Keywords: nerve regeneration, alpha-synuclein, nitrated α-synuclein, Parkinson’s disease, methamphetamine, N-nitro-L-arginine, alpha-synuclein aggregation, apoptosis, neurotoxicity, neural regeneration
Abstract
Methamphetamine is an amphetamine-type psychostimulant that can damage dopaminergic neurons and cause characteristic pathological changes similar to neurodegenerative diseases such as Parkinson’s disease. However, its specific mechanism of action is still unclear. In the present study, we established a Parkinson’s disease pathology model by exposing SH-SY5Y cells and C57BL/6J mice to methamphetamine. In vitro experiments were performed with 0, 0.5, 1.0, 1.5, 2.0 or 2.5 mM methamphetamine for 24 hours or 2.0 mM methamphetamine for 0-, 2-, 4-, 8-, 16-, and 24-hour culture of SH-SY5Y cells. Additional experimental groups of SH-SY5Y cells were administered a nitric oxide inhibitor, 0.1 mM N-nitro-L-arginine, 1 hour before exposure to 2.0 mM methamphetamine for 24 hours. In vivo experiments: C57BL/6J mice were intraperitoneally injected with N-nitro-L-arginine (8 mg/kg), eight times, at intervals of 12 hours. Methamphetamine 15 mg/kg was intraperitoneally injected eight times, at intervals of 12 hours, but 0.5-hour after each N-nitro-L-arginine injection in the combined group. Western blot assay was used to determine the expression of nitric oxide synthase, α-synuclein (α-Syn), 5G4, nitrated α-synuclein at the residue Tyr39 (nT39 α-Syn), cleaved caspase-3, and cleaved poly ADP-ribose polymerase (PARP) in cells and mouse brain tissue. Immunofluorescence staining was conducted to measure the positive reaction of NeuN, nT39 α-Syn and 5G4. Enzyme linked immunosorbent assay was performed to determine the dopamine levels in the mouse brain. After methamphetamine exposure, α-Syn expression increased; the aggregation of α-Syn 5G4 increased; nT39 α-Syn, nitric oxide synthase, cleaved caspase-3, and cleaved PARP expression increased in the cultures of SH-SY5Y cells and in the brains of C57BL/6J mice; and dopamine levels were reduced in the mouse brain. These changes were markedly reduced when N-nitro-L-arginine was administered with methamphetamine in both SH-SY5Y cells and C57BL/6J mice. These results suggest that nT39 α-Syn aggregation is involved in methamphetamine neurotoxicity.
Chinese Library Classification No. R459.9; R363; R741
Introduction
Methamphetamine (METH) is a common psychostimulant belonging to amphetamine type. More and more reports have demonstrated that METH abuse can lead to undesirable and potentially fatal conditions in the human nervous system, such as oxidative stress, excitotoxicity, activation of microglia, and toxicity of dopamine neurons (Krasnova and Cadet, 2009; Chao et al., 2017). Studies have shown that people who abuse METH for a long time are more susceptible to Parkinson’s disease (PD) (Callaghan et al., 2010).
Pathological characteristics of PD are the abnormal accumulation and aggregation of alpha-synuclein (α-Syn) in Lewy bodies of the dopaminergic neurons (Abdelmotilib et al., 2017; Emamzadeh, 2017). α-Syn is a soluble protein expressed in the presynaptic and perinuclear regions of the central nervous system (Braak et al., 2000; Segura-Aguilar, 2017). Its structure is highly dependent on the intracellular environment and may exhibit different structures such as monomer, oligomers, fibrils or fibers (Wang et al., 2016). In PD pathology, α-Syn can aggregate forming insoluble fibrin depositions, and leads to the death of nerve cells (Cadet and Krasnova, 2009; Lashuel et al., 2013; Aufschnaiter et al., 2017). Additionally, α-Syn is a main component of Lewy bodies, which are found in the dopaminergic neurons of patients with PD (Recasens and Dehay, 2014). Post-translational modification of α-Syn, including phosphorylation, nitration, acetylation, ubiquitylation and methylation, has been extensively studied. Nitrated α-Syn was found to be an important component of α-Syn aggregation in Lewy bodies of PD patients. The position of tyrosine nitration and oxidation in α-Syn has been disputed. nT39 α-Syn caused a high ratio of oligomerization, and mutations in this residue resulted in high levels of fibrilization (Anderson et al., 2006; Danielson et al., 2009; Lokappa et al., 2014). A study has observed that an abnormal accumulation of nitrated α-Syn at the Tyr39 residue (nT39 α-Syn) is found in the brains of PD patients and in transgenic mice with α-synucleinopathy (Chavarria and Souza, 2013). Under normal physiological conditions, only a small percentage of nT39 α-Syn is found in healthy brains (Hou et al., 2017). Therefore, we speculated that METH increased the expression of nT39 α-Syn in both SH-SY5Y cells and mouse brains in vivo. The purpose of this study is to investigate the role of nT39 α-Syn in METH-induced α-Syn aggregation and neurotoxicity.
Materials and Methods
METH intervention in vitro
The SH-SY5Y cell line was obtained from China Infrastructure of Cell Line Resources. SH-SY5Y cell line is from human neuroblastoma and is often used to study the neurotoxicology of many toxicants (Gómez-Santos et al., 2003). SH-SY5Y cells were cultured in DMEM/F-12 medium (Gibco, Carlsbad, CA, USA) with 10% fetal bovine serum (Gibco) and maintained in a standard humidified incubator with 5% CO2 at 37°C, and the medium was replaced by fresh medium every 2 days. When the plated cells reached approximately 80% confluence the medium was replaced by a non-serum medium and the cells were treated with METH (> 99% purity; National Institutes for Food and Drug Control; Guangzhou, China) at a concentration of 0, 0.5, 1.0, 1.5, 2.0 or 2.5 mM for 24 hours. Alternatively, the cells were treated with 2.0 mM METH for 0, 2, 4, 8, 16 or 24 hours. To examine the effect of the nitric oxide inhibitor, N-nitro-L-arginine (L-NNA), on the effect of METH, SH-SY5Y cells were exposed to L-NNA (0.1 mM) (Selleck Chemicals) for 1 hour followed by treatment with or without 2.0 mM METH for 24 hours. The cell culture morphologies in each group were observed 24 hours later under an inverted microscope (ECLIPSE 80i, Nikon, Tokyo, Japan), and cell culture media were collected for further analysis.
