Abstract
Contactins are a group of cell adhesion molecules that are mainly expressed in the brain and play pivotal roles in the organization of axonal domains, axonal guidance, neuritogenesis, neuronal development, synapse formation and plasticity, axo-glia interactions and neural regeneration. Contactins comprise a family of six members. Their absence leads to malformed axons and impaired nerve conduction. Contactin mediated protein complex formation is critical for the organization of the axon in early central nervous system development. Mutations and differential expression of contactins have been identified in neuro-developmental or neurological disorders. Taken together, contactins are extensively studied in the context of nervous system development. This review summarizes the physiological roles of all six members of the Contactin family in neurodevelopment as well as their involvement in neurological/neurodevelopmental disorders.
Keywords: cell adhesion molecule, Contactins, axonal domain, neurogenesis, synaptogenesis, autism spectrum disorder, neuro-developmental disorder, neurological disease
Introduction
Contactins are a group of six neuronal cell adhesion molecules, contactin-1 to contactin-6. The contactin subfamily comprises six structurally related axon-associated nervous system specific cell adhesion molecules, which belong to the Immunoglobulin (Ig) superfamily (Stoeckli, 2010). Contactins are structurally and functionally rather conserved across species and mainly expressed in neurons (Mohebiany et al., 2014). However, oligodendrocytes and their precursors also express contactins (Colakoglu et al., 2014; Zoupi et al., 2018).
The extracellular components of contactins are structurally very similar, as they all have six Ig-like repeats followed by four fibronectin III-like domains anchored to GPI (glycosylphosphatidylinositol) attached to the outer leaflet of the cell membrane (Figure 1). None of them have an intracellular domain and thus need interacting partners for signal transduction within the cell. Contactins can be localized to both pre- and post-synaptic compartments (Dalva et al., 2007). Contactin-1 and contactin-2 are primarily present at the junctions of myelin and axons and facilitate mutual communication among neurons, oligodendrocytes and astrocytes (Pedraza et al., 2001). Furthermore, a specific subset of contactins and their partners (co-receptors), CNTNAPs (contactin associated proteins) additionally regulate the clustering of voltage-gated ion channels involved in saltatory action potential propagation and control of axonal excitability (Peles and Salzer, 2000). The Contactin-associated proteins (Casprs), which belong to the neurexin family (Bellen et al., 1998), generally occur in a cis-complex with a contactin subfamily members (Rios et al., 2000; Fernandez et al., 2004; Labasque and Faivre-Sarrailh, 2010). Moreover, at specific synapses, contactins and CNTNAPs form complexes within themselves or with each other and take part in establishing synaptic contacts (Rios et al., 2000; Rudenko, 2017), modifying synaptic receptor function and regulating dendritic spine morphology (Stoeckli, 2010). For example, CNTNAP1 forms a complex with Contactin-1, CNTNAP2 with Contactin-2 and CNTNAP4 with Contactin-4 and -5. Contactin-1: CNTNAP1 are involved in the organization of paranodal domains of axons, whereas contactin-2: CNTNAP2 are involved in the organization of juxtaparanodes (Faivre-Sarrailh and Devaux, 2013; Zou et al., 2017). The roles of complexes of contactin 4 and -5 are still to be elucidated.
Figure 1.
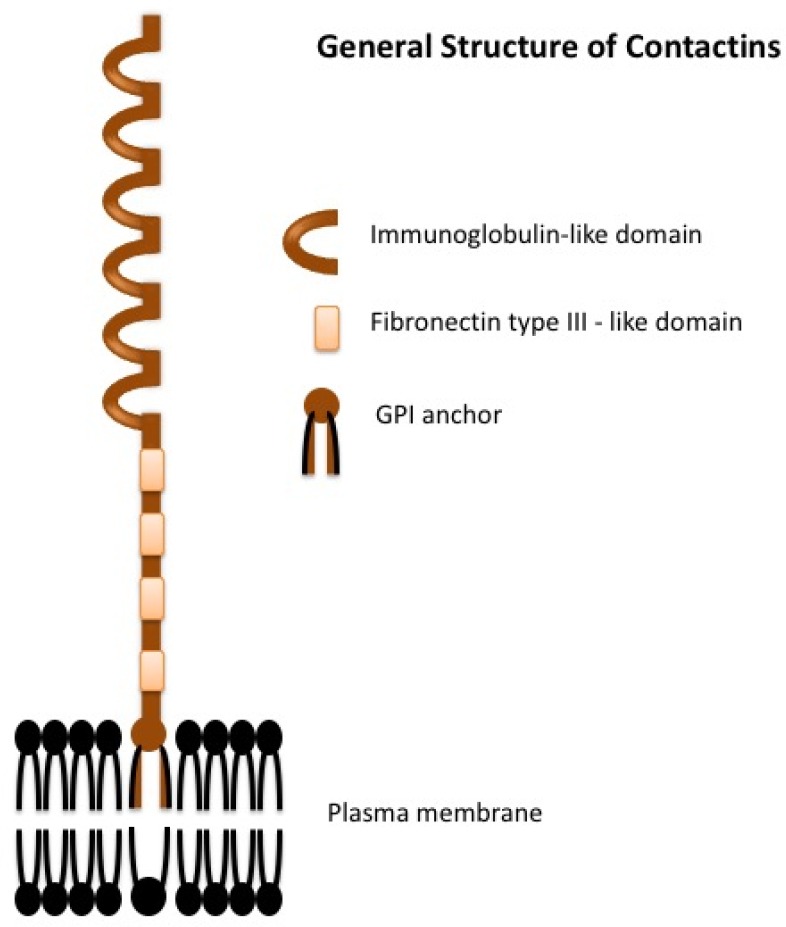
General structure of the contactins.
The six members of the contactin sub-family share a common structure in vertebrates. The extracellular component has immunoglobulin-like domains followed by four fibronectin III-like domains. The protein is attached to the plasma membrane with a glycosylphosphatidylinositol (GPI) anchor and there is no intracellular domain.
The most studied members of the contactin family are contactin-1 (F3 or F9 or contactin) and contactin-2 (Transient Axonal Glycoprotein 1, TAG-1) (Shimoda and Watanabe, 2009). The name of the first protein in the group, contactin-1, was coined in 1988 as ‘contactin’ when it was first characterized as a molecule that interacted with known cell adhesion molecules and found in areas of neuronal contacts (Ranscht, 1988). In comparison, investigations of the other members namely, contactin-3 (BIG-1), contactin-4 (BIG-2), contactin-5 (NB-2) and contactin-6 (NB-3) were performed around ten years later than contactin-1 and -2. Contactins play crucial roles in the organization of axonal domains, axonal guidance, myelination, neuritogenesis, neuronal development, synaptogenesis and axo-glia interactions (Stoeckli et al., 1991; Murai et al., 2002; Poliak and Peles, 2003; Rudenko, 2017). Even though contactin 1-6 have similar roles in the central nervous system (CNS) development process, they are responsible for maintaining separate neuronal circuitries, which is elaborated for each contactin below. Specifically, in the developmental stages, contactin-1 and -2 act as guidance molecules for axonal pathfinding and fasciculation (Shimoda and Watanabe, 2009). They are highly expressed in the adult human brain as well. We found both contactin-1 (unpublished data) and contactin-2 (Chatterjee et al., 2018) to be expressed in the temporal cortex and hippocampus of adults.
The importance of contactins have been elucidated not only in health as described above but also in disease. Recent studies have revealed the role of improper functioning, incorrect cellular localization or genetic aberrations related to contactins in neurological and neurodevelopmental disorders such as autism (Burbach and van der Zwaag, 2009; Kumar and Christian, 2009; van Daalen et al., 2011) and neuro-developmental delay (Fernandez et al., 2004; Roohi et al., 2009). Here, we focus on the recent progresses in the field of contactins and their roles in CNS development and maintenance of CNS physiology (summary in Table 1 and Figure 2). In this review, we will summarize the expression pattern, physiological functions, and the roles in synapse and myelin formation during neurodevelopment for each of the six contactins as well the implications of dysfunction of contactins in neurological disorders.
Table 1.
Contactins: summary of their main characteristics
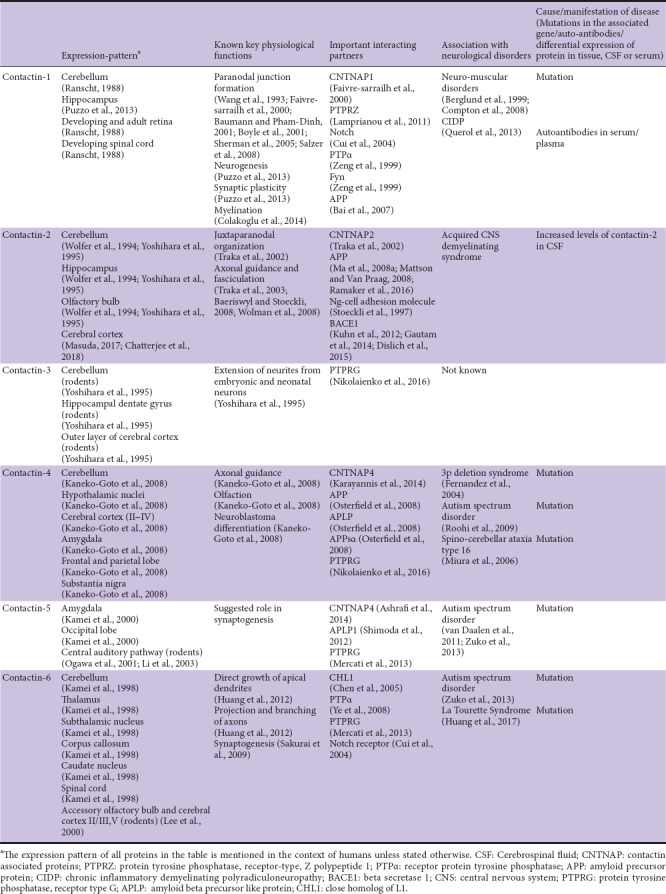
Figure 2.
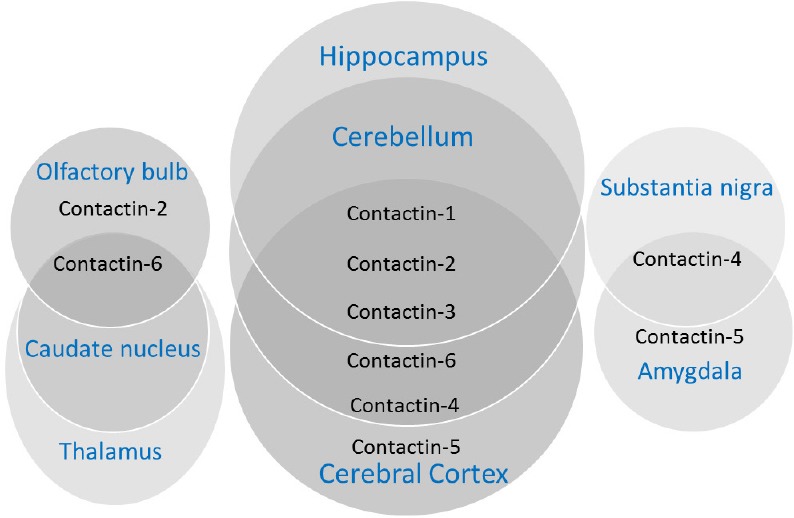
Overlapping and distinct expression patterns of contactins in different areas of brain.
