine, as we used existing secondary data that are publicly available and non-identifiable.
Identification of Breast Cancer Deaths and Mortality
We defined breast cancer deaths as attributed to malignant neoplasms of the breast among women aged 15 and older for the years 2000 through 2015. Breast cancer deaths were identified from the underlying cause-of-death database produced by the CDC’s National Center for Health Statistics (https://wonder.cdc.gov) [28]. The underlying cause-of-death defines attributable deaths as “the disease or injury which initiated the train of events leading directly to death, or the circumstances of the accident or violence which produced the fatal injury” [28]. Data from the underlying cause-of-death are derived from death certificates and include a record for every death of a US resident. We identified the county level total number, crude rates, and age-adjusted rates for breast cancer using the ICD-10 codes for breast cancer (Supplemental Table 1). We included breast cancer-related deaths for all persons aged 15 years or older during the observation period because the Underlying Causes of Death database uses a single reference standard population for ages 15 through 24, and therefore, the inclusion of the 15 to 19 year groups is necessary for the estimation of age-adjusted rates. Age adjusted rates were standardized to the year 2000 US population. We identified breast cancer deaths overall and by race/ethnicity for NH-Black, NH-White, and Hispanic Women.
County-Level Demographic Characteristics
We included the county-level data on age groups, sex, race, household income, population with college education, and Rural-Urban Commuting Area (RUCA) codes as study covariates. We obtained data on county-level proportion of race/ethnic groups for the five most populous racial groups in the US for the 15-year period including: NH-White, NH-Black and Hispanic. Hispanic women consisted of women that identified as having Hispanic, Latino, or Spanish origins including but not limited to: Mexican American, Chicano, Puerto Rican, Cuban, Argentinean, Colombian, Dominican, Nicaraguan, Salvadoran, and Spaniard. We classified counties as Urban or Non-Urban using the 2010 Rural-Urban Commuting Areas (RUCA) classifications. RUCA codes are based on population size and density, urbanization, and primary and secondary worker commuter flow patterns to larger urban areas and clusters [31]. The 10 RUCA codes were then aggregated into a dichotomized variable: (1) Urban (i.e., population centers with 50,000 or more residents) and (2) Non-Urban (i.e., towns or small cities with population centers with less than 50,000 residents) [31, 32]. We obtained all county-level demographic statistics from the 2014 ACS via National Historical Geographic Information System [29]. The 2014 ACS provided aggregated estimates for demographic statistics based on the preceding 5-year period (2010 – 2014).
County-Level Characteristics
We included county-level prevalence of adult obesity, current smokers, mammography screening, physically inactivity as well as the proportion of adults that could not see doctor due to cost, had limited access to healthy foods, were unemployed or uninsured, the total number of primary care physicians, and region as county-level characteristics. We calculated the ratio of primary care physicians per 10,000 persons by dividing the total number of primary care physicians by the county population and then multiplied the quotient by 10,000. Geographic region was defined using the Census definition (i.e., Midwest, Northeast, South, and West) [33]. We obtained county-level characteristics from the 2014 County Health Rankings (CHR) [29, 30]. The CHR consists of nationally representative data collected from a variety of databases which all sample among the total non-institutionalized population over 18 years of age living within households. The CHR gathered and aggregated county level data from different resources such as the National Center for Chronic Disease Prevention and Health Promotion (e.g., data on adult obesity and physical inactivity), Behavioral Risk Factor Surveillance System (e.g., data on smoking and could not see doctor), USDA Food Environment Atlas (e.g., data on limited access to healthy foods), the Medicare/Dartmouth Institute (e.g., data on mammography screening), Bureau of Labor Statistics (e.g., data on unemployment rate), and the Area Health Resources Files (e.g., data on uninsured rate and number of primary care physicians). Detailed descriptions of county-level characteristics are described in Supplemental Table 2.
Geospatial Analysis and Identification of Breast Cancer Mortality Hot Spots
Geographic hot spots for disease can be described as the spatial aggregation of cases in an identifiable subpopulation [34]. While there is no formal and/or commonly accepted approach to identifying spatial disease clustering, there are several geospatial measures that account for the overall rate, county population, and spatial autocorrelation of designated areas of interest [35]. Further, the geospatial methods used in our analyses take into account geospatial differences on local (Getis Ord), global and local (LISA), and overall population sizes of each county (Empirical Bayes method). To avoid spurious results garnered from a singular geospatial measure, we used an aggregation of three separate spatial clustering methods to identify counties that were hot spots for breast cancer mortality [31]. We categorized county-level breast cancer mortality into two groups: “hot spots” or “non-significant” based on statistically significant higher observed vs. expected breast cancer mortality rate using the combination of three distinct methods. We considered a county to be a “hot spot” if it was identified as high risk for breast cancer mortality using all three geospatial analyses: 1) fifth quintile of smoothed Empirical Bayes (EB) breast cancer mortality rates, 2) high-high cluster using Local Indicators of Spatial Association (LISA), and 3) as a hot spot defined by Getis-Ord Gi* statistic) [36, 37]. All other US counties were categorized as non-significant.
First, we estimated the smoothed Empirical Bayes (EB) breast cancer mortality rates using the proportion of women with deaths attributed to breast cancer, with smoothing performed using the EB tools in GeoDa 1.6.7.9 (http://geodacenter.asu.edu)[38]. Briefly, the smoothed EB rate method allows for more stable estimation of breast cancer mortality rates by accounting for the overall county population. EB smoothing includes the variance of each county by using the corresponding county population, and thus counties with higher populations have smaller variance and those with smaller populations had larger variance. Further, the EB rates are smoothed using an inverse function of the variance for shrinkage towards the overall mean for breast cancer mortality rates (i.e., counties with larger populations and smaller variance gave higher weights towards the observed weights). We categorized the EB smoothed breast cancer mortality rates into quintiles, and we defined counties as high-risk if the smoothed EB breast cancer mortality rates were in the fifth quintile. Secondly, we used Local Indicators of Spatial Association (LISA) to measure similarity of breast cancer mortality between counties and calculate values both within and across geographic boundaries, additionally identifying spatial outliers [36, 38, 39]. For each US county, we estimated Local Moran’s I Statistic values, using associated z-scores and p-values to assess the magnitude of spatial autocorrelation and statistical significance, respectively[38]. Statistically significant (p value ≤0.05) positive z-scores indicate counties surrounded by areas with similar breast cancer mortality rates – either similarly high or similarly low (positive spatial autocorrelation) [38]. Lastly, we used the Gi* statistic to identify areas where breast cancer mortality rates with either high or low values clustered within the context of the neighboring county [37, 38, 40, 41].
For both the LISA and Gi* analysis we employed 1000 permutations and randomized data using the specific seed number 74 (an arbitrary number, but important for replication of study results). In contrast to LISA, the Gi* statistic are not related to the global statistic of spatial association [32]. Moreover, while the LISA statistic includes a diagnostic for outliers with respect to a measure of global association, the Gi* only examines associations for counties that share borders [32]. Further, the LISA analysis allows for examining local associations while accounting for the global spatial association (can be observed from the counties identified as either high-low or low-high outliers). Further details to the geospatial autocorrelation analysis were introduced by Nassel et al. (2014) and we have published similar analysis that are described elsewhere [30, 31]. We performed all geospatial analyses using GeoDa version 1.6.7.9, and mapping were performed using ArcGIS 10.5.
