Abstract
Background:
Exposure-based therapies are used to treat a variety of trauma- and anxiety-related disorders by generating successful extinction following cue exposure during treatment. The development of adjuvant strategies that accelerate extinction learning, improve tolerability, and increase efficiency of treatment could increase the efficacy of exposure-based therapies. Vagus nerve stimulation (VNS) paired with exposure can enhance fear extinction, in rat models of psychiatric disorders, and chronic administration of VNS reduces anxiety in rats and humans.
Objective:
We tested whether VNS, like other cognitive enhancers, could produce generalization of extinction for stimuli that are not presented during the extinction sessions, but are associated with the fear event.
Methods:
Male Sprague Dawley rats underwent auditory fear conditioning with two easily discriminable auditory stimuli. Following fear conditioning, extinction training consisted of exposure to only one of the conditioned sounds. Half of the rats received VNS and half received sham stimulation during with sound presentations. VNS effects on anxiety were examined in a separate study where VNS was administered prior to testing on the elevated plus maze.
Results:
Sham stimulated rats given 20 presentations of a conditioned stimulus (CS) during the extinction session showed performance that was matched to VNS-treated rats given only 4 presentations of the CS. Despite comparable levels of freezing to the presented CS, only the VNS-treated rats showed a significant decrease in freezing to the CS that was not presented. VNS-induced generalization of extinction was observed only when the two sounds were paired with footshock within the same fear conditioning session; VNS did not promote generalization of extinction when the two sounds were conditioned on different days or in different contexts. On the anxiety test, VNS administration significantly increased time spent in the open arms of the elevated plus maze.
Conclusion:
These results provide evidence that VNS can promote generalization of extinction to other stimuli associated with a specific fear experience. Furthermore, non-contingent VNS appears to reduce anxiety. The ability to generalize extinction and reduce anxiety makes VNS a potential candidate for use as an adjunctive strategy to improve the efficacy and tolerability of exposure-based therapies.
Keywords: extinction, vagus, anxiety, VNS, generalization, fear
Introduction
Exposure-based therapies are used to treat maladaptive fears and compulsions that are brought about by traumatic events [1–8]. The approach is designed to extinguish conditioned fear; a process that depends upon the suppression of old learned associations by the formation of new associations [9–11]. Unfortunately, drop out, non-response, and relapse rates are high [12]. Impaired extinction memory retention has been observed in individuals who suffer from posttraumatic stress disorder [13–19]. Such impairments may contribute to the development of trauma-related disorders, and may interfere with progress in therapy.
Another challenge of exposure-based therapies is that it is not feasible to expose patients to every thought or sensory stimulus that may remind them of the trauma. Frequently, patients cannot recall significant portions of the traumatic event [20–21]. An ideal adjunct to exposure-based therapies would enhance extinction for the cues presented during therapy as well as trauma-related cues that are not presented during therapy. Encouraging recent findings indicate that some memory consolidation-enhancing methods, such as mild stress or administration of the NMDA partial agonist D-cycloserine (DCS), promote such generalization of extinction [22–23]. However, results of studies of cognitive enhancers as adjuncts are mixed [24]. Some researchers consider the use of pharmacological cognitive enhancers during exposure-based therapy risky due to the potential for reinforcing learned negative associations with reminders of the trauma, or with the therapy itself, when patients experience a severe anxiety response [25]. Optimal adjuvant treatments would promote generalization of extinction and concurrently reduce anxiety to improve treatment efficacy and tolerability.
Vagus nerve stimulation (VNS) paired with specific stimuli has emerged as a strategy to enhance memory consolidation [26–27] as well as consolidation of extinction memory in healthy rats [28–30]. Additionally, VNS administration paired with exposure to conditioned cues accelerates the extinction of conditioned fear and attenuates reinstatement in a rat model of PTSD [31]. VNS could also increase tolerability during exposure-based therapies as chronic administration of VNS reduces anxiety in rats [32–33] and humans [34]. These results suggest that VNS, which is FDA approved for epilepsy and treatment-resistant depression in humans, may be a promising adjunct to exposure-based therapies. In previous studies of VNS effects on extinction, VNS was paired with presentation of only a single conditioned cue. Given the memory-enhancing effects of VNS, the present experiments were designed to determine whether VNS-enhanced extinction could produce generalization of extinction. Based on evidence that chronic VNS reduces anxiety, we also tested whether the VNS parameters used to enhance the consolidation of memory and extinction can reduce anxiety.
Materials and Methods
Animals
All procedures were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals, and were approved by the Institutional Animal Care and Use Committee of the University of Texas at Dallas. Eighty-eight male Sprague-Dawley rats (Charles River) weighing 225–250 g on arrival were housed on a 12 h light/dark cycle (lights on at 7:00 am) with access to food and water ad libitum. Before the start of every experiment, rats were handled for five days, five minutes per day.
VNS and Sham Surgery
Surgery protocols are described in detail elsewhere [35]. In brief, rats were anesthetized with isoflurane (2% at an oxygen flow rate of 600–800 ml/min). The left vagus nerve was located at the cervical level and isolated from other tissue. The left vagus nerve was selected to avoid descending stimulation effects on the sinoatrial node. Central activation from the left vagus nerve is bilateral [36]. The cuff was placed around the nerve and secured in place with a suture. The platinum-iridium wires were tunneled subcutaneously behind the ear to the top of the head and connected to the Omnetics connector which was affixed to the skull using acrylic, to make the headcap. Cessation of breathing was used to test for correct implantation and effectiveness of the VNS cuff; following implantation, while under anesthesia, current (0.8mA, 1 second) was applied through the cuff electrode and breathing rate was visually monitored. If cessation of breathing was not observed, the cuff was adjusted or replaced until cessation of breathing was achieved with stimulation. For sham rats, surgery was conducted in the same manner but the cuff electrode was not implanted. During sham surgery, the vagus nerve was isolated from the other tissue and an Omnetics conector was affixed to the skull. Rats were given one week to recover following surgery.
Extinction Generalization
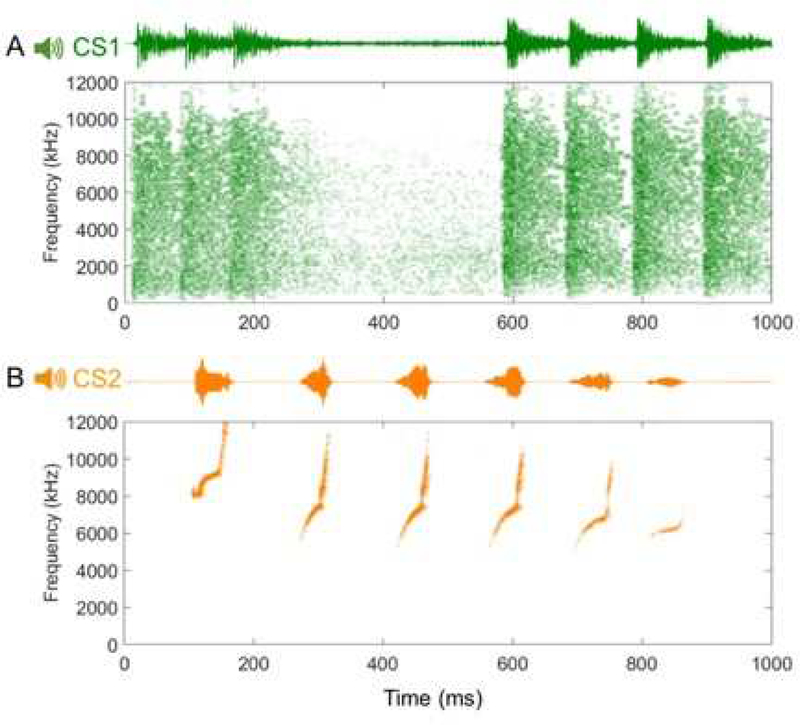
Auditory fear conditioning (AFC) consisted of two discrete, complex auditory stimuli that were paired with a footshock. The stimuli: machine gunfire (CS1) and a marmoset vocalization (CS2), were selected because they activate the auditory cortex more broadly and naturally than pure tones, and our previous work determined that they are perceptually distinct and discriminable [37–38]. Spectrograms of these cues show distinct differences in frequency bandwidth, frequency modulation, rise time, and decay time. Each stimulus was presented at 70dB (Figure 1).
