Abstract
To study the factors associated with poorer health-related quality of life (HRQOL) at 1-year post-allogeneic hematopoietic cell transplantation (alloHCT), a secondary analysis of a prospective feasibility study was performed. PedsQLTM questionnaires were collected in 76 children undergoing alloHCT at baseline (within 30 days before transplantation), day 100, 6-months and 12-months post-transplantation. The global score improved post-HCT (baseline: 67.1, 12-months: 76.6). Females (OR:6.5, 95% CI:1.002–42.17, p=0.04) and patients with low baseline scores (OR: 7.2, 95% CI:1.07–48.63, p=0.04) had lower scores at 12-months post-HCT and suggest a target group for early interventions such as physical exercise, stress management, and cognitive behavior therapy.
Keywords: Allogeneic Hematopoietic Cell Transplantation, Patient reported outcomes, Health-related quality of life
Background
Allogeneic hematopoietic cell transplantation (alloHCT) is increasingly used as a curative option for many malignant and non-malignant hematological conditions. According to a recent publication from the Center for Blood and Marrow Transplant Research (CIBMTR), the number of allogeneic transplantations in the US nearly doubled in the last 15 years (2000: n=4,250; 2015: n=8,351)[1]. Survival post-HCT continues to improve because of better donor selection and availability, and improvement in supportive care during the pre- and post-transplant periods [2]. It is estimated that there will be about half a million transplantation survivors in the US by 2030[3]. As survival rates increase, it is paramount to focus on patients’ health-related quality of life (HRQOL) post-HCT. HRQOL is best measured as directly reported by patients, patient-reported outcome (PRO), the importance of which in patients with acute and chronic medical conditions is being increasingly recognized [4,5]. Despite this growing evidence, PROs are not routinely collected in the clinical practice of HCT.
Very few studies involving pediatric alloHCT recipients have taken a prospective longitudinal approach to study changes in HRQOL in the peri-transplantation period [6,7]. Previous studies have shown that QOL is lower early post-HCT and returns to baseline or better at 1-year post-HCT, but have not investigated specific at-risk groups [8,9]. Therefore, it is important to identify at-risk groups who continue to have poor HRQOL at 12 months post-HCT in order to focus on early interventions. In this study, we aimed to identify the patient-, disease- and transplantation-related factors associated with poor QOL at 12 months post-HCT in a pediatric population undergoing alloHCT.
Materials and Methods
The Center for International Blood and Marrow Transplant Research (CIBMTR) maintains a large international research database with clinical outcomes data of more than 465,000 patients who have undergone HCT. The CIBMTR conducts observational and prospective research to further the field of HCT. From 2011 to 2013, a prospective multi-center study was conducted by the CIBMTR to assess the feasibility of routine PRO collection in patients undergoing alloHCT [10]. This manuscript considers secondary analysis in the pediatric patient subset (2–18 years of age) of that study. PRO data was collected at 4 time-points: at baseline (within 30 days prior to HCT), at 100 days, 6 months, and 12 months post-HCT. Routine clinical data was collected at each of these time-points through the CIBMTR’s proprietary data collection forms. Data regarding patients’ socio-demographics (age, sex, race/ ethnicity, annual household income) were also collected. PRO data was collected using the Pediatric Quality of Life InventoryTM (PedsQLTM) measurement model [11]. The 23-item PedsQLTM generic core scale and 4 multidimensional subscales were used to generate a global QOL score and domain specific scores, respectively. Subscales included 4 domains; physical functioning (8 items), emotional functioning (5 items), social functioning (5 items), and school functioning (5 items). Global and domain specific-scores were computed on a scale of 0–100, where higher scores indicated better functioning. A well-defined cut-off for ill health has been derived from the normal population for self-report (69.7) and proxy-report (65.4) [12]. Children between the ages of 5–18 years completed a self-report, and parents of children aged 2–18 years submitted a proxy-report at each time-point.