An electronic search on Pubmed using search terms-‘CNTN1’, ‘CNTN2’, ‘CNTN3’, ‘CNTN4’, ‘CNTN5’, ‘CNTN6’, ‘Contactin’, ‘Contactin-1’, ‘Contactin-2’, ‘Contactin-3’, ‘Contactin-4’, ‘Contactin-5’, ‘Contactin-6’, ‘Contactin AND multiple sclerosis’, contactin AND Alzheimer’s’, ‘contactin AND diseases’ and ‘contactin AND biomarker’. Articles were included from year 1985 to 2018. The results were further screened by title and abstract to only present physiological functions and diseases related to the CNS.
Contactin-1
Expression pattern and physiological functions
Contactin-1 is a 130 kDa protein, which is present in a membrane-bound and soluble form (Ranscht, 1988). Its orthologs are named contactin/F11 in chicken and F3 in mouse (Williams and Barclay, 1988). The presence of this GPI-anchored molecule on neuronal membranes has been observed in the retina, spinal cord, cerebral cortex, hippocampus and cerebellum (Ranscht, 1988; Massaro et al., 2012). In the postnatal cerebellum, contactin-1 is highly expressed on migrating granule cells where it shows a primary expression in axonal extensions rather than in cell bodies (Virgintino et al., 1999; Faivre-sarrailh et al., 2000). It is also expressed in axons and cell bodies of mossy fibers and Golgi cells (Faivre-Sarrailh et al., 1992). Normal astrocytes do not express contactin-1 (Williams and Barclay, 1988). However, glioblastomas express this protein, which co-localizes with glial fibrillary acidic protein (Eckerich et al., 2006). In addition, contactin-1 expression has been found in oligodendrocytes (Koch et al., 1997).
Contactin-1 is indispensable for early interactions between axons and glia. It bolsters paranodal junction formation by establishing a complex with the transmembrane protein CNTNAP1 (Figure 3). In the axolemma, it interacts with glial neurofascin-155 to establish the axon-glial contacts (NF-155) (Faivre-sarrailh et al., 2000; Boyle et al., 2001; Sherman et al., 2005; Salzer et al., 2008). The role of contactin-1 in paranodal junction formation was shown by knocking out the CNS CNTN1 gene in mice. As a result, paranodal junctions were disrupted due to mislocalization of the shaker-type potassium Kv1.2 channels typically delineating juxtaparanodal regions (Boyle et al., 2001). This suggests that contactin-1 is needed to position Kv1.2 and thus to contribute to the paranodal outward current and thereby proper action potential repolarization during action potential conduction. A spontaneous mutation in BALB/c mice that resulted in a null allele of CNTN1 showed a similar phenotype as the CNTN1 knock-out mice described above (Davisson et al., 2011). After one week, the homozygous animals were smaller in size than littermates and had abnormal locomotion. These mice died after a few weeks which was similar as in the CNTN1 knock-out mice.
Figure 3.
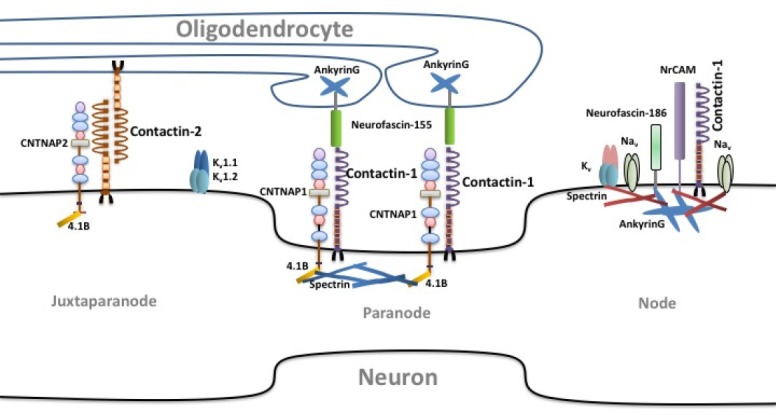
Contactin-1 and contactin-2 in axonal domain organization.
CNTNAP: Contactin associated protein; NaV: sodium channel, voltage-gated, type II; KV: Potassium channel, voltage gated; NrCAM: neuronal cell adhesion molecule.
Besides a role in organization of axonal membranes, contactin-1 also plays an important role in myelination (Colakoglu et al., 2014) and axonal regeneration (Haenisch et al., 2005), where it modulates these processes via axo-glia interaction. The maturation and differentiation of oligodendrocytes from oligodendrocyte precursor cells depends on the interaction of contactin-1 with other proteins, one of them being PTPRZ (protein tyrosine phosphatase, receptor-type, Z polypeptide 1). PTPRZ is expressed primarily by oligodendrocyte precursor cells, astrocytes, and mature oligodendrocytes in the developing and adult nervous systems (Canoll et al., 1996; Harroch et al., 2000; Faissner et al., 2006; Lamprianou et al., 2011). In addition, it was found that F3/Contactin aids in oligodendrocyte generation from progenitor cells by acting as ligand of notch (Cui et al., 2004). Binding of contactin to notch leads to the release of the intracellular domain of notch. Intracellular domain of notch then translocates into the nucleus of oligodendrocyte precursor cells and increases notch1 and notch2 expression, which positively regulates oligodendrogliogenesis (Taylor et al., 2007). Furthermore, contactin-1 regulates myelination by participating in a tripartite complex of contactin1-fyn-PTPα promoting the interaction of PTPα (receptor protein tyrosine phosphatase) and fyn, thus controlling dephosphorylation and subsequent activation of Fyn by PTPα (Zeng et al., 1999; Lamprianou et al., 2011). Regulation of fyn is important as it is a Src family kinase, which regulates myelination by oligodendrocytes (Umemori et al., 1994; Zeng et al., 1999; Cui et al., 2004; Kaneko-Goto et al., 2008). These findings suggest that contactin-1 can be a novel major player in the signal transduction mechanism in myelination pathways of CNS due to its interactions with PTPRZ, notch, PTPα and fyn.
Apart from the role in myelination, contactin-1 has a crucial function in the hippocampus, where it augments synaptic plasticity, neurogenesis, and memory in adult mice (Puzzo et al., 2013). In the hippocampus of aged mice, reduced contactin-1 protein and mRNA was observed in the pyramidal neurons of CA1 and in the granule cells of the dentate gyrus, indicating its potential role in age-related loss of memory and cognition (Shimazaki et al., 1998). Moreover, in aged mice, over-expression of contactin-1 improved hippocampal long-term potentiation and memory compared with wild-type littermates (Puzzo et al., 2015). When F3/contactin-1 was over-expressed within the CA region of hippocampus, it regulated proliferation of neuronal precursors and their commitment towards neuronal fate in a positive manner (Puzzo et al., 2013). Contactin-1 also controls synaptic interactions among cerebellar interneurons. This was found by studying CNTN−/− mice, where granule cell axon guidance and dendritic projections from granule and golgi cells were impaired (Berglund et al., 1999).
Implications in neuro-muscular disorders
A single mutation in the CNTN1 gene is known to lead to deficiency of contactin-1 at the neuro-muscular junction (NMJ) in humans (Compton et al., 2008). The mutation is inherited in an autosomal-recessive manner and the deficiency of contactin-1 disrupts the communication between muscles and nerves resulting in lethal myopathy (Compton et al., 2008). This myopathy is related to the clinical spectrum of congenital myopathies and myasthenic syndromes (Puzzo et al., 2013) that fall under the umbrella of neuro-muscular disorders. However, the phenotype of lethal congenital myopathy could not be replicated in CNTN1 mutant mice. In mice deficient of CNTN1, NMJ morphology was totally normal and they did not show the phenotype of human myopathy (Compton et al., 2008). But CNTN1 knockout mice exhibit myelin detachment in the postnatal paranode which results in a decrease in nerve conduction velocities in CNS and peripheral nervous system (PNS) (Berglund et al., 1999; Virgintino et al., 1999; Boyle et al., 2001; Compton et al., 2008; Sun et al., 2009). These studies suggest that contactin-1 plays an important role in maintaining NMJ function and is thereby indispensable for proper functioning of mature motor neurons.
Implications in chronic inflammatory demyelinating polyradiculoneuropathy
Contactin-1 integrity plays a role in another peripheral nervous system disease, chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) (Querol et al., 2013). Antibodies against the contactin-1/CNTNAP1 complex were present in serum/plasma of a subset of chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) patients. CIDP causes predominantly sensory and motor problems such as limb weakness, distal sensory disturbances, and loss of reflexes. These phenotypes are manifested because the autoantibodies impair cell-cell adhesion mediated by Contactin•CNTNAP complex and their interaction with neurofascin-155, thereby disrupting the paranodal junctions (Labasque et al., 2014). Alternatively, contactin-1 is pre-dominantly involved in myelination and the resulting demyelination in CIDP may be caused by the autoantibodies generated against contactin-1 (Querol et al., 2013; Koike et al., 2017). The identification of auto-antibodies to contactin-1 or contactin-1/CNTNAP1 complex even in small subgroups of CIDP patients suggest that these may play a pathogenic role and that they might serve as diagnostic biomarkers in these patients (Querol et al., 2013; Manso et al., 2016). However, further large cohort studies are required to establish antibodies against contactin-1 as a biomarker for CIDP.
Contactin-2
Expression pattern and physiological functions
Human contactin-2, also known as TAX1, was initially discovered as a glycoprotein that is transiently expressed during development in mice (called TAG-1) (Yamamoto et al., 1986; Dodd et al., 1988; Furley et al., 1990). It was also found in chicken where it is known as axonin-1 (Zuellig et al., 1992). The structure of human contactin-2 is very conserved and has a high degree of similarity to rat TAG-1 (91% identity) and chicken axonin-1 (75% identity) (Hasler et al., 1993; Tsiotra et al., 1993). Moreover, expression patterns of contactin-2 are similar across corresponding developmental stages in human and chicken (Hasler et al., 1993; Kozlov et al., 1995). It is regulated in a space- and time-dependent manner in both neurons and glial cells, the expression being highest during early developmental stages (Stoeckli et al., 1997; Pedraza et al., 2001; Kyriakopoulou et al., 2002). In the adult rodent brain contactin-2 is expressed in the hippocampus, olfactory bulb, and cerebellar granule cells (Wolfer et al., 1994; Yoshihara et al., 1995). In adult fish, however, contactin-2 expression is restricted to nasal retinal ganglion cells (Lang et al., 2001). Axonin-1, its chicken homolog, is primarily present in membranes of developing nerve fiber tracts, while a soluble form is secreted from axons and accumulates in the cerebrospinal fluid and the vitreous fluid of the eye of the chicken (Stoeckli et al., 1991).
