Abstract
Aims
Growth differentiation factor (GDF)‐15 mirrors inflammation and oxidative stress in cardiovascular diseases. Brain natriuretic peptide (BNP) is associated with cardiomyocyte stretch in heart failure (HF). The objective of this study was to evaluate the prognostic impact of plasma GDF‐15 and BNP in acute HF.
Methods and results
We studied a subgroup of patients prospectively recruited in an acute HF registry (follow‐up: 2 years; endpoint: all‐cause mortality). Cox regression multivariate models were built to study the association of GDF‐15 and mortality. Further cross‐classification according to discharge GDF‐15 (mean) and BNP (mean) and association with mortality was studied. We studied 158 patients: seventy‐nine were male, mean age was 75 years, 55.1% had left ventricular ejection fraction < 40%, mean discharge BNP was 1000 pg/mL, and mean GDF‐15 was 3013 ng/mL. Higher BNP and GDF‐15 predicted 2‐year mortality. Patients with GDF‐15 ≥ 3000 ng/mL had a multivariate adjusted 2‐year death risk of 1.86 (1.08–3.18). Patients discharged with both BNP and GDF‐15 above the mean had an adjusted hazard ratio of 4.33 (2.07–9.06) when compared with those with both <mean.
Conclusions
Higher GDF‐15 associated with worse prognosis in acute HF independently of BNP. When both biomarkers GDF‐15 and BNP were elevated at discharge, the 2‐year mortality risk increased over four‐fold. Biomarkers related to different pathophysiological pathways can provide incremental prognostic information in acute HF.
Keywords: Natriuretic peptides, Prognosis, Heart failure, GDF‐15, Mortality
Introduction
Biomarkers reflect biological processes and can provide objective and accurate information with diagnostic and prognostic significance. Their use has emerged as a promising and cost‐effective diagnostic method to facilitate therapeutic decision‐making in acute heart failure (HF).1 Growth differentiation factor 15 (GDF‐15) is a member of the transforming growth factor 𝛽 cytokine superfamily that is highly expressed in inflammatory states.2, 3, 4 There is evidence that GDF‐15 levels are elevated in cardiovascular diseases and in HF.5, 6 Previous observations have shown that GDF‐15 is associated with prognosis in chronic HF irrespective of ejection fraction.6, 7 B‐type natriuretic peptide (BNP) is a robust biomarker with prognostic significance in all spectrum of HF severity and in acute and chronic HF.8, 9, 10, 11, 12 GDF‐15 mirrors inflammatory stress and BNP mirrors mainly wall stretch. We aimed to evaluate the correlation between these two biomarkers and to evaluate if GDF‐15, by reflecting a different pathophysiological pathway, provided incremental prognostic information to BNP in acute HF.
Methods
We studied a subgroup of patients prospectively recruited as part of an acute HF registry that was conducted in the Internal Medicine Department of São João Hospital Center between January 2009 and December 2010. All patients admitted with the primary diagnosis of acute HF were eligible for inclusion in the registry; patients with acute coronary syndrome were excluded, as well as those patients whose symptoms were ultimately attributed, by the attending physician, to causes other than HF. Patients with no structural or function echocardiographic abnormalities were also excluded from the registry. Both de novo and worsening chronic HF as well as patients with systolic dysfunction and those with HF with preserved ejection fraction were included. As part of the registry's protocol, a complete physical examination at admission and in the discharge day was performed to all patients, and patients were drawn a fasting venous blood sample within the first 48 h of hospital admission as well as in the discharge day. BNP determination is a routine laboratory procedure in our hospital; an Abbott chemiluminescent microparticle immunoassay (two‐step immunoassay) is used. Serum creatinine was measured using conventional methods with an Olympus AU5400® automated clinical chemistry analyzer (Beckman‐Coulter®, Krefeld, Germany). Haemoglobin was obtained using an automated blood counter Sysmex® XE‐5000 (Sysmex Europe GmbH, Norderstedt, Germany). GDF‐15 was measured by ELISA (Quantikine Human GDF‐15 immunoassay). Dilutions (1/4 to 1/8) were performed to obtain values within the analytic range of the kit the assay had a 7% of coefficient of variation.
An echocardiogram was performed to all patients during hospitalization. Left ventricular ejection fraction ≥50% was considered preserved systolic function. The patient's treatment strategy, timing of discharge, and discharge medication were at the discretion of the attending physician. Physicians treating acute HF patients were aware of the ongoing registry. The 2008 European Society of Cardiology guidelines were used for the diagnosis of HF.13
The registry's protocol conformed to the ethical guidelines of the Declaration of Helsinki, and it was approved by the local ethics committee.