Patient characteristics were summarized using descriptive statistics. Continuous variables were described using means and standard deviations (SD), and categorical variables with frequencies and proportions. We studied the temporal trends of global PedsQLTM and subscale scores. Scores were further explored using baseline characteristics, and continuous measure comparison was performed between groups using Wilcoxon rank sum test. PRO scores were also compared for cross-informant variance, for patients having both self- and parent-proxy scores available at each time-point. Student’s t-test was utilized to assess statistically significant difference between self- and parent-proxy PRO scores. A score-point difference (Mu0) of 4.3 was allowed due to difference in ill-health cut-off scores (self: 69.7, proxy: 65.4). Univariate logistic regression was performed for each variable to test their effect on the primary outcome of having a self-reported PRO score less than ill-health cut-off (<69.7) at 12 months post-HCT. Odds ratio (OR) and 95% confidence interval (CI) were calculated. Potential predictors included sex (male vs female), ethnicity (Caucasian vs others vs unknown), Karnofsky/ Lansky performance score (≥90 vs <90), disease type (malignant vs non-malignant), use of total body irradiation (yes vs no), HCT- comorbidity index (0 vs ≥1), conditioning intensity (myeloablative vs reduced intensity/ non-myeloablative), graft source (bone marrow vs others), annual household income (<$60,000 vs ≥60,000), and year of transplantation (2011 vs 2012 vs 2013). Age was tested as a categorical variable (grouped as: 2–4 vs 5–7 vs 8–12 vs 13–18 years) for feasibility. A statistical significance (alpha) level of 0.05 was used throughout, and SAS version 9.4 (SAS institute Inc, Cary, NC, USA) was used to perform all statistical analyses.
Results
Patient characteristics
A total of 76 patients completed PedsQLTM report (self or parent-proxy or both) at baseline. The baseline characteristics of these patients are described in table 1. Median age at transplantation was 7 years (range: 2–18 years). Forty-two (55%) patients were males. Patients were predominantly of white/ Caucasian race (82%). Forty-three (57%) patients received HCT for non-malignant diseases. Total body irradiation (TBI) was used for 26 (34%) patients. Post-HCT complications such as acute and chronic GVHD were seen in 39% and 21% of the patients, respectively. Disease relapse or progression was seen in 21% of patients with malignant disease at baseline. Among patients surviving to each time-point, PedsQLTM self -reported measures were completed by 45 (62%) at day 100, 46 (67%) at 6 months and 36 (55%) at 12 months post-HCT. There was no major difference in clinical characteristics of patients who did and did not complete the PRO measures at 12-months post-HCT (supplement table 1, Supplemental Digital Content 1, http://links.lww.com/JPHO/A258).
