Abstract
Language production and cognitive control are complex processes that involve distinct, yet interacting, brain networks. However, the extent to which these processes interact and their neural bases has not been thoroughly examined. Here we investigated the neural and behavioral bases of language production and cognitive control, via a phonological Go No-Go picture naming task. Naming difficulty and cognitive control demands (i.e., conflict monitoring and response inhibition) were manipulated by varying the proportion of naming trials (Go trials) and inhibition trials (No-Go trials) across task runs. The results demonstrated that as task demands increased, participants’ behavioral performance declined (i.e., longer reaction times on naming trials, higher commission error rates on inhibition trials) while brain activation generally increased. Increased activation was found not only in within the language network, but also in domain-general control regions. Additionally, right superior and inferior frontal and left supramarginal gyri were sensitive to increased task difficulty during both language production and response inhibition. We also found both positive and negative brain-behavior correlations. Most notably, increased activation in sensory-motor regions, such as precentral and postcentral gyri, was associated with better behavioral performance, in both successful picture naming and successful inhibition. Moreover, comparing the strength of correlations across conditions indicated that the brain-behavior correlations in sensory-motor regions that were associated with improved performance became stronger as task demands increased. Overall, our results suggest that cognitive control demands affect language production, and that successfully coping with increases in task difficulty relies on both language specific and domain-general cognitive control regions.
Introduction
Speaking, or language production, is a fundamental aspect of communication that involves several processes: activating semantic information, selecting the correct lexical information from the mental lexicon, retrieving phonological information, phonetic encoding, and articulation (Burke & Shafto, 2008; Levelt, 1999; Levelt, Roelofs, & Meyer, 1999; Martin, 2003; Schwartz, Dell, Martin, Gahl, & Sobel, 2006). Although these are conceptually distinct processes, many word production models suggest that these processes are highly interactive (e.g., Dell, 1986). Moreover, language production almost always involves general cognitive abilities such as planning, paying attention, response selection, and monitoring output. However, the interaction between language production and executive components, and their neural bases remain unclear. The goal of the current study was to examine these issues using functional magnetic resonance imaging (fMRI) with a phonological Go No-Go picture naming paradigm.
The Go No-Go paradigm has been widely used as a way to measure aspects of cognitive control such as response inhibition or conflict monitoring. In this task, participants are asked to respond to a particular stimulus or type of stimulus (Go trials) while withholding their responses to other types of stimuli (No-Go trials). Researchers have identified a right lateralized fronto-parietal network that is sensitive to these inhibition and conflict monitoring processes (No-Go trials, Bellgrove, Hester, & Garavan, 2004; de Zubicaray, Andrew, Zelaya, Williams, & Dumanoir, 2000; Niendam et al., 2012; Nieuwenhuis, Yeung, Van Den Wildenberg, & Ridderinkhof, 2003; Simmonds, Pekar, & Mostofsky, 2008). This network includes lateral prefrontal cortex including superior, middle, and inferior frontal gyri which support aspects of cognitive control, anterior cingulate cortex which supports performance monitoring, bilateral parietal regions which guide attention, and pre-supplementary motor area supporting response inhibition (Botvinick, Braver, Barch, Carter, & Cohen, 2001; Botvinick, Cohen, & Carter, 2004; MacDonald, Cohen, Stenger, & Carter, 2000; Ridderinkhof, Van Den Wildenberg, Segalowitz, & Carter, 2004). Additionally, dorsal anterior cingulate and pre-supplementary motor cortices have a role in heteromodal processing in which information from unimodal regions (e.g., somatosensory regions, motor regions) is integrated (Mayer et al., 2012; Ridderinkhof, Ullsperger, Crone, & Nieuwenhuis, 2004; Ridderinkhof, Van Den Wildenberg, et al., 2004). Task difficulty can be manipulated by altering the proportion of Go or No-Go trials to create a response bias. For example, a run that contains a majority of Go trials (e.g., 75%), generates a prepotent response tendency that would need to be inhibited during No-Go trials. Functional MRI studies have localized these response inhibition effects to supplementary motor cortices extending to anterior cingulate cortex, along with right dorsal lateral prefrontal cortex (e.g., Criaud & Boulinguez, 2013; Nieuwenhuis et al., 2003). The Go No-Go paradigm has also been incorporated within the language domain to study the temporal properties of syntactic, semantic, and phonological processes in speech production, using ERP indices such as the N200 or LRP (Rodriguez-Fornells, Schmitt, Kutas, & Münte, 2002; Schmitt, Rodriguez-Fornells, Kutas, & Münte, 2001; Van Turennout, Hagoort, & Brown, 1997). Additionally, Rodriguez-Fornells and colleagues (2005) used a cross language Go No-Go paradigm in bilinguals to explore the interference of phonological information from the nontarget language using functional MRI. They found that cognitive control regions such as left middle frontal gyrus were crucial in inhibiting the production of the nontarget language.
In contrast to cognitive control manipulations, language tasks typically engage a left-lateralized frontal-temporal-parietal network (Geranmayeh et al., 2012; Geranmayeh, Wise, Mehta, & Leech, 2014; Price, 2010). Specifically, semantic processing and conceptually-driven lexical selection involve regions supporting perceptual or feature-based aspects (e.g., ventral and lateral temporal cortices) as well as regions that support semantic integration (e.g., anterior temporal lobe, angular gyrus, Binder, Desai, Graves, & Conant, 2009; Mirman et al., 2015; Pobric, Jefferies, & Ralph, 2007; Price, 2010; Visser, Jefferies, & Ralph, 2010). Lexical processing is supported by posterior middle temporal gyrus (Hickok & Poeppel, 2007; Indefrey & Levelt, 2004), as well as regions that are involved in lexical selection such as left inferior frontal gyri (Hirshorn & Thompson-Schill, 2006; Price, 2010). Neural structures supporting phonological encoding and retrieval include left posterior superior and middle temporal cortices, as well as left supramarginal and posterior aspects of inferior frontal gyri (Indefrey & Levelt, 2000; Poldrack et al., 2001; Poldrack et al., 1999). Finally, articulatory processes involve left anterior insula, and several motor regions including bilateral premotor, motor, and pre-supplementary motor regions (Brown et al., 2009; Chang, Kenney, Loucks, Poletto, & Ludlow, 2009; Price, 2010), as well as subcortical regions such as left putamen and caudate (Chang et al., 2009; Price, 2010; Seghier & Price, 2009). Although distinct regions have been attributed to specific processes, the brain functions as a network. For example, Hickok and Poeppel (2007) and others (Friederici & Alter, 2004) have proposed two pathways for language: a ventral pathway supporting early stages of processing and semantics, and a dorsal pathway involved in language production and syntactic processing.
These previous lines of research on cognitive control and language production largely reflect isolated investigations into one or the other domain. However, language production often involves cognitive control1. For instance, many behavioral and neuroimaging studies have demonstrated that language production involves conflict monitoring and control (Freund, Gordon, & Nozari, 2016; Gauvin, De Baene, Brass, & Hartsuiker, 2016; Hanley, Cortis, Budd, & Nozari, 2016; Nozari, Dell, & Schwartz, 2011; Nozari & Novick, 2017; Riès, Janssen, Dufau, Alario, & Burle, 2011; Shitova, Roelofs, Schriefers, Bastiaansen, & Schoffelen, 2017; Van Maanen & Van Rijn, 2010), engaging language related regions such as left middle temporal cortex (de Zubicaray, McMahon, & Howard, 2015; de Zubicaray, Wilson, McMahon, & Muthiah, 2001) and domain general regions such as pre supplementary motor area, anterior cingulate, and ventrolateral prefrontal cortex (Nozari & Novick, 2017). Language production also involves inhibitory control (Shao, Meyer, & Roelofs, 2013; Shao, Roelofs, Acheson, & Meyer, 2014; Shao, Roelofs, & Meyer, 2012; Tydgat, Diependaele, Hartsuiker, & Pickering, 2012; Tydgat, Stevens, Hartsuiker, & Pickering, 2011), and other types of control processes (Nozari, Freund, Breining, Rapp, & Gordon, 2016). Additionally, other neuroimaging studies have reported that dorsal anterior cingulate cortex and regions in lateral prefrontal cortex such as inferior frontal gyrus assist in aspects of cognitive control required during language production (Fedorenko, Duncan, & Kanwisher, 2012; Piai, Riès, & Swick, 2016; Piai et al., 2013). Language and general cognitive control networks have also been found to overlap in other brain regions such as left parietal lobe (Geranmayeh et al., 2014). Moreover, studies on individuals with aphasia have reported that domain-general cognitive control regions such dorsal anterior cingulate cortex, pre supplementary motor area, and superior frontal gyrus contribute significantly to patients’ recovery from aphasia (Brownsett et al., 2013; Geranmayeh, Chau, Wise, Leech, & Hampshire, 2017). Furthermore, studies have found that manipulating executive demands modulated brain activation associated with language production. For instance, increasing lexical selection demands engaged left inferior frontal gyrus (Kan & Thompson-Schill, 2004; Novick, Trueswell, & Thompson-Schill, 2010; Schnur et al., 2009) and dorsal anterior cingulate cortex (Piai et al., 2013). The resolution of lexical-semantic conflict involved left middle temporal cortex (de Zubicaray et al., 2015; de Zubicaray et al., 2001). Other studies suggest that phonological competition involves left posterior superior temporal gyrus, extending to supramarginal and precentral gyri (e.g., Peramunage, Blumstein, Myers, Goldrick, & Baese-Berk, 2011).
In the present study, we wanted to explore the role of cognitive control in phonological aspects of language production, and their neural bases. To investigate this, we examined how varying cognitive control demands for response inhibition and conflict monitoring affected language production by incorporating the Go No-Go paradigm with a commonly used language production task: Picture naming. Phonological characteristics of the target photograph’s name were used to make Go (overt naming) or No-Go (withhold naming) decisions. In both trial types, participants were required to process phonological aspects of the photographs in order to arrive at correct decisions. This task is similar to the phoneme monitoring task in which participants monitor specific phonemes across words to explore the incremental nature of phonological encoding (Jansma & Schiller, 2004; Sasisekaran & Luc, 2006; Schiller, Jansma, Peters, & Levelt, 2006; Wheeldon & Levelt, 1995). Therefore, we anticipated that this task involved a higher level of monitoring processes compared to a typical picture naming task. We manipulated the proportion of Go and No-Go trials to alter the difficulty in picture naming and response inhibition. We expected that such increases in naming difficulty would elicit more activation in language regions that are sensitive to task demands, such as left inferior frontal gyrus, posterior superior temporal sulcus, supramarginal gyrus, and precentral gyrus (Novick et al., 2010; Peramunage et al., 2011; Roskies, Fiez, Balota, Raichle, & Petersen, 2001; Schnur et al., 2009). As naming difficulty increased, we also expected to observe greater involvement of domain-general control regions such as anterior cingulate cortex and lateral prefrontal cortex. Similarly, the difficulty in withholding responses to No-Go trials should also change with the number of No-Go trials in a run. For the No-Go trials, we hypothesized that inhibition failures and activation in anterior cingulate and right inferior and middle frontal gyri would change as a function of cognitive control demands considering the sensitivity of these regions to task manipulations (Criaud & Boulinguez, 2013; Nieuwenhuis et al., 2003). Moreover, conjunction analyses across trial types can identify brain regions that reflect general task difficulty whether the picture names were overly produced or not. Based on the previous literature, we expected that lateral prefrontal cortex including superior and middle frontal gyri, and anterior cingulate cortex would be involved in such domain-general roles.