GDF‐15 was measured at hospital discharge in a subgroup of consecutive patients. Patients were followed up to 2 years, and the endpoint under analysis was all‐cause mortality.
Statistical analysis
A Spearman correlation coefficient was used to study the correlation between GDF‐15 and BNP, age, creatinine, C‐reactive protein, and haemoglobin. Patients with discharge GDF‐15 below and above the median value were compared: χ2 test for categorical variables, Student's t‐test to compare continuous variables, and a Mann–Whitney U‐test when continuous variables had a highly skewed distribution.
A Cox regression analysis was used to study the prognostic impact of GDF‐15 and BNP. GDF‐15 was analysed both as a continuous and as a categorical variable—dichotomized according to the mean. If was first tested and confirmed that there was a stepwise increase in mortality risk with increasing values of GDF‐15. Multivariate models were built to study the independent association of GDF‐15 with mortality. Variables entering the model were variables differently distributed according to GDF‐15: age, haemoglobin, creatinine, C‐reactive protein, New York Heart Association (NYHA) class, and BNP; gender and left ventricular systolic dysfunction also entered the model. A second model was built also considering evidence‐based HF medications; however, this is not the final model presented because the number of events does not support so many covariates without risk of overfitting. Patients were further cross‐classified according to discharge GDF‐15 (mean) and discharge BNP (mean) in three groups: those with both discharge GDF‐15 and BNP ≥ mean, those with both discharge GDF‐15 and BNP < mean (reference category), and those with only one of BNP or GDF‐15 above the mean (BNP ≥ mean, but not GDF‐15, and GDF‐15 ≥ mean, but not BNP). Independent association with mortality was also studied for this dummy‐coded variable.
Results
We studied 158 patients discharged after an acute HF episode. Seventy‐nine (50%) were male, mean age was 75 years, 87 (55.1%) had left ventricular ejection fraction <40%, mean discharge BNP was 1000.2 pg/mL, and mean (standard deviation) discharge GDF‐15 was 3013.3 (1643.0) ng/mL. During the 2‐year follow‐up, 71 patients (44.9%) died.
Table 1 shows patients' characteristics and comparison between patients with GDF‐15 <3000 and ≥3000 ng/mL (approximately the mean value). Patients with higher GDF‐15 (≥3000 ng/mL) were significantly older and had higher BNP, higher creatinine and C‐reactive protein, and lower haemoglobin; they were discharged on higher NYHA class and less medicated with beta‐blockers. No differences were reported concerning gender, co‐morbidities, ischaemic aetiology of HF, and left ventricular ejection fraction. More patients with elevated discharge GDF‐15 died in the 2‐year follow‐up. GDF‐15 correlated positively with age (ρ = 0.27), BNP (ρ = 0.36), creatinine (ρ = 0.51), and C‐reactive protein (ρ = 0.28) and negatively with haemoglobin (ρ = −0.305).
Table 1.