METH intervention in vivo
Fifty-two specific-pathogen-free healthy adult male C57BL/6J mice weighing 20–26 g and aged 6–8 weeks were obtained from the Laboratory Animal Center of Southern Medical University of China (SCXK2016-0041). Animal care and experimental procedures were approved by the Institutional Animal Care and Use Committee at the Southern Medical University and followed the NIH Guidelines for the Care and Use of Laboratory Animals. Adult mice were kept individually in cages, controlled at 22°C, and on a 12/12-hour dark/light schedule with food and water available ad libitum. The mice were randomly divided into two groups (n = 6 per group) and injected intraperitoneally with a saline control (control group) or METH (8 times, 15 mg/kg, at 12-hour intervals; METH group). The remainder were randomly divided into four experimental groups (10 mice each group): control group, L-NNA alone (L-NNA group), METH (8 times, 15 mg/kg, at 12-hour intervals) alone (METH group) and L-NNA + METH (L-NNA+METH group). The mice in the L-NNA group and L-NNA + METH group were intraperitoneally injected with L-NNA (Selleck Chemicals) at 8 mg/kg (8 times, at 12-hour intervals), and with METH 15 mg/kg half an hour after each injection of L-NNA, respectively. The mice were anesthetized with Nembutal and euthanized by decapitation, then fixed with 4% paraformaldehyde. Brains were removed, and the prefrontal cortex, hippocampus and midbrain regions were dissected out. Each sample was quick-frozen on liquid nitrogen, and kept at −80°C for subsequent study. Samples of three from each group were selected for each test by random sampling.
Western blot assay in cells and brain tissue
Cells or brain tissues treated with or without METH were washed in sterile ice-cold phosphate-buffered saline (PBS) twice and then lysed in RIPA lysis buffer (Beyotime, Nanjing, China) with protease inhibitors and phosphatase inhibitors. Protein concentrations were measured with the bicinchoninic acid protein assay (Chen et al., 2007). The same amount of protein in each lysate was separated by denaturing on 12% or 15% sodium dodecyl sulfate polyacrylamide gel and transferred onto polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). The membranes were incubated at room temperature for 1 hour in 5% nonfat milk or 5% bovine serum albumin in Tris-buffered saline Tween-20 buffer, then incubated with primary antibodies [neuronal nitric oxide synthase (anti-rabbit), α-Syn (anti-rabbit), 5G4 (demonstrated to react with human), nT39 α-Syn (anti-rabbit), caspase-3 and poly ADP-ribose polymerase (PARP) (1:1000 for all; anti-rabbit; Cell Signaling Technology, Boston, MA, USA)] overnight at 4°C. β-Actin (anti-rabbit; 1:2000; Bioss, Beijing, China) was used as a quantitative control. The membranes were washed three times with Tris-buffered saline Tween-20 and incubated with horseradish peroxidase-conjugated secondary antibodies for 1 hour at room temperature. The membranes were washed as above and then developed using chemiluminescence reagents (Thermo Scientific, Waltham, MA, USA). The signal of band intensities was quantified by Gel-Pro analyzer (Media Cybernetics, Inc., Rockville, MD, USA).
Immunofluorescence staining in SH-SY5Y cells
Cells were seeded on confocal dishes, fixed with 4% paraformaldehyde for 20 minutes, permeabilized with 0.2% Triton X-100 (Amresco, Shanghai, China; diluted in PBS) for 15 minutes, incubated with 5% bovine serum albumin (Solarbio, Beijing, China; diluted in PBS) for 45 minutes at room temperature, then incubated with primary antibodies rabbit anti-NeuN (1:150), rabbit anti-nT39 α-Syn (1:50) and human anti-α-Syn 5G4 (1:50) overnight at 4°C. After washing three times with PBS, cells were incubated with secondary antibody [either Cy3-conjugated goat anti-rabbit (1:100; DingGuo, Dalian, China) or TRITC-conjugated goat anti-mouse (1:100; DingGuo, Dalian, China)] for 1 hour at room temperature. The cells were washed as above and then a drop of 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) was added for nuclear staining. Microphotographs were taken using a confocal microscopy (ECLIPSE 80i, Nikon, Tokyo, Japan).
Enzyme-linked immunosorbent assay (ELISA) for dopamine detection in mouse brain tissue
Mouse brain tissues were collected as described previously and protein concentrations were measured by the Micro bicinchoninic acid Kit (Biocolors, Shanghai, China). The levels of dopamine were detected by an ELISA kit (Boston, MA, USA) following the manufacturer’s instructions. The optical density at 450 nm was read with a microplate reader (Cytation 3, BioTek, Nanjing, China), and the sample concentration was determined using a standard curve.
Statistical analysis
All representative data were analyzed from at least three independent experiments. Data are summarized as the mean ± SD. Data analysis for multiple comparisons was performed by two-independent-samples t-tests or one-way analysis of variance followed by least significant difference post hoc test using SPSS 19.0 software (IBM, Armonk, NY, USA). A value of P < 0.05 was considered statistically significant.
Results
METH increases α-Syn aggregation in SH-SY5Y cells
The present findings showed that the expression of α-Syn in SH-SY5Y cells was significantly increased after METH treatment (Figure 1A–D). Monoclonal antibody (α-Syn 5G4) has a strong selectivity for β-sheet-rich α-Syn oligomers (Sengupta et al., 2015), therefore the level of α-Syn 5G4 was chosen to analyze the accumulation of misfolded α-Syn. Compared with the control group, METH exposure caused an obvious aggregation of α-Syn in SH-SY5Y cells observed by immunoblotting and immunofluorescence (Figure 1E–G).
Figure 1.