We were also interested in the relationship between behavioral performance and brain activation in response to task difficulty. Increased task difficulty has typically been associated with increased brain activation, across a broad range of cognitive tasks (Banich, 1998; Barch et al., 1997; Dove, Pollmann, Schubert, Wiggins, & von Cramon, 2000; Rypma & D’Esposito, 1999; Rypma, Prabhakaran, Desmond, Glover, & Gabrieli, 1999; E. E. Smith & Jonides, 1999). However, linking such changes in functional activation to behavior is critical for interpreting activation changes. If increases in activation are associated with declines in behavioral performance, these increases could potentially be interpreted as less efficient processing. On the other hand, if increases in activation are associated with equivalent or improved performance, such increases may serve a beneficial or compensatory role. Moreover, by examining the patterns of such brain-behavior activations across trial types, we can identify whether such relationships are specific to language production or extend more broadly to cognitive control in general.
Methods
Participants
Twenty individuals (10 females; mean age = 22.65 years, age range = 18–34 years, SD = 4.30) participated in this study. All participants had normal or correct-to-normal vision and reported no known psychiatric or neurological illnesses. They were all native English speakers with little knowledge of another language. Before the MRI session, all participants underwent psychometric testing to assess language and basic cognitive skills such as speed, executive functions, and working memory (See Table 1). All participants gave written, informed consent, and all procedures were approved by the Institutional Review Board at the Pennsylvania State University.
Table 1.
Participant demographic and neuropsychological testing information.
| Mean (SD) | 95% Confidence Interval1 | |
|---|---|---|
| Demographic information | ||
| N | 20 | |
| Age | 22.65 (4.3) | |
| Gender (M/F) | 10/10 | |
| Education (Years) | 16.1 (2.36) | |
| Neuropsychological testing | ||
| Mini Mental State Examination (MMSE) | 29.05 (1) | 29.09–29.49 |
| Vocabulary (WAIS III-score) | 51 (7.30) | 55.53–58.84 |
| Verbal Fluency (Total Number of Tokens) | 59.95 (13.35) | 69.20–77.22 |
| Author Recognition2 | 13.1 (8.64) | 10.50–18.10 |
| Magazine Recognition2 | 12.7 (7.76) | 9.60–15.80 |
| Immediate Recall (CVLT, Number of correct items) | 11.25 (2.15) | 11.45–12.47 |
| Delayed Recall (CVLT, Number of correct items) | 10 (2.29) | 10.43–11.63 |
| Simple Speed (ms) | 253.44 (27.25) | 260.43–275.87 |
| Digit Span Forward (Number of correct items, WAIS III) | 10.65 (1.39) | 11.32–12.44 |
| Digit Symbol (WAIS III, ms) | 1298.83 (156.4) | 1204.50–1327.93 |
| Executive Functions | ||
| AX-CPT3: AY RT (ms) | 386.03 (47.71) | |
| AX-CPT: AY ER | 0.27 (0.20) | |
| Stroop Effect (ms, Incongruent Congruent) | 16.98 (70.28) | 15.55–33.49 |
| Task Switching Cost | 56.33 (73.03) |
Values provided are means, with standard deviations in parentheses.
The 95% Confidence Interval was calculated based on four previous experiments in our lab that tested participants with comparable age and education levels using these cognitive assessment (N=76), providing a relatively normative range of scores for reference. Note that participants from the current study scored slightly lower than participants from previous studies on the MMSE, vocabulary, verbal fluency, recall, and digit span forward. This appears to be a combination of sampling variability and site differences. Statistical comparisons across experiments revealed no significant differences in MMSE, or either recall. However, for vocabulary, verbal fluency, and digit span forward, participants from studies conducted at Duke University scored significantly higher than participants from studies conducted at Penn State (ps < .001).
ART and MRT scores are calculated as the number of correct identifications the number of incorrect responses.
AX-CPT is a frequently used task to measure executive function (Braver, Gray, & Burgess, 2007). The performance on AY condition is a reflection of the ability to inhibit incorrect response tendencies. References for tests: MMSE (Crum, Anthony, Bassett, & Folstein, 1993; Folstein, Folstein, & McHugh, 1975); WAIS III (Wechsler, Coalson, & Raiford, 1997); Author & Magazine Recognition Tests (Acheson, Wells, & MacDonald, 2008); Immediate and delayed memory from the California Verbal Learning Test (CVLT, Delis, Kramer, Kaplan, & Ober, 2000; Woods, Delis, Scott, Kramer, & Holdnack, 2006); Stroop Test (Stroop, 1935); Task switching test (Monsell, 2003).
Stimuli and Procedure
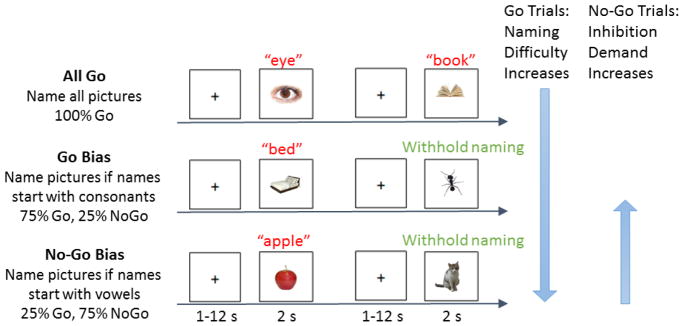
Participants completed a phonological Go No-Go picture naming task in the MRI scanner. Photographs were presented and participants were instructed to overtly name the photograph as quickly and accurately as possible. Task difficulty was manipulated via the proportion of trials that needed to be named or inhibited across three conditions: All Go, Go Bias, and No-Go Bias (See Figure 1 for an illustration of the task). In the All Go condition, participants were required to name all of the photographs (100% Go trials). In the Go Bias condition, participants were instructed to name the photograph only if the name of the photograph started with a consonant (75% Go trials; e.g., nose) but withhold their response if the name started with a vowel (25% No-Go trials; e.g., ear). In the No-Go Bias condition, the Go cue was reversed. Participants were instructed to name the photograph only if the name started with a vowel (25% Go trials; e.g., apple) but withhold their response if the name started with a consonant (75% No-Go trials; e.g., chair). Before scanning, participants practiced overt picture naming while minimizing head movement in a simulation scanner. In the scanner, participants always performed the All Go runs first, prior to being informed about the Go - No-Go manipulation to minimize the possible influence of Go - No-Go task demands. After the All Go condition, participants underwent a practice run and then completed the other two conditions, whose order was counterbalanced across participants. Stimuli were not repeated across practice runs or conditions.
Figure 1.
Task design. An overview of the phonological Go No-Go picture naming task is provided. Examples of Go and No-Go trials for each of the three conditions are shown: All Go, Go Bias, and No-Go Bias. Correct names to the two No-Go trials (withhold naming, in green) are “ant”, and “cat”, respectively. Naming difficulty (Go trials, shown in red) increased from the All Go condition to the Go Bias condition to the No-Go Bias condition. Cognitive control demands in terms of conflict monitoring and response inhibition (No-Go trials) increased from the No-Go Bias condition to the Go Bias condition.
Photographs were taken from two normed databases (Brodeur, Guérard, & Bouras, 2014; Moreno-Martínez & Montoro, 2012) and depicted a broad range of common objects. Additionally, an independent group of 21 healthy, native English-speaking adults was recruited to norm the selected stimuli. Items were included if naming consistency was 67% or higher. A total of 330 colored photographs, 110 unique items per condition (All go, Go Bias, No-Go Bias), were selected for the MRI experiment. For the Go Bias and No-Go Bias conditions, these trials were further divided into 82 trials (75%) of the biased trial type (e.g., Go trials in the Go Bias runs) and 28 trials (25%) of the non-biased trial type (e.g., No-Go trials in the Go Bias runs). The linguistic characteristics of the object names were obtained from the English Lexicon Project (ELP, Balota et al., 2007). Stimuli across the three conditions were similar in terms of word length (F (2, 327) = .75, p = .47), word frequency (F (2, 327) = .17, p = .84), number of phonemes (F (2, 327) = .30, p = .74), number of syllables (F (2, 327) = .33, p = .72), and reaction time (RT) and accuracy in the ELP Lexical Decision Task (F1 (2, 327) = .72, p1 = .49; F2 (2, 327) = 1.43, p2 = .24) and the ELP Word Naming Task (F1 (2, 327) = .44, p1 = .65; F2 (2, 327) = .69, p2 = .50). See Supplemental Table 1 for additional details.
For each trial, a color photograph of an object on a white background was presented and the participant was instructed to respond with its name or withhold a response based on the specific condition requirements. Participants were asked to use one word to name the photograph and not to say anything else. Photographs (duration = 2s) were presented with a variable inter-stimulus interval (ISI, range = 1–12s, average ISI = 3.40s) to optimize the hemodynamic response (Optseq2, Dale, 1999). Participants completed 6 runs, 2 runs per condition in the scanner. Overt verbal responses in the scanner were recorded and filtered using a dual-channel, MR-compatible, fiber optic microphone system (Optoacoustics Ltd., Or-Yehuda, Israel). After the scan, an additional debriefing task was included in which participants were asked to name all the photographs from the Go Bias and No-Go Bias conditions, to verify their identification and naming of the photographs, and to confirm that they knew the name of the objects when a response was withheld (e.g., in the No-Go trials).
Acquisition of MRI Data
MRI data were collected on a 3T Siemens Prisma Fit MRI scanner with a 20-channel head coil. We collected a sagittal T1 weighted localizer image to define a volume for data collection and higher-order shimming. The anterior and posterior commissures were identified for slice selection and shimming. T-1 weighted structural images were then collected using a magnetization-prepared rapid acquisition gradient echo (MP-RAGE) sequence (repetition time [TR] = 2300 ms; echo time [TE] = 2.28 ms; Inversion Time [TI] = 900 ms; flip angle = 8°; echo spacing = 7 ms; acceleration factor = 2; field of view [FOV] = 256 mm2; voxel size = 1 × 1 × 1 mm; 160 contiguous slices).
Functional images sensitive to BOLD contrast were collected using an echo-planar imaging (EPI) sequence (TR = 2500 ms; TE = 25 ms; flip angle = 90°; echo spacing = 0.49 ms; FOV = 240 mm2; voxel size = 3 × 3 × 3 mm; 41 contiguous axial slices, parallel to the AC PC line, interleaved acquisition). Two additional volumes were acquired and deleted at the beginning of each functional run to reach steady state equilibrium. Each of six functional runs was approximately 122 volumes (309 s) in length.
