Abstract
Alzheimer’s disease (AD) is the most common age-related, progressive neurodegenerative disease. It is characterized by memory loss and cognitive decline and responsible for most cases of dementia in the elderly. Late-onset or sporadic AD accounts for > 95% of cases, with age at onset > 65 years. Currently there are no drugs or other therapeutic agents available to prevent or delay the progression of AD. The cellular and molecular changes occurring in the brains of individuals with AD include accumulation of β-amyloid peptide and hyperphosphorylated tau protein, decrease of acetylcholine neurotransmitter, inflammation, and oxidative stress. Aggregation of β-amyloid peptide in extracellular plaques and the hyperphosphorylated tau protein in intracellular neurofibrillary tangles are characteristic of AD. A major challenge is identifying molecular biomarkers of the early-stage AD in patients as most studies have been performed with blood or brain tissue samples (postmortem) at late-stage AD. Subjects with mild cognitive impairment almost always have the neuropathologic features of AD with about 50% of mild cognitive impairment patients progressing to AD. They could provide important information about AD pathomechanism and potentially also highlight minimally or noninvasive, easy-to-access biomarkers. MicroRNAs are dysregulated in AD, and may facilitate the early detection of the disease and potentially the continual monitoring of disease progression and allow therapeutic interventions to be evaluated. Four recent reviews have been published of microRNAs in AD, each of which identified areas of weakness or limitations in the reported studies. Importantly, studies in the last three years have shown considerable progress in overcoming some of these limitations and identifying specific microRNAs as biomarkers for AD and mild cognitive impairment. Further large-scale human studies are warranted with less disparity in the study populations, and using an appropriate method to validate the findings.
Keywords: Alzheimer’s disease, mild cognitive impairment, microRNAs, biomarkers, blood, brain tissue, cerebrospinal fluid, humans, animal models
Introduction
Alzheimer’s disease (AD) is the most common age-related, progressive neurodegenerative disease. It is characterized by memory loss and cognitive decline and accounts for most cases of dementia in the elderly. Currently more than 35 million people globally have AD and it is forecast to affect 1% of all people worldwide by 2050 (Brookmeyer et al., 2007; Querfurth and LaFerla, 2010; Thies and Bleiler, 2011). AD patients have been diagnosed as probable AD based on clinical examination, magnetic resonance imaging, positron emission tomography, cerebrospinal fluid (CSF) assays, and neuropsychological testing which includes cognitive performance (McKhann et al., 1984; McKhann et al., 2011). There are two major forms of AD, early-onset (familial) and late-onset (sporadic). Early-onset AD is rare, whereas late-onset AD accounts for > 95% of cases (Bali et al., 2012). Late-onset AD is defined as AD with age at onset > 65 years, while early-onset AD is AD with age at onset from 30 years to 65 years (Piaceri et al., 2013). Etiology involves a combination of genetic (70%) and environmental factors (30%) (Dorszewska et al., 2016). Many genes have been shown to be involved in the development of late-onset AD including ABCA7, APOE, BIN1 (Barber, 2012). At present there are no drugs or other therapeutic agents available to prevent or delay the progression of AD. No biomarkers have yet been confirmed for the early detection of AD before the onset of irreversible neurological damage (Reddy et al., 2017). Multiple cellular and molecular changes occur in the brains of individuals with AD. These include neuronal and synaptic loss, mitochondrial damage, production and accumulation of β-amyloid peptide (Aβ) and hyperphosphorylated tau protein, decrease of acetylcholine neurotransmitter, inflammation, and oxidative stress. Aggregation of Aβ peptide in extracellular plaques and the hyperphosphorylated tau protein in intracellular neurofibrillary tangles (NFTs) are characteristic of AD (Serrano-Pozo et al., 2011). MicroRNAs are small noncoding RNAs (~22 nucleotides) and involved in each of the cellular changes in AD. They act by binding to the 3′-untranslated region (3′-UTR) of their target mRNAs and interfere with gene regulation and translation, and cause mRNA destabilization or degradation (Guo et al., 2010; Reddy et al., 2017). They have been detected in blood, CSF, saliva and urine, and also in blood cells such as mononuclear cells and erythrocytes.