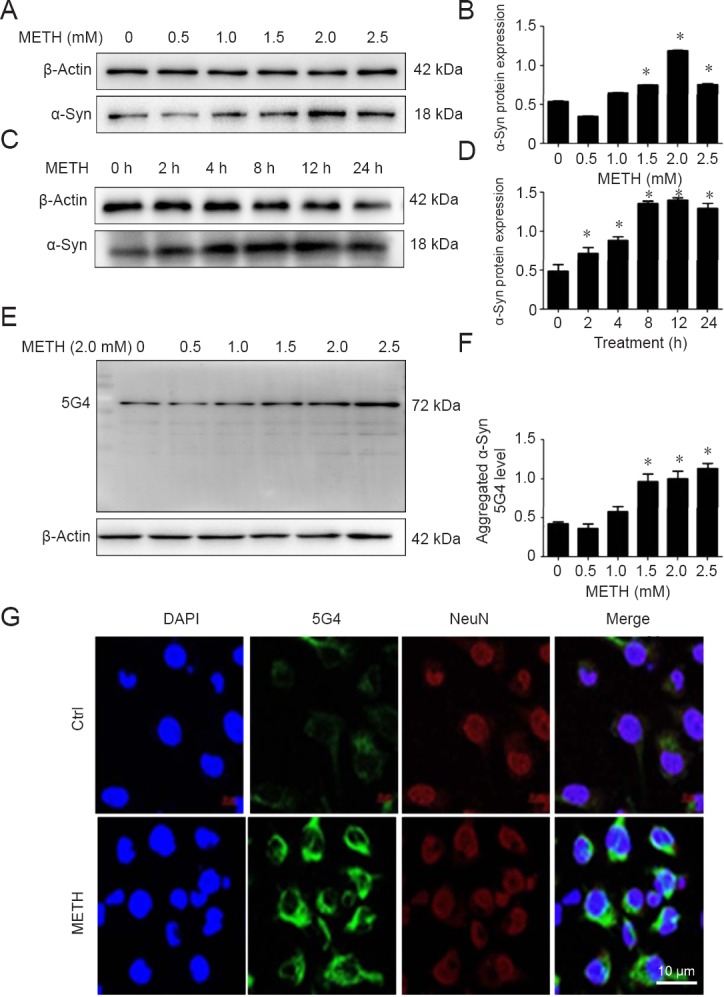
METH increases α-Syn level and aggregation of α-Syn G4 in SH-SY5Y cells.
METH exposure up-regulates α-Syn and aggregation of α-Syn protein expression in a concentration-dependent manner in SH-SY5Y cells. SH-SY5Y cells were treated with 0−2.5 mM METH for 24 hours or 2.0 mM METH for 2−24 hours. (A−D) Western blot assay (A, C) and quantitative analyses (B, D) were performed to determine the protein expression level of α-Syn. (E, F) Western blot assay (E) and quantitative analyses (F) were performed to determine α-Syn 5G4 protein expression. β-Actin was used as the loading control. Relative protein expression was expressed as the optical density ratio to β-actin. (G) α-Syn 5G4 was expressed at a higher level after 2.0 mM METH exposure for 24 hours than in the control group analyzed using a fluorescence microscope. α-Syn 5G4 was stained with anti-aggregated α-Syn, clone 5G4 antibody (green); SH-SY5Y cells were stained with NeuN (red); nuclei were counterstained with DAPI (blue). Scale bar: 10 µm. Data are expressed as the mean ± SD (n = 3 per group; one-way analysis of variance followed by least significant difference post hoc test). *P < 0.05, vs. control group. α-Syn: α-Synuclein; DAPI: 4′,6-diamidino -2-phenylindole; METH: methamphetamine; Ctrl: control; h: hour(s).
METH increases nT39 α-Syn and promotes apoptosis in vitro and in vivo
The nitrated form of α-Syn most associated with cell damage in PD is nT39 α-Syn. To determine whether neuronal cell bodies in vitro and in vivo contain this modified form of α-Syn and whether α-Syn nitration is enhanced by METH exposure, the expression of nT39 α-Syn was identified by western blot assay and immunofluorescence staining. After SH-SY5Y cells were exposed to METH, they showed morphological changes, including a round shape, shrinkage, loose adherence and floating in the medium under the microscope. Western blot assay showed that nT39 α-Syn was rarely detected under control conditions but significantly increased after METH exposure. The nT39 α-Syn protein level in SH-SY5Y cells was 2.5-fold higher after treatment with METH (2.0 mM) for 4 hours than in the control group (Figure 2A–D). Immunofluorescence staining was used to study the expression patterns of nT39 α-Syn in SH-SY5Y cells and similar results were observed (Figure 2E). Caspase-3 and PARP levels were studied to investigate whether the caspase-3 pathway participates in METH-induced neuronal apoptosis. We have found that METH increases the levels of cleaved caspase-3 and cleaved PARP significantly in SH-SY5Y cells (Figure 2F–I). Similar results were observed in the subacute METH mouse in vivo model (Figure 3).
Figure 2.
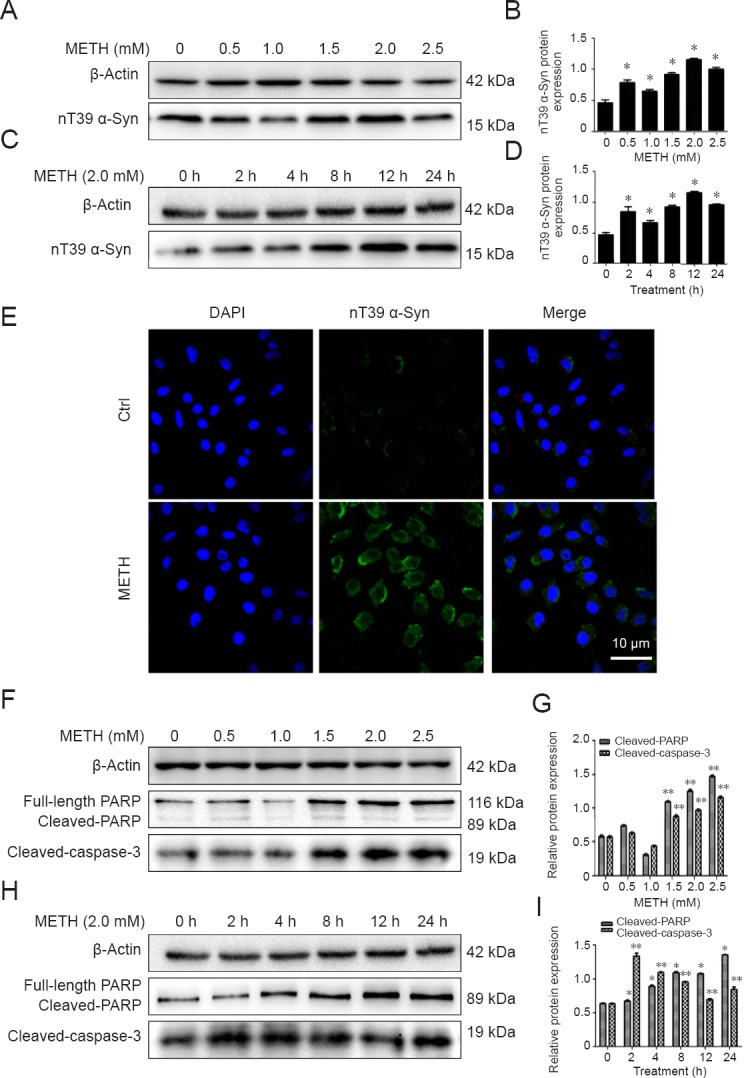
METH increases the expression of α-Syn nitration at tyrosine 39 (nT39 α-Syn) and induces significant apoptosis in SH-SY5Y cells.