Figure 1. Spectrograms of each conditioned stimulus used during AFC. A. Spectrogram for CS1 (machine gun fire). B. Spectrogram for CS2 (marmoset vocalization).
Spectrograms for CS1 and CS2 were used in generalization experiments, as they are easy to discriminate given they have distinct differences in frequency bandwidth, frequency modulation, rise time, and decay time. Duration of stimuli were also different, CS1 lasted 50 seconds while CS2 lasted 39 seconds.
Stimuli interleaved during AFC (co-conditioned)
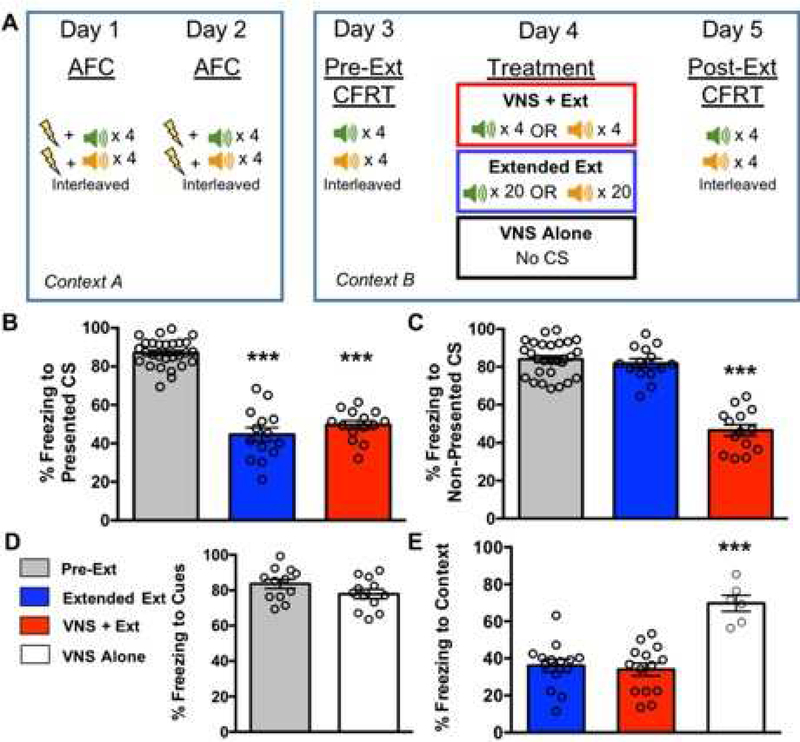
Experiment 1 was designed to determine whether VNS promotes generalization of extinction across auditory cues. Thirty-four rats underwent auditory fear conditioning (AFC) where two discriminable stimuli (CS1 and CS2) were presented during the same conditioning session. Both CS1 and CS2 were coupled with footshocks. Only one of the stimuli was presented during the extinction treatment day but both were presented during test sessions (Figure 4a).
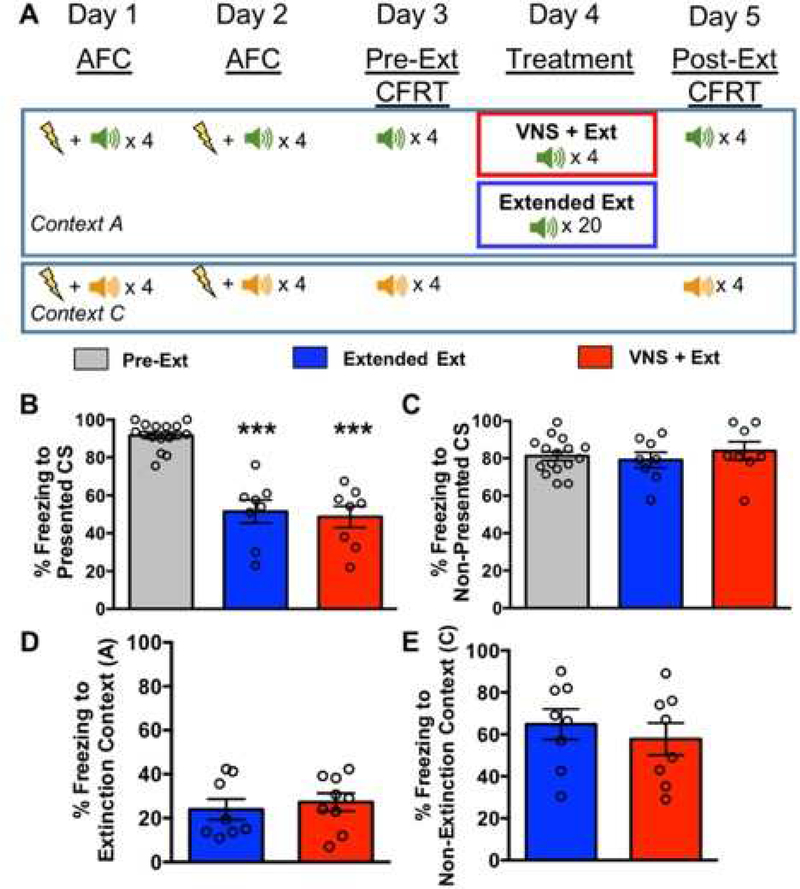
Figure 4. Separating conditioning of CS1 and CS2 by context blocks the VNS+Extinction effect on generalization. A. Timeline for AFC, CFRT, and extinction treatment.
AFC was administered across two days, where CS1 was presented only in Context A, and CS2 was presented only in Context C, but both CS1 and CS2 were presented during the same session. Twenty-four hours after AFC, a Pre-Extinction CFRT was administered for CS1 in Context A, where CS1 was presented four times without reinforcement. On the same day, a Pre-Extinction CFRT was also administered to CS2 in Context C, where CS2 was presented four times without reinforcement. Following the Pre-Extinction CFRT day, rats were subjected to extinction treatment consisting of either: 20 presentations of CS1 in Context A paired with sham stimulation (Extended Extinction), or 4 presentations of CS1 in Context A paired with VNS (VNS+Extinction). Twenty-four hours following extinction, a Post-Extinction CFRT was given to CS1 in Context A, and then on the same day a Post-Extinction CFRT was given for CS2 in Context C. B. Extended Extinction and VNS+Extinction rats show equal reduction in conditioned fear to the Presented CS. Following extinction of CS1 in Context A, Extended Extinction and VNS+Extinction rats showed reduced conditioned fear to the Presented CS versus Pre-Extinction (p=2.3×10−3) and (p=1.2×10−3), respectively. C. Neither Extended Extinction rats or VNS+Extinction rats show reduced freezing to the Non-Presented CS. Freezing to the Non-Presented CS was not reduced after Extinction training for Extended Extinction rats (p=0.38). Similar to Figure 2, freezing to the Non-Presented CS was also not reduced for VNS+Extinction rats versus Pre-Extinction (p=0.40). D. Baseline freezing to the extinction context is not different between groups. Extended Extinction and VNS+Extinction rats spent an equal amount of time freezing to the extinction context during the five minutes prior to presentation of CS1 during the Post-Extinction CFRT (p=0.53). E. Baseline freezing to the non-extinction context is not different between groups. Extended Extinction and VNS+Extinction rats spent an equal amount of time freezing to the non-extinction context during the five minutes prior to CS2 presentation during the Post-Extinction CFRT (p=0.62).