Table 1.
Baseline Characteristics of the Pediatric Cohort Undergoing Allogeneic Hematopoietic Cell Transplantation (n=76)
| Variables | N (%) | |
|---|---|---|
| Median age at transplantation, year (range) | 7 (2–18) | |
| Age groups (years) | 2–4 | 24 (32) |
| 5–7 | 20 (27) | |
| 8–12 | 18 (24) | |
| 13–18 | 14 (18) | |
| Sex | Male | 42 (55) |
| Female | 34 (45) | |
| Race | White/ Caucasian | 62 (82) |
| Other | 13 (17) | |
| Unknown/ declined | 1 (1) | |
| Karnofsky/ Lansky score | ≥ 90 | 67 (88) |
| < 90 | 9 (12) | |
| HCT-comorbidity index | 0 | 52 (68) |
| ≥ 1 | 22 (29) | |
| Missing | 2 (3) | |
| Annual household income | < $20,000 | 15 (20) |
| $20,000– $39,999 | 11 (14) | |
| $40,000– $59,999 | 6 (8) | |
| $60,000– $79,999 | 9 (12) | |
| $80,000– $99,999 | 7 (9) | |
| ≥ $100,000 | 13 (17) | |
| Unknown/ Declined | 15 (20) | |
| Disease type | Malignant | 33 (43) |
| Non-malignant | 43 (57) | |
| Stem cell source | Bone marrow | 45 (60) |
| Peripheral blood | 20 (26) | |
| Cord blood | 11 (14) | |
| TBI use | No | 50 (66) |
| < 1200 cGy | 20 (26) | |
| ≥ 1200 cGy | 6 (8) | |
| Conditioning regimen | Myeloablative | 55 (72) |
| Reduced intensity/ Non-myeloablative | 21 (28) | |
| Graft vs. host disease prophylaxis | CNI + MMF ± other(s) | 12 (16) |
| CNI + MTX ± other(s) | 23 (30) | |
| CNI ± other(s) (not MMF or MTX) | 26 (34) | |
| Ex-vivo T-cell depletion/ CD34 selection | 14 (18) | |
| Unknown | 1 (1) | |
| Year of transplant | 2011 | 9 (12) |
| 2012 | 50 (66) | |
| 2013 | 17 (22) | |
| Median follow-up of survivors, months (range) | 24 (3–46) | |
| Post-HCT complications | Acute graft vs. host disease | 30 (39) |
| Chronic graft vs. host disease | 15 (20) | |
| Relapse/ progression (malignant diseases only) | 7 (21) |
HCT- hematopoietic cell transplant, TBI- total body irradiation, CNI- calcineurin inhibitor, MMF- mycophenolate mofetil, MTX- methotrexate
Temporal trends of PedsQLTM scores
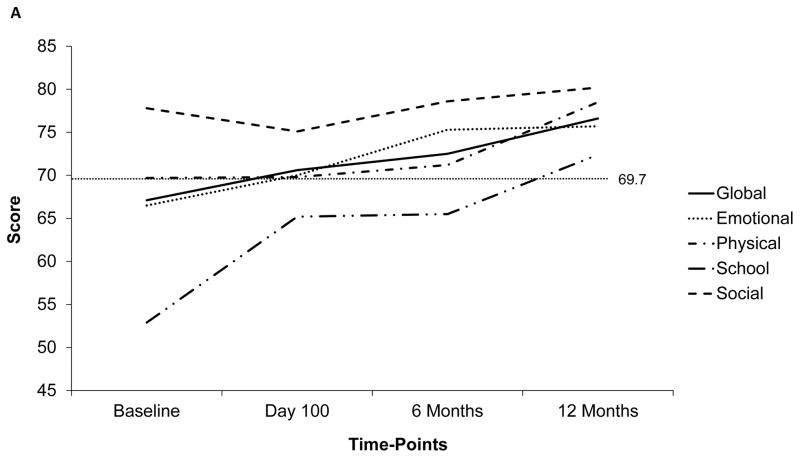
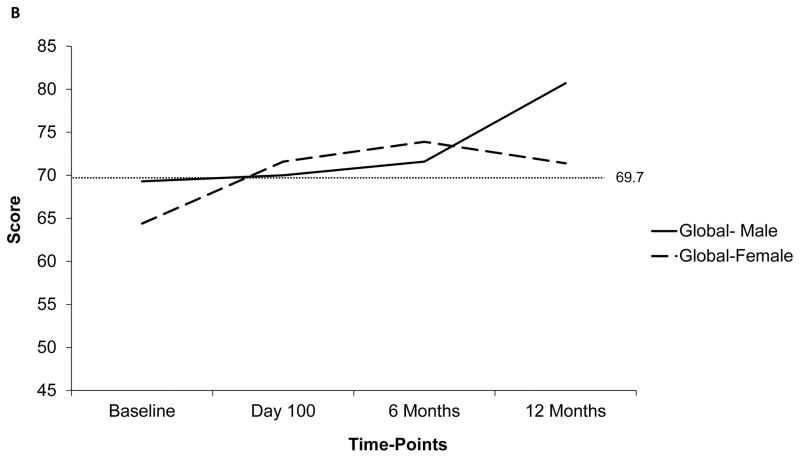
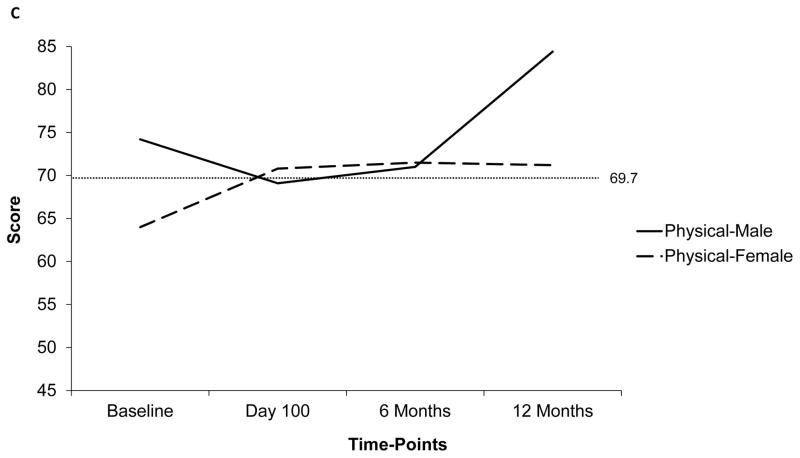
