Abstract
Post-transplant diarrhea is associated with kidney allograft failure and death but its etiology remains unknown in the majority of cases. Because altered gut microbial ecology is a potential basis for diarrhea, we investigated whether post-transplant diarrhea is associated with gut dysbiosis. We enrolled 71 kidney allograft recipients for serial fecal specimen collections in the first 3 months of transplantation and profiled the gut microbiota using 16S rRNA gene V4-V5 deep sequencing. The Shannon diversity index was significantly lower in 28 diarrheal fecal specimens from 25 recipients with post-transplant diarrhea than in 112 fecal specimens from 46 recipients without post-transplant diarrhea. We found lower relative abundance of 13 commensal genera (Benjamini-Hochberg adjusted p value ≤ 0.15) in the diarrheal fecal specimens including the same 4 genera identified in our prior study. The 28 diarrheal fecal specimens were also evaluated by a multiplexed PCR assay for 22 bacterial, viral, and protozoan gastrointestinal pathogens, and 26 specimens were negative for infectious etiologies. Using PICRUSt to predict metagenomic functions, we found diarrheal fecal specimens had a lower abundance of metabolic genes. Our findings suggest that post-transplant diarrhea is not associated with common infectious diarrheal pathogens but with a gut dysbiosis.
1. INTRODUCTION
Post-transplant diarrhea affects 1 in 5 patients in the first year after kidney transplantation (1) and is associated with a decreased quality of life (2) and with allograft failure and death (3). Despite being a common complication, the etiology of post-transplant diarrhea is not identified in a majority of cases. In a study of over 7,000 cases of post-transplant diarrhea, an astounding 85 percent of diarrheal cases were classified as unspecified noninfectious diarrhea (3).
In the absence of a specific infectious etiology, transplant physicians often attribute the etiology of post-transplant diarrhea to immunosuppressive drugs such as mycophenolate mofetil (MMF) (4). Reduction of MMF dosing has thus become a common strategy to treat post-transplant diarrhea, but this commonly used approach can lead to insufficient immunosuppression (4) and an increased risk of acute rejection and allograft loss (5).
Very little is known about the disturbances in the gut microbial communities during an episode of post-transplant diarrhea. In a pilot study comprised of 26 kidney allograft recipients, we found that kidney transplant recipients with post-transplant diarrhea have a lower relative abundance of the genera, Ruminococcus, Dorea, Bacteroides, and Coprococcus, in their diarrheal fecal specimens (6). Our findings led us to consider the idea that post-transplant diarrhea is associated with a decrease in the relative abundance of commensal bacterial taxa. To test our supposition, we characterized the gut microbiota in fecal specimens from an independent cohort of 71 kidney transplant recipients and investigated whether post-transplant diarrhea is associated with gut dysbiosis.
2. MATERIALS AND METHODS
2.1. Transplant Cohort
One hundred and twenty-five patients received a kidney transplant at New York Presbyterian Hospital - Weill Cornell Medical Center between August 2015 and February 2016. We enrolled 107 for serial fecal specimen collections, and 71 of the 107 enrollees provided at least 1 fecal specimen. Demographic and transplant-related information from the 71 transplant recipients are further described in Supporting Information (SI) Materials and Methods. The Institutional Review Board approved this study (Protocol Number: 1207012730) and each subject gave written informed consent.
2.2. Diarrhea Classification and Cohort Selection
Subjects were instructed to provide a fecal specimen within one day of collection. Each fecal specimen was subsequently aliquoted into approximately 200 mg aliquots and stored at −80°C. Fecal specimens were collected at post-transplant week 1, week 2, week 4, week 12, and during episodes of diarrhea. We collected the specimens during the first 3 months of transplantation as little is known about early post-transplant diarrhea and we had close clinical follow up during this time period.
Diarrhea was defined as a complaint of diarrhea and by the World Health Organization definition of 3 or more unusually loose/liquid bowel movements per day (7). Diarrheal fecal specimens were defined as fecal specimens collected within 24 hours of an episode of active post-transplant diarrhea. Further details are described in SI Materials and Methods.
The subjects provided 199 fecal specimens with 193 specimens (97%) collected within one day and 6 (3%) specimens collected within 2 days. Further details of the specimens are found in Figure 1. In addition to the 199 fecal specimens, we analyzed 2 subjects who underwent fecal microbial transplantation, one of which was part of the 71 subjects. These 2 subjects provided an additional 14 fecal specimens.
Figure 1. Flow Chart of the Profiled Fecal Specimens from the Diarrhea Group and the No Diarrhea Group.
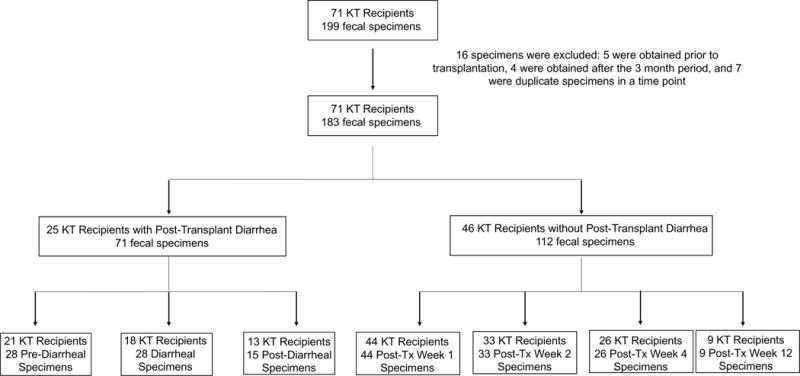
Seventy-one kidney transplant recipients provided 199 fecal specimens and 183 fecal specimens were analyzed. 25 kidney transplant recipients developed post-transplant diarrhea (Diarrhea Group) in the first 3 months following kidney transplantation and provided 71 fecal specimens which were classified as pre-diarrheal specimens, diarrheal specimens (collected at the time of a diarrheal episode), or post-diarrheal specimens. Forty-six kidney transplant recipients did not develop post-transplant diarrhea within the first 3 months of transplantation (No Diarrhea Group) and provided 112 fecal specimens which were collected during post-transplant week 1, post-transplant week 2, post-transplant week 4, or post-transplant week 12. KT: Kidney Transplant.
2.3. 16S rRNA Gene Deep Sequencing and Bioinformatics Analyses
DNA was isolated from the fecal specimens using a phenol-chloroform extraction method involving bead-beater disruption. The 16S rRNA gene V4-V5 variable region was amplified using polymerase chain reaction (PCR) and was sequenced on an Illumina MiSeq platform (250 bp × 250 bp) (6). Multiplexing of samples, taxonomic classification of reads, and bioinformatics analyses are described in SI Materials and Methods.
2.4. Multiplex PCR Gastrointestinal Pathogen Panel
One aliquot from each of the diarrheal fecal specimens was analyzed using the FilmArray® Gastrointestinal Panel (v2.1) (BioFire Diagnostics, LLC) which tests for 22 bacterial, viral, and protozoan diarrheal pathogens (Full list, SI Materials and Methods) (8). Each aliquot was combined with Para-Pak C&S media (Meridian Bioscience, Inc) in a 1:3 ratio (fecal specimen to Para-Pak C&S media) and analyzed using the BioFire FilmArray 2.0 system.
2.5. Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt) Analysis
Sequences were analyzed using the closed-reference operational taxonomic units (OTU) picking protocol (MacQiime 1.9.1) to construct an OTU table. The python functions, normalize_by_copy_number, predict_metagenomes.py, and categorize_by_function.py were used to estimate relative abundances of bacterial genes at KEGG levels (PICRUSt v1.0.0) (9).
2.6. Statistical Analyses
The distributions of continuous variables in any two unpaired groups were compared using Wilcoxon rank sum test; the distribution of continuous variables in any paired groups were compared using Wilcoxon sign rank test. Proportions of categorical variables were compared using Fisher’s exact test. For comparing relative abundances of taxa, multiple comparisons were adjusted for using the Benjamini-Hochberg correction (adjusted P value≤0.15). A hierarchical logistic model using the stan_glmer function in Rstanarm package version 2.17.2 (10) was utilized to identify predictors that distinguish non-diarrheal samples from diarrheal samples from microbial taxa and clinical variables. Repeated measures from the same subject were accounted for using random intercepts per patient ID. We log-transformed taxa abundances and set 0 values to half of the lowest observed abundance of the corresponding taxon. Uninformative priors were chosen for intercepts (Normal distributions with mean 0 and standard deviation of 10) and weakly regularizing priors were chosen for slopes (Normal distributions with mean 0 and standard deviation of 2.5); 8000 posterior samples were generated using the Hamiltonian Monte Carlo algorithm. For metabolic genes derived in PICRUSt, we utilized STAMP to analyze differences at the KEGG level 3, a database for understanding metabolic functions, and at the gene level (Welch’s t-test, Benjamini-Hochberg adjustment) (11). Analyses were performed in R 3.1.1 or in STAMP.