Two days of AFC were conducted in Context A (electric grid floor). During AFC, CS1 and CS2 were randomly interleaved with an interstimulus interval (ISI) between 120 and 240 seconds. Four presentations of each CS coupled with a footshock (0.8mA, 1 second) were administered on each day of AFC. As in our previous studies [27–30], we aimed to minimize timing predictability by administering the footshock at a random time during each CS presentation. Twenty-four hours following Day 2 of AFC, rats were given a conditioned fear response test (CFRT) to determine whether fear conditioning was equivalent across the two cues and across the two groups before VNS. The CFRT was carried out in Context B (peppermint smell, black Plexiglas floor), where each CS was presented four randomly interleaved times, without reinforcement. Videos were recorded via webcam and the amount of time spent freezing during presentations of each CS was scored by two experimenters who were blind to treatment conditions, and used as the measure of conditioned fear. On the following day, rats were given extinction training in Context B, where only one CS was presented and paired with either VNS or sham stimulation (Presented CS); the other CS was not presented on extinction treatment day (Non-Presented CS). The CS selected as the Presented CS was counter-balanced. VNS-treated rats received four presentations of the CS that overlapped with VNS (VNS+Extinction group; n=14). The parameters used for VNS were the same as those used to enhance memory consolidation and the consolidation of extinction of conditioned fear [28–31] and they are within the range of stimulation parameters approved by the FDA for seizure prevention in humans [33]. For seizure prevention, a 30 sec train of VNS is given every 5 min throughout the day and night. Here, the “paired” VNS was delivered at an intensity of 0.4 mA, with a pulse width of 100 µs and frequency of 20 Hz for a single 30 sec train, starting 150 ms before the onset of each 30-sec tone [29]. We previously found that 20 presentations of the CS paired with sham stimulation and four presentations of the CS paired with VNS generate equivalent levels of extinction [30]. To compare generalization, the Presented CS was extinguished to the same degree across groups by giving sham-treated rats 20 presentations of the CS paired with sham stimulation (Extended Extinction group; n=14). To assess any effects of VNS alone on extinction, a third group of rats were subjected to the same AFC and CFRT procedures but remained in the home cage on extinction day and received equal amounts of VNS in lieu of extinction (VNS Alone group; n=6). Twenty-four hours later, levels of conditioned fear to Context B and to each CS were tested again by placing all rats into Context B, recording baseline freezing to the context for five minutes, then presenting rats with four randomly interleaved presentations of each CS. The CFRT session was recorded by webcam and baseline freezing to Context B, freezing during CS1, and freezing during CS2 were scored by two blind experimenters. The CFRTs could allow for extinction learning because they involve four non-reinforced exposures to both stimuli on each test day. However, we find a significant effect when only four of the exposures are paired with VNS [28–31]. Although we have not observed locomotor or other performance effects that may obscure measurement of conditioned fear, taking the CFRT before and after VNS treatment prevents mistaking of immediate performance disturbances for VNS effects on extinction. Testing retention 24 hours later allows for a measure of VNS effects on the consolidation of the extinction memory. Furthermore, with this approach, it is unlikely that the VNS serves as a safety signal because fear is measured in the absence of VNS.
Stimuli separated by time during AFC
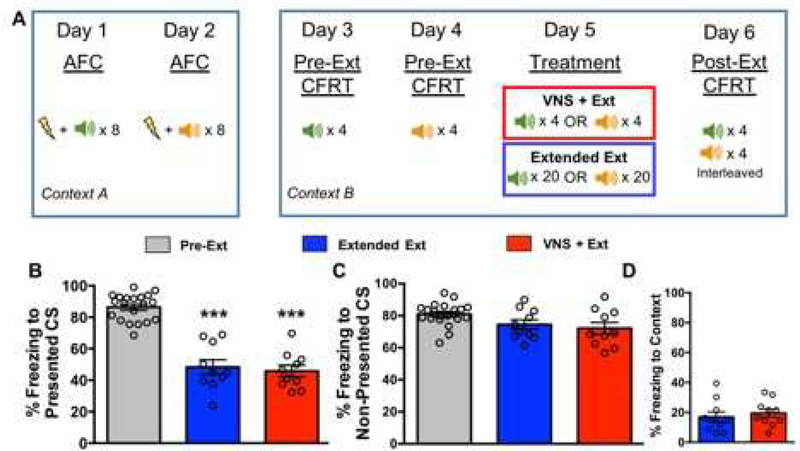
To determine if VNS led to generalization of extinction for cues learned on separate days, we performed Experiment 2 where CS1 was coupled with a footshock and, twenty-four hours later, CS2 was coupled with a footshock (Figure 5a). Experiment 2 was preregistered on Open Science Framework (osf.io/wk7re). Twenty rats were subjected to two days of AFC in Context A (neutral smell, electric grid floor). On Day 1 of AFC, rats were presented with only CS1, which was presented 8 times coupled with a footshock (0.8mA, 1 second). On Day 2, CS2 was presented 8 times coupled with a footshock (0.8mA, 1 second). Twenty-four hours following AFC, rats underwent a pre-extinction CFRT in Context B (peppermint smell, black acrylic floor). Rats were presented with four unreinforced presentations of CS1. On the following day, rats underwent another pre-extinction CFRT where this time they were presented with four unreinforced presentations of CS2. Both CFRT days were recorded with a Logitech camera. Percent of time spent freezing during presentations of CS1 and CS2 were scored by two blind experimenters and used as the measure of conditioned fear. On Day 5, rats underwent extinction to either CS1 or CS2 paired with either sham stimulation or VNS (counter-balanced across groups). Extinction was performed the same way as in Experiment 2; the CS was presented four times if paired with VNS (VNS+Extinction group, n=10), or 20 times if paired with sham stimulation (Extended Extinction group, n=10). Twenty-four hours after the extinction treatment session, rats were placed into Context B where freezing to the context was recorded for five minutes, then four presentations of each CS were randomly interleaved. This post-extinction CFRT session was recorded and freezing to Context B, CS1, and CS2 were scored by two blind experimenters and used as a measure of conditioned fear.
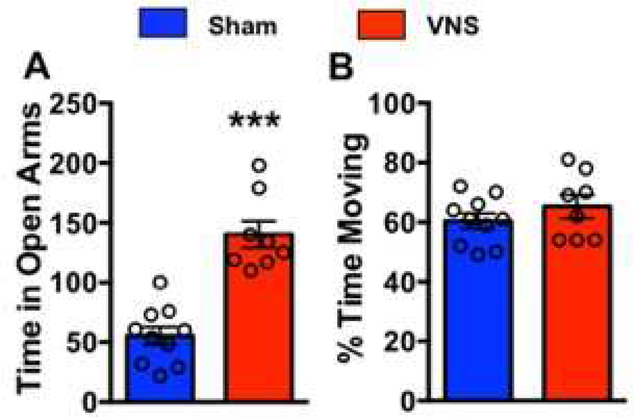
Figure 5. VNS reduces anxiety.
Rats were given one non-contingent train of VNS or sham stimulation in their home cage. Ten minutes later, they were removed from their home cage and placed on the elevated plus maze (EPM). VNS-treated rats spent significantly more time in the open arms of the EPM when compared to sham-treated rats (p=2.1×10−3), indicating a VNS-induced reduction in anxiety. The percent of time spent moving during EPM testing was similar between groups, indicating no general locomotor effect.
Stimuli separated by context during AFC
To determine if VNS led to generalization of extinction for cues learned in different contexts, we performed Experiment 3 where AFC to CS1 occurred in Context A, and AFC to CS2 occurred in Context C within one hour (Figure 6a). Experiment 3 was preregistered on Open Science Framework (osf.io/wk7re). Sixteen rats were subjected to AFC for CS1 in Context A (neutral smell, shock grid floor) and AFC for CS2 in Context C (peppermint smell, shock grid floor, different light position, different room). On the first day of AFC, CS1 was coupled with a footshock (0.8mA, 1 second) four times, and then rats were returned to their home cages. After 15 minutes in the home cage, rats were transferred into Context C where CS2 was coupled with a footshock four times. On Day 2, rats were again placed into Context C and exposed to four presentations of CS2 coupled with a footshock and then, after 15 minutes, transferred to Context A and exposed to four presentations of CS1 coupled with a footshock. Twenty-four hours later, rats underwent a pre-extinction CFRT to CS1 in Context A, where CS1 was presented four times without reinforcement. Rats were returned to their home cages for 15 minutes then transferred to Context C where they underwent another pre-extinction CFRT to CS2, where CS2 was presented four times without reinforcement. Test sessions were recorded by a Logitech camera and freezing to each CS was scored by two experimenters who were blind to treatment conditions. On Day 4 rats underwent extinction to CS1, in Context A, paired with VNS or sham treatment. Extinction was performed the same way as in Experiments 2 and 3; VNS-treated rats received four presentations of CS1 paired with VNS (VNS+Extinction group; n=8) whereas sham-treated rats received 20 presentations of CS1 (Extended Extinction group; n=8). Twenty-four hours following the extinction session, rats were given a post-extinction CFRT for each CS. Rats were first placed in Context A, where baseline freezing to the context was recorded for five minutes, then CS1 was presented four times without reinforcement and freezing behavior was recorded. After 15 minutes in the home cage, rats were transferred to context C where baseline freezing to the context was recorded for five minutes, then four unreinforced presentations of CS2 were given. Test sessions were recorded by a Logitech camera and freezing to context A, context C, CS1, and CS2 were scored by two blind experimenters as measures of conditioned fear.