The temporal trends of PedsQLTM scores (global and subscale) at the 4 time-points are depicted in figure 1a. The mean global score was below the ill-health cut-off at baseline (67.1±17.8), but improved post-HCT (76.6±20.3 at 12 months). The subscale score trajectories were dissimilar to those for the global score. Particularly, the scores for school and studies was the lowest at baseline (52.9±28.1), and continued to be low including at the 6-month time-point (day 100: 65.2±25.1, 6 months: 65.5±19.9, 12 months: 72.4±20). In comparison, the scores for physical (baseline: 69.7±23.6, day 100: 69.8±23.1, 6 months: 71.2±22.9, 12 months: 78.5±22.7) and social health (baseline: 77.8±19.8, day 100: 75.1±15.7, 6 months: 78.6±17.3, 12 months: 80.2±19) stayed above the ill-health cut-off at all time-points. Scores were further explored in relation to patient, disease-, and transplant-related factors. The most significant difference in temporal trends was noted when comparing scores between males and females. Figure 1b and 1c depict the global and physical health scores according to patient sex, respectively. Notably, the scores were down-trending for females at 12-months.
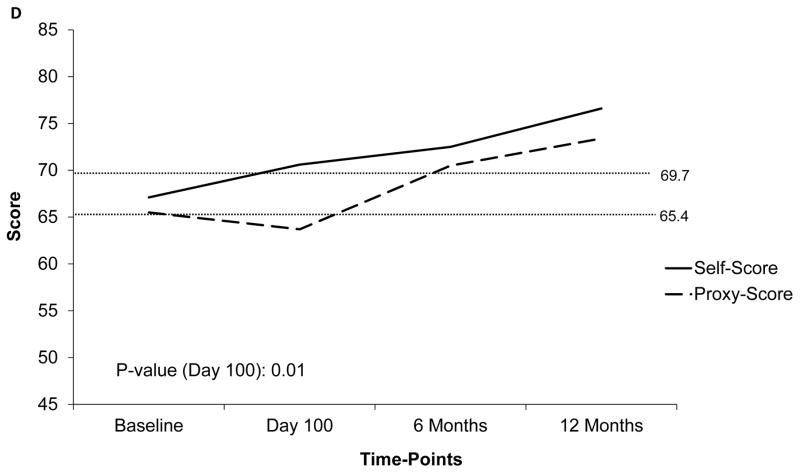
Figure 1.
Temporal trends of PedsQLTM scores at 4 time-points based on available data. Dotted line represents the ill-health cut-off for self-reported (69.7) and parent-proxy (65.4) PedsQLTM scores. Y-Axis has been truncated (range: 45– 85) to emphasize on the score range
a) Trend of mean global and subscale self-reported PedsQLTM scores at 4 time-points; b) Trend of mean global self-reported PedsQLTM scores at 4 time-points according to patient sex; c) Trend of mean physical function subscale self-reported PedsQLTM scores at 4 time-points; d) Comparison of self-reported and parent-proxy mean global PedsQLTM scores at each time-point
Predictors of PedsQLTM score <69.7 at 12 months post-HCT
Results of univariate analysis are summarized in table 2. Unadjusted odds of having global self-reported PedsQLTM score <69.7 at 12 months post-HCT were significantly higher in females compared to male patients (OR 6.5, 95% CI: 1.002, 42.17, p=0.04), Low baseline self-score (<69.7) was also significantly associated with a low 12-months score (OR: 7.2, 95% CI: 1.07, 48.63, p=0.04). When studying the subscale scores, only poor physical function score (<69.7) at 12-month post-HCT was predicted by low baseline score (OR 10.0, 95% CI: 1.56, 64.2, p=0.01) and female sex (OR 9.1, 95% CI: 1.39, 59.6, p=0.02) in univariate analysis. None of the baseline predictors were significantly associated with poor emotional, school, or social health at 12-months post-HCT. We compared the baseline scores between males and females using a continuous measure (data not shown) and did not find any significant difference (p=0.24).