3. RESULTS
3.1. Characteristics of the Kidney Transplant Cohort
Seventy-one kidney transplant recipients provided 199 fecal specimens within the first 3 months after transplantation. Twenty-five transplant recipients developed post-transplant diarrhea within the first 3 months after transplantation and provided 71 fecal specimens (Diarrhea Group). Forty-six transplant recipients did not develop post-transplant diarrhea at any time during the first 3 months of transplantation and provided 112 fecal specimens (No Diarrhea Group). Further details of the fecal specimens are found in Figure 1 and a summary of clinical demographics and transplant related characteristics is listed in Table 1. We performed 16S rRNA gene deep sequencing on DNA isolated from the fecal specimens and obtained a mean (±SD) of high-quality 16S rRNA gene sequences of 25,133±13,858 per specimen.
Table 1.
Characteristics of the Study Cohorts with or without Post-Transplant Diarrhea
| Characteristics | Diarrhea Group | No Diarrhea Group | P value b |
|---|---|---|---|
| N=25 subjects a | N=46 subjects a | ||
| Age Median (Interquartile Range) | 56 (44, 63) | 53 (44, 65) | 0.96 |
| Female, N (%) | 15 (60%) | 19 (41%) | 0.15 |
| Hispanic Ethnicity, N (%) | 3 (12%) | 6 (13%) | 0.99 |
| Race | |||
| White, N (%) | 9 (36%) | 26 (57%) | 0.14 |
| African American, N (%) | 8 (32%) | 11 (24%) | 0.58 |
| Asian, N (%) | 4 (16%) | 4 (9%) | 0.44 |
| Other | 4 (16%) | 5 (11%) | 0.71 |
| Cause of End Stage Renal Disease | |||
| Hypertension/Diabetes Mellitus | 5 (20%) | 12 (26%) | 0.77 |
| Diabetes Mellitus | 2 (8%) | 2 (4%) | 0.61 |
| Hypertension | 3 (12%) | 8 (17%) | 0.74 |
| Polycystic Kidney Disease | 3 (12%) | 4 (9%) | 0.69 |
| Systemic Lupus Erythematosus | 4 (16%) | 2 (4%) | 0.18 |
| IgA Nephropathy | 2 (8%) | 5 (11%) | 0.99 |
| Focal Segmental Glomerulosclerosis | 2 (8%) | 4 (9%) | 0.99 |
| Other | 4 (16%) | 9 (20%) | 0.99 |
| History of Diabetes Mellitus, N (%) | 7 (28%) | 15 (33%) | 0.79 |
| Body Mass Index ≥ 30 kg/m2, N (%) | 5 (20%) | 17 (37%) | 0.18 |
| Simultaneous Liver-Kidney Transplantation, N (%) | 1 (4%) | 0 (0%) | 0.35 |
| Living Donor Transplantation, N (%) | 12 (48%) | 36 (78%) | 0.02 |
| Antibiotics in the Year Prior to Transplantation, N (%) | 13 (52%) | 26 (57%) | 0.80 |
| Probiotics in the Year Prior to Transplantation, N (%) | 5 (20%) | 5 (11%) | 0.31 |
| Vegetarian Diet, N (%) | 1 (4%) | 1 (2%) | 0.99 |
| History of Lactose Intolerance, N(%) | 7 (28%) | 10 (22%) | 0.57 |
| History of Inflammatory Bowel Disease, N (%) | 1 (4%) | 0 (0%) | 0.35 |
| History of Gastric Bypass or Colon Resection, N (%) | 4 (16%) | 5 (11%) | 0.71 |
| History of C. difficile Infection, N (%) | 3 (12%) | 1 (2%) | 0.12 |
| Immunosuppressive Therapy | |||
| Induction Therapy | |||
| Anti-thymocyte globulin, N (%) | 12 (48%) | 35 (76%) | 0.02 |
| Basiliximab, N (%) | 13 (52%) | 11 (24%) | 0.02 |
| Maintenance Therapy | |||
| Tacrolimus, N (%) | 23 (92%) | 46 (100%) | 0.12 |
| Belatacept, N (%) | 2 (8%) | 0 (0%) | 0.12 |
| Mycophenolate Mofetil, N (%) | 24 (96%) | 45 (98%) | 0.99 |
| Mycophenolate 2000 mg Daily Dosage, N (%) | 18 (72%) | 24 (52%) | 0.12 |
| Prednisone maintenance, N (%) | 10 (40%) | 11 (24%) | 0.18 |
| Antibiotic Prophylaxis | |||
| Perioperative Cefazolin, N (%) | 21 (84%) | 39 (85%) | 0.99 |
| Trimethoprim/Sulfamethoxazole, N (%) | 23 (92%) | 44 (96%) | 0.61 |
Among the 71 study participants who provided serial fecal specimens, 25 experienced post-transplant diarrhea within the first three months of transplantation and the remaining 46 participants remained diarrhea free during the 3 months following transplantation. The median number of serial fecal specimens collected from these patients was 3 per study participant.
P value was calculated using Fisher’s exact test for categorical values and Wilcoxon rank sum test for continuous values.
Among the 25 subjects in the Diarrhea Group, fecal specimens were not available from 7 recipients at the time of the diarrheal episode. A total of 28 diarrheal fecal specimens were available at the time of diarrheal episode from 18 subjects in the Diarrhea Group. We compared the gut microbial composition of these 28 diarrheal fecal specimens from the Diarrhea Group to the microbial profiles of 112 specimens from the 46 subjects in the No Diarrhea Group.
3.2. Lower Microbial Diversity and Decreased Abundance of Commensal Bacterial Taxa in the Diarrheal Fecal Specimens
The median Shannon diversity index (12) was lower in the 28 diarrheal fecal specimens from the 18 subjects in the Diarrhea Group than in the 112 fecal specimens from the 46 subjects in the No Diarrhea Group (2.4 vs. 3.1, P=3×10−7, Wilcoxon rank-sum test). Nonmetric multidimensional scaling (NMDS) was performed on the 28 diarrheal fecal specimens from the 18 subjects in the Diarrhea Group and the 112 fecal specimens from the 46 subjects in the No Diarrhea Group, using Bray-Curtis dissimilarity index. The diarrheal fecal specimens separated from the fecal specimens from the No Diarrhea Group by NMDS analysis (Supplementary Figure 1).
Evaluation of the most common taxa (>1% mean relative abundance among all fecal specimens) was performed at the genus levels. The relative abundance of Eubacterium, Anaerostipes, Coprococcus, Romboutsia, Ruminococcus, Dorea, Faecalibacterium, Fusicatenibacter, Oscillibacter, Ruminiclostridium, Blautia, Bifidobacterium, and Bacteroides were significantly lower and the relative abundance of Enterococcus, Escherichia, and Lachnoclostridium were higher in the diarrheal fecal specimens than in the fecal specimens from the No Diarrhea Group (16 significantly different taxa at the genus level) (Table 2). A heatmap of the 24 genera is shown with 13 genera being significantly lower in the diarrheal fecal specimens and 3 genera being significantly higher in the diarrheal fecal specimens compared to the fecal specimens from the No Diarrhea Group (Figure 2).
Table 2.