METH exposure increased nT39 α-Syn and apoptosis in SH-SY5Y cells. SH-SY5Y cells were treated with 0−2.5 mM METH for 24 hours or 2.0 mM METH for 2−24 hours. (A–D) Western blot assay (A, C) and quantitative analyses (B, D) were performed to determine the protein expression levels of nT39 α-Syn. (E) nT39 α-Syn was expressed at a higher level after 2.0 mM METH exposure for 24 hours than in the control group analyzed using a fluorescence microscope. nT39 α-Syn was stained with anti-α-Syn (nitro-Tyr39) antibody (green); nuclei were counterstained with DAPI (blue). Scale bar: 10 µm. (F–I) Western blot assay (F, H) and quantitative analyses (G, I) were performed to determine cleaved PARP and cleaved caspase-3 protein expression levels. Relative protein expression was expressed as the optical density ratio to β-actin. β-Actin was used as the loading control. Data are expressed as the mean ± SD (n = 3 per group; one-way analysis of variance followed by least significant difference post hoc test). *P < 0.05, **P < 0.01, vs. control group (0 mM METH or 0 h group). α-Syn: α-Synuclein; DAPI: 4′,6-diamidino-2-phenylindole; PARP: poly ADP-ribose polymerase; METH: methamphetamine; Ctrl: control; h: hour(s).
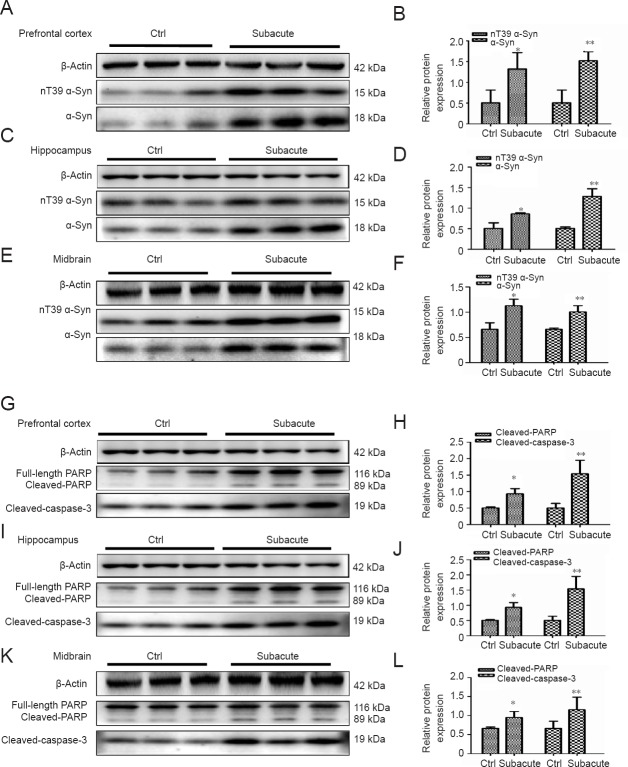
Figure 3.
METH increases the expression of α-Syn nitration at tyrosine 39 (nT39 α-Syn) and induces significant apoptosis in male mouse brain tissues.
Male mice were divided randomly into control and subacute intoxication groups. The prefrontal cortex (A, B, G, H), hippocampus (C, D, I, J) and midbrain (E, F, K, L) tissues were harvested at 24 hours after the last dose. Western blot assay (A, C, E) and quantitative analyses (B, D, F) were performed to determine the protein expression levels of nT39 α-Syn and α-Syn. β-Actin was used as the loading control. Western blot assay (G, I, K) and quantitative analyses (H, J, L) were performed to determine cleaved PARP and cleaved caspase-3 protein expression levels. Relative protein expression was expressed as the optical density ratio to β-Actin. β-Actin was used as the loading control. Data are expressed as the mean ± SD (n = 3 per group; one-way analysis of variance followed by least significant difference post hoc test). *P < 0.05, **P < 0.01, vs. control group. α-Syn: α-Synuclein; PARP: poly ADP-ribose polymerase; METH: methamphetamine; Ctrl: control.
L-NNA decreases METH-induced nT39 α-Syn and apoptosis in vitro and in vivo
To investigate whether L-NNA can affect the expression of nT39 α-Syn induced by METH, we analyzed the expression of α-Syn, α-Syn 5G4 and nT39 α-Syn. As shown in the results, L-NNA attenuated 70% of the elevated nT39 α-Syn expression and reduced the α-Syn levels significantly compared with those induced by METH in SH-SY5Y cells and mouse brain tissues. We also examined levels of apoptotic markers, cleaved caspase-3 and cleaved PARP. It is noted above that METH treatment increased the expression levels of these markers. The L-NNA only group showed very little change from the controls. However, in the L-NNA combined with METH group, the levels of cleaved caspase-3 and cleaved PARP were much reduced compared with those caused by METH alone (Figures 4 and 5).