Statistical Analysis
We compared regional (i.e., Midwest vs. Northeast vs. South vs. West) differences in hot spot breast cancer categories (EB, LISA, Gi*, and Breast Cancer Mortality hot spot Category) using chi-square and Fisher’s exact tests, as appropriate. We performed Fisher’s exact tests for analyses with small cell sizes, and calculated Monte Carlo estimates of exact p values using 10,000 samples with the random seed number 74. We compared differences in demographic and county-level characteristics between hot spot and non-significant counties using the Wilcoxon rank-sum test. We presented the medians and interquartile ranges for the sociodemographic and county-level characteristics due to the non-parametric distribution of continuous variables. Due to a large number of counties examined, there were many associations considered significant at α = 0.05. Therefore, in order to observe the magnitude of correlation between hot spots and county-level characteristics we additionally examined the correlation of demographic and county-level characteristics with being a county-level breast cancer mortality hot spot using a Spearmen correlation (positive values indicate positive correlation) (ρ). We obtained the mean crude and age-adjusted mortality rates standardized to the 2000 US standard population. We additionally estimated the crude and age-adjusted breast cancer mortality rates for the entire contiguous US. Lastly, to examine differences in breast cancer mortality and associated characteristics by race, we stratified all results by race/ethnicity. We performed statistical analyses using SAS version 9.4. All statistical tests were two-sided and p-values ≤ 0.05 were considered statistically significant.
RESULTS
Mortality Rates by Hot Spot Areas
Between 2000 and 2015 there were 653,630 total deaths attributed to breast cancer (Table 1), corresponding to a mean age-adjusted breast cancer mortality rate of 29.2 per 100,000 6women (95% CI: 29.1 – 29.4). Age-adjusted mortality was higher in hot spot counties (34.6 per 100,000 women, 95% CI: 33.0 – 36.2) when compared with the remaining US counties (29.1 per 100,000 women, 95% CI: 29.0 – 29.3). Mortality rates were highest among NH-Black women (39.3, 95% CI: 39.0 – 39.6), compared with NH-White (29.3, 95% CI: 29.1 – 29.4) and Hispanic (18.1, 95% CI: 17.9 – 18.3) women.
Table 1:
Breast Cancer Mortalitya in the United States by Hot Spot Category, 2000 – 2015, excluding Alaska and Hawaii.
| Breast Cancer Deathsb |
Mean Crude Breast Cancer Mortality per 100,000 womenc (95% CI) |
Mean Age- Adjusted Breast Cancer Mortality per 100,000 womenc (95% CI) |
|
|---|---|---|---|
| Entire United States | |||
| Hot Spot (n = 80) | 8,209 | 44.5 (42.0 – 47.0) | 34.6 (33.0 – 36.2) |
| Non-Significant (n = 3,028) |
645,421 | 32.8 (32.6 – 33.1) | 29.1 (29.0 – 29.3) |
| Overall (n = 3,108)d | 653,630 | 32.9 (32.7 – 33.1) | 29.2 (29.1 – 29.4) |
|
Among NH-Black Women |
|||
| Hot Spot (n = 119) | 6,049 | 44.2 (42.7 – 45.8) | 44.7 (43.3 – 46.2) |
| Non-Significant (n = 2,989) | 83,703 | 35.8 (35.4 – 36.2) | 38.9 (38.6 – 39.3) |
| Overall (n = 3,108)e | 89,752 | 36.3 (35.9 – 36.6) | 39.3 (39.0 – 39.6) |
| Among Hispanic Women | |||
| Hot Spot (n = 83) | 8,806 | 18.5 (18.1 – 18.9) | 21.6 (21.2 – 22.0) |
| Non-Significant (n = 3,025) |
23,635 | 12.2 (12.0 – 12.4) | 17.2 (17.0 – 17.4) |
| Overall (n = 3,108)f | 32,441 | 13.4 (13.3 – 13.6) | 18.1 (17.9 – 18.3) |
|
Among NH-White Women |
|||
| Hot Spot (n = 57) | 30,268 | 46.6 (45.4 – 47.8) | 29.1 (28.9 – 29.2) |
| Non-Significant (n = 3,051) | 479,420 | 37.0 (36.8 – 37.3) | 33.2 (32.5 – 33.9) |
| Overall (n = 3,108)g | 509,688 | 37.5 (37.2 – 37.8) | 29.3 (29.1 – 29.4) |
Deaths per 100,000 women.
Total number of deaths, identified using International Classification of Diseases, 10th Version codes for breast cancer.
Weighted by county population of women aged 18+ (weighted by NH-Black, Hispanic, and NH-White populations for race/ethnicity stratified mortality rates).
The row that indicates “overall mortality rate” should be interpreted as the county-level mean mortality rate per 100,000 women for all women
NH-Black women
Hispanic women
NH-White women.
All Race/Ethnicities
Among all women, we identified 80 of 3108 (2.57%) hot spot counties for breast cancer mortality with the majority (72.50%, p value <0.001) located in the southern US region (Table 2, Figure 1). Hot spot maps of each specific method (Gi* statistic, LISA, and EB) and stratified by race can be found in Supplemental Figures 1 – 12. Counties designated as hot spots had a higher proportion of NH-Black population (27.8% vs. 1.9%, p value <0.001, ρ correlation = 0.14), lower proportion of Hispanics (1.8% vs. 3.5%, p value <0.001, ρ correlation = −0.11), lower proportion of NH-White population (65.7% vs. 85.9%, p value <0.001, ρ correlation = −0.07), higher adult obesity (33.9% vs. 30.6%, p <0.001, ρ correlation = 0.12), lower proportion of adults with college education (13.7% vs. 18.0%, p <0.001, ρ correlation = −0.11), higher population with low income (27.8% vs. 20.5%, p <0.001, ρ correlation = 0.14), higher physical inactivity (33.1% vs. 28.1, p <0.001, ρ correlation = 0.13), and higher unemployment (9.4% vs. 7.5%, p <0.001, ρ correlation = 0.11) (Table 3).
Table 2:
Comparison of Breast Cancer Mortality Geospatial Hot Spota by Counties within United States Region, stratified by race/ethnicity years 2000 – 2015.
| Midwest Countiesb (N = 1,055) |
Northeast Counties b (N = 217) |
Southern Countiesb (N = 1,422) |
Western Counties b (N = 414) |
Total (N = 3108) |
P valuec |
||
|---|---|---|---|---|---|---|---|
| Presented as N (%)d | N (%)e | ||||||
| Among All Women | |||||||
| Breast Cancer Hot Spota | 18 (22.50) | 4 (5.00) | 58 (72.50) | 0 (0.0) | 80 (2.57) | <0.001 | |
| LISA High-High Cluster | 87 (37.66) | 29 (12.55) | 112 (48.48) | 3 (1.3) | 231 (7.43) | <0.001 | |
| EB 5thQuintile | 279 (44.87) | 42 (6.75) | 260 (41.80) | 41 (6.59) | 622 (20.01) | <0.001 | |
| Gi* Hot spot | 69 (19.49) | 15 (4.24) | 268 (75.71) | 2 (0.56) | 354 (11.39) | <0.001 | |
|
Among NH-Black Women | |||||||
| Breast Cancer Hot Spota | 1 (0.84) | 1 (0.84) | 117 (98.32) | 0 (0.0) | 119 (3.83) | <0.001 | |
| LISA High-High Cluster | 1 (0.69) | 1 (0.69) | 143 (98.62) | 0 (0.0) | 145 (4.67) | <0.001 | |
| EB 5th Quintile | 104 (16.72) | 35 (5.63) | 473 (76.05) | 10 (1.61) | 622 (20.01) | <0.001 | |
| Gi* Hot Spot | 26 (5.15) | 49 (9.70) | 412 (81.58) | 18 (3.56) | 505 (16.25) | <0.001 | |
|
Among Hispanic Women | |||||||
| Breast Cancer Hot Spota | 0 (0.0) | 4 (4.82) | 51 (61.45) | 28 (33.73) | 83 (2.67) | <0.001 | |
| LISA High-High Cluster | 2 (1.68) | 4 (3.36) | 63 (52.94) | 50 (42.02) | 119 (3.83) | <0.001 | |
| EB 5th Quintile | 98 (15.76) | 89 (14.31) | 257 (41.32) | 178 (28.62) | 622 (20.01) | <0.001 | |
| Gi* Hot Spot | 16 (5.59) | 51 (17.83) | 110 (38.46) | 109 (38.11) | 286 (9.20) | <0.001 | |
|
Among NH-White Women | |||||||
| Breast Cancer Hot Spota | 13 (22.81) | 27 (47.37) | 13 (22.81) | 4 (7.02) | 57 (1.83) | <0.001 | |
| LISA High-High Cluster | 68 (35.98) | 37 (19.58) | 62 (32.80) | 22 (11.64) | 189 (6.08) | <0.001 | |
| EB 5th Quintile | 261 (41.96) | 45 (7.23) | 234 (37.62) | 82 (13.18) | 622 (20.01) | <0.001 | |
| Gi* Hot Spot | 144 (41.62) | 43 (12.43) | 136 (39.31) | 23 (6.65) | 346 (11.13) | <0.001 | |
Defined as counties estimated as a hot spot by all three geospatial methods (local indicators of spatial association, empirical Bayes, and Gi*).