Table 2.
Univariate analyses of factors associated with having low global self-reported PedsQLTM score (<69.7) at 12-months post-HCT in pediatric patients undergoing allogeneic HCT from 2011–2013
| Characteristics | Crude odds ratio (95% confidence interval) for having global self-score <69.7 | P value |
|---|---|---|
| Age (years) | 0.65 | |
| 5–7 | 1 | |
| 8–12 | 2.0 (0.27, 14.78) | |
| 13–18 | 0.76 (0.1, 5.96) | |
| Race/ Ethnicity | 0.88 | |
| White/ Caucasian | 1 | |
| Others | 1.21 (0.09, 15.66) | |
| Sex | 0.04 | |
| Male | 1 | |
| Female | 6.5 (1.002, 42.17) | |
| HCT comorbidity index | 0.82 | |
| 0 | 1 | |
| ≥ 1 | 0.82 (0.15, 4.5) | |
| Disease type | 0.33 | |
| Non-malignant | 1 | |
| Malignant | 2.29 (0.42, 12.5) | |
| TBI use | 0.52 | |
| No | 1 | |
| Yes | 1.71 (0.32, 9.11) | |
| Conditioning regimen | 0.57 | |
| Myeloablative | 1 | |
| RIC/ NMA | 1.78 (0.24, 13.41) | |
| Graft source | 0.82 | |
| Bone marrow | 1 | |
| Others (Peripheral blood, Cord blood) | 0.82 (0.15, 4.5) | |
| Annual household income | 0.79 | |
| ≥ $60,000 | 1 | |
| < $60,000 | 2.0 (0.24, 16.61) | |
| Unknown | 1.5 (0.19, 11.53) | |
| Year of transplant | 0.98 | |
| 2012 | 1 | |
| 2011 | 1.2 (0.09, 16.4) | |
| 2013 | 0.96 (0.14, 6.7) | |
| Baseline global score | 0.04 | |
| ≥69.7 | 1 | |
| <69.7 | 7.2 (1.07, 48.63) |
HCT- hematopoietic cell transplant, TBI- total body irradiation, RIC- reduced intensity conditioning, NMA- non-myeloablative conditioning
Temporal trends of parent-proxy scores and Cross-informant variance
Additionally, we studied the cross-informant variance comparing self and parent-proxy score at each time-point for patients who had both scores available at each timepoint (Figure 1d). Parent-proxy scores were noted to be lower than self-reported scores at each time-point; 65.5±20.2 at baseline, 63.7±21.1 at day 100, 70.5±19.7 at 6 months, and 73.4±19.6 at 12 months post-HCT. Significant difference in global score was noted between self and parent-proxy score only at day 100 (p=0.01). To compare the self and proxy scores further, we attempted to replicate the results of univariate analysis using 12-month low global parent-proxy score (<65.4) as the outcome (data not shown). Low baseline global proxy score was significantly associated with poor global parent-proxy score at 12-months post-HCT (p=0.016). However, patient sex (p=0.08) did not significantly predict the low parent-proxy score at 12-months post-HCT.
Discussion
Herein, we have reported the longitudinal changes in global and individual domains of HRQOL in pediatric patients undergoing allogeneic HCT. Overall, the self-reported global PedsQLTM scores were worse at baseline and continued to improve post-HCT, which was reassuring. Our results were comparable to Felder-Puig and colleagues, who used PedsQLTM measures at 2 timepoints pre-HCT (4–6 weeks before, 7 days before) and 5 timepoints post-HCT (day 10, 28, 100, 180, 360) [6]. The scores declined immediately post-HCT (day 10) but steadily improved and were better at 12-months compared to pre-HCT scores, similar to our study. While studying the subscale score trajectory in our study, we noted that patients reported having good physical and social function throughout the course. Patients are usually out of school during the transplantation process, which would account for the poor school and studies scores up to 6 months post-HCT. Similar subscale score trajectory, especially for social function was reported by Bhatia et al, who used PedsQLTM measures in children undergoing HCT for sickle cell diseases [13]. However, none of these studies explored the scores in relation to patient-, disease-, or transplant-related factors or assessed the factors associated with poor HRQOL at 12-months post-HCT.