Although, contactin-2 and contactin-1 have similar functions in the brain, the sub-cellular localizations are different. The primary area of contactin-2 expression on axons is juxtaparanode, where it is needed for the clustered distribution of Shaker-type Kv1.1 potassium channels (Figure 3). On the other hand, contactin-1 is primarily expressed on the paranode (Traka et al., 2002; Poliak and Peles, 2003). Contactin-2 is highly expressed on growth cones of extending axons and secreted from the axons into the extracellular matrix and cerebrospinal fluid (Baeriswyl and Stoeckli, 2008; Wolman et al., 2008). Furthermore, contactin-2 is also present on the synaptic plasma membrane in a complex with CNTNAP2 (Lu et al., 2016).
The soluble form of contactin-2 acts as a guiding molecule for the outgrowth of neurites (Stoeckli et al., 1991), and thus plays a role in axon extension initiation, axonal guidance and fasciculation (Baeriswyl and Stoeckli, 2008; Wolman et al., 2008). Evidence for impaired learning and memory as well as for sensory dysfunction was found in CNTN2−/− mice (Savvaki et al., 2008). Absence of CNTN2 led to a reduced number of mitral cells, i.e., the projection neurons of the olfactory bulb. This resulted into decreased odor discrimination and diminished long-term social memory formation (Bastakis et al., 2015). The pivotal role of contactin-2 in learning and memory might explain the role of contactin-2 in dementing disorders such as Alzheimer’s disease (AD). Contactin-2 is a ligand of amyloid precursor protein (Ma et al., 2008a, b) and beta secretase 1 (BACE1) (Kuhn et al., 2012). It is well known that amyloid precursor protein processing is at the core of Alzheimer’s disease (AD). By binding with amyloid precursor protein, contactin-2 enhances the production of the amyloid precursor protein intracellular domain in a gamma-secretase-dependent manner (Ma et al., 2008a). This suggests that Contactin-2 influences amyloid precursor protein processing and amyloid precursor protein intracellular domain release with concomitant amyloid beta formation, which is an important player in AD pathogenesis (Konietzko, 2012).
Furthermore, contactin-2 has also roles in neural regeneration. Up-regulation of contactin-2 expression was observed in the zebra fish spinal cord caudally to the site where a lesion was set indicating a role in spinal cord regeneration. This was further supported by the fact that during spinal cord injury antisense morpholinos blocking the expression of contactin-2 impeded axon re-growth beyond the lesion site (Lin et al., 2012).
Implications in demyelinating diseases
Recently, contactin-2 has been found to be involved in oligodendrocyte maturation (Zoupi et al., 2018). Contactin-2 expression was associated with regulation of genes responsible for myelination and oligodendrocyte proliferation (Zoupi et al., 2018). Apart from regeneration, contactin-2 might also have implications in demyelination and may have use as a biomarker for developmental demyelinating neurological diseases. Even though in the absence of CNTN2, demyelination was not observed in mice (Zoupi et al., 2018), it was found to be differentially expressed in the CSF of children suffering from a demyelinating disease, multiple sclerosis (Singh et al., 2015). In addition, a previous proteomic study found that CSF contactin-2 was higher in abundance, among few other neuronal proteins, in children with multiple sclerosis than in children with monophasic acquired CNS demyelinating syndrome (ADS) (Singh et al., 2015).
Contactin-3
Expression pattern and physiological functions
The cDNA cloning and characterization of contactin-3, also called brain-derived immunoglobulin superfamily protein 1 (BIG-1) or plasmacytoma-associated neuronal glycoprotein (PANG), was first reported in 1994 in rats (Connelly et al., 1994; Yoshihara et al., 1994). Contactin-3 appeared structurally closely related to TAG-1/axonin-1 and F3/Fll. Contactin-3 is highly expressed within the adult rat brain in specific subsets of neurons such as granule cells of the hippocampal dentate gyrus, neurons in the outer layer of cerebral cortex and Purkinje cells of the cerebellum of mice (Yoshihara et al., 1995). Purkinje cell-specific localization of contactin-3 is distinct from the expression patterns of contactin-1 and contactin-2 in the sense that contactin-1 and -2 expression were not seen in Purkinje cells (Yoshihara et al., 1995; Hansford et al., 2003). Another difference is that expression of contactin-3 is highest in the adult mouse brain, very low in embryonic brain and absent in peripheral tissues, which is also in contrast to contactin-1 and -2 (Yoshihara et al., 1995). There are no studies of human contactin-3 yet which would reveal the expression pattern of this protein.
Similar to contactin-1 and contactin-2, contactin-3 exists in two forms, i.e., membrane-bound and secreted. Even though the distinct roles of membrane-bound and soluble forms are not clear, the membrane bound forms might be responsible for intercellular communication, axonal domain organization and axo-glia interaction whereas the soluble form may act a guiding molecule for axonal guidance and fasciculation. The authors of the original study (Yoshihara et al., 1994) also found a unique spliced form of BIG-1 cDNA in rats that consists of a signal peptide followed by one immunoglobulin like domain. This form was not found among alternatively spliced forms in related TAG-1/axonin-1 and F3/Fll subgroups and is thus unique for contactin-3. This new spliced form may give rise to a unique soluble form of contactin-3 with a possibly different role as a guidance molecule.
The physiological functions of contactin-3 appear to be similar to TAG-1/axonin-1(contactin-2) and F3/Fll (contactin-1) in that it supports the extension of neurites from embryonic and neonatal neurons. It is possible that contactin-3 may have a role in the elongation of axons and the connections between specific types of neurons (Yoshihara et al., 1995). Much is still to be elucidated regarding this protein, especially its role in neurological disorders.
Implication in autism spectrum disorder
CNTN3, the gene encoding contactin-3 is associated with autism spectrum disorder (ASD). A study of families in which parents shared ancestors aimed to identify inherited factors in autism found that a rare loci mapped to CNTN3 (Morrow et al., 2008). Furthermore, a GWAS study found a large subset of autism-related genes, including CNTN3 that were involved in the outgrowth and guidance of axons and dendrites (Hussman et al., 2011). This study highlighted that disruptions in axonal guidance and fasciculation are manifested in autism.
Contactin-4
Expression-pattern and physiological functions
Contactin-4, also known as brain-derived immunoglobulin super-family protein 2 (BIG-2), was first cloned in the rat (Hansford et al., 2003). Contactin-4 expression was found in testis, thyroid, small intestine, uterus and brain (Yoshihara et al., 1995). Unlike contactin-1, -2 and -3, the expression of contactin-4 is not highest in brain (Hansford et al., 2003). It was found that all contactin-4 expressing tissues manifested a transcript of approximately 4.7 kb, whereas testis expressed an extra transcript of approximately 1.8 kb, which was possibly a splice variant of the CNTN4 gene. Within the brain the highest expression was seen in the paracentral gyrus of the cerebral cortex, amygdala, thalamus, cerebellum, and parietal and frontal lobes. Contactin-4 expression was found in pyramidal neurons of layers Vb and VIa, and all types of interneurons (Oguro-Ando et al., 2017). In addition, contactin-4 was also found to be expressed in retinal ganglion cells (Osterhout et al., 2015). However, its expression in the hippocampus was weak, localized specifically dentate gyrus granule cells whereas it was rather strong in olfactory sensory neurons (Kaneko-Goto et al., 2008). In contrast to the hippocampal expression of contactin-4, contactin-1 expression was found overall in the hippocampus, contactin-2 expression was seen in CA3 pyramidal cells and contactin-5 expression was predominant in CA1 pyramidal cells (Yoshihara et al., 1995).
The protein was shown to be responsible for neurite-promoting effects, like other contactins. Given the expression of contactin-4 in retinal ganglion cells s, it has been found to be important for target-specific axon arborization in the visual system of mice in a complex with amyloid precursor protein (Osterhout et al., 2015). It has been identified as an important factor needed by a subset of retinal ganglion cells to connect to the accessory optic system (Osterhout et al., 2015). Contactin-4 acts as an axonal guidance molecule for the formation of the olfactory odor map as well in the olfactory bulb of mice (Kaneko-Goto et al., 2008). This was supported by the fact that mice devoid of contactin-4 showed ectopic innervations of multiple glomeruli by olfactory sensory neurons expressing a particular odorant receptor. Apart from the role of contactin-4 in olfaction and vision, in humans it was found responsible for the differentiation of neuroblastoma cell lines, which are derived from embryonic neural crest cells (Kaneko-Goto et al., 2008).
Processing of APP can be modulated by contactin-4 indicating a potential role in neural development and disease, at least in chicken, although the exact mechanism is unknown. Contactin-4 may be involved in AD in a manner similar to contactin-2.
Implications in neuro-developmental disorders
Though the number of studies focusing on the CNTN4 gene and contactin-4 is limited, the available studies indicate that they are associated with neuro-developmental disorders. Human CNTN4 locus 3p26.2–3p26.3 is involved in 3p deletion syndrome having hallmarks including developmental delay, postnatal growth retardation, and dysmorphic features (Fernandez et al., 2004). CNTN4 disruption has been observed in a few patients with ASD (Roohi et al., 2009). ASD is a set of complex neurodevelopmental disorders with impairments in learning, verbal and non-verbal abilities and social interactions. A patient with a severe autistic phenotype with a maternally inherited ~535 kb deletion within the 5′ UTR of CNTN4 has been recently identified using comparative genome hybridization (Cottrell et al., 2011). In addition to deletion, duplications in CNTN4 in ASD has been identified as well (Glessner et al., 2009; Nava et al., 2013). A point mutation in the 3’UTR of CNTN4 on chromosome 3p26.2–3p26.3 was also found to be associated with spino-cerebellar ataxia type 16 (SCA16) in patients, which involves cerebellar degeneration (Miura et al., 2006).
It has been postulated that aberrations in the process of local dendritic and synaptic protein synthesis play pivotal roles in autistic-like phenotypes as they lead to improper synapse functions (Kelleher and Bear, 2008; Zuko et al., 2013). Many genes encoding such pre- and post-synaptic proteins are associated with ASD (Zuko et al., 2013). Copy number variations or disruptions in CNTNs possibly affect the local synaptic protein synthesis leading to abrupt neuronal transmission, although the exact role of contactins in ASD is not yet clear. Since, there are evident clinical symptoms related to visual (Simmons et al., 2009) and olfactory impairments (Rozenkrantz et al., 2015) in patients with ASD, it can be further speculated that abnormalities in CNTN4 might be one of the underlying causes, given the role of contactin-4 in the formation of optical and olfactory neuronal circuitry.