Behavioral Data Analyses
Responses were coded based on both the recordings from the scanner session and the debriefings after the scanner session. In the All Go condition, responses were marked as correct if the participant provided the exact target name (e.g., chicken for chicken) or its plural form (e.g., chickens for chicken), or an acceptable alternative word that corresponded to the photograph (e.g., hen for chicken). Responses were marked as incorrect if the response did not match the photograph (e.g., ice for tea), or if no response was provided.
For Go trials in the other conditions, responses were similarly coded, however acceptable alternative words that matched the photograph also had to have the same onset category (vowel/consonant) as the target word (e.g., raven for crow but not spaceman for astronaut). For No-Go trials in both the Go Bias condition and the No-Go Bias condition, they were marked as correct if no response was provided in the scanner and the response in the debriefing task indicated that they knew the name of the object in the photograph.
An error analysis was also conducted across three types of errors: incorrect responses as defined above; commission errors (failures to inhibit a response during a No-Go trial when the response matched the photograph and its onset category), omission errors (no response was provided in the scanner for a Go trial). Errors were treated as categorical variables and analyzed separately using generalized logistic mixed-effect modeling, employing the glmer function in the lme4 package (Bates, Mächler, Bolker, & Walker, 2014) in the R environment (Venables & Smith, 2006). For each trial type, errors of that type were coded as 1s and other trials were coded as 0s. We obtained p values for regression coefficients using the lmerTest package (Kuznetsova, Brockhoff, & Christensen, 2017). This approach has the advantage of taking into account individual data points, allowing intercepts and slopes to be random across participants and allowing different items to have random intercepts. As recommended by Barr, Levy, Scheepers, and Tily (2013), linear mixed-effect models generalize best when they include the maximal random effects structure justified by the design. Therefore, for each type of error, a full model including the task condition as the independent variable and the error as the dependent variable was run while allowing different participants to have random intercepts and slopes and different items to have random intercepts. Additionally, for commission errors, a model adding the error rates in the AY condition in the AX-CPT (an index of inhibition ability) as the participant level variable was also conducted to further validate our manipulation.
Error rates were also calculated for each type of errors. The overall error rate (overall ER) was calculated by dividing the total number of errors by the total number of trials in that condition. The incorrect response rate in each condition was calculated by dividing the number of incorrect responses by the total number of trials. Note that since commission errors could only occur on No-Go trials, and the number of No-Go trials varied across conditions, the commission error rate (commission ER) in each condition was calculated by dividing the number of commission errors by the number of No-Go trials in that condition. Similarly, since omission errors could only be observed on Go trials, and the number of Go trials in each condition varied, the omission error rate (omission ER) in each condition was calculated by dividing the number of omission errors by the number of Go trials in that condition.
Reaction times (RTs) to Go trials were calculated using customized PRAAT scripts. The PRAAT scripts identified response onsets by searching the recordings for pitch deviations from the pitch of the filtered auditory signal. These onsets were then manually verified by using both the audio and visual speech stream. The reaction times were calculated as the difference between the photograph onsets (from E-Prime output) and the response onsets. Only trials with correct responses and reaction times within 2.5 SDs were included in further analyses. Reaction times were also analyzed using mixed-effect regression modelling, employing the lmer function in the lme4 package (Bates et al., 2014) in the R environment (Venables & Smith, 2006). Task condition was included as the categorical independent variable and reaction time was included as the continuous dependent variable, while allowing different participants to have random intercepts and slopes and different items to have random intercepts.
fMRI Data Analyses
A quality assurance tool was used to analyze data quality including the number of potentially clipped voxels, mean signal fluctuation to noise ratio (SFNR), and per-slice variation (Glover et al., 2012). Additionally, both the structural and the functional images were visually inspected for artifacts and blurring. The skull and other coverings were stripped from the structural brain images using Optimized Brain Extraction for Pathological Brains (optiBET: Lutkenhoff et al., 2014). We used FSL (version 5.0.9), with FEAT (fMRI expert analysis tool) version 6.0 (S. M. Smith et al., 2004; Woolrich, Behrens, Beckmann, Jenkinson, & Smith, 2004), to carry out preprocessing and statistical analyses. Preprocessing steps included motion correction (FSL MCFLIRT), B0 unwarping with field mapping, slice timing correction, spatial smoothing (FWHM = 5 mm), high-pass filtering, coregistration (first to the participants’ own brain-extracted structural image, then to the MNI space using the MNI 152 T1 2 mm template), and normalization. We used a double-gamma hemodynamic response function to model the BOLD signal for each event and only correct trials were included in the analyses. We conducted first level analyses on each participant’s individual runs. To remove the residual effects of motion that remained after motion correction, standard motion parameters were included in the first level models as confound EVs. We then combined analyses from the previous step across runs and participants in group-level analyses using FMRIB’s local analysis of mixed effects (FLAME 1+2, Beckmann, Jenkinson, & Smith, 2003; Woolrich et al., 2004). Within FSL, we first identified regions that were responsive to our basic trial types (Go trials in All Go runs, Go trials in the biased runs, No-Go trials in the biased runs) compared with the implicit baseline using each participant’s individual runs as inputs. We then compared functional activation differences across task conditions for both Go trials (All Go < Go Bias < No-Go Bias) and No-Go trials (No-Go Bias < Go Bias). All significant activations were determined using a two-step process in which Z (Gaussianised T/F) statistical images were initially thresholded at the voxel level (p < .01). Clusters of identified voxels were then corrected for multiple comparisons (p < .05, corrected) based on Gaussian random field theory (Worsley, 2001) in which each cluster's estimated significance level was compared with the cluster probability threshold, and then only clusters whose estimated significance exceeded the threshold were included in the results (Hayasaka & Nichols, 2003). Additionally, results from comparisons between conditions were masked to ensure that significant differences were based on significant positive hemodynamic responses (e.g., Go trials: All Go < Go Bias < No-Go Bias, masked with significant activation of Go trials in the No-Go Bias condition).
To assess brain-behavior relations, we conducted three correlational analyses on Go trials and No-Go trials separately. First, we correlated participants’ overall reaction times and brain activation to all of the Go trials in a whole-brain analysis (No-Go Bias, Go Bias, and All Go). This approach allowed us to examine the relationship between overall speed and functional activation across individuals. We hypothesized that brain regions that showed a positive correlation (i.e., longer reaction times associated with more activation on Go trials) may indicate less efficient processing across individuals, while regions that showed a negative correlation (i.e., shorter reaction times associated with more activation on Go trials) would reflect a potentially beneficial recruitment. Second, we correlated reaction time and brain activation for Go trials as a function of naming difficulty in a second whole-brain analysis. That is, we correlated changes in RT from the easier condition (All Go) to the harder condition (No-Go Bias) with changes in brain activation between these two conditions. Positive correlations (i.e., larger reaction time increases associated with larger increases in activation on Go trials) would indicate larger individual change score differences and functional activation as a function of naming difficulty (e.g., reflecting reduced processing efficiency when difficulty increased across individuals), while negative correlations (i.e., larger reaction time increases associated with smaller increases in activation on Go trials) would suggest a potential beneficial function of increases in activation across individuals. Third, we calculated correlations between reaction time and activation to Go trials within each condition, in order to compare the strength of these correlations and to observe if the correlation strength changed as a function of task difficulty (All Go < Go Bias < No-Go Bias). We hypothesized that the strength of both positive and negative brain-behavior correlations to Go trials may increase as task difficulty increased.
Three similar correlation analyses were also conducted on commission error rate and brain activation for No-Go trials, again using whole-brain analyses. All reported brain regions were identified according to the Harvard-Oxford Structural Atlas (Desikan et al., 2006). Reported coordinates are in MNI space, and results are overlaid on a representative brain in MNI space.
Additionally, to estimate the potential effect of power differences due to the unbalanced number of critical trials across runs, we randomly2 selected equal number of critical trials from all conditions for both Go trials and No-Go trials and statistically compared the results from this analysis to the main analysis that included all correct trials (e.g., we selected an equal number of Go trials from the All Go condition and the Go Bias condition to match the number of Go trials in the No-Go Bias condition, and selected an equal number of No-Go trials from the No-Go Bias condition to match the number of No-Go trials in the Go Bias condition).
Results
Behavioral Results
Error Rates
A mixed logistic regression was conducted on the number of overall errors to explore the differences across the three conditions (All Go, Go Bias, No-Go Bias). Results showed that the overall error rates were not significantly different across the three conditions (All Go vs Go Bias: β = −.47, SE = .35, p = .18; All Go vs No-Go Bias: β = −.55, SE = .32, p = .09; Go Bias vs No-Go Bias: β = −.08, SE = .35, p = .82). See Table 2 for behavioral data.
Table 2.
Behavioral results (number of errors, reaction times, and error rates).
| Overall Errors | Incorrect Responses | Commission Errors | Omission Errors | Reaction Time(ms) | |
|---|---|---|---|---|---|
| All Go | 7.5 (4.5) | 3.4 (3.4) | 4.1 (3.3) | 1217 (114) | |
| Go Bias | 5.5 (3.9) | 2.7 (1.6) | 1.3 (1.1) | 1.6 (2.3) | 1269 (119) |
| No-Go Bias | 5.3 (2.8) | 3.0 (2.2) | 0.4 (0.9) | 1.9 (1.7) | 1430 (150) |
|
| |||||
| Overall Error Rate | Incorrect Response Rate | Commission Error Rate | Omission Error Rate | ||
| All Go | 6.8 % (4.1 %) | 3.1 % (3.1 %) | 3.7 % (3.0 %) | ||
| Go Bias | 5.0 % (3.5 %) | 2.5 % (1.4 %) | 4.5 % (4.0 %) | 1.9 % (2.8 %) | |
| No-Go Bias | 4.8 % (2.6 %) | 2.7 % (2.0 %) | 0.5 % (1.1 %) | 6.7 % (5.9 %) | |
Values provided are the mean number of errors, mean RT, and mean percentage of errors, with standard deviations in parentheses. Overall Error Rate = Total number of errors/Total number of trials, Incorrect Response Rate = Number of incorrect responses/Total number of trials, Commission Error Rate = Number of commission errors/Number of No-Go trials, Omission Error Rate = Number of omission errors/Number of Go trials. Because the denominator for each error rate is different, the overall error rate does not equal to the sum of three types of error rates. See Behavioral Data Analyses section for more details.