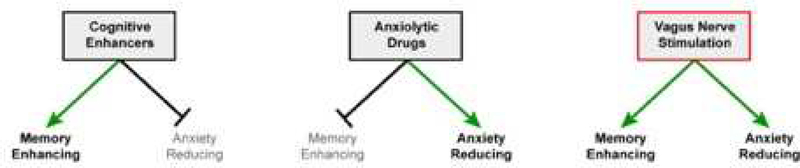
Figure 6. Dual benefits of VNS.
Cognitive enhancers show promise as adjuncts to exposurebased therapies; however, most pharmaceutical enhancers of cognition do not improve tolerability of the therapy by reducing anxiety. Anxiolytic drugs may improve tolerability but they are not effective, possibly because they interfere with the consolidation of new extinction memories. However, VNS enhances memory consolidation and accelerates extinction, and it also reduces anxiety. This unique combination of effects suggests that VNS may offer a desirable alternative to drugs as an adjunct therapy.
VNS effects on anxiety
To determine if the parameters of VNS given during extinction were sufficient to reduce anxiety, we administered VNS or sham stimulation to eighteen rats prior to testing on the elevated plus maze (EPM). In order to prevent a possible neophobia effect before EPM testing, rats were given a single 30 sec train of VNS or sham stimulation per day, spread across four days. Rats were connected to the AM systems stimulator using the previously described parameters (20Hz, 0.4mA, 30 seconds, 100µs pulse width), however stimulation was not triggered in response to an event. Rather, a single non-contingent train of VNS was given in the home cage. Sham-treated rats were connected to the stimulator in the same way as VNS-treated rats but did not receive stimulation. On Day 4, rats underwent testing on the EPM ten minutes after being disconnected from the stimulator.
The EPM was used to measure anxiety [39]. Rats were placed onto an elevated plusshaped maze (10.0cm wide, 50.0cm long, elevated 55.0cm off the floor), with walls (30.0cm tall) on two opposing arms and no walls on the other opposing arms. Time spent in the open arms, time spent in the closed arms, and time spent in the center of the maze were recorded during the ten-minute test. An entry into an arm was scored when the rat’s full body, excluding the tail, was in the arm at one time. All behavior was recorded and scored using AnyMaze video tracking software. Time spent in the open arms was used as a measure of risk taking, and the percent of the total time spent moving was taken as a control measure of general locomotion.
Statistical Analyses
Data for extinction generalization experiments were analyzed using a two-factor repeated measures ANOVA with a Greenhouse-Geisser correction followed by a Tukey’s post hoc test for multiple comparisons. Context freezing was analyzed using a one-way ANOVA with a Tukey’s post hoc test for multiple comparisons or an unpaired samples t-test. Data for VNS effects on anxiety were analyzed using an unpaired samples t-test.
Statistically significant effects were defined as those with p values that were <0.05. All error bars represent standard error of the mean. Individual data points are represented on each graph as circles. Criteria for exclusion of rats from the analysis was performance > 2 standard deviations away from the mean on any task. Six rats were excluded from analysis for failure to express conditioned fear following AFC (freezing less than 50 percent to the CS after tone-shock couplings). Exclusion of these rats did not alter results.
Results
VNS+Extinction leads to generalization of extinction for co-conditioned stimuli
VNS paired with exposure promotes extinction of a fear conditioned stimulus. Here, we tested the hypothesis that VNS, like other cognitive enhancers, would promote generalization of extinction for multiple fear-related stimuli learned within a session. In Experiment 1, rats underwent AFC to two distinct auditory stimuli interleaved during the fear conditioning sessions. All rats exhibited similar levels of conditioned fear to both CS1 and CS2 at the CFRT given prior to extinction training (Pre-Extinction). Rats underwent either VNS+Extinction (n=14), where a train of VNS was paired with presentations of one of the conditioned cues a total of four times and the other CS was not presented, or Extended Extinction (n=14) where the conditioned cue was presented without VNS a total of 20 times and the other CS was not presented. The CFRT measured 24 hours after the Extinction Treatment session was compared across the two groups. A two-factor repeated measures ANOVA indicated a significant effect of treatment group across days (F(3, 64)=47.00 p=3.6×10−8). Following 20 presentations of either CS1 or CS2 paired with sham stimulation, Extended Extinction rats showed a reduction in conditioned fear to the CS presented during extinction (Presented CS) versus Pre-Extinction freezing (p=2.3×10−4). Four presentations of CS1 or CS2 paired with VNS was also sufficient to reduce freezing to the Presented CS in VNS+Extinction rats versus Pre-Extinction freezing (p=3.2×10−4) (Figure 2b).
Figure 2. VNS+Extinction leads to generalization of extinction for both the CS that was presented and the CS that was not presented during extinction training. A. Timeline for AFC, conditioned fear response testing, and extinction treatment.
AFC was administered across two days where both CS1 and CS2 were presented and paired with footshocks on each day. The order of CS presentation was random and interleaved such that each rat was administered 4 presentations of each CS on each day. Following AFC, rats underwent a pre-extinction conditioned fear response test (CFRT) where they were presented with four presentations of each CS to measure conditioned fear. Twenty-four hours later, rats underwent treatment consisting of either: 20 extinction trials of only CS1 or only CS2, paired with sham stimulation (Extended Extinction), 4 extinction trials of only CS1 or only CS2, paired with VNS (VNS+Extinction), or no exposures to either CS but equivalent amounts of VNS in the home cage (VNS Alone). A day later, rats underwent another CFRT to assess levels of conditioned fear to both stimuli. B. Extended Extinction and VNS+Extinction rats show equal reduction in conditioned fear to the Presented CS. Following 20 unreinforced presentations of a CS, Extended Extinction rats showed reduced freezing in response to the CS versus freezing during Pre-Extinction (p=2.3×10−4). VNS+Extinction rats also showed reduced freezing in response to the Presented CS versus Pre-Extinction (p=3.2×10−4). C. Only VNS+Extinction leads to generalization of extinction for the Non-Presented CS. Extended Extinction did not show reduced fear in response to the Non-Presented CS versus the Pre-Extinction freezing response (p=0.15). In contrast, VNS+Extinction rats showed a reduction in freezing response to presentation of the Non-Presented CS versus the Pre-Extinction freezing response, even though it was never presented during extinction (p=2.1×10−4). D. VNS Alone does not lead to extinction of either CS. Following VNS in the home cage in lieu of extinction, VNS Alone rats showed no reduction in conditioned fear for either CS versus the Pre-Extinction CFRT (p=0.16). E. Context extinction cannot explain VNS+Extinction generalization. There is no difference in baseline fear to the context between Extended Extinction and VNS+Extinction groups, rats spend equal amounts of time freezing to the context during the Post-Extinction CFRT (p=0.68). VNS Alone rats show elevated freezing to the context versus Extended Extinction (p=2.1×10−3) and VNS+Extinction rats (p=3.8×10−3).
Extended Extinction rats showed no reduction in conditioned fear to the CS that was not presented during extinction (Non-Presented CS) versus Pre-Extinction freezing (p=0.15). In contrast, VNS+Extinction rats showed a significant reduction in freezing during exposure to the Non-Presented CS when compared to Pre-Extinction freezing to the same CS (VNS + Ext vs. Pre-Extinction, p=2.1×10−4) (Figure 2c). This indicates that VNS+Extinction led to generalization of extinction whereas Extended Extinction did not.
VNS is likely to enhance fear extinction by engaging neuromodulatory networks to support extinction-related plasticity. However, it is possible that VNS simply reduces fear or anxiety leading to reduced time spent freezing in response to either CS. To determine if VNS administration had a non-extinction-specific effect, such as a general reduction in expression of fear, a matched amount of VNS was administered in the home cage in lieu of extinction (VNS Alone, n=6). A two-tailed t-test indicated no significant effect of VNS Alone (VNS Alone vs. Pre-Extinction freezing, t(11)=1.8 p=0.16), demonstrating that VNS Alone was not sufficient to reduce conditioned fear to either CS (Figure 2d). These findings are consistent with previous results and indicate that VNS reduces conditioned fear by enhancing fear extinction [28].