In this study, female patients were noted to have poor HRQOL at 12 months post-HCT compared to males according to their self-reported PRO measures. We also found significant association of low global and physical function scores at baseline with having low 12-months PRO scores. However, when exploring the patients with low scores at baseline, there was no significant difference between males and females. Two prior studies that have explored associations between patient sex and HRQOL in pediatric patients undergoing HCT have failed to demonstrate a significant relationship [14,15]. In contrast, Meade et al. reported females having significantly lower physical, psychological, and autonomy domain scores compared to males using KIDSCREEN-27 questionnaire in Australian adolescents (n=403) suggesting gender vulnerability [16]. Similarly, girls from 13 European countries also had lower scores in same domains when compared with boys (total n= 22,827) [17]. Lower HRQOL have also been reported in females with chronic health conditions such as sickle cell disease and cystic fibrosis irrespective of PRO tools [18,19]. A Study using PedsQLTM questionnaires in Norwegian children noted lower emotional subscale scores in females compared to males [20]. In our study, we did not see any significant difference between males and females in terms of their baseline self-reported scores, underlying diagnosis, or cumulative incidence of post-HCT complications such as acute or chronic GVHD, or disease relapse/ progression. Therefore, we speculate that the worse HRQOL reported by females at 12-months is not necessarily due to severity of their health, but rather associated with how they perceive and cope with their chronic health condition. Due to small sample size we were unable to further explore the causes of worse HRQOL in females at 12-months compared to males in our study. We also noted that patients with low global and physical function scores were at significantly higher risk for having lower HRQOL at 12-months post-HCT. Baseline scores lower than ill-health cut-off have been previously reported and are likely secondary to physical deconditioning from prior treatment and anxiety of undergoing an intense procedure [13]. However, post-HCT improvement in HRQOL is expected and has been reported [6,7]. While it is concerning that a small patient subset still reported having poor HRQOL at 12-months timepoint, this finding requires further evaluation. While studying other baseline characteristics, unlike the results of previous publications, our study did not document significant association of younger patient age (<3 years), and lower socio-economic status with poor HRQOL post-HCT [14,21].
We also studied the cross-informant variance in PRO scores between patients and parents. Self-reports are considered standard in adult PRO measures, however parent-proxy reports are utilized in pediatric population especially when children are either sick, too young, or have cognitive impairment to complete the questionnaires [11]. The ability to use proxy score as a surrogate for self-report has been a topic of debate, however it is recommended to use both scores in the pediatric population [11]. In general, parents as proxies are known to score lower than self-report mainly because of difference in perception of the illness and expectations regarding short-term and long-term complications and overall prognosis. In our study, parents scored their children lower than the patient themselves at each time-point; however, the only significant difference in scoring was noted at day 100. Moreover, the higher odds in females of having low 12-month PRO score were not replicated when parent-proxy PRO measure was taken into account. While these findings are interesting, we were not able to explore the causes for cross-informant variance due to small number of patient-parent pairs.
There are limitations to the study that need to be recognized. This is a secondary data analysis of a pilot study, which was performed to test feasibility of routine PRO collection; therefore, the sample size is small, and caution should be exercised in drawing strong conclusions. Due to the small sample size and lack of adequate events, we were not able to perform a multivariable analysis using pre- and post-HCT time dependent variables to study the predictors of poor QOL at 12 months post-HCT.
Our study results are important in clinical practice as HRQOL is getting recognized as an important treatment outcome post-HCT along with overall survival and disease-free survival. PRO scores returned to baseline or improved from baseline at 12-months post-HCT in majority of patients. However, certain subgroups especially females and patients with lower scores had significantly lower HRQOL at 12 months and suggest a target group for early interventions. These patients may benefit from delivering interventions such as 1) physical exercise, 2) stress management/ cognitive behavior therapy, 3) virtually joining school classrooms, and 4) early psychosocial assessment/ maintaining social connections directed towards improving specific HRQOL components of physical, emotional, school, and social function, respectively [4]. We anticipate future studies to identify ways to effectively deliver these interventions using time and resources in judicial manner. Further research in the pediatric population is warranted using a larger cohort to further understand the impact of patient sex on post-HCT HRQOL. Our study also advocates for routine longitudinal PRO collection in HCT practice in order to identify an at-risk population early in their transplantation course, to whom interventions should be directed to avoid long-term decline in HRQOL.