We also conducted a mixed-logistic regression on the number of commission errors between the Go Bias and No-Go Bias conditions. The results showed that the number of commission errors was significantly higher in the Go Bias condition than in the No-Go Bias condition (β = −3.24, SE = .95, p < .001), suggesting that cognitive control demands in terms of conflict monitoring and response inhibition were higher during the Go Bias condition, consistent with the intended effect of our response-bias manipulation. Additionally, the number of commission errors in the Go Bias condition was also significantly positively predicted by the error rates on the AY condition of the AX-CPT, a measure of inhibition (Braver et al., 2007), suggesting that these two measures of inhibition were consistent across individuals (β = 2.49, SE = 1.09, p = .02). Moreover, the mixed logistic regression on omission errors showed that the Go Bias condition elicited fewer omission errors compared to the No-Go Bias condition (β = 1.77, SE = .52, p < .001), confirming that our manipulation biased participants toward not naming during the No-Go Bias condition.
Reaction Times
A generalized linear mixed-effect model was conducted on Go trial RTs to explore the differences across the three conditions (All Go, Go Bias, No-Go Bias). Results showed that the RTs in the No-Go Bias condition were significantly longer than the All Go condition (β = .21, SE = .04, p < .001) and the Go
Bias condition (β = .17, SE = .03, p < .001), while the RTs in the All Go condition and the Go Bias condition were not significantly different from each other (β = .05, SE = .03, p = .13). These results confirmed the gradual increase of reaction times as the number of Go trials decreased and suggest that the No-Go Bias condition was the most difficult and had the highest language production demands.
Neuroimaging Results
Head Motion
Because participants produced overt speech in the scanner, we conducted a repeated measures ANOVA on the average amount of head motion across three conditions (All Go: 0.19 mm, Go Bias: 0.19 mm, No-Go Bias: 0.24 mm). First, all estimates of motion were less than the standard inclusion criteria for task-based fMRI studies (i.e., < ½ voxel, < 1.5mm). Moreover, the main effect of condition was only marginally significant (F (2, 38) = 3.6, p = .06), and trending in the opposite direction than one would anticipate. Further pairwise comparisons did not show any significant differences between any of the conditions (ps > .1). This suggests that the potential effects of motion were comparable across conditions.
Activation to Basic Trial Types
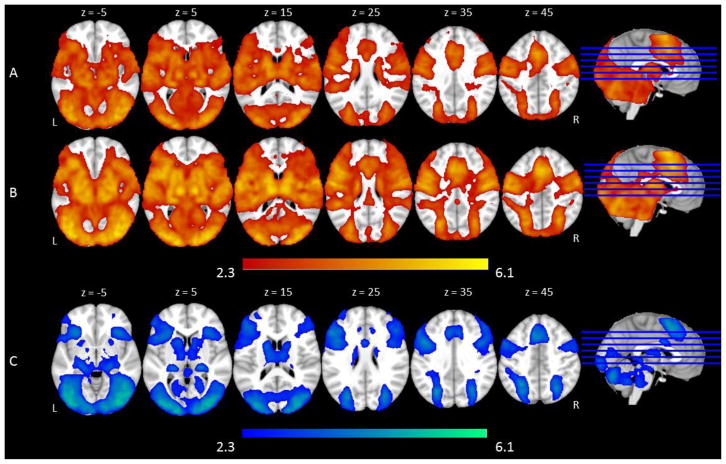
The Go trials in the All Go condition elicited activation in established regions that are involved in language processing including bilateral inferior frontal gyri, bilateral superior temporal gyri, and other motor and visual regions such as right cerebellum, and bilateral lateral occipital cortex (See Supplemental Table 2 and Figure 2A). Go trials in the Bias conditions (collapsing across Go Bias and No-Go Bias) elicited activation in similar regions3, but patterns of activation were more extensive and also included bilateral frontal pole (See Supplemental Table 2 and Figure 2B). The No-Go trials elicited activation in established regions that are involved in cognitive control such as bilateral frontal cortices (e.g., frontal pole, middle and inferior frontal gyri), and in visual regions such as bilateral lateral occipital cortex (See Supplemental Table 2 and Figure 2C). We were specifically interested in the brain responses to Go and No-Go trials as a function of task difficulty. Therefore, analyses comparing activation differences across different conditions on the two trial types are reported below.
Figure 2.
fMRI activation to basic trial types. Shown is an overview of the regions in which there was significant activation in (A) Go trials in the All Go condition, (B) Go trials in the Go Bias and No-Go Bias conditions, and (C) No-Go trials in the Go Bias and No-Go Bias conditions. Slices are depicted in increments of 10 mm, starting at z = 5 and ending at z = 45.
Go Trials Comparison
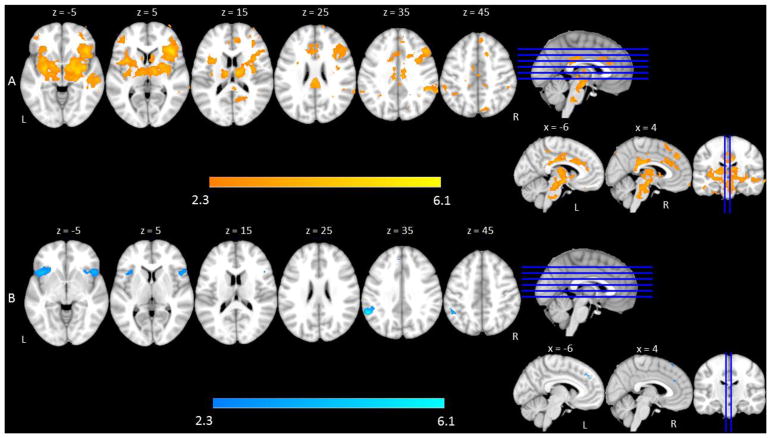
To explore how the brain responds to changes in language production difficulty, a parametric linear trend analysis was conducted for Go trials across the three conditions (All Go < Go Bias < No-Go Bias). Results revealed that Go trials in the No-Go Bias condition elicited greater activation than Go trials in other conditions in left frontal pole, right superior and inferior frontal gyri (pars triangularis, pars opercularis), bilateral orbitofrontal cortex, bilateral insula, bilateral temporal pole, right posterior superior and middle temporal gyri, left anterior cingulate, bilateral posterior cingulate gyrus, bilateral supramarginal and bilateral precuneus (See Table 3 and Figure 3A).
Table 3.
fMRI activation as a function of task difficulty for Go trials and No-Go trials.
| Hemisphere | Voxels | Coordinates (mm)
|
Z value | |||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Go Trials Comparison (All Go < Go Bias < No-Go Bias) | ||||||
| Frontal Pole | Right | 19543 | 44 | 48 | 6 | 3.81 |
| Frontal Pole | Left | −42 | 44 | 2 | 3.54 | |
| Inferior Frontal Gyrus | Right | 52 | 30 | 4 | 3.09 | |
| Middle Frontal Gyrus | Right | 44 | 28 | 36 | 2.62 | |
| Orbital Frontal Cortex | Right | 36 | 26 | −4 | 4.34 | |
| Orbital Frontal Cortex | Left | −36 | 24 | −6 | 3.2 | |
| Superior Frontal Gyrus | Right | 10 | 22 | 62 | 2.48 | |
| Insular Cortex | Right | 40 | 16 | −4 | 3.74 | |
| Insular Cortex | Left | −38 | 8 | −8 | 3.87 | |
| Temporal Pole | Right | 32 | 8 | −40 | 2.93 | |
| Temporal Pole | Left | −32 | 6 | −42 | 3.29 | |
| Temporal Fusiform Cortex | Right | 28 | 0 | −42 | 3.64 | |
| Temporal Fusiform Cortex | Left | −32 | −2 | −42 | 3.64 | |
| Posterior Superior Temporal Gyrus | Right | 46 | −26 | −4 | 3.63 | |
| Posterior Middle Temporal Gyrus | Right | 66 | −28 | −6 | 5.92 | |
| Anterior Cingulate Gyrus | Left | 2603 | −2 | 22 | 34 | 5.15 |
| Posterior Cingulate Gyrus | Right | 67 | 8 | −36 | 46 | 3.8 |
| Posterior Cingulate Gyrus | Left | 12 | −6 | −44 | 18 | 2.99 |
| Posterior Inferior Temporal Gyrus | Right | 36 | 48 | −24 | −24 | 3.23 |
| Anterior Supramarginal Gyrus | Left | 385 | −54 | −36 | 46 | 2.97 |
| Posterior Supramarginal Gyrus | Right | 576 | 64 | −40 | 38 | 3.05 |
| Precuneus Cortex | Left | 146 | −6 | −38 | 46 | 3.51 |
| Precuneus Cortex | Right | 164 | 8 | −74 | 46 | 3.78 |
| No-Go Trials Comparison (No-Go Bias < Go Bias) | ||||||
| Frontal Pole | Right | 39 | 22 | 60 | 30 | 4.78 |
| Anterior Cingulate Gyrus | Left | 15 | −6 | 36 | 20 | 2.76 |
| Paracingulate Gyrus | Right | 11 | 4 | 34 | 26 | 2.78 |
| Paracingulate Gyrus | Left | 47 | −4 | 44 | 28 | 3.66 |
| Superior Frontal Gyrus | Right | 48 | 10 | 28 | 64 | 4.13 |
| Orbital Frontal Cortex | Right | 759 | 30 | 22 | −14 | 4.85 |
| Operculum Frontal Cortex | Right | 44 | 20 | −2 | 3.67 | |
| Insular Cortex | Right | 42 | 18 | −6 | 3.02 | |
| Inferior Frontal Gyrus | Right | 52 | 16 | 4 | 3.28 | |
| Orbital Frontal Cortex | Left | 858 | −32 | 20 | −16 | 5.33 |
| Operculum Frontal Cortex | Left | −46 | 18 | −4 | 3.66 | |
| Insular Cortex | Left | −40 | 18 | −6 | 4.41 | |
| Inferior Frontal Gyrus | Left | −52 | 16 | −2 | 2.73 | |
| Middle Temporal Gyrus | Left | 10 | −56 | −36 | −10 | 2.98 |
| Posterior Supramarginal Gyrus | Left | 347 | −58 | −50 | 36 | 6.11 |
Figure 3.
fMRI activation as a function of task difficulty. Shown is an overview of regions that comprise (A) Increased naming difficulty (Go trials): All Go < Go Bias < No-Go Bias, and (B) Increased cognitive control demands in terms of response inhibition (No-Go trials): No-Go Bias < Go Bias.
No-Go Trials Comparison
To explore how the brain responded to changes in cognitive control aspects of language production (i.e., No-Go trials), a direct comparison was made between No-Go trials in the Go Bias condition and No-Go trials in the No-Go Bias condition. No-Go trials in the Go Bias condition elicited greater activation than No-Go trials in the No-Go Bias condition in left frontal pole; right superior frontal gyrus; bilateral orbitalfrontal cortex, which extended to bilateral insula and inferior frontal gyri; bilateral cingulate cortex, left middle temporal gyrus, and left posterior supramarginal gyrus (See Table 3 and Figure 3B). Additionally, a conjunction analysis was conducted to identify the overlap between these Go trial and No-Go trial comparisons to identify regions that reflected general difficulty across both language production and response inhibition. Regions that were responsive to both types of task difficulty included right superior and inferior frontal gyri, right frontal orbital cortex, left supramarginal gyrus, and left insula.