Genus Level Gut Microbial Composition in the Fecal Specimens from the Diarrhea Group and the No Diarrhea Group
| Bacterial Taxonomy Genus | Median Relative Abundance Fecal Specimens from the Diarrhea Group (N = 28 specimens, 18 subjects) |
Median Relative Abundance Fecal Specimens from the No Diarrhea Group (N = 112 specimens, 46 subjects) |
P value | BH Adjusted P value |
|---|---|---|---|---|
| Eubacterium | 0.002 | 0.017 | 1.5E-09 | 3.7E-08 |
| Anaerostipes | 0.000 | 0.005 | 2.7E-08 | 2.4E-07 |
| Coprococcus | 0.000 | 0.004 | 3.0E-08 | 2.4E-07 |
| Romboutsia | 0.000 | 0.014 | 4.2E-06 | 2.5E-05 |
| Ruminococcus | 0.007 | 0.025 | 8.3E-06 | 4.0E-05 |
| Dorea | 0.000 | 0.007 | 3.4E-05 | 1.3E-04 |
| Enterococcus | 0.002 | 0.000 | 1.3E-04 | 4.1E-04 |
| Faecalibacterium | 0.000 | 0.019 | 1.4E-04 | 4.1E-04 |
| Fusicatenibacter | 0.000 | 0.006 | 0.001 | 0.003 |
| Oscillibacter | 0.001 | 0.008 | 0.001 | 0.003 |
| Ruminiclostridium | 0.005 | 0.021 | 0.002 | 0.004 |
| Blautia | 0.097 | 0.166 | 0.02 | 0.04 |
| Bifidobacterium | 0.000 | 0.002 | 0.05 | 0.09 |
| Bacteroides | 0.003 | 0.006 | 0.06 | 0.10 |
| Escherichia | 0.000 | 0.000 | 0.06 | 0.10 |
| Lachnoclostridium | 0.018 | 0.012 | 0.10 | 0.15 |
| Holdemanella | 0.000 | 0.000 | 0.13 | 0.18 |
| Gemmiger | 0.002 | 0.017 | 0.17 | 0.23 |
| Coprobacillus | 0.000 | 0.001 | 0.44 | 0.52 |
| Streptococcus | 0.010 | 0.005 | 0.45 | 0.52 |
| Erysipelatoclostridium | 0.045 | 0.057 | 0.45 | 0.52 |
| Lactobacillus | 0.000 | 0.000 | 0.56 | 0.61 |
| Intestinibacter | 0.001 | 0.002 | 0.78 | 0.81 |
| Unspecified Erysipelotricaceae | 0.001 | 0.002 | 0.87 | 0.87 |
DNA was isolated from the 140 fecal specimens and the 16S rRNA gene V4-V5 variable region was amplified using PCR and sequenced on an Illumina MiSeq platform. Sequences were grouped and classified at the genus level. For each taxon, median relative abundances were calculated for the 28 diarrheal fecal specimens from the 18 patients who had diarrhea in the first 3 months of transplantation and for the 112 fecal specimens from the 46 patients who did not have diarrhea in the first 3 months of transplantation. P value was calculated by Wilcoxon rank sum test and adjusted p value was calculated using Benjamini-Hochberg (BH) correction. In bold are the genera that discriminated the two groups at a BH adjusted P value ≤0.15.
Figure 2. Heatmap of the Most Abundant Bacterial Genera by Diarrhea Group Status.
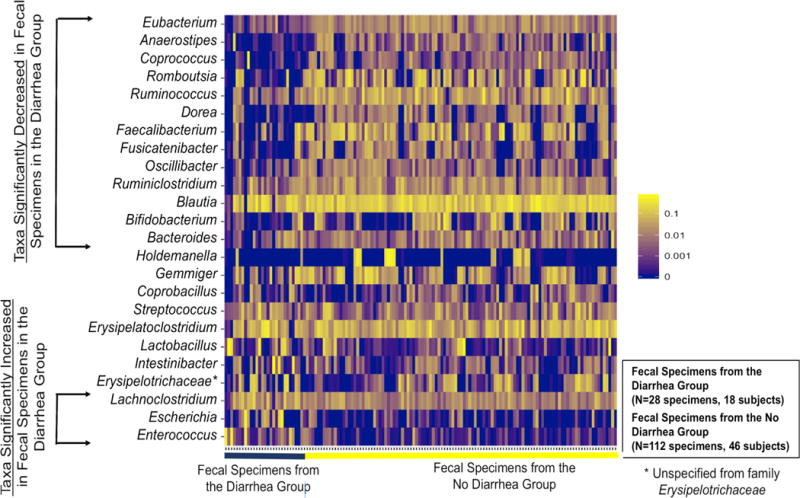
On the x-axis are the 140 fecal specimens ordered by the 28 diarrheal fecal specimens from the 18 subjects in the Diarrhea Group (black line) and the 112 fecal specimens from the 46 subjects in the No Diarrhea Group (yellow line). On the y-axis are the top 24 genera with a >1% mean relative microbial abundance. The 13 genera significantly lower in the diarrheal fecal specimens compared to fecal specimens from the No Diarrhea Group are represented at the top portion of the y-axis (in order of significance) and the 3 genera significantly higher in the diarrheal fecal specimens compared to fecal specimens from the No Diarrhea Group are represented at the bottom portion of the y-axis (in order of significance). The intensity of the yellow color represents the relative abundance and is log-scaled.
To account for the contribution of multiple samples from a single subject, we also separately evaluated the first fecal specimen associated with diarrhea from each of the 18 subjects in the Diarrhea Group (N=18 specimens) and compared the microbial profiles of these samples to the profiles in fecal specimens from each of the 46 subjects in the No Diarrhea Group, matched for time from day of collection after kidney transplantation (N=46 specimens). Twelve of the 16 genera identified as significant in Table 2 still remained significantly different between the Diarrhea Group and the No Diarrhea Group (Supplementary Table S1). Given that antibiotics influence the gut microbiota (13), we performed additional analysis after exclusion of 21 post-transplant fecal specimens collected from patients after exposure to antibiotics beyond routine antibiotic prophylaxis. We compared the microbial profiles of 18 diarrheal fecal specimens to the microbial profiles of 100 fecal specimens from the No Diarrhea Group. Thirteen of the 16 genera identified as significant in Table 2 still remained significantly different between the Diarrhea Group and the No Diarrhea Group (Supplementary Table S2).
3.3. Parallel Pathogen Testing of Diarrheal Fecal Specimens Using a Multiplex PCR Assay
In order to investigate whether post-transplant diarrhea was caused by common gastrointestinal pathogens, we evaluated a separate aliquot of the 28 diarrheal fecal specimens using the FilmArray® Gastrointestinal Panel (8). This assay utilizes multiplex PCR to detect 22 diarrheal bacterial, viral, and protozoan pathogens such as Clostridiodes (formerly Clostridium) difficile, diarrheagenic Escherichia coli, and norovirus (Complete list in SI Materials and Methods).
All but 2 diarrheal fecal specimens tested negative using this panel. Two specimens from 2 different subjects tested positive for Yersinia enterocolitica. We did not find evidence of Y. enterocolitica via 16S rRNA gene sequencing.
3.4. Comparison of Gut Microbiota Based on Mycophenolate Mofetil Dosing
We conducted further analysis of the fecal specimens based on MMF dosage (1000 mg/day vs. 2000 mg/day) and by group (Diarrhea Group vs. No Diarrhea Group) status. Analysis of MMF dosage restricted to the Diarrhea Group showed no significant difference at the genus level in the diarrheal fecal specimens from the subjects prescribed 2000 mg/day of MMF compared to the subjects prescribed 1000 mg/day of MMF (Supplementary Table S3). Analysis of MMF dosage restricted to the No Diarrhea Group revealed one genus, unspecified Erysipelotrichaceae, that was significantly different in the fecal specimens from the subjects prescribed 2000 mg/day of MMF compared to the subjects prescribed 1000 mg/day of MMF (Supplementary Table S4). In contrast, analysis restricted to the MMF dosage of 1000 mg/day showed that eleven of the 16 genera identified as significant in Table 2 remained significantly different between the diarrheal fecal specimens from the Diarrhea Group and the fecal specimens from the No Diarrhea Group (Supplementary Table S5). In a similar way, analysis restricted to the MMF dosage of 2000 mg/day revealed that thirteen of the 16 genera identified as significant in Table 2 remained significantly different between the diarrheal fecal specimens from the Diarrhea Group and the fecal specimens from the No Diarrhea (Supplementary Table S6).
3.5. Bacterial Taxa Associated with Diarrhea Identified by Hierarchical Bayesian Logistic Regression
To account for the contribution of multiple samples from a single subject and to address clinical variables, a hierarchical Bayesian logistic regression (with random intercepts per patient ID) was performed to assess each of the genera’s independent association with diarrheal specimens. We utilized all of the genera assessed in Table 2 as well as the following clinical variables listed in Table 1: age, gender, African American race, living donor transplantation, anti-thymocyte globulin therapy, perioperative cefazolin surgical prophylaxis, and trimethoprim/sulfamethoxazole Pneumocystis jiroveci prophylaxis. We confirmed nine of the 16 genera identified as significant in Table 2 (i.e. with a parameter estimate posterior intervals that did not cross 0) (Table 3). The logistic regression also identified five additional taxa (Gemmiger, Streptococcus, Erysipelatoclostridium, Intestinibacter, and Unspecified Erysipelotricaceae) that were not significant by univariate analysis in Table 2.
Table 3.