Since VNS accelerates extinction, it is possible that reduced freezing to the Non-Presented CS is due to enhanced extinction of Context B. To determine if VNS-dependent generalization of extinction was due to enhanced learning that Context B is safe, baseline freezing to Context B was measured for five minutes prior to any CS presentation during the Post-Extinction CFRT. A one-way ANOVA indicated a significant effect across groups (F(2, 31) =19.21 p=3.7×10−6). However, VNS+Extinction and Extended Extinction demonstrated equivalent freezing to context (p=0.68) (Figure 2e). This similarity indicates that the VNS effect on extinction generalization cannot be explained by VNS enhancement of extinction to Context B. VNS Alone rats showed elevated freezing to the extinction context, likely because they were left in the homecage instead of undergoing extinction training in Context B. Together, these findings indicate that VNS does not reduce conditioned fear to the Non-Presented CS through an enhancement of context extinction, or a general VNS-induced reduction in fear expression. Note that the comparable freezing to context in VNS+Extinction and Extended Extinction animals is consistent with the hypothesis that VNS enhances and generalizes extinction because the time in the extinction context was five times greater for Extended Extinction rats on the extinction treatment day.
Experiment 2: VNS-induced generalization of extinction does not extend to stimuli that are conditioned on separate days
To determine if fear memories that were acquired at different times were susceptible to VNS-induced generalization of extinction, we coupled CS1 alone with a footshock during the first AFC session and then coupled CS2 alone with a footshock twenty-four hours later. As in Experiment 1, an extinction session consisted of only one CS given with VNS (n=10) or sham stimulation (n=10). A two-factor repeated measures ANOVA indicated a significant effect of group across days (F(2, 37)=26.65 p=6.8×10−7). All rats exhibited similar levels of conditioned fear to both CS1 and CS2 at the Pre-Extinction CFRT. Following 20 presentations of either CS1 or CS2 paired with sham stimulation, Extended Extinction rats showed a reduction in freezing in response to the Presented CS versus Pre-Extinction freezing (p=7.1×10−3). Four presentations of CS1 or CS2 paired with VNS (VNS+Extinction) was also sufficient to significantly reduce freezing to the Presented CS versus Pre-Extinction freezing (p=4.4×10−3). The degree of extinction was comparable to that observed with Extended Extinction (Figure 3b).
Figure 3. Separating CS1 and CS2 conditioning by twenty-four hours blocks the VNS effect on generalization. A. Timeline for AFC, CFRT, and extinction treatment.
AFC was administered across two days where CS1 was presented on 1 day and CS2 was presented twenty-four hours later. A CFRT to CS1 was administered on day 3 where CS1 was presented four times without reinforcement to measure conditioned fear to CS1. The next day, a CFRT was administered to CS2 where CS2 was presented four times without reinforcement to measure conditioned fear to CS2. Twenty-four hours later, rats underwent extinction consisting of either: 20 extinction trials of CS1 or CS2 paired with sham stimulation (Extended Extinction), or 4 extinction trials of CS1 or CS2 paired with VNS (VNS+Extinction). Following extinction, a Post-Extinction CFRT was administered where four presentations of each CS were presented and randomly interleaved to assess conditioned fear to each CS following extinction. B. Extended Extinction and VNS+Extinction rats show equal reduction in conditioned fear to the Presented CS. Following 20 presentations of the Presented CS, both Extended Extinction and VNS+Extinction rats showed reduced freezing to the Presented CS (p=7.1×10−3 vs PreExtinction) and (p=4.4×10−3 vs. Pre-Extinction), respectively. C. Neither Extended Extinction rats or VNS+Extinction rats show reduced freezing to the Non-Presented CS. After extinction training, freezing to the Non-Presented CS was not reduced for Extra Extinction rats (p=0.12). In contrast to Figure 1, freezing to the Non-Presented CS was not reduced in (p=0.29). D. Baseline freezing to the context is not different between groups. Extended Extinction and VNS+Extinction rats spent equivalent amounts of time freezing to the extinction context during the five minutes prior to CS presentation at the Post-Extinction CFRT (p=0.55).
Extended Extinction rats did not show a reduction in conditioned fear to the Non-Presented CS versus Pre-Extinction freezing (p=0.12), similar to results of Experiment 1 with interleaved AFC. However, in contrast with the results from Experiment 1, administration of VNS during extinction was not sufficient to reduce freezing to the Non-Presented CS (VNS + Ext freezing vs. Pre-Extinction freezing: p=0.29) (Figure 3c).
An unpaired t-test indicated no significant difference in freezing to the context between Extended Extinction and VNS + Extinction (t(9)=0.59 p=0.56) (Figure 3d). Taken together, these findings suggest that VNS does not promote generalized extinction of conditioned fear when the original fear learning does not occur at the same time.
Experiment 3: VNS-induced generalization of extinction does not extend to stimuli that are conditioned in separate contexts
We next tested whether fear memories learned during a single session but across two contexts were susceptible to VNS-induced generalization of extinction. We paired CS1 with footshocks in Context A and then, on the same day, paired CS2 with footshocks in Context C. During extinction, only CS1 was presented in Context A, paired with VNS (n=8) or sham stimulation (n=8). A two-factor repeated measures ANOVA indicated a significant effect of group across days (F(2, 29)=19.16 p=5.0×10−6). All rats exhibited similar levels of conditioned fear to both CS1 and CS2 at the Pre-Extinction CFRT. Similar to Experiment 1 using interleaved AFC and Experiment 2 using separated AFC, following 20 presentations of CS1 paired with sham stimulation, Extended Extinction rats showed a reduction in conditioned fear to the Presented CS (p=2.3×10−3). Four presentations of CS1 paired with VNS was also sufficient to reduce freezing to the Presented CS in VNS+Extinction rats versus Pre-Extinction freezing (p=1.2×10−3) (Figure 4b).
After AFC in two distinct contexts, Extended Extinction rats did not show a reduction in conditioned fear for the Non-Presented CS (Pre- vs. Post-Extinction freezing; p=0.38), as seen in Experiments 1 and 2. When AFC was separated by time, VNS+Extinction rats did not show reduced conditioned fear of the Non-Presented CS (p=0.40) (Figure 4c). Unpaired t-tests indicated no significant difference in freezing to the Extinction Context between Extended Extinction and VNS+Extinction (t(7)=0.53 p=0.60) (Figure 4d) or in freezing to the Non-Extinction context (t(7)=0.67 p=0.52) (Figure 4e). These findings suggest that VNS does not generalize extinction of conditioned fear when the original fear learning does not happen in the same context.
Experiment 4: VNS reduces anxiety
Previous research indicates that chronic VNS is anxiolytic [34]. We aimed to see if the VNS given during extinction training could produce an anxiolytic effect. We administered VNS or sham stimulation prior to testing on the EPM. In order to prevent VNS-related neophobia on the day of EPM testing, rats were given one non-contingent stimulation in their home cage/day for four days. Ten minutes following the single, non-contingent stimulation, rats were disconnected from the stimulator. On the fourth day, rats were placed on the EPM for ten minutes immediately after being disconnected from the stimulator. During the EPM session, time spent in the open arms, time spent in the closed arms, and total time spent moving were recorded. An unpaired t-test indicated a significant increase in time spent in the open arms in VNS-treated rats versus sham (t(16)=6.35 p=3.1×10−3). Total time spent moving was not different between groups, indicating no gross locomotor effects. These results indicate that a 30-sec train of VNS can reduce anxiety within minutes.
Discussion
Exposure-based therapies are founded on the premise that unreinforced exposure to conditioned cues leads to extinction of learned associations [13]. Exposure therapy is used to treat disorders such as PTSD, phobia, obsessive-compulsive disorder, and addiction. Unfortunately, these therapies show a high incidence of non-response, dropout, and relapse [12]. Various explanations have been offered, including the observation that patients with anxiety-related disorders show impairments in recall of extinction learning [15–19]. Furthermore, it is not feasible to expose patients to every thought or sensory stimulus that may remind them of the trauma. Some recent findings indicate that methods that enhance memory consolidation, such as mild stress or administration of the NMDA partial agonist D-cycloserine (DCS), promote generalization of extinction to reminders that are not presented during therapy [22–23]. Therefore, other tools for enhancement of memory consolidation could be useful adjuvants for exposure-based therapies. In order to improve treatment efficacy and reduce relapse, an ideal adjunct would promote extinction to the totality of cues associated with the trauma experience while reducing anxiety to improve tolerability.