Contactin-5/NB-2
Expression pattern and physiological functions
NB-2, the contactin-5 ortholog in rodents, was first identified in rat brain in 1996 along with NB-3 (Ogawa et al., 1996), a contactin-6 ortholog. cDNAs encoding two splicing isoforms of human CNTN5, the gene encoding contactin-5, were identified later in 2000 (Kamei et al., 2000). The expression pattern of human contactin-5 was found to be very similar to contactin-3; high expression of contactin-5 was seen in the amygdala and occipital lobe in the human brain, whereas the expression was low in the corpus callosum, caudate nucleus, and spinal cord (Kamei et al., 2000). In rodents, strong expression of contactin-5 was in the thalamus, caudate putamen and weaker expression was seen in the cerebral cortex (Kleijer et al., 2015). Interestingly, in rodents, NB-2/contactin-5 is expressed preferentially in the central auditory pathway including the ventral cochlear nucleus, ventral acoustic stria, lateral and medial superior olivary complex, superior paraolivary nucleus, medial nucleus of the trapezoid body, ventrolateral lemniscus, and central nucleus of the inferior colliculus highlighting its pivotal role in the auditory system (Ogawa et al., 2001; Li et al., 2003; Toyoshima et al., 2009).
Similar to other contactins, contactin-5 is involved in cell adhesion during development but it might be the only contactin sub-family member responsible for maintaining neural circuitry in the auditory system (Ogawa et al., 2001; Li et al., 2003). Furthermore, contactin-5 has been implicated in dendritic morphogenesis (Peng et al., 2017). Complex of contactin-5 with its partner CNTNAP4 acts as a scaffold on inter-neurons where dendrites of direction-selective ganglion cells can fasciculate (Ashrafi et al., 2014; Peng et al., 2017).
Implication in ASD
There are indications of an involvement of CNTN5 in ASD (Zuko et al., 2013). Copy number variations in CNTN5 gene in cohorts of multiplex and simplex families suffering from ASD have been reported (Hussman, 2011; van Daalen et al., 2011; Mercati et al., 2013). It is known that many children suffering from ASD manifest pre- and post-stimulus superior temporal gyrus auditory oscillatory abnormalities and delayed auditory responses (Edgar et al., 2015). The involvement of CNTN5 in ASD may be linked to the primary physiological function of contactin-5 in the maintenance of neuronal circuitry within auditory system.
Contactin-6/NB-3
Expression pattern and physiological functions
The last member of the contactin family, contactin-6, is functionally similar to contactin-4 and contactin-5 as it is involved in brain development like the former two (Mercati et al., 2013, 2017). Evolutionarily, it is close to contactin-3 and located at the same position on chromosome 3 (Kamei et al., 1998). The cDNA of human CNTN6 was isolated from the cerebellum and the expression of contactin-6 was found to be highest in the cerebellum followed by the thalamus, sub-thalamic nucleus, corpus callosum, caudate nucleus and the spinal cord (Kamei et al., 1998). Contactin-6 expression has also been widely studied in rodents where it is known as NB-3 (Takeda et al., 2003). Pronounced expression of NB-3 has been seen in the developing cerebellum after birth. Here its expression increased until adulthood whereas in the cerebrum, the expression reached its maximum on postnatal day 7 and then declined (Lee et al., 2000). In situ hybridization demonstrated that NB-3 mRNA was preferentially expressed in the accessory olfactory bulb, layers II/III and V of the cerebral cortex, piriform cortex, anterior thalamic nuclei, locus coeruleus of the pons and mesencephalic trigeminal nucleus, and in Purkinje cells of the cerebellum (Lee et al., 2000). The expression of NB-3 co-localizes here with the presynaptic vGLUT1 indicating that NB-3 is localized pre-synaptically at glutamatergic synapses between parallel fibers and Purkinje cells (Sakurai et al., 2009, 2010).
Contactin-6 has an important role in guidance of neuronal tracts during development. For example, it was shown to direct the growth of apical dendrites of deep layer cortical pyramidal neurons and to regulate the projection and branching of axons of the corticospinal tract during development (Huang et al., 2012). Very low levels of contactin-6 lead to increased death of granule cells and decreased synapse density between parallel fibers and Purkinje cells during cerebellum development, suggesting that contactin-6 is important for both survival of granule cells and synapse formation (Sakurai et al., 2009). Contactin-6 interacts with close homolog of L1 (CHL1) and protein tyrosine phosphatase alpha where it is involved in controlling apical dendrite orientation in the visual cortex (Ye et al., 2008). CHL1 is an important neural cell adhesion molecule involved in synaptic remodeling and cell migration (Chen et al., 2005). Protein tyrosine phosphatase alpha is a receptor protein tyrosine phosphatase which plays a principal role in nervous system development (Zeng et al., 1999). Contactin-6 also interacts with protein tyrosine phosphatase receptor-gamma (Peles et al., 1997; Bouyain and Watkins, 2010). This latter finding confirms its importance of this contactin for neural circuit construction. In addition, contactin-6 was recently found to play a major protective role in apoptosis (Zuko et al., 2016). This idea was suggested by the observation that the population of apoptotic cells increased in the cortex of CNTN6–/– mice compared to wild-type controls. Lastly, due to its interaction with the Notch receptor (Hu et al., 2006), contactin-6 can also play a role in oligodendrocyte development in a manner similar to contactin-1 (Cui et al., 2004).
CNTN6 knock-out mice also showed a significant reduction in Cux1+ (marker for upper cortical layer) projection neurons in cortical layers II–IV and increased projection of FoxP2+ (marker for deep cortical layer) projection neurons in layer VI of visual cortex were observed in as compared to wildtype mice (Zuko et al., 2016). Moreover, parvalbumin+ (inhibitory) interneurons were decreased. These findings corroborate the view that contactin-6 is important for developmental functions involving cell survival, migration and fasciculation (Takeda et al., 2003).
Implications in ASD and Tourette syndrome
Like CNTN3, CNTN4 and CNTN5, copy number variations have been identified in the CNTN6 gene in ASD in humans (Saus et al., 2010; van Daalen et al., 2011). Recently, a large scale European genome-wide association study study has identified CNTN6 duplication as one of the genetic risk factors for Tourette syndrome (Huang et al., 2017). Cerebellum is at the core of motor and coordination functions, which are disturbed in neuro-developmental disorders such as ASD and Tourette syndrome (Tobe et al., 2010; Becker et al., 2012; Caligiore et al., 2017). Since the expression of contactin-6 is highest in the cerebellum, it can be speculated that loss of proper functioning of this protein may have implications in neuronal circuitry dysfunction in the cerebellum thus affecting motor functions in ASD and Tourette syndrome.
Conclusion
Contactins are cell adhesion molecules that are highly expressed in the brain and are involved in the physiology and pathophysiology of the CNS (See Table 1 for summary). The six members of the Contactin family have some common functions during development but maintain exclusive neuronal circuitries. Almost all members are major players in axonal guidance, fasciculation and synaptic integrity, although the sub-polpulation of neurons where they are expressed may be different. Many non-overlapping roles of contactins have been demonstrated in recent years. For example, contactin-1 and contactin-2 are expressed on paranodes and juxta-paranodes in association with their interaction partners CNTNAP1 and CNTNAP2 respectively, wherein they are involved in axonal domain organization. Given their role in myelination, both contactin-1 and contactin-2 are implicated in demyelinating diseases. However, the cellular and molecular pathways where the next member, contactin-3 is involved are still largely unknown. Contactin-4 is a key guiding molecule in olfactory odour map formation and correct wiring of optic neurons, whereas contactin-5 has been implicated in circuitry of the auditory neurons. Contactin-6 is involved in oligodendrocyte development and synapse formation similar to contactin-1. Given the roles of contactin-4-6 in key CNS developmental processes, mutations in the genes encoding them lead to complex neurodevelopmental disorders such as ASD. In summary, further studies are warranted to completely understand the cellular and molecular mechanisms mediated by contactins which may provide therapeutic leads for neuro-developmental disorders.
Additional file: Open peer review report 1 (89.4KB, pdf) .
Footnotes
Conflicts of interest: The authors declare no conflicts of interest relevant to this work.
Financial support: This project was funded by the Center for Nanoscale Microscopy and Molecular Physiology and the European Neuroscience Campus Network, an Erasmus Mundus Joint Doctoral Program (cycle 5/2014/P-04) (to MC).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Dario Siniscalco, Second University of Naples, Italy.
P-Reviewer: Siniscalco D; C-Editors: Zhao M, Yu J; T-Editor: Liu XL
Funding: This project was funded by the Center for Nanoscale Microscopy and Molecular Physiology and the European Neuroscience Campus Network, an Erasmus Mundus Joint Doctoral Program (cycle 5/2014/P-04) (to MC).