EB = empirical Bayes.
US regions as determined by the US Census Bureau.
Midwest counties are in the states of IN, IL, IA, MI, MN, MO, NE, ND, OH, SD, and WI.
Northeast counties are in the states of CT, ME, NH, NJ, NY, PA, RI, and VT.
Southern counties are in the states of AL, AR, DE, DC, FL, GA, KY, LA, MD, MS, NC, OK, SC, TN, TX, VA, and WV.
Western counties are in the states of AZ, CA, CO, ID, MT, NM, NV, OR, UT, WA, and WY.
p values determined using chi-square or Fisher’s exact tests (exact p values calculated using Monte Carlo estimation).
Denotes row percentages.
Denotes column percentages.
Figure 1:
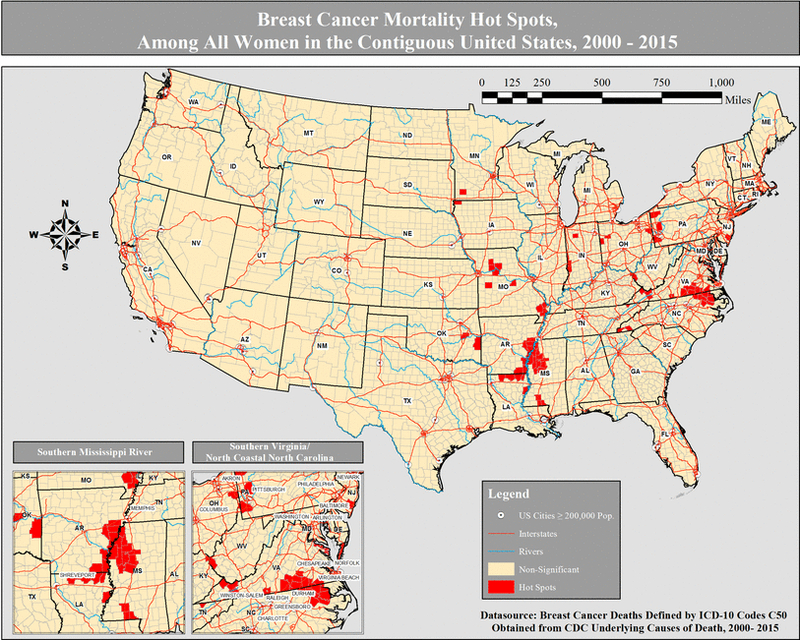
Breast cancer mortality hot spots, among all women in the contiguous United States, 2000 – 2015.
Table 3:
County-Level Characteristics Comparisons by Breast Cancer Mortality Hot Spot Category, Among All Women 2000 – 2015.
| Hot Spota (N = 80) |
Non-Significant (N = 3,028) |
P valueb | ρc | |
|---|---|---|---|---|
| Presented as Median (IQR)d |
||||
| Race | ||||
| % NH-White | 65.7 (41.4 – 94.2) | 85.9 (67.6 – 94.0) | <0.001 | −0.07 |
| % NH-Black | 27.8 (2.4 – 52.5) | 1.9 (0.5 – 9.4) | <0.001 | 0.14 |
| % Hispanic | 1.8 (1.2 – 3.1) | 3.5 (1.7 – 8.8) | <0.001 | −0.11 |
| % Female Sex | 51.1 (49.6 – 52.1) | 50.4 (49.5 – 51.1) | <0.001 | 0.06 |
| % Age | ||||
| <18 | 22.0 (20.0 – 24.2) | 22.9 (20.9 – 24.6) | 0.05 | −0.04 |
| 18 – 29 | 13.9 (12.7 – 15.1) | 14.0 (12.3 – 16.0) | 0.77 | −0.01 |
| 30 – 44 | 17.5 (16.4 – 18.4) | 17.7 (16.3 – 19.0) | 0.26 | −0.02 |
| 45 – 64 | 28.8 (27.0 – 29.9) | 28.0 (26.3 – 29.6) | 0.10 | 0.03 |
| 65 – 79 | 12.9 (11.5 – 14.5) | 12.1 (10.3 – 13.9) | 0.005 | 0.05 |
| 80+ | 4.4 (3.8 – 5.1) | 4.2 (3.4 – 5.2) | 0.09 | 0.03 |
| % Completed college | 13.7 (12.1 – 17.2) | 18.0 (14.0 – 23.9) | <0.001 | −0.11 |
| % Household income <$20,000 | 27.8 (23.3 – 34.5) | 20.5 (16.4 – 25.4) | <0.001 | 0.14 |
| County-level characteristics | ||||
| % Obesity | 33.9 (31.4 – 36.9) | 30.6 (28.3 – 33.1) | <0.001 | 0.12 |
| % Smoking | 22.6 (20.2 – 26.5) | 19.4 (14.4 – 24.1) | <0.001 | 0.09 |
| % Could not see doctor due to cost | 17.1 (13.2 ––21.5) | 12.6 (5.0 – 17.4) | <0.001 | 0.09 |
| % Limited access to healthy foods | 6.3 (3.4 – 12.0) | 6.1 (3.4 – 10.2) | 0.36 | 0.02 |
| % Mammography screening | 58.0 (51.6 – 63.0) | 61.4 (55.9 – 66.7) | 0.003 | −0.05 |
| % Physical inactivity | 33.1 (29.4 – 34.6) | 28.1 (24.5 – 31.2) | <0.001 | 0.13 |
| % Unemployment | 9.4 (7.7 – 11.3) | 7.5 (5.7 – 9.3) | <0.001 | 0.11 |
| % Uninsured | 23.0 (20.1 – 26.0) | 21.4 (16.8 – 26.1) | 0.02 | 0.04 |
| PCPe per 10,000 persons | 4.3 (2.5 – 6.0) | 4.9 (3.1 – 7.0) | 0.02 | −0.04 |
| Non-Urban | 68.8 (50.8 – 88.4) | 59.1 (32.9 – 87.0) | 0.006 | 0.05 |
Defined as counties estimated as a hot spot by all three geospatial methods (local indicators of spatial association, empirical Bayes, and Gi*).
Significance determined using Wilcoxon test, p values <0.05.
Spearman correlation with being a county-level breast cancer mortality hot spot.
IQR = interquartile range.
PCP = Primary care physicians
NH-Black Women
Among NH-Black women, we identified 119 (3.83%) hot spot counties for breast cancer mortality with 117 (98.32%, p value <0.001) of those located in the southern US (Figure 2). When compared with non-significant counties, hot spots for NH-Blacks had a higher proportion of NH-Black residents (34.6% vs. 1.8%, p value <0.01, ρ correlation = 0.29), adult obesity (34.9% vs. 30.5%, p value <0.01, ρ correlation = 0.21), those that could not visit doctor due to cost (17.2% vs. 12.5%, p value <0.01, ρ correlation = 0.13), household incomes less than $20,000 (27.7% vs. 20.4%, p value <0.01, ρ correlation = 0.16), physical inactivity (32.4% vs. 28.0%, p value <0.01, ρ correlation = 0.16), unemployment (9.7% vs. 7.4%, p value <0.01, ρ correlation = 0.14), and uninsured residents (25.3% vs. 21.3%, p value, ρ correlation = 0.12) (Table 4). In addition, counties that were hot spots for NH-Blacks had lower proportion of adults with college education (14.3% vs. 18.0%, p value <0.01, ρ correlation = −0.11).