VNS has emerged as a potential strategy to promote neural plasticity, enhance memory, and reduce conditioned fear when delivered coincidentally with cue presentation during fear extinction [29–31], and chronic VNS has anxiolytic effects [32]. We tested whether VNS, like other cognitive enhancing strategies, would promote generalization of extinction and accelerate extinction to multiple fear-related cues in rats. We also tested whether the amount of VNS given to promote extinction was sufficient to reduce anxiety.
The results of these studies provide evidence that VNS paired with exposure to the CS can enhance the consolidation of extinction of conditioned fear, not only for the CS that is paired with VNS, but also for another CS that is associated with the same fear experience. VNS-enhanced extinction generalized to a conditioned cue that was acquired during the same fear conditioning session, indicating that VNS-paired extinction training specifically enhances extinction of learned associations acquired within a session. VNS effects on extinction generalization cannot be explained by enhanced learning of the extinction context, as VNS+Extinction and Extended Extinction rats showed equivalent reductions of freezing responses to the extinction context. Both groups froze less in the context than rats given VNS in lieu of extinction (VNS Alone), indicating that both groups extinguished fear of the context to an equivalent degree, despite the fact that the Extended Extinction group spent more time in the context during the extinction session. VNS Alone rats did not show extinction of conditioned fear of the presented auditory cue or the context, nor did they show generalization of extinction, suggesting that the VNS-induced generalization effect is not due to a general VNS-related reduction in freezing, fear, or anxiety. Taken together, these results indicate that the VNS effects on freezing responses are due to the generalization of extinction of conditioned fear.
Importantly, when presentations of each stimulus occurred on different days, or in different contexts, VNS-enhanced extinction did not promote generalization. This finding suggests that VNS does not act to reduce all conditioned fear. Because fear is an adaptive biological process that protects individuals from danger [40], it is important for trauma sufferers to extinguish conditioned fear of cues that are associated with maladaptive fears without interfering with all learned fears. Memories must be bound by time and place to be susceptible to extinction generalization. Interestingly, optogenetic studies indicate that neuronal ensembles for similar experiences that happen closely in time can overlap. However, following the passage of time or the changing of context, memories are represented by separate neuronal ensembles, indicating that memory engram cells are timing and context-specific [41–43]. The present findings indicate that VNS affects memories that are bound by time, place, and neuronal ensemble, when the effects of Extended Extinction are more limited.
The results of the present investigation do not explain why Extended Extinction does not lead to the same generalization effect as VNS+Extinction. Presenting five times as many presentations of the CS during extinction paired with sham stimulation (Extended Extinction) is sufficient to generate similar levels of freezing to the Presented CS compared to VNS+Extinction. However, Extended Extinction rats do not show generalization of extinction for the Non-Presented CS. Taken together, these findings indicate that there is a qualitative difference in VNS-influenced extinction and extinction achieved following five times as many exposures paired with sham stimulation, despite the comparable levels of freezing to the Presented CS. The degree of memory displayed at the CFRT does not reveal all of the facets of extinction memory, such as how long-lasting or broadly-reaching it is. In addition to facilitating extinction of conditioned fear, previous studies demonstrate that VNS-enhanced extinction is less susceptible to reinstatement of conditioned fear [31], indicating that the neural plasticity supporting VNS-enhanced extinction memory is more rapid and robust. Here, we report evidence that VNS-enhanced extinction is also more broadly tuned. Generalization of learning is seen with other memory enhancing treatments such as administration of histone deacetylase inhibitors [44].
Similarly, generalization of extinction is seen with other methods that enhance memory consolidation, such as mild stress and administration of D-cycloserine [22–23]. VNS can enhance memory consolidation in rats and humans and it also facilitates experience-dependent plasticity [23–24; 27; 45–54]. We have observed similarities and differences in plasticity related to VNS-enhanced and Extended Extinction [28, 30]. For example, VNS administration during extinction training predisposes field potentials in the basolateral complex of the amygdala (BLA) to LTP following high-frequency stimulation of the infralimbic region (IL) of the prefrontal cortex, whereas the same stimulation of the IL did not produce LTP in the BLA of animals given Extended Extinction training with sham stimulation [30]. Follow-up work examined the potential of a VNS-induced effect on meta-plasticity that could set the stage for these alterations in LTP. VNS-induced molecular changes include a reduction in expression of the activity-regulated cytoskeleton-associated protein (Arc) in the BLA [28]. These VNS-induced effects indicate that there can be lasting synaptic and behavioral changes that are not achieved with extra training.
In brain regions involved in fear extinction, administration of VNS alters levels of neuromodulators important for learning and memory. VNS rapidly activates the locus coeruleus [48] and increases levels of norepinephrine in the amygdala [46], and intra-BLA infusions of norepinephrine enhance the consolidation of extinction memory in rats [55]. Similarly, a 2007 study showed that a higher intensity VNS (2 mA) administered in 30 sec trains every 5 min for 3 hours increased levels of brain-derived neurotrophic factor (BDNF) in the prefrontal cortex [56].
BDNF is important for memory consolidation and fear extinction [57–58]. BDNF engages downstream effectors and pathways related to plasticity, including Arc expression [59], which is altered following VNS-paired extinction [28]. Both chronic (30 sec on every 5 min for 14 days) and acute (30 sec on every 5 min for 2 hours) VNS increase phosphorylation of several sites on the BDNF receptor, tyrosine receptor kinase B (TrkB) [60]. Furthermore, multiple studies have demonstrated that infusion of BDNF into the prefrontal cortex is sufficient to generate extinction of fear, even without training [61–62]. Thus, it is possible that BDNF contributes to generalization of extinction seen in response to VNS+Extinction, but not Extended Extinction. However, the duration of acute VNS that affected BDNF or its receptor was greater than the duration of stimulation used in the current studies. Furthermore, VNS alone did not promote extinction. Thus, if four 30 sec trains of VNS increases BDNF, that BDNF is not sufficient to produce the observed generalization of extinction.
Administration of VNS prior to anxiety testing produced anxiolytic effects. We found that VNS given in the home cage immediately before testing on the EPM increased time spent in the open arms. The anxiolytic effect of VNS cannot explain the generalization of extinction because rats were given equivalent amounts of VNS when fear conditioning occurred on different days and in different contexts, yet VNS-treated rats maintained a fear response to the CS that was not presented during extinction trials. Furthermore, VNS alone was not sufficient to reduce freezing in response to conditioned cues. However, evidence that VNS reduces anxiety indicates promise for VNS as an adjunct to exposure-based therapies, where dropout and noncompliance rates are high [12], and avoidance behavior is a major symptom. The unique combination of memory consolidation enhancing and anxiety reducing effects gives VNS an advantage over currently available pharmacological adjuncts. Though some research involving currently available cognitive enhancers shows promise for use during exposure-based therapies [22], they do not improve tolerability and they may reinforce associations of aversive anxiety responses with reminders of the trauma. In contrast, options like benzodiazepines that reduce anxiety interfere with memory consolidation that is required for extinction of conditioned fear [52–53]. Strategies that can both enhance memory consolidation and reduce anxiety would be ideal because they would enhance the consolidation of extinction memory while improving tolerability of exposure-based therapy. VNS appears to provide this unique combination (Figure 6).
Here, we found that VNS paired with exposure to a conditioned stimulus led to generalization of extinction of stimuli that co-occurred during AFC, whereas Extended Extinction training did not. VNS effects were not explained by enhanced contextual extinction or a direct effect of VNS on the expression of fear. Co-occurrence of stimuli during AFC was necessary for extinction generalization, indicating that VNS influenced extinction in an experience-dependent manner, only for conditioned cues that were associated with each other in both time and context. We have found that VNS produces a rapid and robust extinction of conditioned fear in rats [28–31]. This extinction enhancement could overcome extinction impairments seen in patients with anxiety-related disorders. VNS could also be effective in increasing tolerability because chronic administration of VNS reduces anxiety in rats [33] and humans [33], and we found here that VNS also has an immediate anxiolytic effect. Additionally, we report here that VNS can lead to generalization of extinction, which may make exposure based therapies more efficient and prevent cue-induced relapses elicited by reminders not addressed during exposure-based therapy. These results indicate that VNS, which has been used in tens of thousands of patients with drug-resistant epilepsy [63], has promise as an adjunct treatment to enhance the efficacy and improve the tolerability of exposure-based therapies.