Supplementary Material
Acknowledgments
Source of Funding:
The CIBMTR is supported primarily by Public Health Service Grant/Cooperative Agreement 5U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 4U10HL069294 from NHLBI and NCI.
CIBMTR Support List:
The CIBMTR is supported primarily by Public Health Service Grant/Cooperative Agreement 5U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 4U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-17-1-2388 and N0014-17-1-2850 from the Office of Naval Research; and grants from *Actinium Pharmaceuticals, Inc.; *Amgen, Inc.; *Amneal Biosciences; *Angiocrine Bioscience, Inc.; Anonymous donation to the Medical College of Wisconsin; Astellas Pharma US; Atara Biotherapeutics, Inc.; Be the Match Foundation; *bluebird bio, Inc.; *Bristol Myers Squibb Oncology; *Celgene Corporation; Cerus Corporation; *Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Gamida Cell Ltd.; Gilead Sciences, Inc.; HistoGenetics, Inc.; Immucor; *Incyte Corporation; Janssen Scientific Affairs, LLC; *Jazz Pharmaceuticals, Inc.; Juno Therapeutics; Karyopharm Therapeutics, Inc.; Kite Pharma, Inc.; Medac, GmbH; MedImmune; The Medical College of Wisconsin; *Mediware; *Merck & Co, Inc.; *Mesoblast; MesoScale Diagnostics, Inc.; Millennium, the Takeda Oncology Co.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; *Neovii Biotech NA, Inc.; Novartis Pharmaceuticals Corporation; Otsuka Pharmaceutical Co, Ltd. – Japan; PCORI; *Pfizer, Inc; *Pharmacyclics, LLC; PIRCHE AG; *Sanofi Genzyme; *Seattle Genetics; Shire; Spectrum Pharmaceuticals, Inc.; St. Baldrick’s Foundation; *Sunesis Pharmaceuticals, Inc.; Swedish Orphan Biovitrum, Inc.; Takeda Oncology; Telomere Diagnostics, Inc.; and University of Minnesota. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
Footnotes
Corporate Members
Conflicts of Interest: Authors have no conflict of interest to disclose
References
- 1.D’Souza A, Lee S, Zhu X, et al. Current Use and Trends in Hematopoietic Cell Transplantation in the United States. 2017;23(9):1417–1421. doi: 10.1016/j.bbmt.2017.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horowitz MM. Thomas’ hematopoietic cell transplantation. 3. Hoboken, NJ: Blackwell Publishing; 2004. Uses and growth of hematopoietic cell transplantation; pp. 9–15. [Google Scholar]
- 3.Majhail NS, Tao L, Bredeson C, et al. Prevalence of hematopoietic cell transplant survivors in the United States. Biology of Blood and Marrow Transplantation. 2013;19:1498–1501. doi: 10.1016/j.bbmt.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bevans M, El-Jawahri A, Tierney DK, et al. National Institutes of Health Hematopoietic Cell Transplantation Late Effects Initiative: The Patient-Centered Outcomes Working Group Report. Biology of Blood and Marrow Transplantation. 2017;23:538–551. doi: 10.1016/j.bbmt.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Panepinto JA. Health-related quality of life in sickle cell disease. Pediatric blood & cancer. 2008;51:5–9. doi: 10.1002/pbc.21557. [DOI] [PubMed] [Google Scholar]
- 6.Felder-Puig R, Di Gallo A, Waldenmair M, et al. Health-related quality of life of pediatric patients receiving allogeneic stem cell or bone marrow transplantation: results of a longitudinal, multi-center study. Bone Marrow Transplant. 2006;38:119–126. doi: 10.1038/sj.bmt.1705417. [DOI] [PubMed] [Google Scholar]
- 7.Parsons SK, Shih M, DuHamel KN, et al. Maternal perspectives on children’s health-related quality of life during the first year after pediatric hematopoietic stem cell transplant. J Pediatr Psychol. 2005;31:1100–1115. doi: 10.1093/jpepsy/jsj078. [DOI] [PubMed] [Google Scholar]