Figure 2:
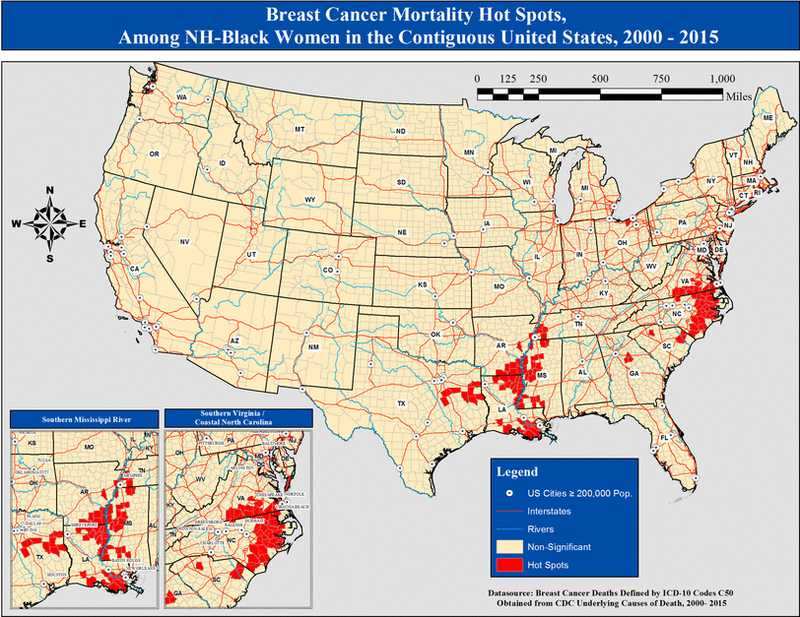
Breast cancer mortality hot spots, among all NH-Black women in the contiguous United States, 2000 – 2015.
Table 4:
County-Level Characteristics Comparisons by Breast Cancer Mortality Hot Spot Category, Stratified by Race/Ethnicity years 2000 – 2015
| Race/Ethnicity | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| NH-Blacks | Hispanics | NH-Whites | |||||||
|
| |||||||||
| Hot Spota (N = 119) |
Non- Significant (N = 2,989) |
ρb | Hot Spota (N = 83) |
Non- Significant (N = 3,025) |
ρb | Hot Spota (N = 57) |
Non- Significant (N = 3,051) |
ρb | |
|
| |||||||||
| Presented as Median (IQR)c |
Presented as Median (IQR)c |
Presented as Median (IQR)c |
|||||||
|
|
|||||||||
| Race | |||||||||
| % NH-White | 58.8 (45.1 – 66.1) | 86.6 (69.2 – 94.2) | −0.23 | 42.2 (19.5 – 58.4) | 86.4 (68.4 – 94.2) | −0.23 | 79.2 (60.9 – 93.0) | 85.8 (67.2 – 94.0) | −0.03 |
| % NH-Black | 34.6 (25.8 – 50.9) | 1.8 (0.5 – 8.0) | 0.29 | 1.4 (0.5 – 4.1) | 2.1 (0.5 – 10.6) | −0.03 | 3.9 (1.4 – 9.6) | 2.0 (0.5 – 10.1) | 0.05 |
| % Hispanic | 3.0 (2.0 – 6.0) | 3.4 (1.7 – 8.7) | −0.03 | 50.2 (27.6 – 70.4) | 3.3 (1.7 – 7.7) | 0.26 | 6.9 (1.7 – 16.7) | 3.4 (1.7 – 8.4) | 0.03 |
| % Female Sex | 51.2 (50.2 – 52.1) | 50.4 (49.5 – 51.1) | 0.11 | 50.4 (49.0 – 51.0) | 50.4 (49.6 – 51.2) | −0.01 | 51.1 (50.4 – 51.4) | 50.4 (49.5 – 51.1) | 0.06 |
| % Aged 80+ | 3.8 (3.1 – 4.3) | 4.2 (3.4 – 5.2) | −0.07 | 3.6 (2.9 – 4.5) | 4.2 (3.4 – 5.2) | −0.07 | 4.7 (3.9 – 5.9) | 4.2 (3.4 – 5.2) | 0.05 |
| % Completed college | 14.3 (12.0 – 18.6) | 18.0 (14.1 – 24.0) | −0.11 | 16.2 (12.1 – 24.4) | 17.9 (14.0 – 23.7) | −0.02 | 19.5 (14.9 – 27.4) | 17.9 (13.9 – 23.6) | 0.04 |
| % Household income <$20,000 | 27.7 (22.1 – 33.9) | 20.4 (16.4 – 25.3) | 0.16 | 23.4 (18.2 – 29.6) | 20.6 (16.5 – 25.6) | 0.06 | 19.9 (16.9 – 25.2) | 20.7 (16.5 – 25.7) | −0.01 |
| County-level characteristics | |||||||||
| % Adult obesity | 34.9 (32.7 – 37.2) | 30.5 (28.3 – 33.0) | 0.21 | 28.8 (24.5 – 29.9) | 30.8 (28.4 – 33.2) | −0.13 | 28.3 (25.9 – 31.9) | 30.7 (28.4 – 33.2) | −0.06 |
| % Adult smoking | 22.2 (19.2 – 25.6) | 19.3 (14.3 – 24.1) | 0.08 | 14.0 (0.0 – 18.8) | 19.7 (14.7 – 24.2) | −0.12 | 19.9 (16.3 – 24.3) | 19.5 (14.4 – 24.1) | 0.01 |
| % Could not see doctor due to cost | 17.2 (13.8 – 21.5) | 12.5 (4.6 – 17.3) | 0.13 | 13.6 (0.0 – 17.7) | 12.7 (5.8 – 17.5) | 0.02 | 13.2 (10.4 – 17.7) | 12.7 (5.0 – 17.5) | 0.02 |
| % Limited access to healthy foods | 6.2 (3.4 – 10.9) | 6.1 (3.4 – 10.3) | 0.01 | 10.5 (5.9 – 17.1) | 6.1 (3.4 – 10.1) | 0.09 | 5.0 (2.3 – 7.5) | 6.2 (3.4 – 10.4) | −0.05 |
| % Mammography screening | 61.3 (54.6 – 66.6) | 61.4 (55.8 – 66.7) | −0.01 | 55.4 (48.8 – 60.9) | 61.5 (56.0 – 66.7) | −0.11 | 59.1 (56.1 – 62.5) | 61.4 (55.8 – 66.7) | 0.04 |
| % Physical inactivity | 32.4 (29.4 – 34.2) | 28.0 (24.4 – 31.1) | 0.16 | 25.9 (23.3 – 27.8) | 28.3 (24.6 – 31.5) | −0.10 | 27.7 (24.2 – 30.8) | 28.1 (24.5 – 31.4) | −0.01 |
| % Unemployment | 9.7 (7.7 – 11.2) | 7.4 (5.7 – 9.2) | 0.14 | 7.1 (5.6 – 8.9) | 7.5 (5.8 – 9.3) | 0.02 | 8.5 (7.3 – 10.5) | 7.5 (5.8 – 9.3) | 0.06 |
| % Uninsured | 25.3 (21.9 – 28.0) | 21.3 (16.7 – 25.9) | 0.12 | 29.5 (24.9 – 33.2) | 21.3 (16.7 – 25.9) | 0.17 | 20.1 (14.8 – 25.7) | 21.5 (16.9 – 26.1) | −0.03 |
| PCP per 10,000 persons | 4.0 (2.7 – 5.5) | 4.9 (3.1 – 7.0) | −0.05 | 4.2 (2.9 – 6.3) | 4.9 (3.1 – 7.0) | −0.04 | 5.9 (4.4 – 7.6) | 4.9 (3.1 – 7.0) | 0.04 |
| Non-Urbane | 60.8 (38.5 – 82.9) | 59.4 (33.1 – 88.2) | 0.00 | 31.8 (10.7 – 60.7) | 60.1 (34.4 – 87.8) | −0.09 | 37.3 (5.8 – 67.1) | 59.7 (33.8 – 88.2) | −0.07 |
Defined as counties estimated as a hot spot by all three geospatial methods (local indicators of spatial association, empirical Bayes, and Gi*).