Highlights.
Vagus nerve stimulation (VNS) promotes generalization of fear extinction.
VNS induced generalization is only present for memories of co-occurring stimuli.
Acute amounts of VNS reduce innate anxiety.
VNS can promote extinction, generalize extinction, and reduce anxiety to improve therapy.
Acknowledgments:
We would like to thank Phillip Gonzalez and Michelle Schmutz for their work manufacturing cuff electrodes. We thank Ashleigh Chuah, Eva Recoussine, Brandon Currie, Kinnari Karia, Amber Mawji, and Kathleen Callahan for their assistance conducting behavioral studies. We also thank Jonathan Ploski and Stephen Maren for their feedback on experimental design.
This work was sponsored by the Defense Advanced Research Projects Agency (DARPA) Biological Technologies Office (BTO) Electrical Prescriptions (ElectRx) program under the auspices of Dr. Doug Weber and Dr. Eric Van Gieson and through the Space and Naval Warfare Systems Center, Pacific. Grant/Contract No. DARPA-BAA-14–38 and DARPA-BAA-15–06 and by the NIMH, MH099655.
Footnotes
Conflict of interest:
This work has not been published and has not been submitted for publication elsewhere while under consideration. Authors: Noble, Meruva, and Hays declare no potential conflicts of interest. Dr. Kilgard is a paid consultant for and shareholder of MicroTransponder. Drs. Kilgard and McIntyre are authors of a patent entitled “Enhancing Fear Extinction using Vagus Nerve Stimulation”. Dr. Rennaker is an owner of Vulintis Inc. and Optokinetics and is a paid consultant for Konan Medical USA; none of these financial interests are related to this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- (1).Rauch S, Foa E (2006) Emotional processing theory (EPT) and exposure therapy for PTSD. Journal of Contemporary Psychotherapy 36: 1–7. [Google Scholar]
- 2).Rosen CS, Chow HC, Finney JF, Greenbaum MA, Moos RH, Sheikh JI, Yesavage JA (2004) VA practice patterns and practice guidelines for treating posttraumatic stress disorder. Journal of traumatic stress 17:213–222. [DOI] [PubMed] [Google Scholar]
- 3).Rothbaum BO, Hodges L, Watson BA, Kessler GD, Opdyke D (1999) Virtual reality exposure therapy for PTSD Vietnam veterans: A case study. Behaviour Research and Therapy 34: 477–481. [DOI] [PubMed] [Google Scholar]
- 4).Kushner MG, et al. , (2007) D-cycloserine augmented exposure therapy for obsessive-compulsive disorder. Biological Psychiatry 62: 835–838 [DOI] [PubMed] [Google Scholar]
- 5).Foa EB, et al. , (2005) Randomized, placebo-controlled trial of exposure and ritual prevention, clomipramine, and their combination in the treatment of obsessive-compulsive disorder. American Journal of Psychiatry 162: 151–161. [DOI] [PubMed] [Google Scholar]
- 6).Riggs DS, Foa EB (1993) Obsessive-compulsive disorder In: Barlow DH, editor. Clinical Handbook of Psychological Disorders, New York: Guilford Press, 189–239. [Google Scholar]
- 7).Rothbaum BO, Hodges L, Watson BA, Kessler GD, Opdyke D (1996) Virtual reality exposure therapy in the treatment of fear of flying: A case report. Behaviour Research Therapy 34: 477–481. [DOI] [PubMed] [Google Scholar]
- 8).Wiederhold BK, Jang DP, Gevritz RG, Kim SI, Kim IY, Wiederhold MD (2002) The treatment of fear of flying: a controlled study of imaginal and virtual reality graded exposure therapy. IEEE Transactions on Information 6: 1–10. [DOI] [PubMed] [Google Scholar]
- 9).Bouton ME (2004) Context and behavioral processes in extinction. Learning & memory 11:485– 494. [DOI] [PubMed] [Google Scholar]
- 10).Sotres-Bayon F, Cain CK, LeDoux JE (2006) Brain mechanisms of fear extinction: historical perspectives on the contribution of prefrontal cortex. Biological Psychiatry 60: 329–336. [DOI] [PubMed] [Google Scholar]
- 11).Quirk GJ, Mueller D (2008) Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology 33: 56–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Schottenbauer MA, Glass CR, Arnkoff DB, Tendick V, Gray SH (2008) Nonresponse and dropout rates in outcome studies on PTSD: review and methodological considerations. Psychiatry 71: 134–68. [DOI] [PubMed] [Google Scholar]
- 13).Powers MB, Halpern JM, Ferenschak MP, Gillihan SJ, Foa EB (2010) A meta-analytic review of prolonged exposure for posttraumatic stress disorder. Clinical Psychology Review 30: 635–641. [DOI] [PubMed] [Google Scholar]
- 14).Davis M, Myers KM, Chhatwal J, Ressler KJ (2006) Pharmacological treatments that facilitate extinction of fear: relevance to psychotherapy. NeuroRx 3: 82–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Milad MR, Orr SP, Lasko NB, Chang Y, Rauch SL, Pitman RK (2008) Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. J Psychiatr Res 42: 515– 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Milad MR et al. , (2009) Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biological Psychiatry 66: 1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Rothbaum BO, Davis M (2003) Applying learning principles to the treatment of post-trauma reactions. Ann N Y Acad Sci 1008: 112–21. [DOI] [PubMed] [Google Scholar]
- 18).Milad MR, et al. , (2013) Deficits in conditioned fear extinction in obsessive-compulsive disorder and neurobiological changes in the fear circuit. JAMA Psychiatry 70: 608–618. [DOI] [PubMed] [Google Scholar]
- 19).Powers MB, Smits J, Otto MW, Sanders C, Emmelkamp PM (2009) Facilitation of fear extinction in phobic participants with a novel cognitive enhancer: a randomized placebo controlled trial of yohimbine augmentation. Journal of Anxiety Disorders 23:350–356. [DOI] [PubMed] [Google Scholar]
- 20).McNally RJ (1996) Perceptual implicit memory for trauma-related information in posttraumatic stress disorder. Cognition and Emotion 10: 551–556. [Google Scholar]
- 21).McNally RJ (1997) Implicit and explicit memory for trauma-related information in PTSD. Annals of the Academy of Sciences 821: 219–224. [DOI] [PubMed] [Google Scholar]
- 22).Byrne SP, Rapee RM, Richardson R, Malhi GS, Jones M, Hudson JL (2015) D-cycloserine enhances generalization of fear extinction in children. Depression and Anxiety 32: 408–414. [DOI] [PubMed] [Google Scholar]
- 23).Drexler MS, Hamacher-Dang TC, Wolf OT (2017) Stress before extinction learning enhances and generalizes extinction memory in a predictive learning task. Neurobiology of Learning and Memory 141: 143–149. [DOI] [PubMed] [Google Scholar]
- 24).Mataiz-Cols et al. , (2017) D-cycloserine augmentation of exposure-based cognitive behavior therapy for anxiety, obsessive-compulsive, and posttraumatic stress disorders. A systematic review and meta-analysis of individual patient data. JAMA Psychiatry 74: 501–510. [DOI] [PubMed] [Google Scholar]
- 25).Litz BT, Salters-Pedneault K, Steenkamp M, Hermos JA, Bryant RA, Otto MW (2012) A randomized placebo-controlled trial of d-cycloserine and exposure therapy for post-traumatic stress disorder. Journal Psychiatry Research 46: 1184–90. [DOI] [PubMed] [Google Scholar]
- 26).Clark KB, Krahl S, Smith DC, Jensen RA (1995) Post-training unilateral vagal stimulation enhances retention performance in the rat. Neurobiology of Learning and Memory 63:213–216. [DOI] [PubMed] [Google Scholar]
- 27).Clark KB, Narikotu DK, Smith DC, Browning RA, Jensen RA (1999) Enhanced recognition memory following vagus nerve stimulation in human subjects. Nature Neuroscience 2:94–98. [DOI] [PubMed] [Google Scholar]