Brain-Behavior Correlations
Because we were also interested in how these patterns of brain activation related to behavioral performance in response to task difficulty, we conducted correlational analyses between behavioral performance and brain activation on Go trials and No-Go trials separately, specifically examining effects of task difficulty.
Go Trials: Brain Activation - Reaction Time
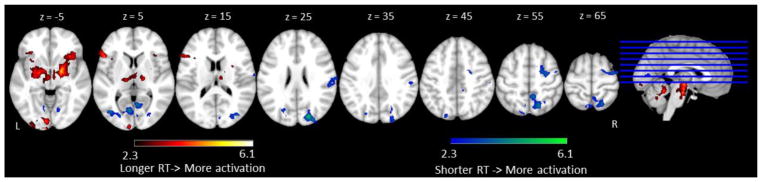
First, correlation analyses were conducted between overall reaction time and brain activation across participants for all of the Go trials in three conditions (No-Go Bias, Go Bias, All Go). Across all of the Go trials, positive correlations between RT and activation were found in bilateral orbital frontal cortex, extending subcortically, and left occipital pole extending to left lingual gyrus and the occipital portion of fusiform gyrus. These findings suggest that individuals with slower response times had more activation in these regions. On the other hand, we also found negative correlations in which individuals with faster RTs had more activation in right precentral and postcentral gyri extending anteriorly to right superior frontal gyrus and posteriorly to right supramarginal gyrus, and in bilateral precuneus extending to right lingual gyrus. These results suggest that individuals who had greater recruitment of sensory-motor, frontal control regions, and some higher order visual processing regions had better naming performance (See Table 4 and Figure 4).
Table 4.
Correlation between reaction time and fMRI activation across all of the Go trials (No-Go Bias, Go Bias, and All Go).
| Hemisphere | Voxels | Coordinates (mm)
|
Z value | |||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Positive Correlation | ||||||
| Orbital Frontal Cortex | Right | 5531 | 40 | 28 | −6 | 3.46 |
| Orbital Frontal Cortex | Left | −36 | 22 | −12 | 3.51 | |
| Insular Cortex | Right | 34 | 22 | −6 | 3.78 | |
| Pallidum | Left | −18 | −6 | −8 | 4.52 | |
| Pallidum | Right | 20 | −6 | −6 | 5.15 | |
| Brain-Stem | Right | 4 | −16 | −22 | 4.93 | |
| Occipital Fusiform Gyrus | Left | 777 | −34 | −82 | −12 | 3.82 |
| Lingual Gyrus | Left | −4 | −82 | −14 | 3.55 | |
| Occipital Pole | Left | −8 | −102 | −2 | 3.48 | |
| Negative Correlation | ||||||
| Superior Frontal Gyrus | Right | 693 | 22 | −4 | 62 | 4.07 |
| Precentral Gyrus | Right | 24 | −6 | 50 | 4.73 | |
| Postcentral Gyrus | Right | 472 | 66 | −16 | 22 | 3.44 |
| Anterior Supramarginal Gyrus | Right | 60 | −22 | 28 | 3.52 | |
| Operculum Parietal Cortex | Right | 58 | −28 | 26 | 3.47 | |
| Precuneus Cortex | Right | 1336 | 8 | −48 | 54 | 4.19 |
| Superior Parietal Lobule | Right | 20 | −48 | 68 | 3.51 | |
| Superior Lateral Occipital Cortex | Right | 18 | −64 | 58 | 4.58 | |
| Superior Lateral Occipital Cortex | Left | −22 | −82 | 42 | 4.15 | |
| Lingual Gyrus | Right | 974 | 6 | −62 | 4 | 4.17 |
| Lingual Gyrus | Left | −8 | −72 | 0 | 4.48 | |
| Inferior Lateral Occipital Cortex | Left | −46 | −82 | 0 | 3.41 | |
| Inferior Lateral Occipital Cortex | Right | 874 | 34 | −82 | 0 | 3.64 |
| Cuneal Cortex | Right | 22 | −72 | 30 | 3.78 | |
| Occipital Pole | Right | 30 | −90 | 30 | 3.75 | |
Figure 4.
The correlation between reaction time and fMRI activation across all of the Go trials (No-Go Bias, Go Bias, and All Go). Regions in which longer reaction times were associated with increases in activation are shown in red and regions in which faster reaction times were associated with increases in activation are shown in blue. Slices are depicted in increments of 10 mm, starting at z = 5 and ending at z = 65.
To examine the relationship between RT and fMRI activation in response to naming difficulty (All Go < Go Bias < No-Go Bias), we further conducted correlational analyses comparing changes in RT and brain activation from the All Go condition to the No-Go Bias condition. Positive correlations (larger increases in reaction times associated with larger increases in activation) were found in right frontal pole and left middle frontal gyrus. This result suggests that those individuals who were slowed the most across conditions also had larger increases in activation as a function of increased task difficulty in these regions, suggesting less efficient neural recruitment. On the other hand, these patterns could also reflect participants’ attempts to adapt to the changes in task difficulty. Negative correlations (smaller increases in reaction time correlated with larger increases in activation as a function of naming difficulty) were found in left precentral and postcentral gyri (extending to left supplementary motor cortex and left superior frontal gyrus), bilateral lingual gyrus and cerebellum. Consistent with the results of the negative correlations across all of the Go trials, these negative correlation results suggest that as production difficulty increased, increased recruitment of sensory-motor and frontal regions was associated with better naming performance across individuals (See Supplementary Table 3 and Supplementary Figure 2).
Additionally, we were interested in whether the strength of these brain-behavioral correlations changed as a function of increased naming difficulty (All Go < Go Bias < No-Go Bias). However, no differences in the strength of correlations across the different conditions were found.
No-Go Trials: Brain Activation - Commission Error Rate
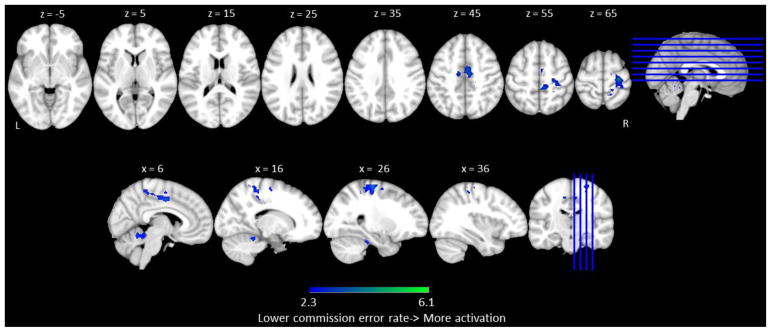
Similar to the analyses with the Go trials, we conducted three types of brain-behavior correlation analyses on No-Go trial performance and activation. The commission error rate was used as the index of behavioral performance for No-Go trials because it represents the ability to inhibit responses correctly. First, correlation analyses were conducted between commission error rate and brain activation across all of the No-Go trials in both conditions (collapsing across Go Bias and No-Go Bias). No significant positive correlation was found. Negative correlations (i.e., lower commission error rates associated with increased activation to No-Go trials across individuals) were found in right anterior cingulate which extended into right precentral, postcentral and supplementary motor cortices, and in bilateral cerebellum. These results indicate that increases in activation in sensory-motor and cognitive control regions was associated with participants who were more successful in response inhibition (See Table 5 and Figure 5)4.
Table 5.
Correlation between commission error rate and fMRI activation across all of the No-Go trials (Go Bias and No-Go Bias).
| Hemisphere | Voxels | Coordinates (mm)
|
Z value | |||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Positive Correlation | ||||||
| No Significant Correlation | ||||||
| Negative Correlation | ||||||
| Superior Frontal Gyrus | Right | 1232 | 24 | −4 | 62 | 2.39 |
| Anterior Cingulate Gyrus | Right | 6 | −6 | 44 | 3.88 | |
| Supplementary Motor Cortex | Right | 8 | −12 | 46 | 3.69 | |
| Precentral Gyrus | Right | 26 | −22 | 64 | 4.19 | |
| Postcentral Gyrus | Right | 30 | −32 | 62 | 3.45 | |
| Cerebellum | Right | 526 | 4 | −52 | −14 | 3.25 |
| Cerebellum | Left | −10 | −40 | −18 | 4.1 | |
Figure 5.
The correlations between commission error rate and fMRI activation across all of the No-Go trials (Go Bias and No-Go Bias). Regions in which lower commission error rates were associated with increases in activation are shown in blue. Axial view slices are depicted in increments of 10, starting at z = 5 and ending at z = 65. Sagittal view slides are depicted in increments of 10, starting at x = 6 andending at x = 36. No significant positive correlations were found.
A second set of commission ER-fMRI activation correlation analyses were conducted on No-Go trials to examine potential correlations between changes in commission error rate and changes in fMRI activation from the No-Go Bias condition to the Go Bias condition (No-Go Bias < Go Bias). However, no significant positive or negative brain-behavior correlations were found.
Lastly, we also explored whether the strength of correlations between commission error rate and fMRI activation differed between the two bias conditions. As there were no significant positive correlations, we limited our analysis to the negative correlations. The negative brain-commission ER correlation was stronger in the Go Bias condition than the No-Go Bias condition in left postcentral gyrus, extending to left posterior superior temporal gyrus and left planum temporale (See Supplemental Table 4).
Power Analysis
To rule out the potential effect of power differences due to the unbalanced number of critical trials across runs, we randomly selected equal number of critical trials from all conditions and compared fMRI activation between an analysis including all critical trials and one including equal numbers of trials across conditions. For both Go trials and No-Go trials, there were no significant differences between these analyses. Furthermore, we compared two similar sets of analyses of brain activation as a function of increased task difficulty for both Go trials and No-Go trials (e.g., Go trials: All Go < Go Bias < No-Go Bias; No-Go trials: No-Go Bias < Go Bias). There were no significant differences between the analysis including all critical trials and the one including equal numbers of trials across conditions (See Supplemental Figure 1). These results suggest that differences in the number of trials per condition were not driving any differences that we observed.
Discussion
The primary goal of this experiment was to investigate the influence of cognitive control (in terms of response inhibition and conflict monitoring) on the phonological aspects of language production by using a phonological Go No-Go picture naming task. We manipulated task difficulty for both naming pictures and inhibiting responses by varying the proportions of Go trials (overt naming) and No-Go trials (withhold naming). Previously, researchers have used language Go No-Go paradigms to study the temporal and spatial properties of speech production (e.g., Rodriguez-Fornells et al., 2002; Rodriguez-Fornells et al., 2005; Schmitt et al., 2001; Van Turennout et al., 1997). However, here we used this paradigm to test the interaction between language production and cognitive control. We predicted that as language production difficulty increased, increased activation would be observed in not only language related regions such as left inferior frontal gyrus, posterior superior temporal sulcus, supramarginal gyrus, and precentral gyrus, but also in regions related to cognitive control such as anterior cingulate cortex and lateral and superior prefrontal cortex. Moreover, we examined whether the engagement of such regions was specific to language production or whether it was related to domain-general cognitive control. We were also interested in the relationships between behavioral performance and fMRI activation, the effect of task difficulty on these relations, and whether these brain-behavior relations were specific to language production, cognitive control processes such as conflict monitoring and response inhibition, or both.