Hierarchical Bayesian Logistic Regression to Evaluate Taxa Associated with Diarrheal Status
| Characteristic | Median Point Estimate | Lower Posterior Uncertainty Interval (10%) | Upper Posterior Uncertainty Interval (90%) |
|---|---|---|---|
| Eubacterium | −3.5 | −5.3 | −2.0 |
| Anaerostipes | −2.3 | −3.4 | −1.4 |
| Coprococcus | −1.5 | −2.5 | −0.6 |
| Romboutsia | −1.2 | −2.0 | −0.4 |
| Ruminococcus | −1.7 | −3.3 | −0.3 |
| Dorea | 0.4 | −0.5 | 1.3 |
| Enterococcus | 0.4 | −0.5 | 1.4 |
| Faecalibacterium | −0.4 | −1.1 | 0.3 |
| Fusicatenibacter | −0.2 | −1.1 | 0.7 |
| Oscillibacter | 0.3 | −1.0 | 1.6 |
| Ruminiclostridium | −2.2 | −3.8 | −0.6 |
| Blautia | −2.5 | −4.2 | −1.1 |
| Bifidobacterium | −0.1 | −0.9 | 0.6 |
| Bacteroides | 0.9 | −0.3 | 2.1 |
| Escherichia | 1.3 | 0.5 | 2.1 |
| Lachnoclostridium | 7.2 | 5.0 | 9.8 |
| Holdemanella | 0.4 | −0.6 | 1.3 |
| Gemmiger | 1.3 | 0.5 | 2.3 |
| Coprobacillus | 0.1 | −0.8 | 1.0 |
| Streptococcus | 1.5 | 0.0 | 3.2 |
| Erysipelatoclostridium | −3.3 | −5.0 | −1.7 |
| Lactobacillus | 0.2 | −0.6 | 1.1 |
| Intestinibacter | 1.7 | 0.9 | 2.5 |
| Unspecified Erysipelotricaceae | 1.0 | 0.2 | 1.7 |
| Age | 0.0 | −0.1 | 0.1 |
| Female Gender | 0.9 | −1.3 | 2.9 |
| African American Race | 0.5 | −1.6 | 2.8 |
| Living Donor Transplantation | −1.9 | −4.0 | 0.2 |
| Anti-thymocyte Globulin Induction | −1.6 | −3.6 | 0.4 |
| Perioperative Cefazolin PPX | −1.3 | −3.8 | 1.2 |
| Trimethoprim/Sulfamethoxazole PPX | 0.1 | −2.8 | 2.8 |
| Intercept | −3.6 | −16.7 | 8.7 |
A total of 183 specimens collected from the study cohort of 71 kidney transplant recipients and classified as either diarrheal fecal specimens (1) or non-diarrheal fecal specimens (0) were included in the analyses. In view of the non-normal distribution of the relative abundances of the taxa, log transformation was performed and any value of 0 was assigned half of the lowest value abundance in the specific taxon. The hierarchical model considered fixed effects (all of the genera that were analyzed in Table 2 as well as clinical variables) and for random intercepts per subject (to account for multiple samples from one subject). The median point estimate, the lower posterior uncertainty interval (10%), and upper posterior uncertainty interval (90%) are listed for each characteristic. In bold are the characteristics whose posterior uncertainty intervals did not cross zero. positive parameter values indicate that a large value for the corresponding variable independently increases the probability of a sample to have diarrheal status (conversely, negative values indicate that large values of the corresponding variable indicates non-diarrheal status). PPX: prophylaxis.
3.6. Changes in the Gut Microbial Abundances Within the Diarrhea Group and Within the No Diarrhea Group
Within the Diarrhea Group, we determined the relative abundances of taxa in pre-diarrheal specimens, diarrheal specimens, and post-diarrheal specimens and analyzed the data using paired specimens. There was one taxa, Ruminococcus, that significantly decreased from pre-diarrheal specimens to diarrheal specimens (Supplementary Table S7). There were no taxa that were significantly different from diarrheal specimens to post-diarrheal specimens (Supplementary Table S8) and no taxa that were significantly different form pre-diarrheal specimens to post-diarrheal specimens (Supplementary Table S9).
Within the No Diarrhea Group, we determined the relative abundances of taxa in post-transplant week 1, week 2, and week 4 specimens and analyzed the data using paired specimens. The relative abundances of Blautia and Fusicatenbacter significantly increased and the relative abundances of Ruminiclostridium, Oscillibacter, and Anaerostipes significantly decreased from post-transplant week 1 to post-transplant week 2 (Supplementary Table S10). The relative abundances of Eubacterium and Dorea significantly increased from post-transplant week 2 to post-transplant week 4 (Supplementary Table S11).
3.7. Changes in the Abundances of Bacterial Taxa After Fecal Microbial Transplantation
We evaluated two kidney transplant recipients who had a history of Clostridium difficile infections and underwent donor allogeneic fecal microbial transplantation (FMT). Notably, both had persistent diarrhea despite repeated negative C. difficile testing prior to FMT. In both cases, diarrhea resolved in the first month after FMT. Microbiome profiles in these 2 recipients revealed an overall increase in the abundances of the 13 taxa that were found to be significantly lower in diarrheal fecal specimens and an overall decrease in the abundances of the 3 taxa that were found to be significantly higher in diarrheal fecal specimens after FMT (Figure 3).
Figure 3. Serial Microbiome Profiles Before and After Fecal Microbial Transplantation in 2 Kidney Transplant Recipients with Recurrent Episodes of Diarrhea.

Each panel represents a unique patient with each bar within the panel representing the microbiome profile in a single stool specimen. On the x axis, day 0 is the day the patient underwent fecal microbial transplantation (FMT); the relative abundances (y axis) of the 13 taxa that are decreased in diarrheal fecal specimens (Gray), the relative abundances of the 3 taxa that are increased in the diarrheal fecal specimens (Magenta), and the relative abundance of all other taxa (Yellow) are shown. The donor microbiome profile is shown to the right of Patient 1. Testing for C. difficile toxin B by PCR assay (Xpert® C. difficile/Epi, Cepheid, Sunnyvale, CA) is indicated by arrows above the graph. Following FMT from an allogeneic donor, the relative abundance of the 13 taxa that are significantly lower in the diarrheal fecal specimens together increased after FMT whereas the relative abundance of the 3 taxa that are significantly higher in the diarrheal fecal specimens together decreased after FMT.
3.8. Lower Metabolism-Associated Bacterial Genes in the Diarrheal Fecal Specimens
We utilized PICRUSt (9) to predict the metagenome functions in the 28 diarrheal fecal specimens from the 18 subjects in the Diarrhea Group and the 112 fecal specimens from the 46 subjects in the No Diarrhea Group. At KEGG Level 3 that included 328 KEGG pathways, we identified 21 different KEGG pathways that were significantly different between the two groups (BH adjusted p value≤0.15, minimum threshold of 10,000 occurrences per KEGG pathway). The 15 pathways that were decreased in the diarrheal fecal specimens included 9 metabolism-related pathways; 3 genetic information processing pathways; and 3 uncharacterized pathways. Among the 6 pathways that were increased in the diarrheal fecal specimens, 2 were environmental information processing pathways, 1 was a metabolism-related pathway, 1 was a genetic information processing pathway, and 2 were uncharacterized pathways (Figure 4A).
Figure 4. Metabolic Pathways and Bacterial Genes Distinguishing the Diarrheal Fecal Specimens from the Fecal Specimens in the No Diarrhea Group.