Comparison between acute HF patients with HFrEF and HFpEF
| Characteristics | All patients (n = 158) | GDF‐15 < 3000 ng/mL (n = 91) | GDF‐15 ≥ 3000 ng/mL (n = 67) | P‐value |
|---|---|---|---|---|
| Male, n (%) | 79 (50.0) | 46 (50.5) | 33 (49.3) | 0.87 |
| Age, mean (SD) | 75 (13) | 73 (14) | 78 (12) | 0.02 |
| Atrial fibrillation, n (%) | 86 (54.4) | 51 (56.0) | 35 (52.2) | 0.71 |
| Diabetes mellitus, n (%) | 57 (36.1) | 32 (35.2) | 25 (37.3) | 0.78 |
| Arterial hypertension history, n (%) | 114 (72.2) | 64 (70.3) | 50 (74.6) | 0.52 |
| Ischaemic aetiology, n (%) | 74 (46.8) | 40 (44.0) | 34 (50.7) | 0.40 |
| Left ventricular systolic function < 40% | 87 (55.1) | 57 (57.1) | 35 (52.2) | 0.54 |
| Discharge NYHA ≥III, n (%) | 30 (19.0) | 11 (12.1) | 19 (28.4) | 0.009 |
| Discharge haemoglobin (g/dL), mean (SD) | 12.4 (2.1) | 12.8 (1.7) | 12.0 (2.4) | 0.02 |
| Discharge creatinine (mg/dL), mean (SD) | 1.44 (0.71) | 1.19 (0.36) | 1.77 (0.92) | <0.001 |
| Discharge BNP (pg/mL), median (IQR) | 599.6 (260.2–1205.4) | 444.1 (209.2–869.0) | 800.7 (390.8–1513.8) | 0.001 |
| Discharge high‐sensitivity C‐reactive protein (mg/L), median (IQR) | 12.3 (5.5–24.6) | 10.7 (4.5–21.4) | 15.5 (9.2–28.6) | 0.003 |
| Acetylsalicylic acid, n (%) | 92 (58.2) | 52 (57.1) | 40 (59.7) | 0.78 |
| Statin, n (%) | 97 (61.4) | 55 (60.4) | 42 (62.7) | 0.82 |
| Beta‐blocker at discharge, n (%) | 115 (72.8) | 72 (79.1) | 43 (64.2) | 0.04 |
| ACE‐I and/or ARB at discharge, n (%) | 134 (84.8) | 79 (86.8) | 55 (82.1) | 0.41 |
| Mineralocorticoid receptor antagonists, n (%) | 44 (27.8) | 24 (26.4) | 20 (29.9) | 0.63 |
| 2‐year death | 71 (44.9) | 32 (35.2) | 39 (58.2) | 0.004 |
ACE‐I, angiotensin‐converting enzyme inhibitors; ARB, angiotensin receptor blockers; BNP, B‐type natriuretic peptide; GDF‐15, Growth differentiation factor 15; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; IQR, interquartile range; NYHA, New York Heart Association; SD, standard deviation.
Patients with discharge BNP ≥ 1000 pg/mL had an: age‐, gender‐, left ventricular systolic dysfunction‐, discharge NYHA class‐, creatinine‐, C‐reactive protein‐, haemoglobin‐, and GDF‐15; independent risk of 2‐year mortality of 2.26 (95% confidence interval: 1.30–3.94), and the age‐, gender‐, left ventricular systolic dysfunction‐, discharge NYHA class‐, creatinine, C‐reactive protein‐, haemoglobin‐, and BNP‐adjusted risk for GDF‐15 ≥ 3000 ng/mL was 1.86 (95% confidence interval:1.08–3.18). Table 2 shows the final multivariate model. Figure 1 shows the Kaplan–Meier survival curves in patients discharged with GDF‐15 < 3000 ng/mL and those with higher GDF‐15 values. When BNP and GDF‐15 were analysed as continuous variables, similar independent associations with mortality were obtained (Table 3). When angiotensin‐converting enzyme inhibitors and/or angiotensin receptor blockers, beta‐blockers, and mineralocorticoid receptor antagonists were included in the model, both GDF‐15 and BNP remained independently associated with the outcome (data not shown).
Table 2.
Association of discharge BNP and discharge GDF‐15 level with 2‐year mortality: multivariate model
| HR (95% CI) | P‐value | |
|---|---|---|
| Discharge GDF‐15 ≥ 3000 ng/mL | 1.86 (1.08–3.18) | 0.02 |
| Discharge BNP ≥1000 pg/mL | 2.26 (1.30–3.94) | 0.004 |
| Age (per year) | 1.01 (0.99–1.04) | 0.30 |
| Male | 1.52 (0.88–2.65) | 0.14 |
| Discharge creatinine (per mg/dL) | 1.06 (0.74–1.52) | 0.76 |
| Discharge haemoglobin (per g/dL) | 0.91 (0.80–1.02) | 0.12 |
| Discharge C‐reactive protein (per mg/L) | 1.00 (0.99–1.01) | 0.73 |
| Left ventricular systolic dysfunction<40% | 0.72 (0.40–1.29) | 0.27 |
| Discharge NYHA class ≥III | 0.84 (0.45–1.57) | 0.58 |
BNP, B‐type natriuretic peptide; CI, confidence interval; GDF‐15, growth differentiation factor 15; HR, hazard ratio; NYHA, New York Heart Association.
Figure 1.