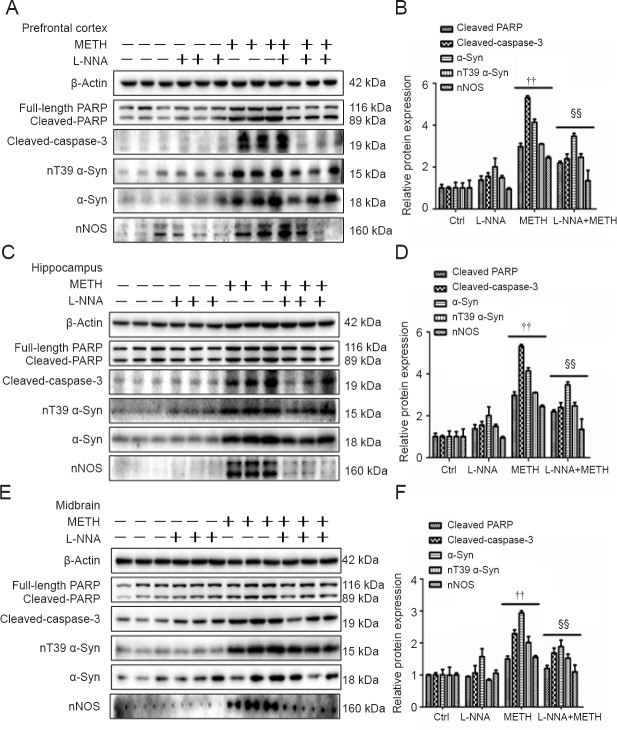
Figure 4.
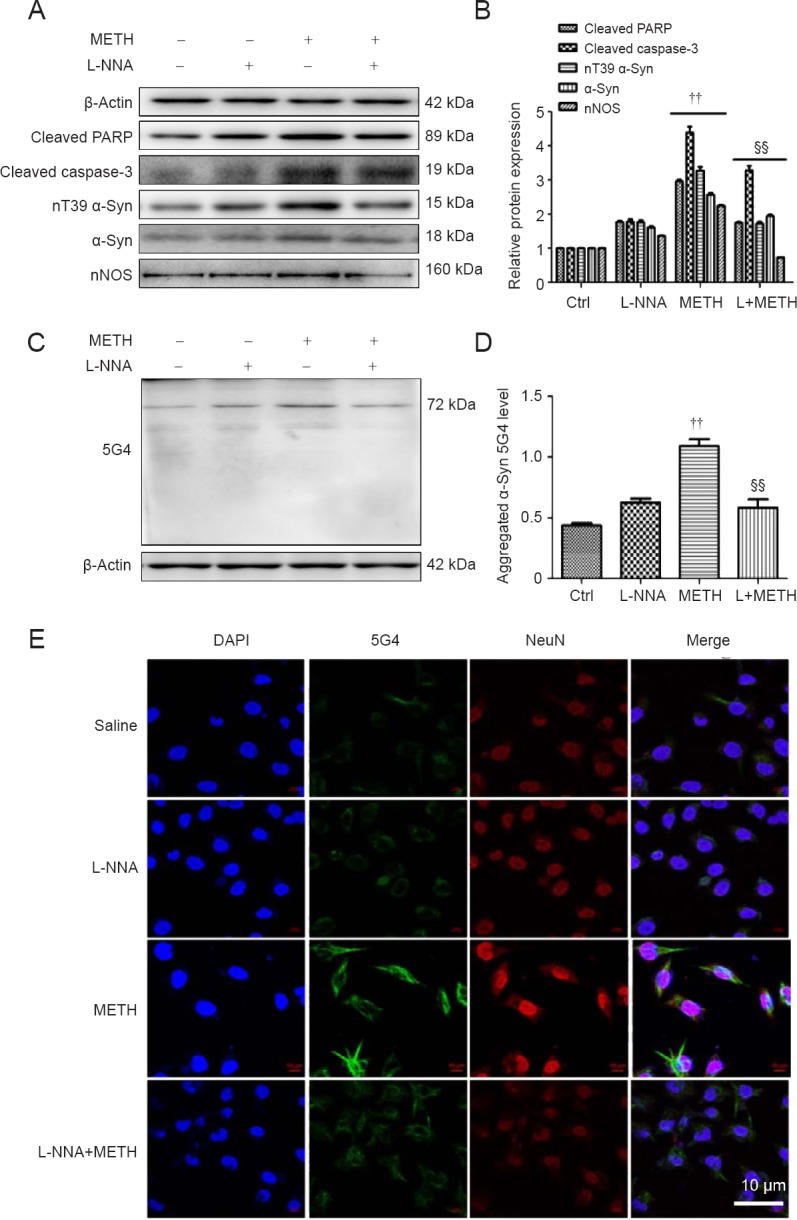
L-NNA decreases METH-induced nT39 α-Syn, α-Syn aggregation and apoptosis in SH-SY5Y cells.
(A, B) Western blot assay (A) and quantitative analyses (B) were performed to determine cleaved PARP, cleaved caspase-3, nT39 α-Syn, α-Syn and nNOS protein expression levels. (C, D) Western blot assay (C) and quantitative analyses (D) were performed to determine α-Syn 5G4 protein expression levels in SH-SY5Y cells. Relative protein expression was expressed as the optical density ratio to β-actin. β-Actin was used as the loading control. (E) α-Syn 5G4 was expressed at a higher level after 2.0 mM METH exposure for 24 hours than in the control group under a fluorescence microscope. α-Syn 5G4 was stained with anti-aggregated α-Syn, clone 5G4 antibody (green); SH-SY5Y cells were stained with NeuN (red); nuclei were counterstained with DAPI (blue). The nitric oxide synthase inhibitor L-NNA reduced METH-induced nT39 α-Syn, α-Syn aggregation and apoptosis in SH-SY5Y cells. Data are expressed as the mean ± SD (one-way analysis of variance). ††P < 0.01, vs. control and L-NNA groups; §§P < 0.01, vs. METH-treated group. α-Syn: α-Synuclein; DAPI: 4′,6-diamidino -2- phenylindole; METH: methamphetamine; Ctrl: control; L-NNA: N-nitro-L-arginine; nNOS: neuronal nitric oxide synthase.
Figure 5.
L-NNA decreases METH-induced nT39 α-Syn, α-Syn aggregation and apoptosis in male mouse brain tissues.
Male mice were randomly divided into four groups (n = 3 per group): control, L-NNA alone, METH alone and L-NNA + METH. The prefrontal cortex (A, B), hippocampus (C, D) and midbrain (E, F) tissues were harvested at 24 hours after the last dose. Western blot assay (A, C, E) and quantitative analyses (B, D, F) were performed to determine cleaved PARP, cleaved caspase-3, nT39 α-Syn, α-Syn and nNOS protein expression levels. The nitric oxide synthase inhibitor L-NNA reduced METH-induced nT39 α-Syn, α-Syn aggregation and apoptosis in mouse brain tissues. Relative protein expression was expressed as the optical density ratio to β-actin. β-Actin was used as the loading control. Data are expressed as the mean ± SD (one-way analysis of variance). ††P < 0.01, vs. control and L-NNA groups; §§P < 0.01, vs. METH-treated group. α-Syn: α-Synuclein; METH: methamphetamine; Ctrl: control; L-NNA: N-nitro-L-arginine; nNOS: neuronal nitric oxide synthase.