References
- 1.Ashrafi S, Betley JN, Comer J, Brenner-Morton S, Bar V, Shimoda Y, Watanabe K, Peles E, Jessell T, Kaltschmidt J. Neuronal Ig/Caspr recognition promotes the formation of axoaxonic synapses in mouse spinal cord. Neuron. 2014;81:120–129. doi: 10.1016/j.neuron.2013.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baeriswyl T, Stoeckli ET. Axonin-1/TAG-1 is required for pathfinding of granule cell axons in the developing cerebellum. Neural Dev. 2008;3:7. doi: 10.1186/1749-8104-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bai Y, Markham K, Chen F, Weerasekera R, Watts J, Horne P, Wakutani Y, Bagshaw R, Mathews PM, Fraser PE, Westaway D, St George-Hyslop P, Schmitt-Ulms G. The in vivo brain interactome of the amyloid precursor protein. Mol Cell Proteomics. 2007;7:15–34. doi: 10.1074/mcp.M700077-MCP200. [DOI] [PubMed] [Google Scholar]
- 4.Bastakis GG, Savvaki M, Stamatakis A, Vidaki M, Karagogeos D. Tag1 deficiency results in olfactory dysfunction through impaired migration of mitral cells. Development. 2015;142:4318–4328. doi: 10.1242/dev.123943. [DOI] [PubMed] [Google Scholar]
- 5.Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev. 2001;81:871–927. doi: 10.1152/physrev.2001.81.2.871. [DOI] [PubMed] [Google Scholar]
- 6.Becker EBE, Zuliani L, Pettingill R, Lang B, Waters P, Dulneva A, Sobott F, Wardle M, Graus F, Bataller L, Robertson NP, Vincent A. Contactin-associated protein-2 antibodies in non-paraneoplastic cerebellar ataxia. J Neurol Neurosurg Psychiatry. 2012;83:437–440. doi: 10.1136/jnnp-2011-301506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bellen HJ, Lu Y, Beckstead R, Bhat MA. Neurexin IV, caspr and paranodin--novel members of the neurexin family: encounters of axons and glia. Trends Neurosci. 1998;21:444–449. doi: 10.1016/s0166-2236(98)01267-3. [DOI] [PubMed] [Google Scholar]
- 8.Berglund EO, Murai KK, Fredette B, Sekerková G, Marturano B, Weber L, Mugnaini E, Ranscht B. Ataxia and abnormal cerebellar microorganization in mice with ablated contactin gene expression. Neuron. 1999;24:739–750. doi: 10.1016/s0896-6273(00)81126-5. [DOI] [PubMed] [Google Scholar]
- 9.Bouyain S, Watkins DJ. The protein tyrosine phosphatases PTPRZ and PTPRG bind to distinct members of the contactin family of neural recognition molecules. Proc Natl Acad Sci U S A. 2010;107:2443–2448. doi: 10.1073/pnas.0911235107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyle ME, Berglund EO, Murai KK, Weber L, Peles E, Ranscht B. Contactin orchestrates assembly of the septate-like junctions at the paranode in myelinated peripheral nerve. Neuron. 2001;30:385–397. doi: 10.1016/s0896-6273(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 11.Burbach JPH, van der Zwaag B. Contact in the genetics of autism and schizophrenia. Trends Neurosci. 2009;32:69–72. doi: 10.1016/j.tins.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Caligiore D, Mannella F, Arbib MA, Baldassarre G. Dysfunctions of the basal ganglia-cerebellar-thalamo-cortical system produce motor tics in Tourette syndrome. PLoS Comput Biol. 2017;13:e1005395. doi: 10.1371/journal.pcbi.1005395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Canoll PD, Petanceska S, Schlessinger J, Musacchio JM. Three forms of RPTP-beta are differentially expressed during gliogenesis in the developing rat brain and during glial cell differentiation in culture. J Neurosci Res. 1996;44:199–215. doi: 10.1002/(SICI)1097-4547(19960501)44:3<199::AID-JNR1>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 14.Chatterjee M, Campo M, Del Morrema THJ, De Waal M, Van Der Flier WM, Hoozemans JJM, Teunissen CE. Contactin-2, a synaptic and axonal protein, is reduced in cerebrospinal fluid and brain tissue in Alzheimer's disease. Alzheimers Res Ther. 2018;10:52. doi: 10.1186/s13195-018-0383-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen QY, Chen Q, Feng GY, Lindpaintner K, Chen Y, Sun X, Chen Z, Gao Z, Tang J, He L. Case-control association study of the close homologue of L1 (CHL1) gene and schizophrenia in the Chinese population. Schizophr Res. 2005;73:269–274. doi: 10.1016/j.schres.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Colakoglu G, Bergstrom-Tyrberg U, Berglund EO, Ranscht B. Contactin-1 regulates myelination and nodal/paranodal domain organization in the central nervous system. Proc Natl Acad Sci U S A. 2014;111:E394–403. doi: 10.1073/pnas.1313769110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Compton AG, Albrecht DE, Seto JT, Cooper ST, Ilkovski B, Jones KJ, Challis D, Mowat D, Ranscht B, Bahlo M, Froehner SC, North KN. Mutations in contactin-1, a neural adhesion and neuromuscular junction protein, cause a familial form of lethal congenital myopathy. Am J Hum Genet. 2008;83:714–724. doi: 10.1016/j.ajhg.2008.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Connelly MA, Grady RC, Mushinski JF, Marcu KB. PANG, a gene encoding a neuronal glycoprotein, is ectopically activated by intracisternal A-type particle long terminal repeats in murine plasmacytomas. Proc Natl Acad Sci U S A. 1994;91:1337–1341. doi: 10.1073/pnas.91.4.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cottrell CE, Bir N, Varga E, Alvarez CE, Bouyain S, Zernzach R, Thrush DL, Evans J, Trimarchi M, Butter EM, Cunningham D, Gastier-Foster JM, McBride KL, Herman GE. Contactin 4 as an autism susceptibility locus. Autism Res. 2011;4:189–199. doi: 10.1002/aur.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cui XY, Hu QD, Tekaya M, Shimoda Y, Ang BT, Nie DY, Sun L, Hu WP, Karsak M, Duka T, Takeda Y, Ou LY, Dawe GS, Yu FG, Ahmed S, Jin LH, Schachner M, Watanabe K, Arsenijevic Y, Xiao ZC. NB-3/Notch1 pathway via Deltex1 promotes neural progenitor cell differentiation into oligodendrocytes. J Biol Chem. 2004;279:25858–25865. doi: 10.1074/jbc.M313505200. [DOI] [PubMed] [Google Scholar]
- 21.Dalva MB, McClelland AC, Kayser MS. Cell adhesion molecules: signalling functions at the synapse. Nat Rev Neurosci. 2007;8:206–220. doi: 10.1038/nrn2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davisson MT, Bronson RT, Tadenev AL, Motley WW, Krishnaswamy A, Seburn KL, Burgess RW. A spontaneous mutation in contactin 1 in the mouse. PLoS One. 2011;107:e29538. doi: 10.1371/journal.pone.0029538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dislich B, Wohlrab F, Bachhuber T, Müller SA, Kuhn PH, Hogl S, Meyer-Luehmann M, Lichtenthaler SF. Label-free quantitative proteomics of mouse cerebrospinal fluid detects β-site APP cleaving enzyme (BACE1) protease substrates in vivo. Mol Cell Proteomics. 2015;14:2550–2563. doi: 10.1074/mcp.M114.041533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dodd J, Morton SB, Karagogeos D, Yamamoto M, Jessell TM. Spatial regulation of axonal glycoprotein expression on subsets of embryonic spinal neurons. Neuron. 1988;1:105–116. doi: 10.1016/0896-6273(88)90194-8. [DOI] [PubMed] [Google Scholar]
- 25.Eckerich C, Zapf S, Ulbricht U, Müller S, Fillbrandt R, Westphal M, Lamszus K. Contactin is expressed in human astrocytic gliomas and mediates repulsive effects. Glia. 2006;53:1–12. doi: 10.1002/glia.20254. [DOI] [PubMed] [Google Scholar]
- 26.Edgar JC, Fisk Iv CL, Berman JI, Chudnovskaya D, Liu S, Pandey J, Herrington JD, Port RG, Schultz RT, Roberts TP. Auditory encoding abnormalities in children with autism spectrum disorder suggest delayed development of auditory cortex. Mol Autism. 2015;6:69. doi: 10.1186/s13229-015-0065-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faissner A, Heck N, Dobbertin A, Garwood J. DSD-1-Proteoglycan/Phosphacan and receptor protein tyrosine phosphatase-beta isoforms during development and regeneration of neural tissues. Adv Exp Med Biol. 2006;557:25–53. doi: 10.1007/0-387-30128-3_3. [DOI] [PubMed] [Google Scholar]
- 28.Faivre-Sarrailh C, Devaux JJ. Neuro-glial interactions at the nodes of Ranvier: implication in health and diseases. Front Cell Neurosci. 2013;7:196. doi: 10.3389/fncel.2013.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faivre-sarrailh C, Gauthier F, Denisenko-nehrbass N, Le Bivic A, Rougon G, Girault J. The glycosylphosphatidyl inositol-anchored adhesion molecule f3/contactin is required for surface transport of paranodin / contactin-associated protein (caspr) Cell. 2000;149:491–502. doi: 10.1083/jcb.149.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faivre-Sarrailh C, Gennarini G, Goridis C, Rougon G. F3/F11 cell surface molecule expression in the developing mouse cerebellum is polarized at synaptic sites and within granule cells. J Neurosci. 1992;12:257–267. doi: 10.1523/JNEUROSCI.12-01-00257.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernandez T, Morgan T, Davis N, Klin A, Morris A, Farhi A, Lifton RP, State MW. Disruption of contactin 4 (CNTN4) results in developmental delay and other features of 3p deletion syndrome. Am J Hum Genet. 2004;74:1286–1293. doi: 10.1086/421474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Furley a J, Morton SB, Manalo D, Karagogeos D, Dodd J, Jessell TM. The axonal glycoprotein TAG-1 is an immunoglobulin superfamily member with neurite outgrowth-promoting activity. Cell. 1990;61:157–170. doi: 10.1016/0092-8674(90)90223-2. [DOI] [PubMed] [Google Scholar]
- 33.Gautam V, D’Avanzo C, Hebisch M, Kovacs DM, Kim DY. BACE1 activity regulates cell surface contactin-2 levels. Mol Neurodegener. 2014;9:4. doi: 10.1186/1750-1326-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glessner JT, Wang K, Cai G, Korvatska O, Kim CE, Wood S, Zhang H, Estes A, Brune CW, Bradfield JP, Imielinski M, Frackelton EC, Reichert J, Crawford EL, Munson J, Sleiman PM, Chiavacci R, Annaiah K, Thomas K, Hou C, et al. Autism genome-wide copy number variation reveals ubiquitin and neuronal genes. Nature. 2009;459:569–573. doi: 10.1038/nature07953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haenisch C, Diekmann H, Klinger M, Gennarini G, Kuwada JY, Stuermer CAO. The neuronal growth and regeneration associated Cntn1 (F3/F11/Contactin) gene is duplicated in fish: Expression during development and retinal axon regeneration. Mol Cell Neurosci. 2005;28:361–374. doi: 10.1016/j.mcn.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 36.Hansford LM, Smith SA, Haber M, Norris MD, Cheung B, Marshall GM. Cloning and characterization of the human neural cell adhesion molecule, CNTN4 (alias BIG-2) Cytogenet Genome Res. 2003;101:17–23. doi: 10.1159/000073412. [DOI] [PubMed] [Google Scholar]