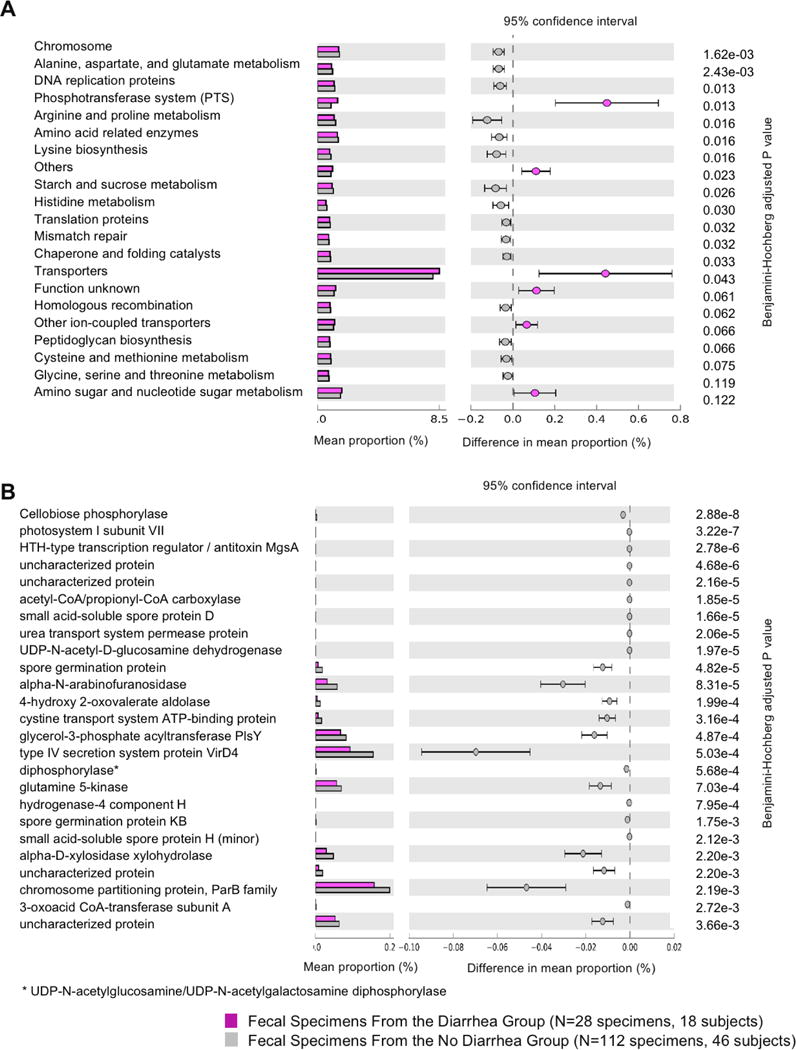
PICRUSt analysis was performed on the 28 diarrheal fecal specimens from the 18 subjects in the Diarrhea Group and the 112 fecal specimens from the 46 subjects in the No Diarrhea Group. Panel A. The top 21 metabolic KEGG 3 pathways significantly different between the diarrheal fecal specimens and the fecal specimens from the No Diarrhea Group are shown. On the left are the relative mean abundances of the different KEGG pathways with the diarrheal fecal specimens in magenta and the fecal specimens in the No Diarrhea Group in gray. On the right are the mean differences between the diarrheal fecal specimens and the fecal specimens in the No Diarrhea Group, by KEGG pathways, with the magenta point representing a significantly higher abundance in the diarrheal fecal specimens and the gray point representing a significantly higher abundance in the fecal specimens in the No Diarrhea Group. Each point is accompanied by a 95% confidence interval indicated by the error bars. The metabolic pathways are sorted by BH adjusted p value. Panel B. The top 25 genes significantly different between the diarrheal fecal specimens and the fecal specimens from the No Diarrhea Group are shown. On the left are the relative mean abundances of the different genes with the diarrheal fecal specimens in magenta and the fecal specimens in the No Diarrhea Group in gray. On the right are the mean differences between the diarrheal fecal specimens and the fecal specimens in the No Diarrhea Group, by bacterial genes, with the gray points representing a significantly higher abundance in the fecal specimens in the No Diarrhea Group. Each point is accompanied by a 95% confidence interval indicated by the error bars. The genes are sorted by BH adjusted p value.
Among the 6921 predicted genes in the total population, 810 genes were significantly different between the two groups (BH adjusted p value≤0.15). The top 25 genes that were significantly different between the two groups were all genes decreased in the diarrheal fecal specimens with 10 genes related to metabolism functions, 3 genes related to environmental-information processing functions, and 12 genes related to unclassified functions (Figure 4B). Detailed analysis is described in SI Results.
4. DISCUSSION
The current study offers new insights into the gut dysbiosis that occurs during post-transplant diarrhea. Our study has identified the following: diarrheal fecal specimens are characterized by lower microbial diversity and lower abundance of commensal bacterial taxa; most early post-transplant diarrhea is not associated with common infectious diarrheal pathogens.
In a pilot study, we previously found a lower microbial diversity in fecal specimens associated with post-transplant diarrhea as well as a lower relative abundance of the commensal bacterial genera, Ruminococcus, Dorea, Coprococcus, and Bacteroides (6). In the current validation study, we confirm and extend these previous findings. We report the relative abundances of Ruminococcus, Dorea, Coprococcus, and Bacteroides to be lower in diarrheal fecal specimens and identify additional commensal bacterial taxa whose abundances were decreased in post-transplant diarrhea as well as Enterococcus and Escherichia whose abundances were increased.
Interestingly, many of the 13 taxa identified as significantly lower in post-transplant diarrheal fecal specimens belong to the Lachnospiraceae family and Ruminococcaceae family and belong to Clostridium Cluster XIVa and IV, which is collectively known as the Commensal Clostridia (14). Among many other important functions, these bacterial taxa contribute butyrate to colonocytes and contribute to overall gut health (14). For example, bacteria in the Faecalibacterium genus degrade glucose, fructose, fructo-oligosaccarides and complex molecules such as pectin, and bacteria in the Dorea genus perform glucose fermentation (15). Altogether, these data suggest that decreased commensal bacterial taxa in the gut create a dysfunctional metabolic state indicating and potentially leading to diarrhea. Consistent with this, a similar lower abundance of commensal bacteria during diarrhea has also been reported in non-transplant recipients. In a study of 39 individuals with C. difficile-associated diarrhea, 36 subjects with C. difficile negative diarrhea, and 40 healthy volunteers, the genera, Blautia, Faecalibacterium, Anaerostipes, Ruminococcus, Dorea, and Coprococcus, were depleted in both the C. difficile-associated diarrhea group and the C. difficile negative diarrhea group compared to the healthy control group (16). It is worth noting that our observational translational study can not determine whether diarrhea caused the observed dysbiosis or the observed dysbiosis caused diarrhea. Nevertheless, it is interesting to point out that in the 2 cases of fecal microbial transplantation for recurrent C. difficile infections, resolution of diarrhea correlated with an overall increase in the relative abundances of the 13 taxa identified as significantly lower in diarrheal specimens and with an overall decrease in the relative abundances of the 3 taxa identified as significantly higher in diarrheal specimens.
At the level of 25,000 sequences per specimen, 16S rRNA gene deep sequencing data may not capture lower abundances of bacterial pathogens, does not distinguish between diarrheagenic and non-diarrheagenic E. coli, and does not evaluate for viral and protozoan pathogens. We overcame this limitation by using a multiplexed PCR assay that detects 22 infectious diarrheal etiologies. We demonstrated that 26 of the 28 diarrheal fecal specimens were negative for bacterial, viral, and protozoan diarrheal pathogens while only 2 were positive for Y. enterocolitica. It is important to note that 16S rRNA gene deep sequencing may not have identified Y. enterocolitica DNA because of the technique’s insensitivity at the species level as well as sequencing depth. In our study, the FilmArray® Gastrointestinal Panel complemented 16S rRNA gene deep sequencing and supports the hypothesis that early post-transplant diarrhea is mostly non-infectious.
To further explore the potential mechanisms by which decreased commensal bacterial taxa contribute to diarrhea, we utilized PICRUSt (9) to predict the metagenomic functions of the bacterial communities. KEGG level 3 analysis revealed significant decreases in metabolic pathways such as sucrose and starch metabolism and amino sugar and nucleotide sugar metabolism in the diarrheal fecal specimens. The predicted metagenomic data suggest that post-transplant diarrhea may be a state of altered metabolic homeostasis with diminished ability to digest complex sugars. As an example, the top gene that was significantly different between diarrheal fecal specimens and the fecal specimens from the No Diarrhea Group was cellobiose phosphorylase which degrades disaccharide cellobiose into alpha-D-glucose 1-phosphate and D-glucose (17). Cellobiose has been found in rat models to induce diarrhea (18). Alpha-N-arabinofuranosidase is also significantly lower in the diarrheal fecal specimens. Alpha-N-arabinofuranosidase is involved in the hydrolysis of terminal arabinofuranoside bonds in hemicellulose homopolysaccharides and heteropolysaccharides (19). Together, cellobiose phosphorylase and alpha-N-arabinofuranosidase are involved in the degradation of complex polysaccharides, supporting the idea that the post-transplant diarrhea is characterized by decreased abundance of commensal bacteria taxa that are involved in key metabolism in the gut.
Non-infectious post-transplant diarrhea is commonly attributed to immunosuppressive medications such as MMF (4, 5). As a consequence, transplant physicians routinely reduce MMF dosing in order to treat post-transplant diarrhea. The current study does not directly address the microbiota changes due to MMF, as almost all subjects were on MMF. However, our data suggest that the taxa identified as significantly different between the diarrheal fecal specimens and fecal specimens in the No Diarrhea Group remained significantly different when stratified either by 1000 mg MMF dosage or by 2000 mg MMF dosage (Supplementary Tables S5-S6). In contrast, within the Diarrhea Group or within the No Diarrhea Group, there was only one significantly different taxon between the 1000 mg MMF dosage and 2000 mg MMF dosage (Supplementary Tables S3-S4). These results are supportive of the interpretation that changes in the gut microbiota may not be attributed solely to MMF dosage.
There are several limitations to our study. Our study focused on diarrhea within the first 3 months of transplantation. Late chronic post-transplant diarrhea has been attributed to infectious etiologies like norovirus (20) and whether our findings are also applicable to late post-transplant diarrhea requires additional investigation. We were not able to obtain fecal specimens at all serial time points and during every episode of post-transplant diarrhea. Limited data regarding the diet of subjects is a limitation since diet is known to influence the gut microbiota (21). Our data highlights decreased commensal bacterial diversity during diarrhea but does not address whether diarrhea caused the gut dysbiosis or whether the dysbiosis caused the diarrhea. Another limitation is that we utilized a prediction system to postulate bacterial metagenome functions. Future studies focused on the metagenome of post-transplant diarrhea are needed to confirm whether bacterial metabolism genes and metabolites are associated with post-transplant diarrhea.