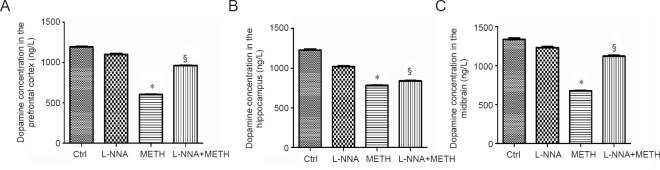
L-NNA raises dopamine level reduced by METH in mouse brain tissues
As shown in Figure 6, the DA levels in mouse brain tissues were significantly reduced in the METH group compared with the controls. However, in the L-NNA + METH group, the decrease in levels of DA was less than that in the METH group. There was no significant difference in DA levels between the control and L-NNA groups. These results showed that L-NNA could protect DA levels in mouse brain tissues from the effect of METH.
Figure 6.
L-NNA reduces the loss of DA level induced by METH in mouse brain tissues.
Male mice were divided into four groups randomly (n = 3 per group): control, L-NNA alone, METH alone and L-NNA + METH. The prefrontal cortex (A), hippocampus (B) and midbrain (C) tissues were harvested at 24 hours after the last dose. The level of dopamine was measured by enzyme linked immunosorbent assay. Data are expressed as the mean ± SD (analysis of variance followed by least significant difference post hoc test). *P < 0.05, vs. control group; §P < 0.05, vs. METH group. METH: Methamphetamine; Ctrl: control; L-NNA: N-nitro-L-arginine.
Discussion
α-Syn is a 140-amino acid protein that exists widely in the body, particularly in the brain. Furthermore, it is a major component of Lewy bodies, which are found in dopaminergic neurons of PD patients. This protein has various functions, which have been implicated in the maintenance of synaptic structure, neuroplasticity, integrating presynaptic membrane signaling, exerting a heat shock protein-like function, learning, memory, cell differentiation and the regulation of dopamine uptake (Emamzadeh, 2016). However, abnormal expression of α-Syn leads to pathological lesions. Several reports have proven that over-expression of α-Syn is strongly associated with the pathogenesis of PD (Baksi et al., 2016; Kinghorn et al., 2017).
METH, an illegal but widely abused drug, is believed to cause neurotoxicity due to aggregation of α-Syn (Klongpanichapak et al., 2006; Wu et al., 2014; Flack et al., 2017). There have been many reports that METH can result in the degeneration of neurons in rodents and primates (Gou et al., 2015; Killinger and Moszczynska, 2016). After discovering that METH exposure can induce a PD-like neuropathology, it has been used extensively as a model of drug-based Parkinson syndrome (Todd et al., 2016). Oxidative stress is one of the major mediators of excitotoxicity, neuroinflammation and apoptosis in METH-based Parkinson syndrome (Thrash et al., 2009). Our previous studies have shown that harmful generation of nitric oxide and α-Syn accumulation were caused by METH. The consequent S-nitrosylation of protein disulfide isomerase results in dysfunction and a tendency to increase α-Syn accumulation (Wu et al., 2014). A related study has shown that the endoplasmic reticulum has high amounts of protein disulfide isomerase, which has many important functions; in oxidation, reduction, isomerization and molecular chaperone activity. Protein disulfide isomerase plays a very important role in neurodegenerative diseases. Nevertheless, the mechanism of METH-induced α-Syn aggregation remains unclear. The current study investigated whether METH induces the nitration of α-Syn and aggregation of α-Syn.
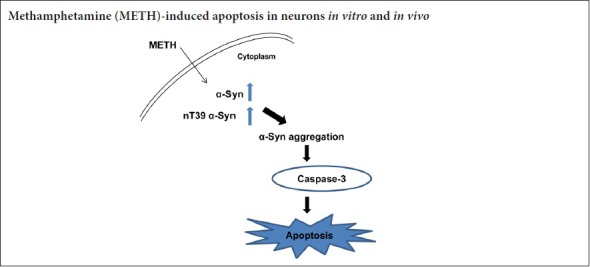
In the previous study, we found that METH exposure caused a significant increase in the generation of nitric oxide synthase. The present study shows that METH caused a significant increase in nT39 α-Syn. A related study has demonstrated that the expression of nitric oxide synthase produces a chronic, high level of neurotoxic nitric oxide, and this results in α-Syn nitration at tyrosine 39 (Ali and Itzhak, 1998). Post-translational modification of α-Syn containing nT39 α-Syn often appears in dopaminergic neurons of the brains of PD patients and other patients with neurodegenerative diseases characterized by Lewy bodies (Gao et al., 2008; Mak et al., 2010). Based on these data, we hypothesized that the regulation of nT39 α-Syn protein might control the PD-like pathology induced by METH exposure. Consistent with our hypothesis, we found that METH upregulated the formation of nT39 α-Syn and α-Syn aggregation in both SH-SY5Y cells and mouse brains. We also discovered that the inhibition of nT39 α-Syn formation clearly attenuated METH-induced α-Syn aggregation and neurotoxicity. These findings indicated that nT39 α-Syn plays a very important part in METH-induced α-Syn aggregation in dopaminergic neurons. We also showed that the upregulation of nT39 α-Syn contributed to dopaminergic neuronal apoptosis by inducing caspase-3 activity, which is consistent with published reports that post-translational modification mediated the METH-induced apoptosis of SH-SY5Y cells by activating the caspase pathway (Wu et al., 2014; Huang et al., 2015). Therefore, our data demonstrate that post-translational modification of α-Syn plays a significant role in not only neuronal α-Syn aggregation but also neuronal apoptosis induced by METH. These findings are consistent with the conclusion that METH abusers have a high risk of PD (Callaghan et al., 2012).
The results of this study are summarized as below: (1) nT39 α-Syn increased after METH treatment in SH-SY5Y cells and mouse brains, (2) the increased level of nT39 α-Syn involved dopaminergic α-Syn accumulation and neurotoxicity in SH-SY5Y cells and mouse brains, (3) L-NNA markedly attenuated METH-induced nT39 α-Syn accumulation and neurotoxicity in SH-SY5Y cells and mouse brains and (4) L-NNA counteracted the METH induced reductions in DA levels in mouse brain tissues. These findings indicate that nT39 α-Syn plays a significant role in neurotoxicity induced by METH in neurons.