In general, we predicted that increases in difficulty in both language production and response inhibition would elicit increases in brain activity and declines in behavioral performance, as suggested by previous studies (de Zubicaray et al., 2015; de Zubicaray et al., 2001; Kan & Thompson-Schill, 2004; Novick et al., 2010; Piai et al., 2013; Schnur et al., 2009). As predicted, behavioral results showed that Go trial reaction times in the No-Go Bias condition, where naming should be the most difficult, were significantly longer compared to Go trials in the other conditions. We also found that increased difficulty in naming pictures (Go trials: All Go < Go Bias < No-Go Bias) was associated with more activation in right prefrontal regions (such as frontal pole, superior and inferior frontal gyri, and orbitofrontal cortex), anterior and posterior cingulate gyri, right posterior superior and middle temporal gyri, and bilateral supramarginal gyri (See Table 3 and Figure 3A for more details). These results are consistent with previous studies focusing on brain mechanisms of cognitive control and language production (Botvinick et al., 2004; Geranmayeh et al., 2012; Geranmayeh et al., 2014; Indefrey & Levelt, 2004; MacDonald et al., 2000).
The prefrontal cortex and cingulate have well-established roles in cognitive control and conflict monitoring in general (Botvinick et al., 2004; MacDonald et al., 2000), and also in language control during production (Abutalebi et al., 2008; Abutalebi & Green, 2008; Fedorenko & Thompson-Schill, 2014; Kan & Thompson-Schill, 2004; Piai et al., 2016; Riès et al., 2011; Schnur et al., 2009). Within these regions, the role of right inferior frontal gyrus in inhibition, particularly as measured by the Go No-Go paradigm, has also been well documented (Aron, Robbins, & Poldrack, 2004). While these right prefrontal regions are not part of the core language production network, their activation suggests that regions related to general cognitive control were recruited to a greater extent in response to increased naming difficulty.
In addition to cognitive control regions, increased naming difficulty for Go trials also elicited activation in both the typical language network (e.g., left insula, temporal pole, and supramarginal gyrus), and homologous right hemisphere regions. Left insula and supramarginal gyrus have been associated with language production processes and phonological processing (Hickok & Poeppel, 2007; Indefrey & Levelt, 2000, 2004; Poldrack et al., 2001; Poldrack et al., 1999), while temporal cortex has long been associated with semantic processes. Interestingly the increases in homologous right hemisphere activation in response to increased naming difficulty, have also been previously observed by others (e.g., Bench et al., 1993; de Zubicaray et al., 2001). Taken together, the changes we observed in language production regions and general control regions indicated that the control process in language production involved both domain-specific and domain-general resources.
The phonological Go No-Go picture naming paradigm also allowed us to examine behavioral and brain responses to changes in conflict monitoring and response inhibition via the No-Go trials. Response inhibition demands were the highest in the condition with the fewest No-Go trials (Go Bias condition), and consistent with this, participants exhibited a higher commission error rate in this condition compared to the condition with lower response inhibition demands (No-Go Bias condition). Moreover, the average commission error rate of these trials in the Go Bias condition was significantly positively correlated with the error rate on a separate behavioral assessment of inhibitory control (i.e., the AY condition of the AX-CPT, Braver et al., 2007), again consistent with the idea that the commission error rate reflects inhibition and conflict monitoring demands. In general, No-Go trials engaged bilateral frontal regions that have been associated with cognitive control such as bilateral frontal pole and middle and inferior frontal gyri (Simmonds et al., 2008), and bilateral lateral occipital cortex that is associated with processing visual objects (Grill-Spector, Kourtzi, & Kanwisher, 2001; Grill-Spector et al., 1999). This pattern of results suggests that response inhibition demands may modulate the recruitment of basic sensory processing regions. Moreover, we also found significant differences in No-Go trial activation as a function of the changing response inhibition demands. Increases in response inhibition demands (i.e., No-Go trials: No-Go Bias condition < Go Bias condition) were associated with more extensive fMRI activation in bilateral orbitofrontal cortex, which extended to bilateral superior and inferior frontal gyri, bilateral anterior cingulate cortex, and left posterior supramarginal gyrus, which is consistent with previous studies focusing on brain networks of cognitive control (Botvinick et al., 2001; Botvinick et al., 2004; MacDonald et al., 2000; Nieuwenhuis et al., 2003; Simmonds et al., 2008). Specifically, in the Go No-Go paradigm, the role of anterior cingulate cortex in conflict monitoring has been well established (Botvinick et al., 2001; Botvinick et al., 2004; MacDonald et al., 2000). Additionally, anterior cingulate is one of several domain-general control regions that is involved in speech production, not only in healthy participants (Piai et al., 2013) but also in aphasia recovery (Brownsett et al., 2013; Geranmayeh et al., 2017). In the current task, the anterior cingulate cortex could be involved in monitoring the conflict between the response bias to name pictures and the trial-level demand to not name the picture. This conflict was stronger in the Go Bias condition compared to the No-Go Bias condition, and correspondingly the anterior cingulate cortex showed more activation to No-Go trials during the Go Bias condition. The greater recruitment of left supramarginal gyrus, which has an established role in phonological processing (Indefrey & Levelt, 2000; Poldrack et al., 2001; Poldrack et al., 1999), to No-Go trials in the Go Bias condition, may reflect a greater focus on phonological aspects of target names particularly when naming must be inhibited (i.e., during No-Go trials). Furthermore, as reflected in the conjunction analysis, right superior and inferior frontal gyri and left supramarginal gyrus were sensitive to increases in task difficulty during both Go and No-Go trials, suggesting that recruitment of these regions may reflect task difficulty in general whether or not the item was overtly produced.
To better understand the nature of these fMRI activation patterns, we also examined the relationships between behavioral performance and fMRI activation on both Go trials and No-Go trials. Across all of the Go trials, individuals with longer reaction times showed increases in activation in bilateral orbitofrontal cortex, right frontal pole, left middle frontal gyrus, and left occipital regions (Table 4 and supplemental Table 3). This suggests that participants who named pictures the slowest engaged additional cognitive control and visual regions outside of core language regions. On the other hand, individuals who had better naming performance on naming trials (i.e., faster RTs) had increased activation in sensory-motor regions such as bilateral precentral and postcentral gyri, as well as right superior frontal gyrus, right supramarginal gyrus, and bilateral precuneus. The association between efficient behavioral performance and increased activation, across individuals, in the context of increasing naming difficulty suggests a potential compensatory function of these sensory-motor and frontal regions in language production.
Similarly, across all the No-Go trials, individuals who were more successful inhibitiors (i.e., lower commission error rates) had more activation in core cognitive control regions such as right anterior cingulate gyrus (Botvinick et al., 2004; MacDonald et al., 2000) and sensory-motor regions such as right precentral and postcentral gyri and supplementary motor cortex. Additionally, we found that this negative brain-behavior correlation was stronger when response inhibition demands increased (from No-Go Bias condition to the Go Bias condition). These effects were found in left postcentral gyrus, extending to left posterior superior temporal gyrus and left planum temporale, suggesting that individuals who engaged these regions to a greater extent were more successful at behavioral response inhibition. It is noteworthy that these sensory and motor regions, such as right precentral gyrus, were associated with not only successful naming but also successful inhibition of naming, as suggested in the conjunction analysis. This result suggests that similar regions may contribute to successful language production performance regardless of whether an item is overtly produced.
Although our results highlight the interplay of cognitive control and language production processes, there are a few caveats to our results. First, while the phonological Go No-Go paradigm allowed us to examine this interplay of processes, cognitive control demands were likely much higher in this context compared to naturalistic language production. Moreover, a necessity of the Go No-Go paradigm is that the number of critical trials across different conditions must be unbalanced in order to manipulate response biases, which inevitably leads to differences in power across conditions. However, the conditions with fewer critical trials (e.g., Go trials in No-Go Bias condition, or No-Go trials in Go Bias condition) always elicited more extensive activation than conditions with more critical trials (e.g., No-Go trials in No-Go Bias condition, or Go trials in Go Bias condition). This suggests that any power differences may result in an underestimation of potential effects. Additionally, to assess for the potential influence of these effects, we randomly selected equal numbers of critical trials from all conditions. A comparison of brain activation from our main analysis and this subset analysis revealed that there were no statistically significant differences between these analyses (Supplemental Figure 1).
In conclusion, our results provide new behavioral and neural evidence on the effect of task difficulty on executive aspects of language production. Overall, as task demands increased for both Go trials and No-Go trials, behavioral performance declined (i.e., longer reaction times in Go trials, higher commission error rates in No-Go trials) and brain activation increased. The increased activation involved not only language-specific but also domain-general neural resources. Brain regions such as right superior and inferior frontal gyri and left supramarginal gyrus were recruited during all trials and may reflect general effects of task difficulty, as well as aspects of phonological processing that are required during both overt language production and the inhibition of language production. Critically, the increased activation in sensory motor regions such as right precentral gyrus and frontal regions such as right superior frontal gyrus potentially helped participants maintain their behavioral performance not only during language production, but also as response inhibition demands increased. Overall, these results suggest that cognitive control demands affect language production, and that successfully coping with increases in task difficulty relies on both language specific and domain-general cognitive control regions.
Supplementary Material
Acknowledgments
This project was funded by National Institutes of Health (NIH) grant R01 AG034138 (MTD), the Social Sciences Research Institute, and the Department of Psychology at the Pennsylvania State University. We thank Brandi Whyte and Wenjuan Xu for assistance with data analysis. We also thank the staff and scientists at the Social, Life, and Engineering Sciences Imaging Center (SLEIC) and the Center for Language Science (CLS) where the study was conducted for their support of this project.
Footnotes
There has been some debate about whether such high level resources are specialized for language (Fedorenko, Behr, & Kanwisher, 2011), or domain general (Fedorenko & Thompson-Schill, 2014).
We used the random function in excel to select subsets of trials in each condition that matched the number of correct responses for each participant (Go trial range = 21–27, No-Go trial range = 20–27).
A conjunction analysis was conducted on Go trials in the All Go condition and the Go trials in the Bias conditions. The analysis confirmed that the patterns of activation across these two types overlapped in frontal regions (such as bilateral superior frontal gyri, bilateral inferior frontal gyri), temporal regions (such as bilateral superior temporal gyri), and occipital cortex.
To identify regions that were associated with both successful naming and successful inhibition of naming, a conjunction analysis was conducted on Go trials and No-Go trials in terms of the negative brain-behavioral correlations across all trials. Right precentral gyrus and right superior frontal gyrus were identified as regions that are beneficial for both trial types.