- 28).Alvarez-Dieppa AC, Griffin K, Cavalier S, McIntyre CK (2016) Vagus nerve stimulation enhances extinction of conditioned fear in rats and modulates Arc protein, CaMKII, and GluN2Bcontaining NMDA receptors in the basolateral amygdala. Neural Plasticity 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Peña DF, Engineer ND, McIntyre CK (2013) Rapid remission of conditioned fear expression with extinction training paired with vagus nerve stimulation. Biol Psychiatry 73: 1071–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Peña DF, Childs JE, Willett S, Vital A, McIntyre CK, Kroener S (2014) Vagus nerve stimulation enhances extinction of conditioned fear and modulates plasticity in the pathway from the ventromedial prefrontal cortex to the amygdala. Front Behav Neurosci 8: 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31).Noble LJ, Gonzalez IJ, Meruva VB, Callahan KA, Belfort BD, Ramananthan KR, Meyers E, Kilgard MP, Rennaker RL, McIntyre CK (2017) Effects of vagus nerve stimulation on extinction of conditioned fear and post-traumatic stress disorder symptoms in rats. Translational Psychiatry 7: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32).Shah AP, Carreno FR, Wu H, Chung YA, Frazer A (2016) Role of TrkB in the anxiolytic-like and antidepressant-like effects of vagal nerve stimulation: Comparison with desipramine. Neuroscience 322: 273–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Labiner DM & Ahern GL (2007) Vagus nerve stimulation therapy in depression and epilepsy: therapeutic parameter settings. Acta Neurol Scand 115:23–33 [DOI] [PubMed] [Google Scholar]
- 34).George MS, Ward HE, Ninan PT, Pollack M, Nahas Z, Anderson B, Kose S, Howland RH, Goodman WK, Ballenger JC (2008) A pilot study of vagus nerve stimulation (VNS) for treatmentresistant anxiety disorders. Brain Stimulation 1:112–21. [DOI] [PubMed] [Google Scholar]
- 35).Childs JE, Alvarez-Dieppa AC, McIntyre CK, Kroener S (2015) Vagus nerve stimulation as a tool to induce plasticity in pathways relevant for extinction learning. Journal of Visual Exploration: 53032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).George MS, Sackeim HA, Rush AJ, Marangell LB, Nahas Z, Husain MM, et al. , (2000) Vagus nerve stimulation: a new tool for brain research and therapy. Biological Psychiatry 47: 287–95. [DOI] [PubMed] [Google Scholar]
- 37).Engineer CT, Perez CA, Chen YH, Carraway RS, Reed AC, Shetake JA, Jakkamsetti V, Chang KQ, Kilgard MP (2008) Cortical activity patterns predict speech discrimination ability. Nat Neurosci 11(5):603–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38).Engineer CT, Perez CA, Carraway RS, Chang KQ, Roland JL, Sloan AM, Kilgard MP. Similarity of cortical activity patterns predicts generalization behavior. 2013, PLoS One. 8(10):e78607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Pellow S, Chopin P, File SE, Briley M (1985) Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neuroscience Methods 14: 149–87. [DOI] [PubMed] [Google Scholar]
- 40).Rosen JB, Schulkin J (1998) From normal fear to pathological anxiety. Psychological Review 105: 325–350. [DOI] [PubMed] [Google Scholar]
- 41).Liu X, Ramirez S, Pang PT, Puryear CB, Govindarajam A, Deisseroth K, Tonegowa S (2012) Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature 484: 381–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42).Cai D et al. (2016) A shared neural ensemble links distinct contextual memories encoded close in time. Nature 534:115–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43).Guzowski JF, McNaughton BL, Barnes CA, Worley PF (1999) Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nature 2:1120–1124. [DOI] [PubMed] [Google Scholar]
- 44).Bieszczad KM, Bechay K, Rusche JR, Jacques V, Kudugunti S, Miao W, Weinberger NM, McGaugh JL, Wood MA (2015) Histone deacetylase inhibition via RGFP966 releases the brakes on sensory cortical plasticity and the specificity of memory formation. Journal of Neuroscience 35: 13124–13132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45).Engineer ND, Riley JR, Seale JD, Vrana WA, Shetake HA, Sudanagunta SP, Borland MS, Kilgard MP (2011) Reversing pathological neural activity using targeted plasticity. Nature; 470: 101–06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46).Hassert DL, Miyashita T, Williams CL (2004) The effects of peripheral vagal nerve stimulation at a memory-modulating intensity on norepinephrine output in the basolateral amygdala. Behavioral Neuroscience 118: 79–88. [DOI] [PubMed] [Google Scholar]
- 47).Hays SA, Khodaparast N, Sloan AM, Fayyaz T (2013) The bradykinesia assessment task: an automated method to measure forelimb speed in rodents. Journal of neuroscience 214: 52–61. [DOI] [PubMed] [Google Scholar]
- 48).Hulsey DR, Riley JR, Loerwald KW, Rennaker RL, Kilgard MP, Hays SA (2016) Parametric characterization of neural activity in the locus coeruleus in response to vagus nerve stimulation. Exp Neurol 289: 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49).Khodaparast N, et al. , (2013). Vagus nerve stimulation during rehabilitative training improves forelimb strength following ischemic stroke. Neurobiol Dis 60: 80–8. [DOI] [PubMed] [Google Scholar]
- 50).Kilgard MP (2012) Harnessing plasticity to understand learning and treat disease. Trends Neuroscience 35: 715–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51).Manta S, Dong J, Debonnel G, Blier P (2009) Enhancement of the function of rat serotonin and norepinephrine neurons by sustained vagus nerve stimulation. J Psychiatry Neurosci 34: 272–80. [PMC free article] [PubMed] [Google Scholar]
- 52).Porter BA, Khodaparast N, Fayyaz T, Cheung RJ (2012) Repeatedly pairing vagus nerve stimulation with a movement reorganizes primary motor cortex. Cerebral Cortex 22: 2365–74. [DOI] [PubMed] [Google Scholar]
- 53).Roosevelt RW, Smith DC, Clough RW, Jensen RA, Browning RA (2006) Increased extracellular concentrations of norepinephrine in cortex and hippocampus following vagus nerve stimulation in the rat. Brain Res 1119: 124–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54).Shetake JA, Engineer ND, Vrana WA, Wolf JT (2012) Pairing tone trains with vagus nerve stimulation induces temporal plasticity in auditory cortex. Experimental Neurology 233: 342–9. [DOI] [PubMed] [Google Scholar]
- 55).Berlau DJ, McGaugh JL (2006) Enhancement of extinction memory consolidation: the role of noradrenergic and GABAergic systems within the basolateral amygdala. Neurobiology of Learning and Memory 86: 123–132. [DOI] [PubMed] [Google Scholar]
- 56).Follesa P, et al. , (2007) Vagus nerve stimulation increases norepinephrine concentration and the gene expression of BDNF and bFGF in the rat brain. Brain Research 1179: 28–34. [DOI] [PubMed] [Google Scholar]
- 57).Bramham CR, Messaoudi E (2005) BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Progress in Neurobiology 76:99–125. [DOI] [PubMed] [Google Scholar]
- 58).Chhatwal JP, Stanek-Rattiner L, Davis M, Ressler KJ (2006) Amygdala BDNF signaling is required for consolidation but not encoding of extinction. Nature Neuroscience 9: 870–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59).Ying S, Futter M, Rosenblum K, Webber MJ, Hunt SP, Bliss TVP, Bramham CR (2002) Brainderived neurotrophic factor induces long-term potentiation in intact adult hippocampus: Requirement for ERK activation coupled to CREB and upregulation of Arc synthesis. Journal of Neuroscience 22: 1532–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60).Furmaga H, Carreno FR, Frazer A (2012) Vagal nerve stimulation rapidly activates brain-derived neurotrophic factor receptor TrkB in rat brain. PloS ONE 7: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61).Peters J, Dieppa-Perea LM, Melendez LM, Quirk GJ (2010) Induction of fear extinction with hippocampal-infralimbic BDNF. Science 328: 1288–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62).Rosas-Vidal LE, Do-Monte FH, Sotres-Bayon F, Quirk GJ (2014) Hippocampal-prefrontal BDNF and memory for fear extinction. Neuropsychopharmacology 39:2161–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63).Englot DJ, Chang EF, Auguste KI (2011) Vagus nerve stimulation for epilepsy: a meta-analysis of efficacy and predictors of response. J Neurosurg; 115: 1248–55. [DOI] [PubMed] [Google Scholar]