In the present study, α-Syn nitration at tyrosine 39 is considered as an essential factor for neurodegeneration. We found that L-NNA, an inhibitor of nitric oxide synthase, dramatically reduced the production of nitro-oxidative modifications of α-Syn and neurotoxicity induced by METH in mouse brains. The results suggest that elevated α-Syn nitration contributes to METH-induced dopaminergic toxicity. These results are in agreement with previous studies that showed an aberrant accumulation of nT39 α-Syn induced by a high concentration of METH administration was conducive to neural cell death (Zhang et al., 2013). Therefore, it is concluded that nitration-mediated α-Syn aggregation plays a critical part in METH-induced dopaminergic neurons injury. Since inhibition of post-translational modification obviously protected nerve cells against METH-mediated neurons injury in SH-SY5Y cells and mouse brains, we have a good reason to conclude that inhibition of nT39 α-Syn formation could be a promising treatment for METH-induced dopaminergic neurotoxicity.
Additional file: Open peer review reports 1 (95.9KB, pdf) and 2 (63KB, pdf) .
Footnotes
Conflicts of interest: None declared.
Financial support: This study was supported by the National Natural Science Foundation of China, No. 81373240 (to PMQ) and 81671865 (to PMQ). All authors declare that financial support does not affect the opinion of the article and the objective statistical analysis and report of the research results in this study.
Institutional review board statement: All animal procedures conformed to the Institutional Animal Care and Use Committee at the Southern Medical University of China.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Jin-Tao Li, Kunming Medical University, China; Lorenzo Di Cesare Mannelli, University of Florence, Italy.
C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Qiu Y, Song LP; T-Editor: Liu XL
Funding: This study was supported by the National Natural Science Foundation of China, No. 81373240 (to PMQ) and 81671865 (to PMQ).
References
- 1.Abdelmotilib H, Maltbie T, Delic V, Liu Z, Hu X, Fraser KB, Moehle MS, Stoyka L, Anabtawi N, Krendelchtchikova V, Volpicelli-Daley LA, West A. α-Synuclein fibril-induced inclusion spread in rats and mice correlates with dopaminergic Neurodegeneration. Neurobiol Dis. 2017;105:84–98. doi: 10.1016/j.nbd.2017.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ali SF, Itzhak Y. Effects of 7-nitroindazole, an NOS inhibitor on methamphetamine-induced dopaminergic and serotonergic neurotoxicity in mice. Ann N Y Acad Sci. 1998;844:122–130. [PubMed] [Google Scholar]
- 3.Anderson JP, Walker DE, Goldstein JM, de Laat R, Banducci K, Caccavello RJ, Barbour R, Huang J, Kling K, Lee M, Diep L, Keim PS, Shen X, Chataway T, Schlossmacher MG, Seubert P, Schenk D, Sinha S, Gai WP, Chilcote TJ. Phosphorylation of Ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic Lewy body disease. J Biol Chem. 2006;281:29739–29752. doi: 10.1074/jbc.M600933200. [DOI] [PubMed] [Google Scholar]
- 4.Aufschnaiter A, Kohler V, Büttner S. Taking out the garbage: cathepsin D and calcineurin in neurodegeneration. Neural Regen Res. 2017;12:1776–1779. doi: 10.4103/1673-5374.219031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baksi S, Tripathi AK, Singh N. Alpha-synuclein modulates retinal iron homeostasis by facilitating the uptake of transferrin-bound iron: Implications for visual manifestations of Parkinson's disease. Free Radic Biol Med. 2016;97:292–306. doi: 10.1016/j.freeradbiomed.2016.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braak H, Tredici KD, Gai WP, Braak E. Alpha-synuclein is not a requisite component of synaptic boutons in the adult human central nervous system. J Chem Neuroanat. 2000;20:245–252. doi: 10.1016/s0891-0618(00)00101-0. [DOI] [PubMed] [Google Scholar]
- 7.Cadet JL, Krasnova IN. Cellular and molecular neurobiology of brain preconditioning. Mol Neurobiol. 2009;39:50–61. doi: 10.1007/s12035-009-8051-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Callaghan RC, Cunningham JK, Sajeev G, Kish SJ. Incidence of Parkinson's disease among hospital patients with methamphetamine-use disorders. Mov Disord. 2010;25:2333–2339. doi: 10.1002/mds.23263. [DOI] [PubMed] [Google Scholar]
- 9.Callaghan RC, Cunningham JK, Sykes J, Kish SJ. Increased risk of Parkinson's disease in individuals hospitalized with conditions related to the use of methamphetamine or other amphetamine-type drugs. Drug Alcohol Depend. 2012;120:35–40. doi: 10.1016/j.drugalcdep.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 10.Chao J, Zhang Y, Du L, Zhou R, Wu X, Shen K, Yao H. Molecular mechanisms underlying the involvement of the sigma-1 receptor in methamphetamine-mediated microglial polarization. Sci Rep. 2017;7:11540. doi: 10.1038/s41598-017-11065-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chavarria C, Souza JM. Oxidation and nitration of alpha-synuclein and their implications in neurodegenerative diseases. Arch Biochem Biophys. 2013;533:25–32. doi: 10.1016/j.abb.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 12.Chen YR, Nie SD, Shan W, Jiang DJ, Shi RZ, Zhou Z, Guo R, Zhang Z, Li YJ. Decrease in endogenous CGRP release in nitroglycerin tolerance: Role of ALDH-2. Eur J Pharmacol. 2007;571:44–50. doi: 10.1016/j.ejphar.2007.05.042. [DOI] [PubMed] [Google Scholar]