Spearman correlation with county-level breast cancer hot spot category.
IQR = interquartile range.
Presented as N (Column %)
Hispanic Women
Among Hispanic women, we identified 83 (2.67%) hot spot counties for breast cancer mortality, with 51 (61.45%, p value <0.001) and 28 (33.73%) of those located in the southern and mid-western regions, respectively (Figure 3). When compared with non-significant counties, counties that qualified as hot spots for Hispanics had a higher proportion of Hispanic residents (50.2% vs. 3.3%, p value <0.01, ρ correlation = 0.26) and uninsured residents (29.3% vs. 21.3%, p value, ρ correlation = 0.17) (Table 3). In addition, hot spots for Hispanics had lower proportion of NH-White residents (42.2% vs. 86.4%, p < 0.001, ρ correlation = −0.23), adult obesity (28.8% vs. 30.8%, p <0.001, ρ correlation = −0.13), adult smoking prevalence (14.05 vs. 19.7%, p value <0.001, ρ correlation = −0.12), and mammography screening (55.4% vs. 61.5%, p value <0.01, ρ correlation = −0.11).
Figure 3:
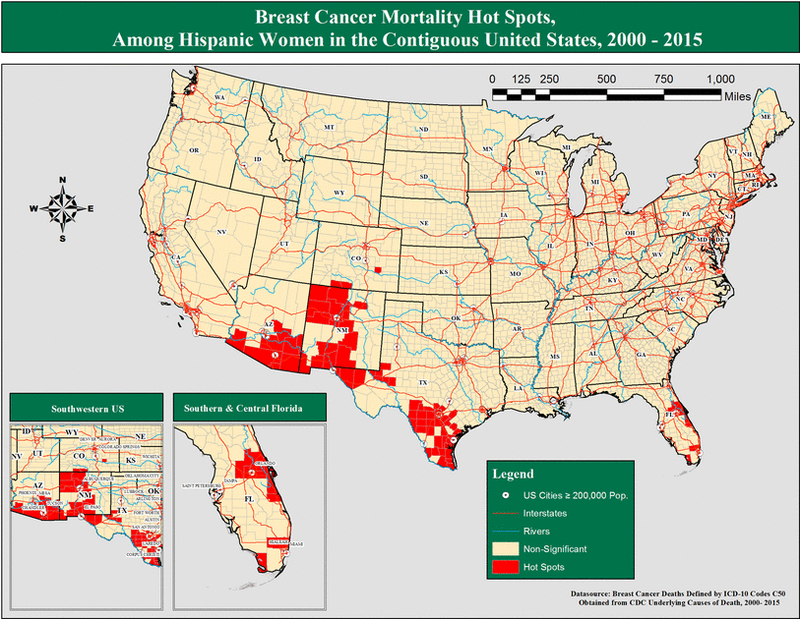
Breast cancer mortality hot spots, among all Hispanic women in the contiguous United States, 2000 – 2015.
NH-White Women
Among NH-White women, we identified 57 (1.83%) hot spot counties for breast cancer mortality, with 27 (47.37%, p value <0.001) of the hot spot counties located in the Northeast (Figure 4). When compared with non-significant counties, counties that qualified as hot spots for NH-White women had a slightly higher proportion of unemployed residents (8.5% vs. 7.5%, p value <0.01, ρ correlation = 0.06), lower proportion of non-urban residents (37.3% vs. 59.7%, p <0.01, ρ correlation = −0.07), and slightly lower obesity (28.3% vs. 30.7%, p value <0.01, ρ correlation = −0.06).
Figure 4:
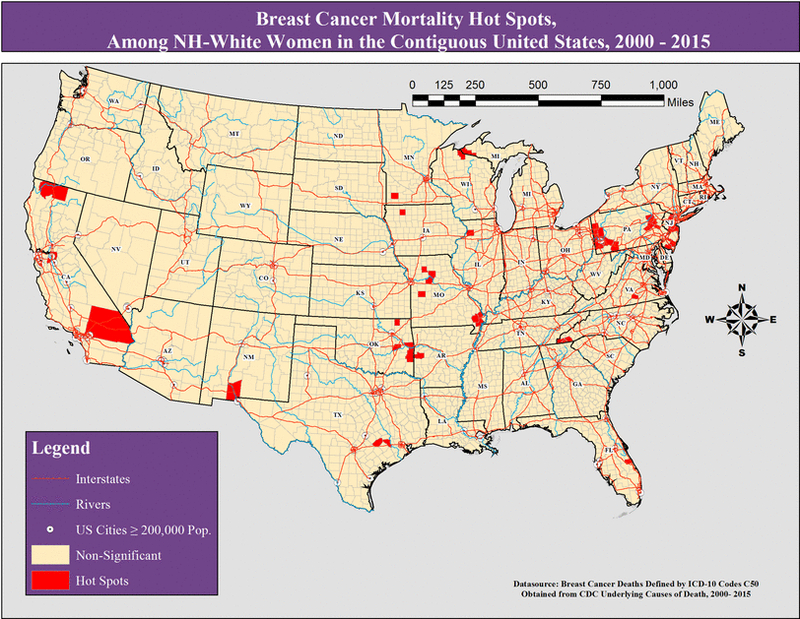
Breast cancer mortality hot spots, among all NH-White women in the contiguous United States, 2000 – 2015.
DISCUSSION
The objectives of our analysis were to evaluate breast cancer mortality hot spots among US counties, identify the county-level characteristics of those counties, and delineate differences in breast cancer mortality hot spots by race. Overall, the age-adjusted breast cancer mortality rate was 18% higher for women living in hot spot counties compared with those in non-significant counties. Hot spot counties also had a higher proportion of NH-Black residents, higher prevalence of obesity, lower education and income, and higher unemployment and smoking prevalence. We observed distinct geographic patterns in hot spots for breast cancer mortality among NH-Black women (rural southern counties near the Mississippi River and counties in the northern coastal North Carolina/southern Virginia areas) and Hispanic women (southwestern US, and central and southern Florida areas), but no geographic patterns were discernable among NH-White women. NH-Black women living in hot spots had a 15% higher risk of breast cancer mortality when compared with NH-Black women living in other geographic areas. Hispanic women living in hot spots had nearly a 26% higher risk of breast cancer mortality when compared with Hispanic women living in other geographic areas. For NH-White women, hot spots were more variably dispersed across the contiguous US, and thus we deem that there were no apparent geographic patterns or significant clustering.
Delineating geospatial patterns of breast cancer mortality, while additionally examining whether these patterns are modified by race and ethnicity, may provide utility in understanding areas where targeted mortality prevention strategies are needed, and may provide clues into etiological and/or prognostic risk factors for breast cancer in those areas. Moreover, limited research has investigated racial disparities in breast cancer mortality while taking into account the geographic variations and county-level characteristics using geospatial epidemiologic methodology [20, 24, 25, 42]. In this study we identified geographic areas of possible intervention for high breast cancer mortality for Hispanic and NH-Black women living in the US. Only a few studies have examined regional patterns of breast cancer mortality among US women [19-21, 23-27, 42, 43]. To our knowledge this is one of the first studies to utilize multiple geospatial analyses for identifying county-level hot spots for high breast cancer mortality among the entire contiguous US.