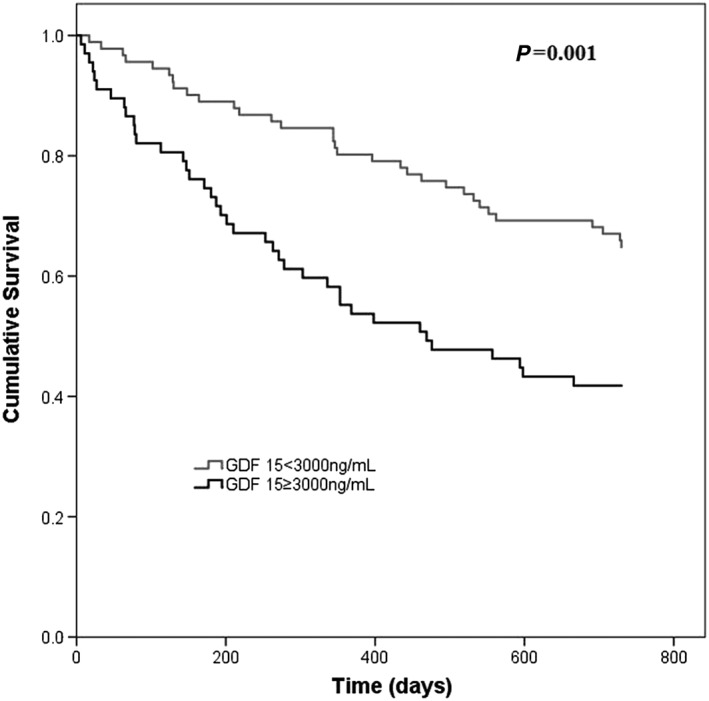
Kaplan–Meier survival curves in patients with discharge growth differentiation factor 15 (GDF‐15) < 3000 and ≥ 3000 ng/mL. Patients with elevated discharge GDF‐15 had higher 2‐year mortality.
Table 3.
Association of discharge BNP and discharge GDF‐15 level (analysed as continuous variables) with 2‐year mortality: multivariate model
| HR (95% CI) | P‐value | |
|---|---|---|
| Discharge GDF‐15 (per 100 ng/mL) | 1.02 (1.00–1.03) | 0.04 |
| Discharge BNP (per 100 pg/mL) | 1.01 (1.00–1.02) | 0.04 |
| Age (per year) | 1.02 (0.99–1.04) | 0.12 |
| Male | 1.59 (0.91–2.75) | 0.10 |
| Discharge creatinine (per mg/dL) | 1.11 (0.77–1.59) | 0.59 |
| Discharge haemoglobin (per g/dL) | 0.88 (0.78–1.00) | 0.05 |
| Discharge C‐reactive protein (per mg/L) | 1.00 (0.99–1.01) | 0.70 |
| Left ventricular systolic function < 40% | 0.88 (0.52–1.49) | 0.64 |
| Discharge NYHA class≥III | 0.97 (0.52–1.81) | 0.92 |
BNP, B‐type natriuretic peptide; CI, confidence interval; GDF‐15, growth differentiation factor 15; HR, hazard ratio; NYHA, New York Heart Association.
Patients discharged with both BNP and GDF‐15 above the mean had a multivariate adjusted hazard ratio of 2‐year death of 4.33 (2.07–90.6), P < 0.001 when compared with the reference category (both BNP and GDF‐15 below the mean). Patients with only one of the variables above the mean had a hazard ratio of 2‐year mortality of 1.76 (0.99–3.14), P = 0.06 (Table 4). Results were similar if evidence‐based HF therapy was included in the final model. Figure 2 shows the Kaplan–Meier survival curves in patients with both GDF‐15 and BNP values at discharge above the mean, those with both parameters below the mean at discharge, and those with only one of the parameters above the mean at hospital discharge.
Table 4.
Two‐year mortality: multivariate model
| HR (95% CI) | P‐value | |
|---|---|---|
| Discharge GDF‐15 and discharge BNP | ||
| GDF‐15 < 3000 ng/mL and BNP < 1000 pg/mL (reference) | 1 | |
| Only one of the variables above the mean | 1.76 (0.99–3.14) | 0.06 |
| GDF‐15 ≥ 3000 ng/mL and BNP ≥ 1000 pg/mL | 4.33 (2.07–9.06) | <0.001 |
| Age (per year) | 1.01 (0.99–1.04) | 0.29 |
| Male | 1.52 (0.88–2.64) | 0.13 |
| Discharge creatinine (per mg/dL) | 1.04 (0.72–1.48) | 0.85 |
| Discharge haemoglobin (per g/dL) | 0.90 (0.80–1.02) | 0.10 |
| Discharge C‐reactive protein (per mg/L) | 1.00 (0.99–1.01) | 0.66 |
| Left ventricular systolic dysfunction < 40% | 0.74 (0.42–1.28) | 0.28 |
| Discharge NYHA class ≥III | 0.81 (0.43–1.53) | 0.52 |
BNP, B‐type natriuretic peptide; CI, confidence interval; GDF‐15, growth differentiation factor 15; HR, hazard ratio; NYHA, New York Heart Association.