In summary, we report that post-transplant diarrhea early after transplantation is associated with lower diversity of commensal bacterial taxa and lower microbial diversity and is uncommonly associated with diarrheal infectious pathogens. Moreover, post-transplant diarrhea is characterized by a predicted lower abundance of metabolism-associated bacterial genes. Future validation studies are needed to further investigate the gut microbiota dysbiosis present in the post-transplant diarrhea and options to prevent and/or treat this common complication.
Supplementary Material
Table S1: Genus Level Gut Microbial Composition in the First Diarrheal Specimens and in Time-Matched Fecal Specimens from the No Diarrhea Group. Data analysis was restricted to the first 18 fecal specimens associated with diarrhea from the 18 subjects in the Diarrhea Group and 46 time-matched fecal specimens from the 46 subjects in the No Diarrhea Group. DNA was isolated from the fecal specimens and the 16S rRNA gene V4-V5 variable region was amplified using PCR and sequenced on an Illumina MiSeq platform. Sequences were grouped and classified at the genus level. For each taxon, median relative abundances were calculated in each group. P value was calculated by Wilcoxon rank sum test and adjusted P value was calculated using Benjamini-Hochberg (BH) correction. In bold are the genera that discriminated the two groups at a BH adjusted P value ≤0.15.
Table S2: Genus Level Gut Microbial Composition in the Fecal Specimens in the Diarrhea Group and the No Diarrhea Group after Exclusion of Fecal Specimens Collected After Antibiotic Administration. Twenty-one post-transplant fecal specimens (10 of 28 diarrheal fecal specimens and 11 of 112 fecal specimens from the No Diarrhea Group) were previously exposed to antibiotics besides preoperative antibiotic prophylaxis and Pneumocystis jiroveci prophylaxis and were excluded in this analysis, leaving 18 antibiotic-excluded diarrheal fecal specimens from 13 subjects in the Diarrhea Group and 101 antibiotic-excluded fecal specimens from 46 subjects in the No Diarrhea Group for data analysis. DNA was isolated from the fecal specimens and the 16S rRNA gene V4-V5 variable region was amplified using PCR and sequenced on an Illumina MiSeq platform. Sequences were grouped and classified at the genus level. For each taxon, median relative abundances were calculated in each group. P value was calculated by Wilcoxon rank sum test and adjusted P value was calculated using Benjamini-Hochberg (BH) correction. In bold are the genera that discriminated the two groups at a BH adjusted P value ≤0.15.
Table S3: Genus Level Gut Microbial Composition in Diarrheal Fecal Specimens Associated with 2000 mg/day MMF Dosage and with 1000 mg/day MMF Dosage. Among the 28 fecal specimens in the Diarrhea Group, 23 specimens from 13 subjects were associated with a dosing of 2000 mg/day of mycophenolate mofetil, 4 specimens from 4 subjects were associated with a dosing of 1000 mg/day, and 1 specimen was not associated with mycophenolate mofetil. DNA was isolated from the fecal specimens and the 16S rRNA gene V4-V5 variable region was amplified using PCR and sequenced on an Illumina MiSeq platform. Sequences were grouped and classified at the genus level. For each taxon, median relative abundances were calculated in each group. P value was calculated by Wilcoxon rank sum test and adjusted P value was calculated using Benjamini-Hochberg (BH) correction.
Table S4: Genus Level Gut Microbial Composition in Fecal Specimens from the No Diarrhea Group by 2000 mg/day MMF Dosage and by 1000 mg/day MMF Dosage. Among the 112 fecal specimens in the No Diarrhea Group, 61 specimens from 25 subjects were associated with a dosing of 2000 mg/day of mycophenolate mofetil, 50 specimens from 21 subjects were associated with a dosing of 1000 mg/day, and 1 specimen was not associated with mycophenolate mofetil. DNA was isolated from the fecal specimens and the 16S rRNA gene V4-V5 variable region was amplified using PCR and sequenced on an Illumina MiSeq platform. Sequences were grouped and classified at the genus level. For each taxon, median relative abundances were calculated in each group. P value was calculated by Wilcoxon rank sum test and adjusted P value was calculated using Benjamini-Hochberg (BH) correction. In bold is the genus that discriminated the two groups at a BH adjusted P value ≤0.15.
Table S5: Genus Level Gut Microbial Composition in Diarrheal Fecal Specimens and Fecal Specimens from the No Diarrhea Group with 1000 mg/day MMF Dosage. Among the 54 fecal specimens associated with 1000 mg/day of mycophenolate mofetil dosage, there were 4 specimens from 4 subjects in the Diarrhea Group and 50 specimens from 21 subjects in the No Diarrhea Group. DNA was isolated from the fecal specimens and the 16S rRNA gene V4-V5 variable region was amplified using PCR and sequenced on an Illumina MiSeq platform. Sequences were grouped and classified at the genus level. For each taxon, median relative abundances were calculated in each group. P value was calculated by Wilcoxon rank sum test and adjusted P value was calculated using Benjamini-Hochberg (BH) correction. In bold are the genera that discriminated the two groups at a BH adjusted P value ≤0.15.
Table S6: Genus Level Gut Microbial Composition in Diarrheal Fecal Specimens and Fecal Specimens from the No Diarrhea Group with 2000 mg/day MMF Dosage. Among the 84 fecal specimens associated with 2000 mg/day of mycophenolate mofetil dosage, there were 23 specimens from 13 subjects in the Diarrhea Group and 61 specimens from 25 subjects in the No Diarrhea Group. DNA was isolated from the fecal specimens and the 16S rRNA gene V4-V5 variable region was amplified using PCR and sequenced on an Illumina MiSeq platform. Sequences were grouped and classified at the genus level. For each taxon, median relative abundances were calculated in each group. P value was calculated by Wilcoxon rank sum test and adjusted P value was calculated using Benjamini-Hochberg (BH) correction. In bold are the genera that discriminated the two groups at a BH adjusted P value ≤0.15.
Table S7: Genus Level Gut Microbial Composition in Pre-Diarrheal Fecal Specimens and Diarrheal Fecal Specimens. Within the Diarrhea Group, 14 subjects had paired pre-diarrheal specimens and diarrheal specimens. DNA was isolated from the fecal specimens and the 16S rRNA gene V4-V5 variable region was amplified using PCR and sequenced on an Illumina MiSeq platform. Sequences were grouped and classified at the genus level. For each taxon, median relative abundances were calculated in each group. P value was calculated by Wilcoxon signed rank test and adjusted P value was calculated using Benjamini-Hochberg (BH) correction. In bold is the genus that discriminated the two groups at a BH adjusted P value ≤0.15.
Table S8: Genus Level Gut Microbial Composition in Diarrheal Fecal Specimens and Post-Diarrheal Fecal Specimens. Within the Diarrhea Group, 9 subjects had paired pre-diarrheal specimens and diarrheal specimens. DNA was isolated from the fecal specimens and the 16S rRNA gene V4-V5 variable region was amplified using PCR and sequenced on an Illumina MiSeq platform. Sequences were grouped and classified at the genus level. For each taxon, median relative abundances were calculated in each group. P value was calculated by Wilcoxon signed rank test and adjusted P value was calculated using Benjamini-Hochberg (BH) correction. A p value was unable to be calculated in one genus, Holdemanella.
Table S9: Genus Level Gut Microbial Composition in Pre-Diarrheal Fecal Specimens and Post-Diarrheal Fecal Specimens. Within the Diarrhea Group, 10 subjects had paired pre-diarrheal specimens and post-diarrheal specimens. DNA was isolated from the fecal specimens and the 16S rRNA gene V4-V5 variable region was amplified using PCR and sequenced on an Illumina MiSeq platform. Sequences were grouped and classified at the genus level. For each taxon, median relative abundances were calculated in each group. P value was calculated by Wilcoxon signed rank test and adjusted P value was calculated using Benjamini-Hochberg (BH) correction.
Table S10: Genus Level Gut Microbial Composition in Post-Transplant Week 1 Specimens and Post-Transplant Week 2 Specimens from the No Diarrhea Group. Within the No Diarrhea Group, 32 subjects had paired post-transplant (post-Tx) week 1 and post-Tx week 2 specimens. DNA was isolated from the fecal specimens and the 16S rRNA gene V4-V5 variable region was amplified using PCR and sequenced on an Illumina MiSeq platform. Sequences were grouped and classified at the genus level. For each taxon, median relative abundances were calculated in each group. P value was calculated by Wilcoxon signed rank test and adjusted P value was calculated using Benjamini-Hochberg (BH) correction. In bold are the genera that discriminated the two groups at a BH adjusted P value ≤0.15.