Prior studies did not offer granular insight to the geographic areas most burdened with breast cancer mortality while delineating racial differences. For example, a prior analysis that supports our observed southern Mississippi River hot spots for breast cancer mortality utilized the same data source (national vital statistics data) although at a different spatial resolution (state-level) to examine changes in breast cancer by race between two time periods; 1986 through 1990 compared with 1991 through 1995 [19]. In this study, Canto et al (2001) reported that among states ranked in the top decile for breast cancer mortality in 1990, White women experienced a 9.85% reduction in mortality rate compared to only a 2.71% reduction in breast cancer mortality for Black women [19]. Mokdad et al (2017) similarly observed that from 1980 through 2014 clusters of high rates remained in the southern belt and along the Mississippi River [24]. Another study performed by Chien et al (2013) similarly utilized national mortality data between 1982 and 2004, to determine geographic disparities in breast cancer mortality by using the structured additive regression (STAR) model (i.e., similar to the current study’s Gi* analysis) [20]. In contrast to our results, the authors observed that among Blacks and Whites the highest risk areas for breast cancer mortality were located in the Northeast US; showing up to a 2-fold increased risk of breast cancer mortality in these area [20]. In addition, approximately 67% and 25% of all US counties were categorized as high risk for White and African American women, respectively [20]. Possible explanations for the differences in our results may be: 1) regional differences in breast cancer mortality may have changed over past few decades, and 2) the use of more conservative cluster detection methodology using three separate statistical approaches in the current study.
We identified two specific hot spot areas for breast cancer mortality among NH-Black women: 1) rural southern counties near the Mississippi River and 2) counties in the northern coastal North Carolina/southern Virginia region. We also identified two unique areas of hot spots for Hispanic women: 1) in the southwestern states of Arizona, New Mexico, and southern Texas, and 2) in counties located in central to southern Florida area. Several factors likely contribute to racial differences in breast cancer mortality and in particular, the observed geographic clustering. However, studies suggest that the most significant causes relate to lack of adequate and timely screening necessary for early detection, and lack of access to adjuvant chemotherapy and/or surgical interventions [2, 6, 44-55]. Fedewa et al. (2011) reported that NH-Blacks were 25% to 106% more likely to have delayed breast cancer chemotherapy after 30-, 60-, and 90-days following breast cancer diagnosis [48]. Further, we previously observed that NH-Black women were 42% more likely to have late-stage breast cancer diagnosis, 45% less likely to receive surgical treatment after breast cancer diagnosis, 15% less likely to receive radiation therapy after breast cancer, and had a 39% higher risk of breast cancer mortality when compared with NH-White women [6]. In the current study, counties that were hot spots for breast cancer mortality also had county-level-characteristics that are likely barriers to receipt of screening and treatment. For instance, we observed that breast cancer mortality hot spot counties for NH-Black and Hispanic women were more likely to have lower household income and higher unemployment, and hot spot counties for NH-Black women had a higher proportion of adults that could not visit the doctor within the past year due to cost, and population with higher uninsured rates. These findings indicate that counties identified as hot spots for breast cancer mortality in the US, also had significant barriers to timely and quality healthcare due to lack of income and health insurance. Public health strategies focused on improving breast cancer screening among Hispanic women and reducing barriers to affordable treatment for NH-Black women may go a long way in preventing excess breast cancer deaths in the US.
We must note that the “southern Mississippi River” areas identified as hot spots in the current analysis have consistently been shown to be associated with poorer health outcomes using geospatial analyses in previous studies investigating varied diseases [56-61]. For example, studies have shown that the southern Mississippi River region has higher risks for lung cancer [60], coronary heart disease and stroke [61], and sepsis [58]. Consistent poverty and lower access to healthcare are likely significant contributors to the health disparities affecting this region, and targeted healthcare strategies focused on this geographic region are likely to have significant impact.
The results and discussion of this study should be interpreted with respect to a few limitations. First, the underlying causes of death file suppressed county-level data representing fewer than ten events, and as a result we were unable to ascertain estimates if counties had less than ten breast cancer deaths over the total fifteen-year period. Thus, counties with less than ten events were far more likely to be in areas of low clustering or non-significance during our analysis on geospatial autocorrelation. In addition, the geospatial hot spots when stratified by race were dependent on both prevalence of disease and number of persons living within a county. An inherent limitation is that the non-significant areas among NH-Blacks and Hispanic women may simply represent areas with lower populations of these specific minority groups. However, this still provides important public health information regarding hot spot areas where targeted cancer prevention efforts may be more efficiently focused. By utilizing multiple geospatial methods to account for the county population size (i.e., empirical Bayes smoothed rates), global spatial associations (Getis-Ord analysis), and local spatial associations (i.e., Local Moran’s I – LISA analysis), we reduced the likelihood of systematic bias in identifying hot spots. In general, it is reported that the latency period of breast cancer is between 8 and 15 years [62–64]. However, we examined breast cancer mortality using an ecologic study design, and thus our results are subject to temporality bias and ecological fallacy. Further, the objective of our study were not to ascertain causal inference, but rather to assess association between county-level factors and breast cancer mortality using similar dates: we used aggregated county demographic and characteristic data ranging from 2010 through 2014 and mortality data for years 2000 through 2015. Although we did not have data on specific breast cancer subtypes (e.g., estrogen receptor positive (ER+), negative (ER-), or triple negative), Hispanic subgroups, or individual level factors associated with breast cancer mortality in the current analysis, future studies focused on etiology would benefit from inclusion of these factors.
Additionally, there were benefits to the current study analyses that lead to more generalizable results. First, our results are likely less spurious than other studies that have employed fewer geospatial similar methods due to our conservative criteria for categorizing hot spots areas. Secondly, our results were derived from nationally representative sources including the underlying cause of death file from the CDC, ACS, and CHR. The ACS, CHR, and CDC datasources are nationally representative samples that have been used in studies examining sociodemographic and county-level characteristics [58, 65].
CONCLUSION
The results of this study suggest that breast cancer mortality for NH-Black and Hispanic American women are geographically clustered in specific regions in the US. Specifically, for NH-Black women the high-risk areas of breast cancer mortality are located in the rural south of counties near the Mississippi River and counties in the northern coastal North Carolina/southern Virginia areas, and for Hispanic women, the hot spot areas are located in the southwestern US, and central and southern Florida areas. Targeted cancer prevention strategies, including transportation, increased availability of affordable screening, and quality treatment facilities in these specific areas are likely to be highly efficient in reducing disparities in breast cancer mortality in the US.
Supplementary Material
Local indicators of spatial association (LISA) for breast cancer mortality, among NH-Black women in the contiguous United States, years 2000 – 2015.
Breast cancer mortality using spatial Empirical Bayes (EB) smoothed rates quintiles, among NH-Black women in the Contiguous United States, years 2000 – 2015.
Getis-Ord (Gi*) for breast cancer mortality hot spots, among Hispanic women in the contiguous United States, years 2000 – 2015.
Local indicators of spatial association (LISA) for breast cancer mortality, among Hispanic women in the contiguous United States, years 2000 – 2015.
Breast cancer mortality using spatial Empirical Bayes (EB) smoothed rates quintiles, among Hispanic women in the Contiguous United States, years 2000 – 2015.
Getis-Ord (Gi*) for breast cancer mortality hot spots, among NH-White women in the contiguous United States, years 2000 – 2015.
Local indicators of spatial association (LISA) for breast cancer mortality, among NH-White women in the contiguous United States, years 2000 – 2015.
Breast cancer mortality using spatial Empirical Bayes (EB) smoothed rates quintiles, among NH-White women in the Contiguous United States, years 2000 – 2015.
Supplemental Table 1: ICD-10 codes for identification of breast cancer.
Supplemental Table 2: Detailed definitions and technical information for 2014 County Health Rankings (CHR) county-level characteristics used in study analysis. All variables are county-level proportions.