Patients were cross‐classified according to discharge BNP and discharge GDF‐15.
Figure 2.
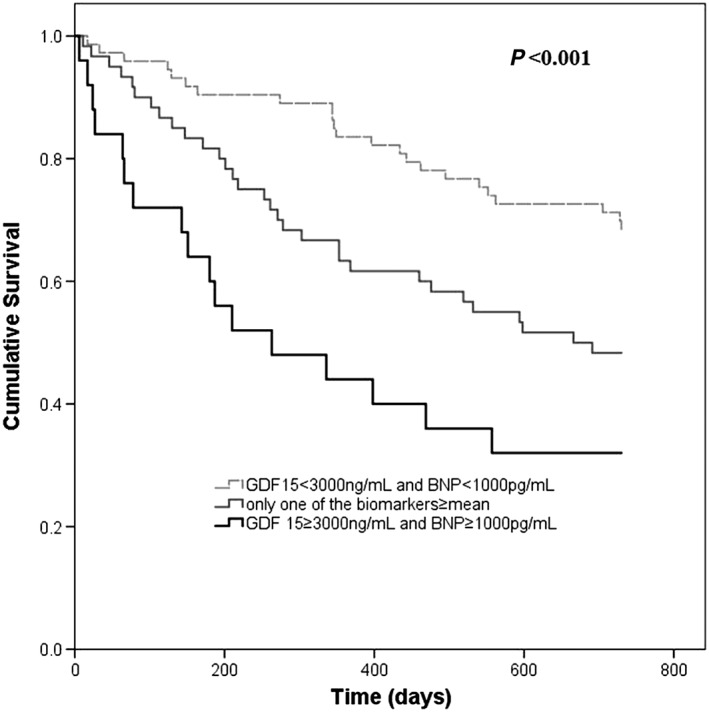
Kaplan–Meier survival curves according to discharge BNP and growth differentiation factor 15 (GDF‐15): reference category: both biomarkers < mean; other categories—only one of the biomarkers ≥ mean and both biomarkers ≥ mean. Patients with both BNP and GDF‐15 < mean had a clear survival benefit while those with both biomarkers ≥ mean had higher 2‐year mortality.
Discussion
Our results provide evidence that a multi‐marker approach to stratify patients after an acute HF episode based on different biomarkers that mirror diverse physiopathological systems in HF is promising. These results give subtract to the growing concept that the development of strategies based on individual characteristics have the potential to better tailor HF therapy in an individual level. In our acute HF population, we observed that patients with higher GDF‐15 had an almost double long‐term mortality when compared with patients with lower levels; patients with higher BNP had a more than double mortality risk; and patients with both biomarkers above the median had more than a four‐fold higher death risk.
GDF‐15 is a marker of inflammation; it is a member of the transforming growth factor family, which is overexpressed in response to myocardial stress.14 GDF‐15 expression is induced by myocardial stress and remodelling and is expressed in myocytes in response to cardiac ischaemia and pressure overload.15 Elevated levels of GDF‐15 have been reported in acute coronary syndromes and chronic HF.6, 7, 16
In our study, we simultaneously measured GDF‐15 and BNP in acute HF. We provide evidence of the incremental prognostic utility of GDF‐15 over and on top of BNP. The additional prognostic value suggests that beyond the haemodynamic wall stress (BNP), the inflammatory stress (GDF‐15) may play an important role in acute HF. Our study sample represents a real‐world acute HF population of elderly patients with elevated prevalence of HF with preserved ejection fraction and high co‐morbidity burden.