Table S11: Genus Level Gut Microbial Composition in Post-Transplant Week 2 Specimens and Post-Transplant Week 4 Specimens from the No Diarrhea Group. Within the No Diarrhea Group, 24 subjects had paired post-transplant (post-Tx) week 2 and Post-tx week 4 specimens. DNA was isolated from the fecal specimens and the 16S rRNA gene V4-V5 variable region was amplified using PCR and sequenced on an Illumina MiSeq platform. Sequences were grouped and classified at the genus level. For each taxon, median relative abundances were calculated in each group. P value was calculated by Wilcoxon signed rank test and adjusted P value was calculated using Benjamini-Hochberg (BH) correction. In bold are the genera that discriminated the two groups at a BH adjusted P value ≤0.15.
Figure S1: Microbial Composition in the Diarrheal Fecal Specimens Is Dissimilar To The Composition In the Fecal Specimens in the No Diarrhea Group. 28 diarrheal specimens from the 18 patients in the Diarrhea Group and 112 fecal specimens from the 46 patients in the No Diarrhea Group were analyzed. Rarefaction was performed to the lower number of reads over all the specimens. Non-metric dimension scaling was performed based on the distant matrix using the Bray-Curtis dissimilarity matrix. Each magenta point represents a fecal specimen from the Diarrhea Group and each gray point represents a fecal specimen from the No Diarrhea Group.
Acknowledgments
The research work was funded, in part, by a K23 AI 124464 award from the National Institute of Allergy and Infectious Diseases to John Lee; by an R37 AI 051652 from the National Institute of Allergy and Infectious Diseases to Manikkam Suthanthiran; and a Chinese American Medical Society Summer Research Fellowship to Lisa Zhang.
We thank BioFire Diagnostics for providing the FilmArray® Gastrointestinal Panels we utilized in this study. We thank Dr. Joseph Schwartz for providing his input on biostatistical analyses and Catherine Snopkowski, Carol Li, and Liana Perry for their meticulous research assistance. We thank Johanna Martin for expert assistance in editing the manuscript.
ABBREVIATIONS
- bp
base pair
- FMT
fecal microbial transplantation
- MMF
mycophenolate mofetil
- NMDS
nonmetric multidimensional scaling
- OTU
operational taxonomic unit
- PCR
polymerase chain reaction
- PICRUSt
Phylogenetic Investigation of Communities by Reconstruction of Unobserved States
- SI
Supporting Information
Footnotes
DISCLOSURE
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. Carl Crawford receives support from MERCK, Rebiotix, and Seres. Lars Westblade receives research support from Accelerate Diagnostics, Inc. The other authors have no conflicts of interest to disclose.
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article.
References
- 1.Mycophenolate mofetil in renal transplantation: 3-year results from the placebo-controlled trial. European Mycophenolate Mofetil Cooperative Study Group. Transplantation. 1999;68(3):391–396. [PubMed] [Google Scholar]
- 2.Ekberg H, Kyllonen L, Madsen S, Grave G, Solbu D, Holdaas H. Increased prevalence of gastrointestinal symptoms associated with impaired quality of life in renal transplant recipients. Transplantation. 2007;83(3):282–289. doi: 10.1097/01.tp.0000251923.14697.f5. [DOI] [PubMed] [Google Scholar]
- 3.Bunnapradist S, Neri L, Wong W, Lentine KL, Burroughs TE, Pinsky BW, et al. Incidence and risk factors for diarrhea following kidney transplantation and association with graft loss and mortality. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2008;51(3):478–486. doi: 10.1053/j.ajkd.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 4.Aulagnon F, Scemla A, DeWolf S, Legendre C, Zuber J. Diarrhea after kidney transplantation: a new look at a frequent symptom. Transplantation. 2014;98(8):806–816. doi: 10.1097/TP.0000000000000335. [DOI] [PubMed] [Google Scholar]
- 5.Bunnapradist S, Lentine KL, Burroughs TE, Pinsky BW, Hardinger KL, Brennan DC, et al. Mycophenolate mofetil dose reductions and discontinuations after gastrointestinal complications are associated with renal transplant graft failure. Transplantation. 2006;82(1):102–107. doi: 10.1097/01.tp.0000225760.09969.1f. [DOI] [PubMed] [Google Scholar]
- 6.Lee JR, Muthukumar T, Dadhania D, Toussaint NC, Ling L, Pamer E, et al. Gut microbial community structure and complications after kidney transplantation: a pilot study. Transplantation. 2014;98(7):697–705. doi: 10.1097/TP.0000000000000370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Organization WH. The treatment of diarrhoea: a manual for physicians and other senior health workers. 2005 [Google Scholar]
- 8.Khare R, Espy MJ, Cebelinski E, Boxrud D, Sloan LM, Cunningham SA, et al. Comparative evaluation of two commercial multiplex panels for detection of gastrointestinal pathogens by use of clinical stool specimens. Journal of clinical microbiology. 2014;52(10):3667–3673. doi: 10.1128/JCM.01637-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langille MG, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nature biotechnology. 2013;31(9):814–821. doi: 10.1038/nbt.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gabry J. B G rstanarm: Bayesian applied regression modeling via StanR package version 2.11.1. 2016 https://CRAN.R-project.org/package=rstanarm.
- 11.Parks DH, Tyson GW, Hugenholtz P, Beiko RG. STAMP: statistical analysis of taxonomic and functional profiles. Bioinformatics. 2014;30(21):3123–3124. doi: 10.1093/bioinformatics/btu494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Magurran AE. Biological diversity. Current biology: CB. 2005;15(4):R116–118. doi: 10.1016/j.cub.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS biology. 2008;6(11):e280. doi: 10.1371/journal.pbio.0060280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopetuso LR, Scaldaferri F, Petito V, Gasbarrini A. Commensal Clostridia: leading players in the maintenance of gut homeostasis. Gut pathogens. 2013;5(1):23. doi: 10.1186/1757-4749-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rajilic-Stojanovic M, de Vos WM. The first 1000 cultured species of the human gastrointestinal microbiota. FEMS microbiology reviews. 2014;38(5):996–1047. doi: 10.1111/1574-6976.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antharam VC, Li EC, Ishmael A, Sharma A, Mai V, Rand KH, et al. Intestinal dysbiosis and depletion of butyrogenic bacteria in Clostridium difficile infection and nosocomial diarrhea. Journal of clinical microbiology. 2013;51(9):2884–2892. doi: 10.1128/JCM.00845-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ayers WA. Phosphorolysis and synthesis of cellobiose by cell extracts from Ruminococcus flavefaciens. The Journal of biological chemistry. 1959;234:2819–2822. [PubMed] [Google Scholar]
- 18.Jessie Fischer Moinuddin HW-TL. Effects of Feeding Diets Containing Sucrose, Cellobiose or Glucose on the Dry Weights of Clean Gastrointestinal Organs in the Rat. Am J Physiology. 1958;192(2):417–420. doi: 10.1152/ajplegacy.1958.192.2.417. [DOI] [PubMed] [Google Scholar]
- 19.Inacio JM, Correia IL, de Sa-Nogueira I. Two distinct arabinofuranosidases contribute to arabino-oligosaccharide degradation in Bacillus subtilis. Microbiology. 2008;154(Pt 9):2719–2729. doi: 10.1099/mic.0.2008/018978-0. [DOI] [PubMed] [Google Scholar]
- 20.Roos-Weil D, Ambert-Balay K, Lanternier F, Mamzer-Bruneel MF, Nochy D, Pothier P, et al. Impact of norovirus/sapovirus-related diarrhea in renal transplant recipients hospitalized for diarrhea. Transplantation. 2011;92(1):61–69. doi: 10.1097/TP.0b013e31821c9392. [DOI] [PubMed] [Google Scholar]
- 21.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Genus Level Gut Microbial Composition in the First Diarrheal Specimens and in Time-Matched Fecal Specimens from the No Diarrhea Group. Data analysis was restricted to the first 18 fecal specimens associated with diarrhea from the 18 subjects in the Diarrhea Group and 46 time-matched fecal specimens from the 46 subjects in the No Diarrhea Group. DNA was isolated from the fecal specimens and the 16S rRNA gene V4-V5 variable region was amplified using PCR and sequenced on an Illumina MiSeq platform. Sequences were grouped and classified at the genus level. For each taxon, median relative abundances were calculated in each group. P value was calculated by Wilcoxon rank sum test and adjusted P value was calculated using Benjamini-Hochberg (BH) correction. In bold are the genera that discriminated the two groups at a BH adjusted P value ≤0.15.