- 8.Grulke N, Albani C, Bailer H. Quality of life in patients before and after haematopoietic stem cell transplantation measured with the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Core Questionnaire QLQ-C30. Bone Marrow Transplant. 2012;47:473–482. doi: 10.1038/bmt.2011.107. [DOI] [PubMed] [Google Scholar]
- 9.Bevans M, Marden S, Leidy N, et al. Health-related quality of life in patients receiving reduced-intensity conditioning allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2006;38:101–109. doi: 10.1038/sj.bmt.1705406. [DOI] [PubMed] [Google Scholar]
- 10.Shaw BE, Brazauskas R, Millard HR, et al. Centralized patient-reported outcome data collection in transplantation is feasible and clinically meaningful. Cancer. 2017;123:4687–700. doi: 10.1002/cncr.30936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Varni JW, Limbers C, Burwinkle TM. Literature review: health-related quality of life measurement in pediatric oncology: hearing the voices of the children. J Pediatr Psychol. 2007;32:1151–1163. doi: 10.1093/jpepsy/jsm008. [DOI] [PubMed] [Google Scholar]
- 12.Varni JW, Burwinkle TM, Seid M, et al. The PedsQL™* 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambulatory Pediatrics. 2003;3:329–341. doi: 10.1367/1539-4409(2003)003<0329:tpaapp>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 13.Bhatia M, Kolva E, Cimini L, et al. Health-related quality of life after allogeneic hematopoietic stem cell transplantation for sickle cell disease. Biol Blood Marrow Transplant. 2015;21:666–672. doi: 10.1016/j.bbmt.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phipps S, Dunavant M, Lensing S, et al. Acute health-related quality of life in children undergoing stem cell transplant: II. Medical and demographic determinants. Bone Marrow Transplant. 2002;29:435–442. doi: 10.1038/sj.bmt.1703376. [DOI] [PubMed] [Google Scholar]
- 15.Kupst M, Penati B, Debban B, et al. Cognitive and psychosocial functioning of pediatric hematopoietic stem cell transplant patients: a prospective longitudinal study. Bone Marrow Transplant. 2002;30:609–617. doi: 10.1038/sj.bmt.1703683. [DOI] [PubMed] [Google Scholar]
- 16.Meade T, Dowswell E. Adolescents’ health-related quality of life (HRQoL) changes over time: a three year longitudinal study. Health and quality of life outcomes. 2016;14:14. doi: 10.1186/s12955-016-0415-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ravens-Sieberer U, Auquier P, Erhart M, et al. The KIDSCREEN-27 quality of life measure for children and adolescents: psychometric results from a cross-cultural survey in 13 European countries. Quality of Life Research. 2007;16:1347–1356. doi: 10.1007/s11136-007-9240-2. [DOI] [PubMed] [Google Scholar]
- 18.Wrotniak BH, Schall JI, Brault ME, et al. Health-related quality of life in children with sickle cell disease using the child health questionnaire. Journal of Pediatric Health Care. 2014;28:14–22. doi: 10.1016/j.pedhc.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arrington-Sanders R, Michael SY, Tsevat J, et al. Gender differences in health-related quality of life of adolescents with cystic fibrosis. Health and Quality of Life Outcomes. 2006;4:5. doi: 10.1186/1477-7525-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reinfjell T, Diseth TH, Veenstra M, et al. Measuring health-related quality of life in young adolescents: Reliability and validity in the Norwegian version of the Pediatric Quality of Life Inventory™ 4.0 (PedsQL) generic core scales. Health and quality of life outcomes. 2006;4:61. doi: 10.1186/1477-7525-4-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simms S, Kazak AE, Golomb V, et al. Cognitive, behavioral, and social outcome in survivors of childhood stem cell transplantation. J Pediatr Hematol Oncol. 2002;24:115–119. doi: 10.1097/00043426-200202000-00011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.