- 37.Harroch S, Palmeri M, Rosenbluth J, Custer A, Okigaki M, Shrager P, Blum M, Buxbaum JD, Schlessinger J. No obvious abnormality in mice deficient in receptor protein tyrosine phosphatase beta. Mol Cell Biol. 2000;20:7706–7715. doi: 10.1128/mcb.20.20.7706-7715.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hasler TH, Rader C, Stoeckli ET, Zuellig RA, Sonderegger P. cDNA cloning, structural features, and eucaryotic expression of human TAG-1/axonin-1. Eur J Biochem. 1993;211:329–339. doi: 10.1111/j.1432-1033.1993.tb19902.x. [DOI] [PubMed] [Google Scholar]
- 39.Hu QD, Ma QH, Gennarini G, Xiao ZC. Cross-talk between F3/contactin and notch at axoglial interface: A role in oligodendrocyte development. Dev Neurosci. 2006;28:25–33. doi: 10.1159/000090750. [DOI] [PubMed] [Google Scholar]
- 40.Huang AY, Yu D, Davis LK, Sul JH, Tsetsos F, Ramensky V, Zelaya I, Ramos EM, Osiecki L, Chen JA, McGrath LM, Illmann C, Sandor P, Barr CL, Grados M, Singer HS, Nöthen MM, Hebebrand J, King RA, Dion Y, et al. Rare copy number variants in NRXN1 and CNTN6 increase risk for Tourette syndrome. Neuron. 2017;94:1101–1111.e7. doi: 10.1016/j.neuron.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang Z, Yu Y, Shimoda Y, Watanabe K, Liu Y. Loss of neural recognition molecule NB-3 delays the normal projection and terminal branching of developing corticospinal tract axons in the mouse. J Comp Neurol. 2012;520:1227–1245. doi: 10.1002/cne.22772. [DOI] [PubMed] [Google Scholar]
- 42.Hussman JP, Chung RH, Griswold AJ, Jaworski JM, Salyakina D, Ma D, Konidari I, Whitehead PL, Vance JM, Martin ER, Cuccaro ML, Gilbert JR, Haines JL, Pericak-Vance MA. A noise-reduction GWAS analysis implicates altered regulation of neurite outgrowth and guidance in autism. Mol Autism. 2011;2:1. doi: 10.1186/2040-2392-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kamei Y, Takeda Y, Teramoto K, Tsutsumi O, Taketani Y, Watanabe K. Human NB-2 of the contactin subgroup molecules: chromosomal localization of the gene (CNTN5) and distinct expression pattern from other subgroup members. Genomics. 2000;69:113–119. doi: 10.1006/geno.2000.6310. [DOI] [PubMed] [Google Scholar]
- 44.Kamei Y, Tsutsumi O, Taketani Y, Watanabe K. cDNA cloning and chromosomal localization of neural adhesion molecule NB-3 in human. J Neurosci Res. 1998;51:275–283. doi: 10.1002/(SICI)1097-4547(19980201)51:3<275::AID-JNR1>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 45.Kaneko-Goto T, Yoshihara S ichi, Miyazaki H, Yoshihara Y. BIG-2 mediates olfactory axon convergence to target glomeruli. Neuron. 2008;57:834–846. doi: 10.1016/j.neuron.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 46.Karayannis T, Au E, Patel JC, Kruglikov I, Markx S, Delorme R, Héron D, Salomon D, Glessner J, Restituito S, Gordon A, Rodriguez-Murillo L, Roy NC, Gogos JA, Rudy B, Rice ME, Karayiorgou M, Hakonarson H, Keren B, Huguet G, et al. Cntnap4 differentially contributes to GABAergic and dopaminergic synaptic transmission. Nature. 2014;511:236–240. doi: 10.1038/nature13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kelleher RJ, Bear MF. The autistic neuron: troubled translation? Cell. 2008;135:401–406. doi: 10.1016/j.cell.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 48.Kleijer KTE, Zuko A, Shimoda Y, Watanabe K, Burbach JPH. Contactin-5 expression during development and wiring of the thalamocortical system. Neuroscience. 2015;310:106–113. doi: 10.1016/j.neuroscience.2015.09.039. [DOI] [PubMed] [Google Scholar]
- 49.Koch T, Brugger T, Bach A, Gennarini G, Trotter J. Expression of the immunoglobulin superfamily cell adhesion molecule F3 by oligodendrocyte-lineage cells. Glia. 1997;19:199–212. doi: 10.1002/(sici)1098-1136(199703)19:3<199::aid-glia3>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 50.Koike H, Kadoya M, Kaida KI, Ikeda S, Kawagashira Y, Iijima M, Kato D, Ogata H, Yamasaki R, Matsukawa N, Kira JI, Katsuno M, Sobue G. Paranodal dissection in chronic inflammatory demyelinating polyneuropathy with anti-neurofascin-155 and anti-contactin-1 antibodies. J Neurol Neurosurg Psychiatry. 2017;88:465–473. doi: 10.1136/jnnp-2016-314895. [DOI] [PubMed] [Google Scholar]
- 51.Konietzko U. AICD nuclear signaling and its possible contribution to Alzheimer's disease. Curr Alzheimer Res. 2012;9:200–216. doi: 10.2174/156720512799361673. [DOI] [PubMed] [Google Scholar]
- 52.Kozlov S V, Giger RJ, Hasler T, Korvatska E, Schorderet DF, Sonderegger P. The human TAX1 gene encoding the axon-associated cell adhesion molecule TAG-1/axonin-1: genomic structure and basic promoter. Genomics. 1995;30:141–148. doi: 10.1006/geno.1995.9892. [DOI] [PubMed] [Google Scholar]
- 53.Kuhn P-H, Koroniak K, Hogl S, Colombo A, Zeitschel U, Willem M, Volbracht C, Schepers U, Imhof A, Hoffmeister A, Haass C, Roßner S, Bräse S, Lichtenthaler SF. Secretome protein enrichment identifies physiological BACE1 protease substrates in neurons. EMBO J. 2012;31:3157–3168. doi: 10.1038/emboj.2012.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kumar RA, Christian SL. Genetics of autism spectrum disorders. Curr Neurol Neurosci Rep. 2009;9:188–197. doi: 10.1007/s11910-009-0029-2. [DOI] [PubMed] [Google Scholar]
- 55.Kyriakopoulou K, de Diego I, Wassef M, Karagogeos D. A combination of chain and neurophilic migration involving the adhesion molecule TAG-1 in the caudal medulla. Development. 2002;129:287–296. doi: 10.1242/dev.129.2.287. [DOI] [PubMed] [Google Scholar]
- 56.Labasque M, Faivre-Sarrailh C. GPI-anchored proteins at the node of Ranvier. FEBS Lett. 2010;584:1787–1792. doi: 10.1016/j.febslet.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 57.Labasque M, Hivert B, Nogales-Gadea G, Querol L, Illa I, Faivre-Sarrailh C. Specific contactin N-glycans are implicated in neurofascin binding and autoimmune targeting in peripheral neuropathies. J Biol Chem. 2014;289:7907–7918. doi: 10.1074/jbc.M113.528489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lamprianou S, Chatzopoulou E, Thomas JL, Bouyain S, Harroch S. A complex between contactin-1 and the protein tyrosine phosphatase PTPRZ controls the development of oligodendrocyte precursor cells. Proc Natl Acad Sci U S A. 2011;108:17498–17503. doi: 10.1073/pnas.1108774108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lang DM, Warren JT, Klisa C, Stuermer CA. Topographic restriction of TAG-1 expression in the developing retinotectal pathway and target dependent reexpression during axon regeneration. Mol Cell Neurosci. 2001;17:398–414. doi: 10.1006/mcne.2000.0936. [DOI] [PubMed] [Google Scholar]
- 60.Lee S, Takeda Y, Kawano H, Hosoya H, Nomoto M, Fujimoto D, Takahashi N, Watanabe K. Expression and regulation of a gene encoding neural recognition molecule NB-3 of the contactin/F3 subgroup in mouse brain. Gene. 2000;245:253–266. doi: 10.1016/s0378-1119(00)00031-7. [DOI] [PubMed] [Google Scholar]
- 61.Li H, Takeda Y, Niki H, Ogawa J, Kobayashi S, Kai N, Akasaka K, Asano M, Sudo K, Iwakura Y, Watanabe K. Aberrant responses to acoustic stimuli in mice deficient for neural recognition molecule NB-2. Eur J Neurosci. 2003;17:929–936. doi: 10.1046/j.1460-9568.2003.02514.x. [DOI] [PubMed] [Google Scholar]
- 62.Lin JF, Pan HC, Ma LP, Shen YQ, Schachner M. The cell neural adhesion molecule contactin-2 (TAG-1) is beneficial for functional recovery after spinal cord injury in adult Zebrafish. PLoS One. 2012;7:e52376. doi: 10.1371/journal.pone.0052376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lu Z, Reddy MVVVS, Liu J, Kalichava A, Liu J, Zhang L, Chen F, Wang Y, Holthauzen LMF, White MA, Seshadrinathan S, Zhong X, Ren G, Rudenko G. Molecular architecture of contactin-associated protein-like 2 (CNTNAP2) and its interaction with contactin 2 (CNTN2) J Biol Chem. 2016;291:24133–24147. doi: 10.1074/jbc.M116.748236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ma QH, Futagawa T, Yang WL, Jiang XD, Zeng L, Takeda Y, Xu RX, Bagnard D, Schachner M, Furley AJ, Karagogeos D, Watanabe K, Dawe GS, Xiao ZC. A TAG1-APP signalling pathway through Fe65 negatively modulates neurogenesis. Nat Cell Biol. 2008a;10:283–294. doi: 10.1038/ncb1690. [DOI] [PubMed] [Google Scholar]
- 65.Ma QH, Bagnard D, Xiao ZC, Dawe GS. A TAG on to the neurogenic functions of APP. Cell Adh Migr. 2008b;2:2–8. doi: 10.4161/cam.2.1.5790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Manso C, Querol L, Mekaouche M, Illa I, Devaux JJ. Contactin-1 IgG4 antibodies cause paranode dismantling and conduction defects. Brain. 2016;139:1700–1712. doi: 10.1093/brain/aww062. [DOI] [PubMed] [Google Scholar]
- 67.Massaro A, Bizzoca A, Corsi P, Pinto MF, Carratù MR, Gennarini G. Significance of F3/Contactin gene expression in cerebral cortex and nigrostriatal development. Mol Cell Neurosci. 2012;50:221–237. doi: 10.1016/j.mcn.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 68.Masuda T. Contactin-2/TAG-1, active on the front line for three decades. Cell Adh Migr. 2017;11:524–531. doi: 10.1080/19336918.2016.1269998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mattson MP, Van Praag H. TAGing APP constrains neurogenesis. Nat Cell Biol. 2008;10:249–250. doi: 10.1038/ncb0308-249. [DOI] [PubMed] [Google Scholar]