In chronic HF, there is wide evidence showing the independent prognostic value of GDF‐15 in patients with preserved and reduced ejection fraction.6, 7, 16, 17, 18 The prognostic value of GDF‐15 has first been observed by Kempf et al. who measured circulating levels of GDF‐15 in 455 chronic HF patients. Increasing GDF‐15 levels were associated with increasing HF severity. Two‐year mortality increased across GDF‐15 quartiles (10.0%, 9.4%, 33.4%, and 56.2%, respectively, P < 0.001). Even after multivariate adjustment including for N terminal pro brain natriuretic peptide, GDF‐15 remained an independent predictor of mortality.6 Further data from the Val‐HeFT (Valsartan Heart Failure Trial) study support the use of GDF‐15 in chronic HF.16 In Val‐HeFT, GDF‐15 was measured at baseline and after 12 months. Similar to the study by Kempf et al., GDF‐15 levels were associated with features of advanced HF and other biomarkers of neurohormonal activation, inflammation, myocyte injury, and renal dysfunction. In a multiple‐variable Cox regression model that included clinical risk factors, BNP, high‐sensitivity C‐reactive protein, and high‐sensitivity Troponin T, GDF‐15 was an independent death predictor. A recent report showed that GDF‐15 measured within or after an acute HF episode, when included in a multi‐marker approach (including N terminal pro brain natriuretic peptide, high‐sensitivity cardiac troponin T, GDF‐15, and soluble ST2) to determine prognosis, was associated with medium term mortality in acute HF patients.19 Our study expands these previous observations in chronic and acute HF patients, showing that GDF‐15 is independently associated with long‐term mortality. Our results also hint that, by using simultaneously BNP and GDF‐15, a mortality risk gradient can be observed; suggesting that using both biomarkers, we can identify patients in need of a closer follow‐up. To the best of our knowledge, this is the first study in which both GDF‐5 and BNP were considered for patient cross‐classification and their somehow synergic prognostic power tested in a multivariate approach.
GDF‐15 levels were equally elevated in patients with preserved and reduced ejection fraction 3077.2 vs. 2980.1 ng/mL. This observation replicates other studies and supports the important role of inflammation in HF with preserved ejection fraction.7 In our patient population, GDF‐15 levels showed a positive correlation with C‐reactive protein. Patients with higher GDF‐15 levels also tended to have higher C‐reactive protein, and, in fact, inflammatory markers seem to be intimately associated with GDF‐15. Previous observations have suggested an association of higher C‐reactive protein levels with worse prognosis in acute HF, supporting the role of inflammation in the pathophysiology of HF.20, 21
Our study is limited by the relatively small sample size and single centre nature. Despite the small sample size, we were able to detect prognostic differences according to the discharge GDF‐15 level as well as to detect the added prognostic value of GDF‐15 on top of BNP knowledge. Future studies are needed to extend our observations and to determine their generalizability to other populations. We did not evaluate other biomarkers with putative clinical value in the acute HF setting such as high‐sensivity troponin I and soluble ST2. Soluble ST2 acts as a decoy receptor for interleukin‐33, whose binding exerts anti‐inflammatory and anti‐fibrotic effects.22, 23 The elevation of soluble ST2 likely reflects activation of systemic inflammation, and the knowledge of both GDF‐15 and soluble ST2 as different ways of reflecting a common inflammatory pathway would likely be interesting. Also, the use of GDF‐15 as a therapeutic target is yet unknown, and our results do not have direct implications in the management of HF patients; still, a better risk stratification is crucial in HF approach.
Conclusions
The marker of inflammatory stress GDF‐15 is associated with long‐term mortality in acute HF independently of and beyond BNP. Thus, GDF‐15, by reflecting increased wall stiffness from inflammatory injury, may provide complementary pathophysiological information to that of BNP, which reflects haemodynamic wall tension and stress. Our results suggest that the inflammation‐related marker GDF‐15 may be a candidate to future development of anti‐inflammatory therapies for HF patients.
Conflict of interest
None declared.
Funding
This work was supported by the project DOCnet (NORTE‐01‐0145‐FEDER000003), supported by Norte Portugal Regional Operational Programme (NORTE 2020), under the PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund (ERDF).
Bettencourt, P. , Ferreira‐Coimbra, J. , Rodrigues, P. , Marques, P. , Moreira, H. , Pinto, M. J. , Guimarães, J. T. , and Lourenço, P. (2018) Towards a multi‐marker prognostic strategy in acute heart failure: a role for GDF‐15. ESC Heart Failure, 5: 1017–1022. 10.1002/ehf2.12301.