References
- Abutalebi J, Annoni JM, Zimine I, Pegna AJ, Seghier ML, Lee-Jahnke H, … Khateb A. Language control and lexical competition in bilinguals: an event-related fMRI study. Cerebral Cortex. 2008;18(7):1496–1505. doi: 10.1093/cercor/bhm182. [DOI] [PubMed] [Google Scholar]
- Abutalebi J, Green DW. Control mechanisms in bilingual language production: Neural evidence from language switching studies. Language and cognitive processes. 2008;23(4):557–582. [Google Scholar]
- Acheson DJ, Wells JB, MacDonald MC. New and updated tests of print exposure and reading abilities in college students. Behavior research methods. 2008;40(1):278–289. doi: 10.3758/brm.40.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends in cognitive sciences. 2004;8(4):170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Balota DA, Yap MJ, Hutchison KA, Cortese MJ, Kessler B, Loftis B, … Treiman R. The English lexicon project. Behavior Research Methods. 2007;39(3):445–459. doi: 10.3758/bf03193014. [DOI] [PubMed] [Google Scholar]
- Banich MT. The missing link: the role of interhemispheric interaction in attentional processing. Brain and cognition. 1998;36(2):128–157. doi: 10.1006/brcg.1997.0950. [DOI] [PubMed] [Google Scholar]
- Barch DM, Braver TS, Nystrom LE, Forman SD, Noll DC, Cohen JD. Dissociating working memory from task difficulty in human prefrontal cortex. Neuropsychologia. 1997;35(10):1373–1380. doi: 10.1016/s0028-3932(97)00072-9. [DOI] [PubMed] [Google Scholar]
- Barr DJ, Levy R, Scheepers C, Tily HJ. Random effects structure for confirmatory hypothesis testing: Keep it maximal. Journal of memory and language. 2013;68(3):255–278. doi: 10.1016/j.jml.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. 2014 arXiv preprint arXiv:1406.5823. [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. NeuroImage. 2003;20(2):1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Bellgrove MA, Hester R, Garavan H. The functional neuroanatomical correlates of response variability: evidence from a response inhibition task. Neuropsychologia. 2004;42(14):1910–1916. doi: 10.1016/j.neuropsychologia.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Bench C, Frith C, Grasby P, Friston K, Paulesu E, Frackowiak R, Dolan R. Investigations of the functional anatomy of attention using the Stroop test. Neuropsychologia. 1993;31(9):907–922. doi: 10.1016/0028-3932(93)90147-r. [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cerebral Cortex. 2009;19(12):2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108(3):624. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends in cognitive sciences. 2004;8(12):539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Braver TS, Gray JR, Burgess GC. Explaining the many varieties of working memory variation: Dual mechanisms of cognitive control. Variation in working memory. 2007:76–106. [Google Scholar]
- Brodeur MB, Guérard K, Bouras M. Bank of Standardized Stimuli (BOSS) phase II: 930 new normative photos. PLoS ONE. 2014;9(9):e106953. doi: 10.1371/journal.pone.0106953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S, Laird AR, Pfordresher PQ, Thelen SM, Turkeltaub P, Liotti M. The somatotopy of speech: Phonation and articulation in the human motor cortex. Brain and cognition. 2009;70(1):31–41. doi: 10.1016/j.bandc.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownsett SL, Warren JE, Geranmayeh F, Woodhead Z, Leech R, Wise RJ. Cognitive control and its impact on recovery from aphasic stroke. Brain. 2013;137(1):242–254. doi: 10.1093/brain/awt289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke DM, Shafto MA. In: Language and aging. Craik F, Salthouse T, editors. New York: Psychology Press; 2008. [Google Scholar]
- Chang SE, Kenney MK, Loucks TM, Poletto CJ, Ludlow CL. Common neural substrates support speech and non-speech vocal tract gestures. NeuroImage. 2009;47(1):314–325. doi: 10.1016/j.neuroimage.2009.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criaud M, Boulinguez P. Have we been asking the right questions when assessing response inhibition in go/no-go tasks with fMRI? A meta-analysis and critical review. Neuroscience and Biobehavioral Reviews. 2013;37(1):11–23. doi: 10.1016/j.neubiorev.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Crum RM, Anthony JC, Bassett SS, Folstein MF. Population-based norms for the Mini-Mental State Examination by age and educational level. Jama. 1993;269(18):2386–2391. [PubMed] [Google Scholar]
- Dale AM. Optimal experimental design for event-related fMRI. Human brain mapping. 1999;8(2–3):109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zubicaray G, Andrew C, Zelaya F, Williams S, Dumanoir C. Motor response suppression and the prepotent tendency to respond: a parametric fMRI study. Neuropsychologia. 2000;38(9):1280–1291. doi: 10.1016/s0028-3932(00)00033-6. [DOI] [PubMed] [Google Scholar]
- de Zubicaray G, McMahon K, Howard D. Perfusion fMRI evidence for priming of shared feature-to-lexical connections during cumulative semantic interference in spoken word production. Language, cognition and neuroscience. 2015;30(3):261–272. [Google Scholar]
- de Zubicaray G, Wilson S, McMahon K, Muthiah S. The semantic interference effect in the picture-word paradigm: An event-related fMRI study employing overt responses. Human brain mapping. 2001;14(4):218–227. doi: 10.1002/hbm.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test® - Second Edition. San Antonio, Tx: Pearson; 2000. [Google Scholar]
- Dell GS. A spreading-activation theory of retrieval in sentence production. Psychological review. 1986;93(3):283. [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, … Hyman BT. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dove A, Pollmann S, Schubert T, Wiggins CJ, von Cramon DY. Prefrontal cortex activation in task switching: an event-related fMRI study. Cognitive Brain Research. 2000;9(1):103–109. doi: 10.1016/s0926-6410(99)00029-4. [DOI] [PubMed] [Google Scholar]
- Fedorenko E, Behr MK, Kanwisher N. Functional specificity for high-level linguistic processing in the human brain. Proceedings of the National Academy of Sciences. 2011;108(39):16428–16433. doi: 10.1073/pnas.1112937108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Duncan J, Kanwisher N. Language-selective and domain-general regions lie side by side within Broca’s area. Current Biology. 2012;22(21):2059–2062. doi: 10.1016/j.cub.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Thompson-Schill SL. Reworking the language network. Trends in cognitive sciences. 2014;18(3):120–126. doi: 10.1016/j.tics.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Freund M, Gordon B, Nozari N. Conflict-based regulation of control in language production. Paper presented at the Proceedings of the 38th annual conference of the cognitive science society.2016. [Google Scholar]
- Friederici AD, Alter K. Lateralization of auditory language functions: a dynamic dual pathway model. Brain and language. 2004;89(2):267–276. doi: 10.1016/S0093-934X(03)00351-1. [DOI] [PubMed] [Google Scholar]
- Gauvin HS, De Baene W, Brass M, Hartsuiker RJ. Conflict monitoring in speech processing: An fMRI study of error detection in speech production and perception. NeuroImage. 2016;126:96–105. doi: 10.1016/j.neuroimage.2015.11.037. [DOI] [PubMed] [Google Scholar]
- Geranmayeh F, Brownsett SL, Leech R, Beckmann CF, Woodhead Z, Wise RJ. The contribution of the inferior parietal cortex to spoken language production. Brain and Language. 2012;121(1):47–57. doi: 10.1016/j.bandl.2012.02.005. [DOI] [PubMed] [Google Scholar]
- Geranmayeh F, Chau TW, Wise RJ, Leech R, Hampshire A. Domain-general subregions of the medial prefrontal cortex contribute to recovery of language after stroke. Brain. 2017;140(7):1947–1958. doi: 10.1093/brain/awx134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geranmayeh F, Wise RJ, Mehta A, Leech R. Overlapping networks engaged during spoken language production and its cognitive control. Journal of Neuroscience. 2014;34(26):8728–8740. doi: 10.1523/JNEUROSCI.0428-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover GH, Mueller BA, Turner JA, Van Erp TG, Liu TT, Greve DN, … Keator DB. Function biomedical informatics research network recommendations for prospective multicenter functional MRI studies. Journal of Magnetic Resonance Imaging. 2012;36(1):39–54. doi: 10.1002/jmri.23572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill-Spector K, Kourtzi Z, Kanwisher N. The lateral occipital complex and its role in object recognition. Vision research. 2001;41(10):1409–1422. doi: 10.1016/s0042-6989(01)00073-6. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Kushnir T, Edelman S, Avidan G, Itzchak Y, Malach R. Differential processing of objects under various viewing conditions in the human lateral occipital complex. Neuron. 1999;24(1):187–203. doi: 10.1016/s0896-6273(00)80832-6. [DOI] [PubMed] [Google Scholar]
- Hanley JR, Cortis C, Budd MJ, Nozari N. Did I say dog or cat? A study of semantic error detection and correction in children. Journal of experimental child psychology. 2016;142:36–47. doi: 10.1016/j.jecp.2015.09.008. [DOI] [PubMed] [Google Scholar]
- Hayasaka S, Nichols TE. Validating cluster size inference: random field and permutation methods. Neuroimage. 2003;20:2343–2356. doi: 10.1016/j.neuroimage.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nature Reviews Neuroscience. 2007;8(5):393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Hirshorn EA, Thompson-Schill SL. Role of the left inferior frontal gyrus in covert word retrieval: neural correlates of switching during verbal fluency. Neuropsychologia. 2006;44(12):2547–2557. doi: 10.1016/j.neuropsychologia.2006.03.035. [DOI] [PubMed] [Google Scholar]
- Indefrey P, Levelt WJ. The new cognitive neurosciences. 2. MIT press; 2000. The neural correlates of language production; pp. 845–865. [Google Scholar]
- Indefrey P, Levelt WJ. The spatial and temporal signatures of word production components. Cognition. 2004;92(1):101–144. doi: 10.1016/j.cognition.2002.06.001. [DOI] [PubMed] [Google Scholar]
- Jansma BM, Schiller NO. Monitoring syllable boundaries during speech production. Brain and language. 2004;90(1–3):311–317. doi: 10.1016/S0093-934X(03)00443-7. [DOI] [PubMed] [Google Scholar]
- Kan IP, Thompson-Schill SL. Effect of name agreement on prefrontal activity during overt and covert picture naming. Cognitive, Affective, & Behavioral Neuroscience. 2004;4(1):43–57. doi: 10.3758/cabn.4.1.43. [DOI] [PubMed] [Google Scholar]
- Kuznetsova A, Brockhoff PB, Christensen RH. lmerTest package: Tests in linear mixed effects models. Journal of Statistical Software. 2017;82(13):1–26. [Google Scholar]
- Levelt WJ. Models of word production. Trends in cognitive sciences. 1999;3(6):223–232. doi: 10.1016/s1364-6613(99)01319-4. [DOI] [PubMed] [Google Scholar]
- Levelt WJ, Roelofs A, Meyer AS. Multiple perspectives on word production. Behavioral and Brain Sciences. 1999;22(01):61–69. [Google Scholar]