- 70.Mercati O, Huguet G, Danckaert A, André-Leroux G, Maruani A, Bellinzoni M, Rolland T, Gouder L, Mathieu A, Buratti J, Amsellem F, Benabou M, Van-Gils J, Beggiato A, Konyukh M, Bourgeois JP, Gazzellone MJ, Yuen RK, Walker S, Delépine M, et al. CNTN6 mutations are risk factors for abnormal auditory sensory perception in autism spectrum disorders. Mol Psychiatry. 2017;22:625–633. doi: 10.1038/mp.2016.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mercati O, Danckaert A, André-Leroux G, Bellinzoni M, Gouder L, Watanabe K, Shimoda Y, Grailhe R, De Chaumont F, Bourgeron T, Cloëz-Tayarani I. Contactin 4, -5 and -6 differentially regulate neuritogenesis while they display identical PTPRG binding sites. Biol Open. 2013;2:324–334. doi: 10.1242/bio.20133343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miura S, Shibata H, Furuya H, Ohyagi Y, Osoegawa M, Miyoshi Y, Matsunaga H, Shibata A, Matsumoto N, Iwaki A, Taniwaki T, Kikuchi H, Kira J, Fukumaki Y. The contactin 4 gene locus at 3p26 is a candidate gene of SCA16. Neurology. 2006;67:1236–1241. doi: 10.1212/01.wnl.0000238510.84932.82. [DOI] [PubMed] [Google Scholar]
- 73.Mohebiany AN, Harroch S, Bouyain S. New insights into the roles of the contactin cell adhesion molecules in neural development. Adv Neurobiol. 2014;8:165–194. doi: 10.1007/978-1-4614-8090-7_8. [DOI] [PubMed] [Google Scholar]
- 74.Morrow EM, Yoo SY, Flavell SW, Kim TK, Lin Y, Hill RS, Mukaddes NM, Balkhy S, Gascon G, Hashmi A, Al-Saad S, Ware J, Joseph RM, Greenblatt R, Gleason D, Ertelt JA, Apse KA, Bodell A, Partlow JN, Barry B, et al. Identifying autism loci and genes by tracing recent shared ancestry. Science. 2008;321:218–223. doi: 10.1126/science.1157657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Murai KK, Misner D, Ranscht B. Contactin supports synaptic plasticity associated with hippocampal long-term depression but not potentiation. Curr Biol. 2002;12:181–190. doi: 10.1016/s0960-9822(02)00680-2. [DOI] [PubMed] [Google Scholar]
- 76.Nava C, Keren B, Mignot C, Rastetter AS, Chantot-Bastaraud S, Faudet A, Fonteneau E, Amiet C, Laurent C, Jacquette A, Whalen S, Afenjar A, Siffroi JP, Cohen D, Brice A, Héron D, Depienne C. Prospective diagnostic analysis of copy number variants using SNP microarrays in individuals with autism spectrum disorders. Eur J Hum Genet. 2013;22:71–78. doi: 10.1038/ejhg.2013.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nikolaienko RM, Hammel M, Dubreuil V, Zalmai R, Hall DR, Mehzabeen N, Karuppan SJ, Harroch S, Stella SL, Bouyain S. Structural basis for interactions between contactin family members and protein-tyrosine phosphatase receptor type G in neural tissues. J Biol Chem. 2016;291:21335–21349. doi: 10.1074/jbc.M116.742163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ogawa J, Kaneko H, Masuda T, Nagata S, Hosoya H, Watanabe K. Novel neural adhesion molecules in the Contactin/F3 subgroup of the immunoglobulin superfamily: Isolation and characterization of cDNAs from rat brain. Neurosci Lett. 1996;218:173–176. doi: 10.1016/s0304-3940(96)13156-6. [DOI] [PubMed] [Google Scholar]
- 79.Ogawa J, Lee S, Itoh K, Nagata S, Machida T, Takeda Y, Watanabe K. Neural recognition molecule NB-2 of the contactin/F3 subgroup in rat: Specificity in neurite outgrowth-promoting activity and restricted expression in the brain regions. J Neurosci Res. 2001;65:100–110. doi: 10.1002/jnr.1133. [DOI] [PubMed] [Google Scholar]
- 80.Oguro-Ando A, Zuko A, Kleijer KTE, Burbach JPH. A current view on contactin-4, -5, and -6: Implications in neurodevelopmental disorders. Mol Cell Neurosci. 2017;81:72–83. doi: 10.1016/j.mcn.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 81.Osterfield M, Egelund R, Young LM, Flanagan JG. Interaction of amyloid precursor protein with contactins and NgCAM in the retinotectal system. Development. 2008;135:1189–1199. doi: 10.1242/dev.007401. [DOI] [PubMed] [Google Scholar]
- 82.Osterhout JA, Stafford BK, Nguyen PL, Yoshihara Y, Huberman AD. Contactin-4 mediates axon-target specificity and functional development of the accessory optic system. Neuron. 2015;86:985–999. doi: 10.1016/j.neuron.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pedraza L, Huang JK, Colman DR. Viewpoint Organizing Principles of the Axoglial Apparatus isms with nervous systems, and, remarkably, certain of its protein constituents are highly conserved, with ancient origins in evolution that stretch back almost. Neuron. 2001;30:335–344. doi: 10.1016/s0896-6273(01)00306-3. [DOI] [PubMed] [Google Scholar]
- 84.Peles E, Nativ M, Lustig M, Grumet M, Schilling J, Martinez R, Plowman GD, Schlessinger J. Identification of a novel contactin-associated transmembrane receptor with multiple domains implicated in protein-protein interactions. EMBO J. 1997;16:978–988. doi: 10.1093/emboj/16.5.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Peles E, Salzer JL. Molecular domains of myelinated axons. Curr Opin Neurobiol. 2000;10:558–565. doi: 10.1016/s0959-4388(00)00122-7. [DOI] [PubMed] [Google Scholar]
- 86.Peng YR, Tran NM, Krishnaswamy A, Kostadinov D, Martersteck EM, Sanes JR. Satb1 regulates contactin 5 to pattern dendrites of a mammalian retinal ganglion cell. Neuron. 2017;95:869–883.e6. doi: 10.1016/j.neuron.2017.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Poliak S, Peles E. The local differentiation of myelinated axons at nodes of Ranvier. Nat Rev Neurosci. 2003;4:968–980. doi: 10.1038/nrn1253. [DOI] [PubMed] [Google Scholar]
- 88.Puzzo D, Bizzoca A, Loreto C, Guida CA, Gulisano W, Frasca G, Bellomo M, Castorina S, Gennarini G, Palmeri A. Role of F3/contactin expression profile in synaptic plasticity and memory in aged mice. Neurobiol Aging. 2015;36:1702–1715. doi: 10.1016/j.neurobiolaging.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 89.Puzzo D, Bizzoca A, Privitera L, Furnari D, Giunta S, Girolamo F, Pinto M, Gennarini G, Palmeri A. F3/Contactin promotes hippocampal neurogenesis, synaptic plasticity, and memory in adult mice. Hippocampus. 2013;23:1367–1382. doi: 10.1002/hipo.22186. [DOI] [PubMed] [Google Scholar]
- 90.Querol L, Nogales-Gadea G, Rojas-Garcia R, Martinez-Hernandez E, Diaz-Manera J, Suárez-Calvet X, Navas M, Araque J, Gallardo E, Illa I. Antibodies to contactin-1 in chronic inflammatory demyelinating polyneuropathy. Ann Neurol. 2013;73:370–380. doi: 10.1002/ana.23794. [DOI] [PubMed] [Google Scholar]
- 91.Ramaker JM, Swanson TL, Copenhaver PF. Manduca contactin regulates amyloid precursor protein-dependent neuronal migration. J Neurosci. 2016;36:8757–8775. doi: 10.1523/JNEUROSCI.0729-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ranscht B. Sequence of contactin, a 130-kD glycoprotein concentrated in areas of interneuronal contact, defines a new member of the immunoglobulin supergene family in the nervous system. J Cell Biol. 1988;107:1561–1573. doi: 10.1083/jcb.107.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rios JC, Melendez-Vasquez C V, Einheber S, Lustig M, Grumet M, Hemperly J, Peles E, Salzer JL. Contactin-associated protein (Caspr) and contactin form a complex that is targeted to the paranodal junctions during myelination. J Neurosci. 2000;20:8354–8364. doi: 10.1523/JNEUROSCI.20-22-08354.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Roohi J, Montagna C, Tegay DH, Palmer LE, DeVincent C, Pomeroy JC, Christian SL, Nowak N, Hatchwell E. Disruption of contactin 4 in three subjects with autism spectrum disorder. J Med Genet. 2009;46:176–182. doi: 10.1136/jmg.2008.057505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rozenkrantz L, Zachor D, Heller I, Plotkin A, Weissbrod A, Snitz K, Secundo L, Sobel N. A mechanistic link between olfaction and autism spectrum disorder. Curr Biol. 2015;25:1904–1910. doi: 10.1016/j.cub.2015.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rudenko G. Dynamic control of synaptic adhesion and organizing molecules in synaptic plasticity. Neural Plast 2017. 2017:6526151. doi: 10.1155/2017/6526151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sakurai K, Toyoshima M, Takeda Y, Shimoda Y, Watanabe K. Synaptic formation in subsets of glutamatergic terminals in the mouse hippocampal formation is affected by a deficiency in the neural cell recognition molecule NB-3. Neurosci Lett. 2010;473:102–106. doi: 10.1016/j.neulet.2010.02.027. [DOI] [PubMed] [Google Scholar]
- 98.Sakurai K, Toyoshima M, Ueda H, Matsubara K, Takeda Y, Karagogeos D, Shimoda Y, Watanabe K. Contribution of the neural cell recognition molecule NB-3 to synapse formation between parallel fibers and Purkinje cells in mouse. Dev Neurobiol. 2009;69:811–824. doi: 10.1002/dneu.20742. [DOI] [PubMed] [Google Scholar]
- 99.Salzer JL, Brophy PJ, Peles E. Molecular domains of myelinated axons in the peripheral nervous system. Glia. 2008;56:1532–1540. doi: 10.1002/glia.20750. [DOI] [PubMed] [Google Scholar]
- 100.Saus E, Brunet A, Armengol L, Alonso P, Crespo JM, Fernández-Aranda F, Guitart M, Martín-Santos R, Menchón JM, Navinés R, Soria V, Torrens M, Urretavizcaya M, Vallès V, Gratacòs M, Estivill X. Comprehensive copy number variant (CNV) analysis of neuronal pathways genes in psychiatric disorders identifies rare variants within patients. J Psychiatr Res. 2010;44:971–978. doi: 10.1016/j.jpsychires.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 101.Savvaki M, Panagiotaropoulos T, Stamatakis A, Sargiannidou I, Karatzioula P, Watanabe K, Stylianopoulou F, Karagogeos D, Kleopa KA. Impairment of learning and memory in TAG-1 deficient mice associated with shorter CNS internodes and disrupted juxtaparanodes. Mol Cell Neurosci. 2008;39:478–490. doi: 10.1016/j.mcn.2008.07.025. [DOI] [PubMed] [Google Scholar]