- 13.Danielson SR, Held JM, Schilling B, Oo M, Gibson BW, Andersen JK. Preferentially increased nitration of alpha-synuclein at tyrosine-39 in a cellular oxidative model of Parkinson's disease. Anal Chem. 2009;81:7823–7828. doi: 10.1021/ac901176t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emamzadeh FN. Alpha-synuclein structure, functions, and interactions. J Res Med Sci. 2016;21:29. doi: 10.4103/1735-1995.181989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Emamzadeh FN. Role of Apolipoproteins and alpha-Synuclein in Parkinson's Disease. J Mol Neurosci. 2017;62:344–355. doi: 10.1007/s12031-017-0942-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flack A, Persons AL, Kousik SM, Celeste Napier T, Moszczynska A. Self-administration of methamphetamine alters gut biomarkers of toxicity. Eur J Neurosci. 2017;46:1918–1932. doi: 10.1111/ejn.13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gómez-Santos C, Ferrer I, Santidrián AF, Barrachina M, Gil J, Ambrosio S. Dopamine induces autophagic cell death and alpha-synuclein increase in human neuroblastoma SH-SY5Y cells. J Neurosci Res. 2003;73:341–350. doi: 10.1002/jnr.10663. [DOI] [PubMed] [Google Scholar]
- 18.Gao HM, Kotzbauer PT, Uryu K, Leight S, Trojanowski JQ, Lee VMY. Neuroinflammation and oxidation/nitration of α-synuclein linked to dopaminergic neurodegeneration. J Neurosci. 2008;28:7687–7698. doi: 10.1523/JNEUROSCI.0143-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gou H, Wen D, Ma C, Li M, Li Y, Zhang W, Liu L, Cong B. Protective effects of cholecystokinin-8 on methamphetamine-induced behavioral changes and dopaminergic neurodegeneration in mice. Behav Brain Res. 2015;283:87–96. doi: 10.1016/j.bbr.2015.01.028. [DOI] [PubMed] [Google Scholar]
- 20.Hou X, Yuan Y, Sheng Y, Yuan B, Wang Y, Zheng J, Liu C-F, Zhang X, Hu L-F. GYY4137, an H2S slow-releasing donor, prevents nitrative stress and α-synuclein nitration in an MPTP mouse model of Parkinson's disease. Front Pharmacol. 2017;8:741. doi: 10.3389/fphar.2017.00741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang W, Xie WB, Qiao D, Qiu P, Huang E, Li B, Chen C, Liu C, Wang Q, Lin Z, Wang H. Caspase-11 plays an essential role in methamphetamine-induced dopaminergic neuron apoptosis. Toxicol Sci. 2015;145:68–79. doi: 10.1093/toxsci/kfv014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Killinger BA, Moszczynska A. Epothilone D prevents binge methamphetamine-mediated loss of striatal dopaminergic markers. J Neurochem. 2016;136:510–525. doi: 10.1111/jnc.13391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kinghorn KJ, Asghari AM, Castillo-Quan JI. The emerging role of autophagic-lysosomal dysfunction in Gaucher disease and Parkinson's disease. Neural Regen Res. 2017;12:380–384. doi: 10.4103/1673-5374.202934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klongpanichapak S, Govitrapong P, Sharma SK, Ebadi M. Attenuation of cocaine and methamphetamine neurotoxicity by coenzyme Q10. Neurochem Res. 2006;31:303–311. doi: 10.1007/s11064-005-9025-3. [DOI] [PubMed] [Google Scholar]
- 25.Krasnova IN, Cadet JL. Methamphetamine toxicity and messengers of death. Brain Res Rev. 2009;60:379–407. doi: 10.1016/j.brainresrev.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lashuel HA, Overk CR, Oueslati A, Masliah E. The many faces of α-synuclein: from structure and toxicity to therapeutic target. Nat Rev Neurosci. 2013;14:38–48. doi: 10.1038/nrn3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lokappa SB, Suk JE, Balasubramanian A, Samanta S, Situ AJ, Ulmer TS. Sequence and membrane determinants of the random coil helix transition of α-synuclein. J Mol Biol. 2014;426:2130–2144. doi: 10.1016/j.jmb.2014.02.024. [DOI] [PubMed] [Google Scholar]
- 28.Mak SK, McCormack AL, Manning-Boğ AB, Cuervo AM, Di Monte DA. Lysosomal degradation of α-synuclein in vivo. J Biol Chem. 2010;285:13621–13629. doi: 10.1074/jbc.M109.074617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Recasens A, Dehay B. Alpha-synuclein spreading in Parkinson's disease. Front Neuroanat. 2014;8:159. doi: 10.3389/fnana.2014.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Segura-Aguilar J. On the role of endogenous neurotoxins and neuroprotection in Parkinson's disease. Neural Regen Res. 2017;12:897–901. doi: 10.4103/1673-5374.208560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sengupta U, Guerrero-Muñoz MJ, Castillo-Carranza DL, Lasagna-Reeves CA, Gerson JE, Paulucci-Holthauzen AA, Krishnamurthy S, Farhed M, Jackson GR, Kayed R. Pathological interface between oligomeric alpha-synuclein and tau in synucleinopathies. Biol Psychiatry. 2015;78:672–683. doi: 10.1016/j.biopsych.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 32.Thrash B, Thiruchelvan K, Ahuja M, Suppiramaniam V, Dhanasekaran M. Methamphetamine-induced neurotoxicity: the road to Parkinson's disease. Pharmacol Rep. 2009;61:966–977. doi: 10.1016/s1734-1140(09)70158-6. [DOI] [PubMed] [Google Scholar]
- 33.Todd G, Pearson-Dennett V, Wilcox RA, Chau MT, Thoirs K, Thewlis D, Vogel AP, White JM. Adults with a history of illicit amphetamine use exhibit abnormal substantia nigra morphology and parkinsonism. Parkinsonism Relat Disord. 2016;25:27–32. doi: 10.1016/j.parkreldis.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 34.Wang C, Zhao C, Li D, Tian Z, Lai Y, Diao J, Liu C. Versatile structures of α-synuclein. Front Mol Neurosci. 2016;9:48. doi: 10.3389/fnmol.2016.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu XF, Wang AF, Chen L, Huang EP, Xie WB, Liu C, Huang WY, Chen CX, Qiu PM, Wang HJ. S-nitrosylating protein disulphide isomerase mediates α-synuclein aggregation caused by methamphetamine exposure in PC12 cells. Toxicol Lett. 2014;230:19–27. doi: 10.1016/j.toxlet.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 36.Zhang F, Chen L, Liu C, Qiu P, Wang A, Li L, Wang H. Up-regulation of protein tyrosine nitration in methamphetamine-induced neurotoxicity through DDAH/ADMA/NOS pathway. Neurochem Int. 2013;62:1055–1064. doi: 10.1016/j.neuint.2013.03.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.