[Correction added after online publication on 10 September 2018: author name corrected to João Ferreira‐Coimbra]
References
- 1. Chow SL, Maisel AS, Anand I, Bozkurt B, de Boer RA, Felker GM, Fonarow GC, Greenberg B, Januzzi JL Jr, Kiernan MS, Liu PP, Wang TJ, Yancy CW, Zile MR. Role of biomarkers for the prevention, assessment, and management of heart failure: a scientific statement from the American Heart Association. Circulation 2017; 135: 1054–1091. [DOI] [PubMed] [Google Scholar]
- 2. Bootcov MR, Bauskin AR, Valenzuela SM, Moore AG, Bansal M, He XY, Zhang HP, Donnellan M, Mahler S, Pryor K, Walsh BJ, Nicholson RC, Fairlie WD, Por SB, Robbins JM, Breit SN. MIC‐1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF‐beta superfamily. Proc Natl AcadSci U S A 1997; 94: 11514–11519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schlittenhardt D, Schober A, Strelau J, Bonaterra GA, Schmiedt W, Unsicker K, Metz J, Kinscherf R. Involvement of growth differentiation factor15/macrophage inhibitory cytokine‐1 (GDF‐15/MIC‐1) in oxLDL‐induced apoptosis of human macrophages in vitro and in arteriosclerotic lesions. Cell Tissue Res 2004; 318: 325–333. [DOI] [PubMed] [Google Scholar]
- 4. Kempf T, Eden M, Strelau J, Naguib M, Willenbockel C, Tongers J, Heineke J, Kotlarz D, Xu J, Molkentin JD, Niessen HW, Drexler H, Wollert KC. The transforming growth factor‐beta superfamily member growth‐differentiation factor‐15 protects the heart from ischemia/reperfusion injury. Circ Res 2006; 98: 351–360. [DOI] [PubMed] [Google Scholar]
- 5. Wollert KC, Kempf T, Wallentin L. Growth differentiation factor 15 as a biomarker in cardiovascular disease. Clin Chem 2017; 63: 140–151. [DOI] [PubMed] [Google Scholar]
- 6. Kempf, von Haehling S, Peter T, Allhoff T, Cicoira M, Doehner W, Ponikowski P, Filippatos GS, Rozentryt P, Drexler H, Anker SD, Wollert KC. Prognostic utility of growth differentiation factor‐15 in patients with chronic heart failure. J Am Coll Cardiol 2007; 50: 1054–1060. [DOI] [PubMed] [Google Scholar]
- 7. Chan MM, Santhana Krishnan R, Chong JP, Chen Z, Tai BC, Liew OW, Ng TP, Ling LH, Sim D, Leong KT, Yeo PS, Ong HY, Jaufeerally F, Wong RC, Chai P, Low AF, Richards AM, Lam CS. Growth differentiation factor 15 in heart failure with preserved vs. reduced ejection fraction. Eur J Heart Fail 2016; 18: 81–88. [DOI] [PubMed] [Google Scholar]
- 8. Berger R, Huelsman M, Strecker K, Bojic A, Moser P, Stanek B, Pacher R. B‐type natriuretic peptide predicts sudden death in patients with chronic heart failure. Circulation 2002; 105: 2392–2397. [DOI] [PubMed] [Google Scholar]
- 9. AnandIS, Fisher LD , Chiang YT, Latini R, Masson S, Maggioni AP, Glazer RD, Tognoni G, Cohn JN. For the Val‐HeFT Investigators . Changes in brain natriuretic peptide and norepinephrine over time and mortality and morbidity in the Valsartan Heart Failure Trial (Val‐HeFT). Circulation 2003; 107: 1278–1283. [DOI] [PubMed] [Google Scholar]
- 10. Doust JA, Pietrzak E, Dobson A, Glasziou P. How well does B‐type natriuretic peptide predict death and cardiac events in patients with heart failure: systematic review. BMJ 2005; 330: 625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Januzzi JL Jr, Sakhuja R, O'donoghue M, Baggish AL, Anwaruddin S, Chae CU, Cameron R, Krauser DG, Tung R, Camargo CA Jr, Lloyd‐Jones DM. Utility of amino‐terminal pro‐brain natriuretic peptide testing for prediction of 1‐year mortality in patients with dyspnea treated in the emergency department. Arch Intern Med 2006; 166: 315–320. [DOI] [PubMed] [Google Scholar]