Getis-Ord (Gi*) for breast cancer mortality hot spots, among all women in the contiguous United States, years 2000 – 2015.
Local indicators of spatial association (LISA) for breast cancer mortality, among all women in the contiguous United States, years 2000 – 2015.
Breast cancer mortality using spatial Empirical Bayes (EB) smoothed rates quintiles, among all women in the Contiguous United States, years 2000 – 2015.
Getis-Ord (Gi*) for breast cancer mortality hot spots, among NH-Black women in the contiguous United States, years 2000 – 2015.
REFERENCES
- 1.Richardson LC, et al. , Patterns and Trends in Age-Specific Black-White Differences in Breast Cancer Incidence and Mortality - United States, 1999-2014. MMWR Morb Mortal Wkly Rep, 2016. 65(40): p. 1093–1098. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, et al. , Annual Report to the Nation on the Status of Cancer, 1975–2014, Featuring Survival. J Natl Cancer Inst, 2017. 109(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carey LA, et al. , Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA, 2006. 295(21): p. 2492–502. [DOI] [PubMed] [Google Scholar]
- 4.Barry J and Breen N, The importance of place of residence in predicting late-stage diagnosis of breast or cervical cancer. Health Place, 2005. 11(1): p. 15–29. [DOI] [PubMed] [Google Scholar]
- 5.Wang F, et al. , Late-Stage Breast Cancer Diagnosis and Health Care Access in Illinois. Prof Geogr, 2008. 60(1): p. 54–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akinyemiju T, et al. , Racial disparities in individual breast cancer outcomes by hormone-receptor subtype, area-level socio-economic status and healthcare resources. Breast Cancer Res Treat, 2016. 157(3): p. 575–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradley CJ, Given CW, and Roberts C, Disparities in cancer diagnosis and survival. Cancer, 2001. 91(1): p. 178–88. [DOI] [PubMed] [Google Scholar]
- 8.Bradley CJ, Given CW, and Roberts C, Race, socioeconomic status, and breast cancer treatment and survival. J Natl Cancer Inst, 2002. 94(7): p. 490–6. [DOI] [PubMed] [Google Scholar]
- 9.Meliker JR, et al. , Breast and prostate cancer survival in Michigan: can geographic analyses assist in understanding racial disparities? Cancer, 2009. 115(10): p. 2212–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pruitt SL, et al. , Residential racial segregation and mortality among black, white, and Hispanic urban breast cancer patients in Texas, 1995 to 2009. Cancer, 2015. 121(11): p. 1845–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ward E, et al. , Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin, 2004. 54(2): p. 78–93. [DOI] [PubMed] [Google Scholar]
- 12.Dai D, Black residential segregation, disparities in spatial access to health care facilities, and late-stage breast cancer diagnosis in metropolitan Detroit. Health Place, 2010. 16(5): p. 1038–52. [DOI] [PubMed] [Google Scholar]
- 13.Haas JS, et al. , Racial segregation and disparities in breast cancer care and mortality. Cancer, 2008. 113(8): p. 2166–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuo TM, Mobley LR and Anselin L, Geographic disparities in late-stage breast cancer diagnosis in California. Health Place, 2011. 17(1): p. 327–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gumpertz ML, et al. , Geographic patterns of advanced breast cancer in Los Angeles: associations with biological and sociodemographic factors (United States). Cancer Causes Control, 2006. 17(3): p. 325–39. [DOI] [PubMed] [Google Scholar]
- 16.Baquet CR and Commiskey P, Socioeconomic factors and breast carcinoma in multicultural women. Cancer, 2000. 88(5 Suppl): p. 1256–64. [DOI] [PubMed] [Google Scholar]
- 17.Li CI, Malone KE, and Daling JR, Differences in breast cancer stage, treatment, and survival by race and ethnicity. Arch Intern Med, 2003. 163(1): p. 49–56. [DOI] [PubMed] [Google Scholar]
- 18.McBride R, et al. , Within-stage racial differences in tumor size and number of positive lymph nodes in women with breast cancer. Cancer, 2007. 110(6): p. 1201–8. [DOI] [PubMed] [Google Scholar]
- 19.Canto MT, Anderson WF, and Brawley O, Geographic variation in breast cancer mortality for white and black women: 1986–1995. CA Cancer J Clin, 2001. 51(6): p. 367–70. [DOI] [PubMed] [Google Scholar]
- 20.Chien LC, Yu HL, and Schootman M, Efficient mapping and geographic disparities in breast cancer mortality at the county-level by race and age in the U.S.. Spat Spatiotemporal Epidemiol, 2013. 5: p. 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grann V, et al. , Regional and racial disparities in breast cancer-specific mortality. Soc Sci Med, 2006. 62(2): p. 337–47. [DOI] [PubMed] [Google Scholar]
- 22.Markossian TW, Hines RB, and Bayakly R, Geographic and racial disparities in breast cancer-related outcomes in Georgia. Health Serv Res, 2014. 49(2): p. 481–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mobley LR, et al. , Modeling Geospatial Patterns of Late-Stage Diagnosis of Breast Cancer in the US. Int J Environ Res Public Health, 2017. 14(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mokdad AH, et al. , Trends and Patterns of Disparities in Cancer Mortality Among US Counties, 1980–2014. JAMA, 2017. 317(4): p. 388–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scott L, Mobley LR, and Il’yasova D, Geospatial Analysis of Inflammatory Breast Cancer and Associated Community Characteristics in the United States. Int J Environ Res Public Health, 2017. 14(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tatalovich Z, et al. , Geographic disparities in late stage breast cancer incidence: results from eight states in the United States. Int J Health Geogr, 2015. 14: p. 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tian N, Wilson JG, and Zhan FB, Spatial association of racial/ethnic disparities between late-stage diagnosis and mortality for female breast cancer: where to intervene? Int J Health Geogr, 2011. 10: p. 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.CDC. Wide-Ranging Online Data for Epidemiologic Research (CDC-Wonder). 2016. [cited 2016 May 15]; Available from: http://wonder.cdc.gov.
- 29.Manson S, et al. , Minnesota Population Center: National Historical Geographic Information System: Version 2.0, 2017: Minneapolis: University of Minnesota. [Google Scholar]
- 30.Institute, U.o.W.P.H., County Health Rankings & Roadmaps, 2016. [Google Scholar]
- 31.Kirchhoff AC, Hart G, & Campbell EG, Rural and urban primary care physician professional beliefs and quality improvement behaviors. J Rural Health, 2014. 30(3): p. 235–43. [DOI] [PubMed] [Google Scholar]
- 32.Gruca TS, Pyo TH, & Nelson GC, Improving Rural Access to Orthopaedic Care Through Visiting Consultant Clinics. J Bone Joint Surg Am, 2016. 98(9): p. 768–74. [DOI] [PubMed] [Google Scholar]
- 33.Census. Geographic Terms and Concepts - Census Divisions and Census Regions. 2016. [cited 2016 May 15]; Available from: https://www.census.gov/geo/reference/gtc/gtc_census_divreg.html.