- Lutkenhoff ES, Rosenberg M, Chiang J, Zhang K, Pickard JD, Owen AM, Monti MM. Optimized brain extraction for pathological brains (optiBET) PLoS ONE. 2014;9(12):e115551. doi: 10.1371/journal.pone.0115551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AW, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288(5472):1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Martin RC. Language processing: functional organization and neuroanatomical basis. Annual review of psychology. 2003;54(1):55–89. doi: 10.1146/annurev.psych.54.101601.145201. [DOI] [PubMed] [Google Scholar]
- Mayer AR, Teshiba TM, Franco AR, Ling J, Shane MS, Stephen JM, Jung RE. Modeling conflict and error in the medial frontal cortex. Human brain mapping. 2012;33(12):2843–2855. doi: 10.1002/hbm.21405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirman D, Chen Q, Zhang Y, Wang Z, Faseyitan OK, Coslett HB, Schwartz MF. Neural organization of spoken language revealed by lesion-symptom mapping. Nature communications. 2015:6. doi: 10.1038/ncomms7762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsell S. Task switching. Trends in cognitive sciences. 2003;7(3):134–140. doi: 10.1016/s1364-6613(03)00028-7. [DOI] [PubMed] [Google Scholar]
- Moreno-Martínez FJ, Montoro PR. An ecological alternative to Snodgrass & Vanderwart: 360 high quality colour images with norms for seven psycholinguistic variables. PLoS ONE. 2012;7(5):e37527. doi: 10.1371/journal.pone.0037527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cognitive, Affective, & Behavioral Neuroscience. 2012;12(2):241–268. doi: 10.3758/s13415-011-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis S, Yeung N, Van Den Wildenberg W, Ridderinkhof KR. Electrophysiological correlates of anterior cingulate function in a go/no-go task: effects of response conflict and trial type frequency. Cognitive, affective, & behavioral neuroscience. 2003;3(1):17–26. doi: 10.3758/cabn.3.1.17. [DOI] [PubMed] [Google Scholar]
- Novick JM, Trueswell JC, Thompson-Schill SL. Broca’s area and language processing: Evidence for the cognitive control connection. Language and Linguistics Compass. 2010;4(10):906–924. [Google Scholar]
- Nozari N, Dell GS, Schwartz MF. Is comprehension necessary for error detection? A conflict-based account of monitoring in speech production. Cognitive psychology. 2011;63(1):1–33. doi: 10.1016/j.cogpsych.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozari N, Freund M, Breining B, Rapp B, Gordon B. Cognitive control during selection and repair in word production. Language, cognition and neuroscience. 2016;31(7):886–903. doi: 10.1080/23273798.2016.1157194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozari N, Novick J. Monitoring and control in language production. Current Directions in Psychological Science. 2017;26(5):403–410. [Google Scholar]
- Peramunage D, Blumstein SE, Myers EB, Goldrick M, Baese-Berk M. Phonological neighborhood effects in spoken word production: An fMRI study. Journal of cognitive neuroscience. 2011;23(3):593–603. doi: 10.1162/jocn.2010.21489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piai V, Riès SK, Swick D. Lesions to lateral prefrontal cortex impair lexical interference control in word production. Frontiers in human neuroscience. 2016;9:721. doi: 10.3389/fnhum.2015.00721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piai V, Roelofs A, Acheson DJ, Takashima A, De Zubicaray GI, Heim S. anterior cingulate cortex in spoken word production. 2013 doi: 10.3389/fnhum.2013.00832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobric G, Jefferies E, Ralph MAL. Anterior temporal lobes mediate semantic representation: mimicking semantic dementia by using rTMS in normal participants. Proceedings of the National Academy of Sciences. 2007;104(50):20137–20141. doi: 10.1073/pnas.0707383104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Temple E, Protopapas A, Nagarajan S, Tallal P, Merzenich M, Gabrieli JD. Relations between the neural bases of dynamic auditory processing and phonological processing: evidence from fMRI. Journal of Cognitive Neuroscience. 2001;13(5):687–697. doi: 10.1162/089892901750363235. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JD. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuroimage. 1999;10(1):15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: a review of 100 fMRI studies published in 2009. Annals of the New York Academy of Sciences. 2010;1191(1):62–88. doi: 10.1111/j.1749-6632.2010.05444.x. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306(5695):443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Van Den Wildenberg WP, Segalowitz SJ, Carter CS. Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain and cognition. 2004;56(2):129–140. doi: 10.1016/j.bandc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Riès S, Janssen N, Dufau S, Alario FX, Burle B. General-purpose monitoring during speech production. Journal of cognitive neuroscience. 2011;23(6):1419–1436. doi: 10.1162/jocn.2010.21467. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Fornells A, Schmitt BM, Kutas M, Münte TF. Electrophysiological estimates of the time course of semantic and phonological encoding during listening and naming. Neuropsychologia. 2002;40(7):778–787. doi: 10.1016/s0028-3932(01)00188-9. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Fornells A, Van Der Lugt A, Rotte M, Britti B, Heinze HJ, Münte TF. Second language interferes with word production in fluent bilinguals: brain potential and functional imaging evidence. Journal of Cognitive Neuroscience. 2005;17(3):422–433. doi: 10.1162/0898929053279559. [DOI] [PubMed] [Google Scholar]
- Roskies A, Fiez J, Balota D, Raichle M, Petersen S. Task-dependent modulation of regions in the left inferior frontal cortex during semantic processing. Journal of Cognitive Neuroscience. 2001;13(6):829–843. doi: 10.1162/08989290152541485. [DOI] [PubMed] [Google Scholar]
- Rypma B, D’Esposito M. The roles of prefrontal brain regions in components of working memory: effects of memory load and individual differences. Proceedings of the National Academy of Sciences. 1999;96(11):6558–6563. doi: 10.1073/pnas.96.11.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rypma B, Prabhakaran V, Desmond JE, Glover GH, Gabrieli JD. Load-dependent roles of frontal brain regions in the maintenance of working memory. NeuroImage. 1999;9(2):216–226. doi: 10.1006/nimg.1998.0404. [DOI] [PubMed] [Google Scholar]
- Sasisekaran J, Luc F. Phoneme monitoring in silent naming and perception in adults who stutter. Journal of Fluency Disorders. 2006;31(4):284–302. doi: 10.1016/j.jfludis.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Schiller NO, Jansma BM, Peters J, Levelt WJ. Monitoring metrical stress in polysyllabic words. Language and cognitive processes. 2006;21(1–3):112–140. [Google Scholar]
- Schmitt BM, Rodriguez-Fornells A, Kutas M, Münte TF. Electrophysiological estimates of semantic and syntactic information access during tacit picture naming and listening to words. Neuroscience Research. 2001;41(3):293–298. doi: 10.1016/s0168-0102(01)00286-3. [DOI] [PubMed] [Google Scholar]
- Schnur TT, Schwartz MF, Kimberg DY, Hirshorn E, Coslett HB, Thompson-Schill SL. Localizing interference during naming: convergent neuroimaging and neuropsychological evidence for the function of Broca's area. Proceedings of the National Academy of Sciences. 2009;106(1):322–327. doi: 10.1073/pnas.0805874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MF, Dell GS, Martin N, Gahl S, Sobel P. A case-series test of the interactive two-step model of lexical access: Evidence from picture naming. Journal of memory and language. 2006;54(2):228–264. doi: 10.1016/j.jml.2006.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier ML, Price CJ. Reading aloud boosts connectivity through the putamen. Cerebral Cortex. 2009:bhp123. doi: 10.1093/cercor/bhp123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Z, Meyer AS, Roelofs A. Selective and nonselective inhibition of competitors in picture naming. Memory & cognition. 2013;41(8):1200–1211. doi: 10.3758/s13421-013-0332-7. [DOI] [PubMed] [Google Scholar]
- Shao Z, Roelofs A, Acheson DJ, Meyer AS. Electrophysiological evidence that inhibition supports lexical selection in picture naming. brain research. 2014;1586:130–142. doi: 10.1016/j.brainres.2014.07.009. [DOI] [PubMed] [Google Scholar]
- Shao Z, Roelofs A, Meyer AS. Sources of individual differences in the speed of naming objects and actions: The contribution of executive control. Quarterly Journal of Experimental Psychology. 2012;65(10):1927–1944. doi: 10.1080/17470218.2012.670252. [DOI] [PubMed] [Google Scholar]
- Shitova N, Roelofs A, Schriefers H, Bastiaansen M, Schoffelen JM. Control adjustments in speaking: Electrophysiology of the Gratton effect in picture naming. Cortex. 2017;92:289–303. doi: 10.1016/j.cortex.2017.04.017. [DOI] [PubMed] [Google Scholar]
- Simmonds DJ, Pekar JJ, Mostofsky SH. Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia. 2008;46(1):224–232. doi: 10.1016/j.neuropsychologia.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283(5408):1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, … Flitney DE. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of experimental psychology. 1935;18(6):643. [Google Scholar]
- Tydgat I, Diependaele K, Hartsuiker RJ, Pickering MJ. How lingering representations of abandoned context words affect speech production. Acta psychologica. 2012;140(3):218–229. doi: 10.1016/j.actpsy.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Tydgat I, Stevens M, Hartsuiker RJ, Pickering MJ. Deciding where to stop speaking. Journal of memory and language. 2011;64(4):359–380. [Google Scholar]
- Van Maanen L, Van Rijn H. The locus of the Gratton effect in picture word interference. Topics in Cognitive Science. 2010;2(1):168–180. doi: 10.1111/j.1756-8765.2009.01069.x. [DOI] [PubMed] [Google Scholar]
- Van Turennout M, Hagoort P, Brown CM. Electrophysiological evidence on the time course of semantic and phonological processes in speech production. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1997;23(4):787. doi: 10.1037//0278-7393.23.4.787. [DOI] [PubMed] [Google Scholar]
- Venables WN, Smith DM. An Introduction to R. R Foundation for Statistical Computing; Vienna, Austria: 2006. The R development core team. [Google Scholar]
- Visser M, Jefferies E, Ralph ML. Semantic processing in the anterior temporal lobes: a meta-analysis of the functional neuroimaging literature. Journal of Cognitive Neuroscience. 2010;22(6):1083–1094. doi: 10.1162/jocn.2009.21309. [DOI] [PubMed] [Google Scholar]
- Wechsler D, Coalson DL, Raiford SE. WAIS-III: Wechsler adult intelligence scale. Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- Wheeldon LR, Levelt WJ. Monitoring the time course of phonological encoding. Journal of memory and language. 1995;34(3):311–334. [Google Scholar]
- Woods SP, Delis DC, Scott JC, Kramer JH, Holdnack JA. The California Verbal Learning Test second edition: Test-retest reliability, practice effects, and reliable change indices for the standard and alternate forms. Archives of Clinical Neuropsychology. 2006;21(5):413–420. doi: 10.1016/j.acn.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage. 2004;21(4):1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Worsley K. Statistical analysis of activation images. Functional MRI: An introduction to methods. 2001;14:251–270. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.