Table S2: Genus Level Gut Microbial Composition in the Fecal Specimens in the Diarrhea Group and the No Diarrhea Group after Exclusion of Fecal Specimens Collected After Antibiotic Administration. Twenty-one post-transplant fecal specimens (10 of 28 diarrheal fecal specimens and 11 of 112 fecal specimens from the No Diarrhea Group) were previously exposed to antibiotics besides preoperative antibiotic prophylaxis and Pneumocystis jiroveci prophylaxis and were excluded in this analysis, leaving 18 antibiotic-excluded diarrheal fecal specimens from 13 subjects in the Diarrhea Group and 101 antibiotic-excluded fecal specimens from 46 subjects in the No Diarrhea Group for data analysis. DNA was isolated from the fecal specimens and the 16S rRNA gene V4-V5 variable region was amplified using PCR and sequenced on an Illumina MiSeq platform. Sequences were grouped and classified at the genus level. For each taxon, median relative abundances were calculated in each group. P value was calculated by Wilcoxon rank sum test and adjusted P value was calculated using Benjamini-Hochberg (BH) correction. In bold are the genera that discriminated the two groups at a BH adjusted P value ≤0.15.
Table S3: Genus Level Gut Microbial Composition in Diarrheal Fecal Specimens Associated with 2000 mg/day MMF Dosage and with 1000 mg/day MMF Dosage. Among the 28 fecal specimens in the Diarrhea Group, 23 specimens from 13 subjects were associated with a dosing of 2000 mg/day of mycophenolate mofetil, 4 specimens from 4 subjects were associated with a dosing of 1000 mg/day, and 1 specimen was not associated with mycophenolate mofetil. DNA was isolated from the fecal specimens and the 16S rRNA gene V4-V5 variable region was amplified using PCR and sequenced on an Illumina MiSeq platform. Sequences were grouped and classified at the genus level. For each taxon, median relative abundances were calculated in each group. P value was calculated by Wilcoxon rank sum test and adjusted P value was calculated using Benjamini-Hochberg (BH) correction.
Table S4: Genus Level Gut Microbial Composition in Fecal Specimens from the No Diarrhea Group by 2000 mg/day MMF Dosage and by 1000 mg/day MMF Dosage. Among the 112 fecal specimens in the No Diarrhea Group, 61 specimens from 25 subjects were associated with a dosing of 2000 mg/day of mycophenolate mofetil, 50 specimens from 21 subjects were associated with a dosing of 1000 mg/day, and 1 specimen was not associated with mycophenolate mofetil. DNA was isolated from the fecal specimens and the 16S rRNA gene V4-V5 variable region was amplified using PCR and sequenced on an Illumina MiSeq platform. Sequences were grouped and classified at the genus level. For each taxon, median relative abundances were calculated in each group. P value was calculated by Wilcoxon rank sum test and adjusted P value was calculated using Benjamini-Hochberg (BH) correction. In bold is the genus that discriminated the two groups at a BH adjusted P value ≤0.15.
Table S5: Genus Level Gut Microbial Composition in Diarrheal Fecal Specimens and Fecal Specimens from the No Diarrhea Group with 1000 mg/day MMF Dosage. Among the 54 fecal specimens associated with 1000 mg/day of mycophenolate mofetil dosage, there were 4 specimens from 4 subjects in the Diarrhea Group and 50 specimens from 21 subjects in the No Diarrhea Group. DNA was isolated from the fecal specimens and the 16S rRNA gene V4-V5 variable region was amplified using PCR and sequenced on an Illumina MiSeq platform. Sequences were grouped and classified at the genus level. For each taxon, median relative abundances were calculated in each group. P value was calculated by Wilcoxon rank sum test and adjusted P value was calculated using Benjamini-Hochberg (BH) correction. In bold are the genera that discriminated the two groups at a BH adjusted P value ≤0.15.
Table S6: Genus Level Gut Microbial Composition in Diarrheal Fecal Specimens and Fecal Specimens from the No Diarrhea Group with 2000 mg/day MMF Dosage. Among the 84 fecal specimens associated with 2000 mg/day of mycophenolate mofetil dosage, there were 23 specimens from 13 subjects in the Diarrhea Group and 61 specimens from 25 subjects in the No Diarrhea Group. DNA was isolated from the fecal specimens and the 16S rRNA gene V4-V5 variable region was amplified using PCR and sequenced on an Illumina MiSeq platform. Sequences were grouped and classified at the genus level. For each taxon, median relative abundances were calculated in each group. P value was calculated by Wilcoxon rank sum test and adjusted P value was calculated using Benjamini-Hochberg (BH) correction. In bold are the genera that discriminated the two groups at a BH adjusted P value ≤0.15.
Table S7: Genus Level Gut Microbial Composition in Pre-Diarrheal Fecal Specimens and Diarrheal Fecal Specimens. Within the Diarrhea Group, 14 subjects had paired pre-diarrheal specimens and diarrheal specimens. DNA was isolated from the fecal specimens and the 16S rRNA gene V4-V5 variable region was amplified using PCR and sequenced on an Illumina MiSeq platform. Sequences were grouped and classified at the genus level. For each taxon, median relative abundances were calculated in each group. P value was calculated by Wilcoxon signed rank test and adjusted P value was calculated using Benjamini-Hochberg (BH) correction. In bold is the genus that discriminated the two groups at a BH adjusted P value ≤0.15.
Table S8: Genus Level Gut Microbial Composition in Diarrheal Fecal Specimens and Post-Diarrheal Fecal Specimens. Within the Diarrhea Group, 9 subjects had paired pre-diarrheal specimens and diarrheal specimens. DNA was isolated from the fecal specimens and the 16S rRNA gene V4-V5 variable region was amplified using PCR and sequenced on an Illumina MiSeq platform. Sequences were grouped and classified at the genus level. For each taxon, median relative abundances were calculated in each group. P value was calculated by Wilcoxon signed rank test and adjusted P value was calculated using Benjamini-Hochberg (BH) correction. A p value was unable to be calculated in one genus, Holdemanella.
Table S9: Genus Level Gut Microbial Composition in Pre-Diarrheal Fecal Specimens and Post-Diarrheal Fecal Specimens. Within the Diarrhea Group, 10 subjects had paired pre-diarrheal specimens and post-diarrheal specimens. DNA was isolated from the fecal specimens and the 16S rRNA gene V4-V5 variable region was amplified using PCR and sequenced on an Illumina MiSeq platform. Sequences were grouped and classified at the genus level. For each taxon, median relative abundances were calculated in each group. P value was calculated by Wilcoxon signed rank test and adjusted P value was calculated using Benjamini-Hochberg (BH) correction.
Table S10: Genus Level Gut Microbial Composition in Post-Transplant Week 1 Specimens and Post-Transplant Week 2 Specimens from the No Diarrhea Group. Within the No Diarrhea Group, 32 subjects had paired post-transplant (post-Tx) week 1 and post-Tx week 2 specimens. DNA was isolated from the fecal specimens and the 16S rRNA gene V4-V5 variable region was amplified using PCR and sequenced on an Illumina MiSeq platform. Sequences were grouped and classified at the genus level. For each taxon, median relative abundances were calculated in each group. P value was calculated by Wilcoxon signed rank test and adjusted P value was calculated using Benjamini-Hochberg (BH) correction. In bold are the genera that discriminated the two groups at a BH adjusted P value ≤0.15.
Table S11: Genus Level Gut Microbial Composition in Post-Transplant Week 2 Specimens and Post-Transplant Week 4 Specimens from the No Diarrhea Group. Within the No Diarrhea Group, 24 subjects had paired post-transplant (post-Tx) week 2 and Post-tx week 4 specimens. DNA was isolated from the fecal specimens and the 16S rRNA gene V4-V5 variable region was amplified using PCR and sequenced on an Illumina MiSeq platform. Sequences were grouped and classified at the genus level. For each taxon, median relative abundances were calculated in each group. P value was calculated by Wilcoxon signed rank test and adjusted P value was calculated using Benjamini-Hochberg (BH) correction. In bold are the genera that discriminated the two groups at a BH adjusted P value ≤0.15.
Figure S1: Microbial Composition in the Diarrheal Fecal Specimens Is Dissimilar To The Composition In the Fecal Specimens in the No Diarrhea Group. 28 diarrheal specimens from the 18 patients in the Diarrhea Group and 112 fecal specimens from the 46 patients in the No Diarrhea Group were analyzed. Rarefaction was performed to the lower number of reads over all the specimens. Non-metric dimension scaling was performed based on the distant matrix using the Bray-Curtis dissimilarity matrix. Each magenta point represents a fecal specimen from the Diarrhea Group and each gray point represents a fecal specimen from the No Diarrhea Group.