- 34.Wartenberg D, Investigating disease clusters: why, when, and how? J.R. Statist. Soc. A, 2001. 164(Part 1): p. 13–22. [Google Scholar]
- 35.Moore JX, Donnelly JP, Griffin R, Howard G, Safford MM, & Wang HE, Defining Sepsis Mortality Clusters in the United States. Crit Care Med, 2016. 44(7): p. 1380–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anselin L, Local indicators of spatial association - LISA. Geogr Anal, 1995. 27: p. 93–115. [Google Scholar]
- 37.Getis A, & Ord K, The analysis of spatial association by use of distance statistics. Geogr Anal, 1992. 24: p. 189–206. [Google Scholar]
- 38.Nassel AF, Root ED, Haukoos JS, McVaney K, Colwell C, Robinson J, Eigel B, Magid DJ, & Sasson C, Multiple cluster analysis for the identification of high-risk census tracts for out-of-hospital cardiac arrest (OHCA) in Denver, Colorado. Resuscitation, 2014. 85: p. 1667–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anselin L, Exploring spatial data with GeoDa: a workbook. 2004, Urbana-Champaign: University of Illinois. [Google Scholar]
- 40.Li H, Calder CA, & Cressie NAC, Beyond Moran's I: testing for spatial dependence based on the spatial autoregressive model. Geogr Anal, 2007. 39: p. 357–375. [Google Scholar]
- 41.Li H, Calder CA, & Cressie NAC, One-step estimation of spatial dependence parameters: properties and extensions of the APLE statistic. J Multivariate Anal, 2012. 105: p. 68–84. [Google Scholar]
- 42.Schootman M, et al. , The role of poverty rate and racial distribution in the geographic clustering of breast cancer survival among older women: a geographic and multilevel analysis. Am J Epidemiol, 2009. 169(5): p. 554–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schootman M, et al. , Temporal trends in geographic disparities in small-area breast cancer incidence and mortality, 1988 to 2005. Cancer Epidemiol Biomarkers Prev, 2010. 19(4): p. 1122–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Akinyemiju T, Meng Q, and Vin-Raviv N, Race/ethnicity and socio-economic differences in colorectal cancer surgery outcomes: analysis of the nationwide inpatient sample. BMC Cancer, 2016. 16: p. 715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Akinyemiju TF, et al. , Individual and neighborhood socioeconomic status and healthcare resources in relation to black-white breast cancer survival disparities. J Cancer Epidemiol, 2013. 2013: p. 490472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Akinyemiju TF, et al. , Race/ethnicity and socio-economic differences in breast cancer surgery outcomes. Cancer Epidemiol, 2015. 39(5): p. 745–51. [DOI] [PubMed] [Google Scholar]
- 47.Damle RN, et al. , Examination of Racial Disparities in the Receipt of Minimally Invasive Surgery Among a National Cohort of Adult Patients Undergoing Colorectal Surgery. Dis Colon Rectum, 2016. 59(11): p. 1055–1062. [DOI] [PubMed] [Google Scholar]
- 48.Fedewa SA, et al. , Race and ethnicity are associated with delays in breast cancer treatment (2003-2006). J Health Care Poor Underserved, 2011. 22(1): p. 128–41. [DOI] [PubMed] [Google Scholar]
- 49.Fedewa SA, et al. , Delays in adjuvant chemotherapy treatment among patients with breast cancer are more likely in African American and Hispanic populations: a national cohort study 2004-2006. J Clin Oncol, 2010. 28(27): p. 4135–41. [DOI] [PubMed] [Google Scholar]
- 50.Freedman RA, et al. , The association of race/ethnicity, insurance status, and socioeconomic factors with breast cancer care. Cancer, 2011. 117(1): p. 180–9. [DOI] [PubMed] [Google Scholar]
- 51.Hershman DL, et al. , Household net worth, racial disparities, and hormonal therapy adherence among women with early-stage breast cancer. J Clin Oncol, 2015. 33(9): p. 1053–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schmid M, et al. , Racial Differences in the Surgical Care of Medicare Beneficiaries With Localized Prostate Cancer. JAMA Oncol, 2016. 2(1): p. 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shavers VL and Brown ML, Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst, 2002. 94(5): p. 334–57. [DOI] [PubMed] [Google Scholar]
- 54.Sheppard VB, et al. , Disparities in breast cancer surgery delay: the lingering effect of race. Ann Surg Oncol, 2015. 22(9): p. 2902–11. [DOI] [PubMed] [Google Scholar]
- 55.Wang EH, et al. , Disparities in Treatment of Patients With High-risk Prostate Cancer: Results From a Population-based Cohort. Urology, 2016. 95: p. 88–94. [DOI] [PubMed] [Google Scholar]
- 56.Torre LA, Siegel RL, & Jemal A, Lung Cancer Statistics. Adv Exp Med Biol, 2016. 893: p. 1–19. [DOI] [PubMed] [Google Scholar]
- 57.Casper ML, Wing S, Anda RF, Knowles M, & Pollard RA, The shifting stroke belt. Changes in the geographic pattern of stroke mortality in the United States, 1962 to 1988. Stroke, 1995. 26(5): p. 755–60. [DOI] [PubMed] [Google Scholar]
- 58.Moore JX, et al. , Defining Sepsis Mortality Clusters in the United States. Crit Care Med, 2016. 44(7): p. 1380–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chang BA, Pearson WS, and Owusu-Edusei K Jr., Correlates of county-level nonviral sexually transmitted infection hot spots in the US: application of hot spot analysis and spatial logistic regression. Ann Epidemiol, 2017. 27(4): p. 231–237. [DOI] [PubMed] [Google Scholar]
- 60.Moore JX, Akinyemiju T, and Wang HE, Pollution and regional variations of lung cancer mortality in the United States. Cancer Epidemiol, 2017: p. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Karp DN, et al. , Reassessing the Stroke Belt: Using Small Area Spatial Statistics to Identify Clusters of High Stroke Mortality in the United States. Stroke, 2016. 47(7): p. 1939–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aschengrau A, Paulu C, and Ozonoff D, Tetrachloroethylene-contaminated drinking water and the risk of breast cancer. Environ Health Perspect, 1998. 106 Suppl 4: p. 947–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Terry PD, Miller AB, and Rohan TE, Cigarette smoking and breast cancer risk: a long latency period? Int J Cancer, 2002. 100(6): p. 723–8. [DOI] [PubMed] [Google Scholar]
- 64.Petralia SA, et al. , Risk of premenopausal breast cancer in association with occupational exposure to polycyclic aromatic hydrocarbons and benzene. Scand J Work Environ Health, 1999. 25(3): p. 215–21. [DOI] [PubMed] [Google Scholar]
- 65.Akinyemiju T, et al. , Disparities in the prevalence of comorbidities among US adults by state Medicaid expansion status. Prev Med, 2016. 88: p. 196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Local indicators of spatial association (LISA) for breast cancer mortality, among NH-Black women in the contiguous United States, years 2000 – 2015.
Breast cancer mortality using spatial Empirical Bayes (EB) smoothed rates quintiles, among NH-Black women in the Contiguous United States, years 2000 – 2015.
Getis-Ord (Gi*) for breast cancer mortality hot spots, among Hispanic women in the contiguous United States, years 2000 – 2015.
Local indicators of spatial association (LISA) for breast cancer mortality, among Hispanic women in the contiguous United States, years 2000 – 2015.
Breast cancer mortality using spatial Empirical Bayes (EB) smoothed rates quintiles, among Hispanic women in the Contiguous United States, years 2000 – 2015.
Getis-Ord (Gi*) for breast cancer mortality hot spots, among NH-White women in the contiguous United States, years 2000 – 2015.
Local indicators of spatial association (LISA) for breast cancer mortality, among NH-White women in the contiguous United States, years 2000 – 2015.
Breast cancer mortality using spatial Empirical Bayes (EB) smoothed rates quintiles, among NH-White women in the Contiguous United States, years 2000 – 2015.
Supplemental Table 1: ICD-10 codes for identification of breast cancer.
Supplemental Table 2: Detailed definitions and technical information for 2014 County Health Rankings (CHR) county-level characteristics used in study analysis. All variables are county-level proportions.
Getis-Ord (Gi*) for breast cancer mortality hot spots, among all women in the contiguous United States, years 2000 – 2015.
Local indicators of spatial association (LISA) for breast cancer mortality, among all women in the contiguous United States, years 2000 – 2015.
Breast cancer mortality using spatial Empirical Bayes (EB) smoothed rates quintiles, among all women in the Contiguous United States, years 2000 – 2015.
Getis-Ord (Gi*) for breast cancer mortality hot spots, among NH-Black women in the contiguous United States, years 2000 – 2015.