- 12. Bettencourt P, Azevedo A, Pimenta J, Friões F, Ferreira S, Ferreira A. N‐terminal‐pro‐brain natriuretic peptide predicts outcome after hospital discharge in heart failure patients. Circulation 2004; 110: 2168–2174. [DOI] [PubMed] [Google Scholar]
- 13. Dickstein K, Cohen‐Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole‐Wilson PA, Strömberg A, van Veldhuisen DJ, Atar D, Hoes AW, Keren A, Mebazaa A, Nieminen M, Priori SG, Swedberg K, ESC Committee for Practice Guidelines (CPG) . ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur J Heart Fail 2008; 10: 933–989. [DOI] [PubMed] [Google Scholar]
- 14. Frank D, Kuhn C, Brors B, Hanselmann C, Ludde M, Katus HA, Frey N. Gene expression pattern in biomechanically stretched cardiomyocytes: evidence for a stretch‐specific gene program. Hypertension 2008; 51: 309–318. [DOI] [PubMed] [Google Scholar]
- 15. Hagström E, Held C, Stewart RA, Aylward PE, Budaj A, Cannon CP, Koenig W, Krug‐Gourley S, Mohler ER 3rd, Steg PG, Tarka E, Östlund O, White HD, Siegbahn A, Wallentin L, Investigators STABILITY. Growth differentiation factor 15 predicts all‐cause morbidity and mortality in stable coronary heart disease. Clin Chem 2017; 631: 325–333. [DOI] [PubMed] [Google Scholar]
- 16. Anand IS, Kempf T, Rector TS, Tapken H, Allhoff T, Jantzen F, Kuskowski K, Cohn JN, Drexler H, Wollert KC. Serial measurement of growth‐differentiation factor‐15 in heart failure: relation to disease severity and prognosis in the Valsartan Heart Failure Trial. Circulation 2010; 122: 1387–1395. [DOI] [PubMed] [Google Scholar]
- 17. Lok DJ, Klip IT, Lok SI, de la Porte Bruggink‐André PW, Badings E, van Wijngaarden J, Voors AA, de Boer RA, van Veldhuisen DJ, van der Meer P. Incremental prognostic power of novel biomarkers (growth‐differentiation factor‐15, high‐sensitivity C‐reactive protein, galectin‐3, and high‐sensitivity troponin‐T) in patients with advanced chronic heart failure. Am J Cardiol 2013; 112: 831–837. [DOI] [PubMed] [Google Scholar]
- 18. Hage C, Michaëlsson E, Linde C, Donal E, Daubert JC, Gan LM, Lund LH. Inflammatory biomarkers predict heart failure severity and prognosis in patients with heart failure with preserved ejection fraction. A holistic proteomic approach. Circ Cardiovasc Genet 2017; 10: e001633. [DOI] [PubMed] [Google Scholar]
- 19. Demissei BG, Cotter G, Prescott MF, Felker GM, Filippatos G, Greenberg BH, Pang PS, Ponikowski P, Severin TM, Wang Y, Qian M, Teerlink JR, Metra M, Davison BA, Voors AA. A multimarker multi‐time point‐based risk stratification strategy in acute heart failure: results from the RELAX‐AHF trial. Eur J Heart Fail 2017; 19: 1001–1010. [DOI] [PubMed] [Google Scholar]
- 20. Kalogeropoulos AP, Tang WH, Hsu A, Felker GM, Hernandez AF, Troughton RW, Voors AA, Anker SD, Metra M, McMurray JJ, Massie BM, Ezekowitz JA, Califf RM, O'Connor CM, Starling RC, Butler J. High‐sensitivity C‐reactive protein in acute heart failure: insights from the ASCEND‐HF trial. J Card Fail 2014; 20: 319–326. [DOI] [PubMed] [Google Scholar]
- 21. Villacorta H, Masetto AC, Mesquita ET. C‐reactive protein: an inflammatory marker with prognostic value in patients with decompensated heart failure. Arq Bras Cardiol 2007; 88: 585–589. [DOI] [PubMed] [Google Scholar]
- 22. Januzzi JL Jr. ST2 as a cardiovascular risk biomarker: from the bench to the bedside. J Cardiovasc Transl Res 2013; 6: 493–500. [DOI] [PubMed] [Google Scholar]
- 23. Wang TJ, Wollert KC, Larson MG, Coglianese E, McCabe EL, Cheng S, Ho JE, Fradley MG, Ghorbani A, Xanthakis V, Kempf T, Benjamin EJ, Levy D, Vasan RS, Januzzi JL. Prognostic utility of novel biomarkers of cardiovascular stress: the Framingham Heart Study. Circulation 2012; 126: 1596–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]