- 102.Sherman DL, Tait S, Melrose S, Johnson R, Zonta B, Court FA, Macklin WB, Meek S, Smith AJH, Cottrell DF, Brophy PJ. Neurofascins are required to establish axonal domains for saltatory conduction. Neuron. 2005;48:737–742. doi: 10.1016/j.neuron.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 103.Shimazaki K, Hosoya H, Takeda Y, Kobayashi S, Watanabe K. Age-related decline of F3/contactin in rat hippocampus. Neurosci Lett. 1998;245:117–120. doi: 10.1016/s0304-3940(98)00179-7. [DOI] [PubMed] [Google Scholar]
- 104.Shimoda Y, Koseki F, Itoh M, Toyoshima M, Watanabe K. A cis-complex of NB-2/contactin-5 with amyloid precursor-like protein 1 is localized on the presynaptic membrane. Neurosci Lett. 2012;510:148–153. doi: 10.1016/j.neulet.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 105.Shimoda Y, Watanabe K. Contactins: emerging key roles in the development and function of the nervous system. Cell Adh Migr. 2009;3:64–70. doi: 10.4161/cam.3.1.7764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Simmons DR, Robertson AE, McKay LS, Toal E, McAleer P, Pollick FE. Vision in autism spectrum disorders. Vision Res. 2009;49:2705–2739. doi: 10.1016/j.visres.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 107.Singh V, van Pelt ED, Stoop MP, Stingl C, Ketelslegers IA, Neuteboom RF, Catsman-Berrevoets CE, Luider TM, Hintzen RQ. Gray matter-related proteins are associated with childhood-onset multiple sclerosis. Neurol Neuroimmunol neuroinflammation. 2015;2:e155. doi: 10.1212/NXI.0000000000000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stoeckli ET. Neural circuit formation in the cerebellum is controlled by cell adhesion molecules of the Contactin family. Cell Adhes Migr. 2010;4:523–526. doi: 10.4161/cam.4.4.12733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stoeckli ET, Kuhn TB, Duc CO, Ruegg MA, Sonderegger P. The axonally secreted protein axonin-1 is a potent substratum for neurite growth. J Cell Biol. 1991;112:449–455. doi: 10.1083/jcb.112.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Stoeckli ET, Sonderegger P, Pollerberg GE, Landmesser LT. Interference with axonin-1 and NrCAM interactions unmasks a floor-plate activity inhibitory for commissural axons. Neuron. 1997;18:209–221. doi: 10.1016/s0896-6273(00)80262-7. [DOI] [PubMed] [Google Scholar]
- 111.Sun X, Takagishi Y, Okabe E, Chishima Y, Kanou Y, Murase S, Mizumura K, Inaba M, Komatsu Y, Hayashi Y, Peles E, Oda S, Murata Y. A novel caspr mutation causes the shambling mouse phenotype by disrupting axoglial interactions of myelinated nerves. J Neuropathol Exp Neurol. 2009;68:1207–1218. doi: 10.1097/NEN.0b013e3181be2e96. [DOI] [PubMed] [Google Scholar]
- 112.Takeda Y, Akasaka K, Lee S, Kobayashi S, Kawano H, Murayama S, Takahashi N, Hashimoto K, Kano M, Asano M, Sudo K, Iwakura Y, Watanabe K. Impaired motor coordination in mice lacking neural recognition molecule NB-3 of the contactin/F3 subgroup. J Neurobiol. 2003;56:252–265. doi: 10.1002/neu.10222. [DOI] [PubMed] [Google Scholar]
- 113.Taylor MK, Yeager K, Morrison SJ. Physiological Notch signaling promotes gliogenesis in the developing peripheral and central nervous systems. Development. 2007;134:2435–2447. doi: 10.1242/dev.005520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tobe RH, Bansal R, Xu D, Hao X, Liu J, Sanchez J, Peterson BS. Cerebellar morphology in tourette syndrome and obsessive-compulsive disorder. Ann Neurol. 2010;67:479–487. doi: 10.1002/ana.21918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Toyoshima M, Sakurai K, Shimazaki K, Takeda Y, Nakamoto M, Serizawa S, Shimoda Y, Watanabe K. Preferential localization of neural cell recognition molecule NB-2 in developing glutamatergic neurons in the rat auditory brainstem. J Comp Neurol. 2009;513:349–362. doi: 10.1002/cne.21972. [DOI] [PubMed] [Google Scholar]
- 116.Traka M, Dupree JL, Popko B, Karagogeos D. The neuronal adhesion protein TAG-1 is expressed by Schwann cells and oligodendrocytes and is localized to the juxtaparanodal region of myelinated fibers. J Neurosci. 2002;22:3016–3024. doi: 10.1523/JNEUROSCI.22-08-03016.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Traka M, Goutebroze L, Denisenko N, Bessa M, Nifli A, Havaki S, Iwakura Y, Fukamauchi F, Watanabe K, Soliven B, Girault JA, Karagogeos D. Association of TAG-1 with Caspr2 is essential for the molecular organization of juxtaparanodal regions of myelinated fibers. J Cell Biol. 2003;162:1161–1172. doi: 10.1083/jcb.200305078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tsiotra PC, Karagogeos D, Theodorakis K, Michaelidis TM, Modi WS, Furley AJ, Jessell TM, Papamatheakis J. Isolation of the cDNA and chromosomal localization of the gene (TAX1) encoding the human axonal glycoprotein TAG-1. Genomics. 1993;18:562–567. doi: 10.1016/s0888-7543(05)80357-x. [DOI] [PubMed] [Google Scholar]
- 119.Umemori H, Sato S, Yagi T, Aizawa S, Yamamoto T. Initial events of myelination involve Fyn tyrosine kinase signalling. Nature. 1994;367:572–576. doi: 10.1038/367572a0. [DOI] [PubMed] [Google Scholar]
- 120.van Daalen E, Kemner C, Verbeek NE, van der Zwaag B, Dijkhuizen T, Rump P, Houben R, van’t Slot R, de Jonge MV, Staal WG, Beemer FA, Vorstman JAS, Burbach JPH, van Amstel HKP, Hochstenbach R, Brilstra EH, Poot M. Social Responsiveness Scale-aided analysis of the clinical impact of copy number variations in autism. Neurogenetics. 2011;12:315–323. doi: 10.1007/s10048-011-0297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Virgintino D, Ambrosini M, D’Errico P, Bertossi M, Papadaki C, Karagogeos D, Gennarini G. Regional distribution and cell type-specific expression of the mouse F3 axonal glycoprotein: a developmental study. J Comp Neurol. 1999;413:357–372. doi: 10.1002/(sici)1096-9861(19991025)413:3<357::aid-cne1>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 122.Wang H, Kunkel DD, Martin TM, Schwartzkroin PA, Tempel BL. Heteromultimeric K+channels in terminal and juxtaparanodal regions of neurons. Nature. 1993;365:75–79. doi: 10.1038/365075a0. [DOI] [PubMed] [Google Scholar]
- 123.Williams AF, Barclay AN. The immunoglobulin superfamily--domains for cell surface recognition. Annu Rev Immunol. 1988;6:381–405. doi: 10.1146/annurev.iy.06.040188.002121. [DOI] [PubMed] [Google Scholar]
- 124.Wolfer DP, Henehan-Beatty A, Stoeckli ET, Sonderegger P, Lipp HP. Distribution of TAG-1/axonin-1 in fibre tracts and migratory streams of the developing mouse nervous system. J Comp Neurol. 1994;345:1–32. doi: 10.1002/cne.903450102. [DOI] [PubMed] [Google Scholar]
- 125.Wolman MA, Sittaramane VK, Essner JJ, Yost HJ, Chandrasekhar A, Halloran MC. Transient axonal glycoprotein-1 (TAG-1) and laminin-alpha1 regulate dynamic growth cone behaviors and initial axon direction in vivo. Neural Dev. 2008;3:6. doi: 10.1186/1749-8104-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yamamoto M, Boyer a M, Crandall JE, Edwards M, Tanaka H. Distribution of stage-specific neurite-associated proteins in the developing murine nervous system recognized by a monoclonal antibody. J Neurosci. 1986;6:3576–3594. doi: 10.1523/JNEUROSCI.06-12-03576.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ye H, Tan YLJ, Ponniah S, Takeda Y, Wang SQ, Schachner M, Watanabe K, Pallen CJ, Xiao ZC. Neural recognition molecules CHL1 and NB-3 regulate apical dendrite orientation in the neocortex via PTP alpha. EMBO J. 2008;27:188–200. doi: 10.1038/sj.emboj.7601939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yoshihara Y, Kawasaki M, Tamada A, Nagata S, Kagamiyama H, Mori K. Overlapping and differential expression of BIG-2, BIG-1, TAG-1, and F3: four members of an axon-associated cell adhesion molecule subgroup of the immunoglobulin superfamily. J Neurobiol. 1995;28:51–69. doi: 10.1002/neu.480280106. [DOI] [PubMed] [Google Scholar]
- 129.Yoshihara Y, Kawasaki M, Tani A, Tamada A, Nagata S, Kagamiyama H, Mori K. BIG-1: A new TAG-1/F3-related member of the immunoglobulin superfamily with neurite outgrowth-promoting activity. Neuron. 1994;13:415–426. doi: 10.1016/0896-6273(94)90357-3. [DOI] [PubMed] [Google Scholar]
- 130.Zeng L, D’Alessandri L, Kalousek MB, Vaughan L, Pallen CJ. Protein tyrosine phosphatase alpha (PTPalpha) and contactin form a novel neuronal receptor complex linked to the intracellular tyrosine kinase fyn. J Cell Biol. 1999;147:707–714. doi: 10.1083/jcb.147.4.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zou Y, Zhang WF, Liu HY, Li X, Zhang X, Ma XF, Sun Y, Jiang SY, Ma QH, Xu DE. Structure and function of the contactin-associated protein family in myelinated axons and their relationship with nerve diseases. Neural Regen Res. 2017;12:1551–1558. doi: 10.4103/1673-5374.215268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zoupi L, Savvaki M, Kalemaki K, Kalafatakis I, Sidiropoulou K, Karagogeos D. The function of contactin-2/TAG-1 in oligodendrocytes in health and demyelinating pathology. Glia. 2018;66:576–591. doi: 10.1002/glia.23266. [DOI] [PubMed] [Google Scholar]
- 133.Zuellig RA, Rader C, Schroeder A, Kalousek MB, Von Bohlen und Halbach F, Osterwalder T, Inan C, Stoeckli ET, Affolter HU, Fritz A. The axonally secreted cell adhesion molecule, axonin-1. Primary structure, immunoglobulin-like and fibronectin-type-III-like domains and glycosyl-phosphatidylinositol anchorage. Eur J Biochem. 1992;204:453–463. doi: 10.1111/j.1432-1033.1992.tb16655.x. [DOI] [PubMed] [Google Scholar]
- 134.Zuko A, Kleijer KTE, Oguro-Ando A, Kas MJH, Van Daalen E, Van Der Zwaag B, Burbach JPH. Contactins in the neurobiology of autism. Eur J Pharmacol. 2013;719:63–74. doi: 10.1016/j.ejphar.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 135.Zuko A, Oguro-Ando A, Van Dijk R, Gregorio-Jordan S, Van Der Zwaag B, Peter J, Burbach H. Cell adhesion & migration developmental role of the cell adhesion molecule contactin-6 in the cerebral cortex and hippocampus developmental role of the cell adhesion molecule contactin-6 in the cerebral cortex and hippocampus. Cell Adh Migr. 2016;10:378–392. doi: 10.1080/19336918.2016.1155018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