A major challenge is identifying molecular biomarkers characteristic of the early-stage AD in patients as most studies have been performed with blood or brain tissue samples (postmortem) at late-stage AD. Towards this end, subjects with mild cognitive impairment (MCI) almost always have the neuropathologic features of AD (Morris et al., 2001; Morris and Cummings, 2005; Garcia-Ptacek et al., 2016) and could provide important information. About 50% of MCI patients progress to AD (Sewell et al., 2010). Also the clinical-epidemiological relationship between AD and major depressive disorder (MDD) suggests they might have common neurobiological abnormalities (Rodrigues et al., 2014; Mendes-Silva et al., 2016). The pattern of microRNA regulation in each disorder could help with elucidating AD pathomechanism and also elucidate minimally or noninvasive, easy-to-access biomarkers.
The currently available biomarkers of AD are detected either by CSF analysis of Aβ and tau protein levels (Mattsson et al., 2009), brain imaging using positron emission tomography to detect Aβ deposits (Vlassenko et al., 2012), or postmortem gross specimen analysis and histology of brain sections (Braak et al., 2006). All of these are expensive, invasive, require skilled expertise to perform and interpret, or time-consuming (Lusardi et al., 2017), and only available in a small number of cases. The CSF levels of Aβ42 isoform and tau protein and particularly the ratio of tau/Aβ42 and phospho-tau/Aβ42 are useful for predicting the risk of progressing from MCI/very mild dementia to AD (Holzman, 2011; Fagan and Perrin, 2012) and have been used to identify MCI patients diagnosed with probable early AD (Najaraj et al., 2017). However, blood contamination occurs in up to 20% of CSF samples collected by lumbar puncture (Aasebø et al., 2014) and may be a confounding factor affecting Aβ42 levels (Bjerke et al., 2010). Also CSF collection by lumbar puncture may not be easily accomplished in elderly patients due to lumbar disc degeneration with narrowing of the intervertebral spaces.
Increasing evidence suggests that microRNAs are dysregulated in neurodegenerative diseases such as AD, Parkinson’s disease, and stroke. Circulating microRNAs within blood may be characterized and used as minimally invasive diagnostic biomarkers. They may facilitate the early detection of the disease and potentially the continual monitoring of disease progression and allow therapeutic interventions to be evaluated. Four recent reviews have been published on studies of microRNAs in AD. They were by Batistela et al. (2017), Mendez-Silva et al. (2016), Van Giau and An (2016), Wu et al. (2016) and, where reported, included studies up to June 2015 and September 2015. Each of the reviews identified areas of weakness or limitations in the reported studies (Table 1) and the findings were often conflicting. The limitations included small size and heterogeneity of study populations, no postmortem confirmation of AD diagnosis or assay of other biomarkers, variations in analytical methods and platforms used and validation of findings, different methods of normalization, statistical testing and data processing, selection of cognitively normal controls (NC) and no confirmation that they did not develop clinical cognitive impairment. Also Ren et al. (2016) indicated that in many of the previous studies on microRNAs in brain, CSF, and blood from AD patients, most samples were from patients being treated with acetylcholinesterase inhibitor, which could have a confounding effect on analysis, rather than de novo drug-naïve patients. Interestingly, differential correlation analysis using paired microRNAs detected different and more sensitive plasma microRNA biomarkers for MCI than were found by single molecule analysis using a t-test (Kayano et al., 2016). Plasma paired microRNA sets were shown to have high sensitivity and specificity for differentiating MCI from age-matched control, and also differentiated AD from age-matched control. In addition, the paired microRNA sets detected MCI in 7 of 10 elderly patients at asymptomatic stage 0.5–5 years preceding the clinical diagnosis (Sheinerman et al., 2012).
Table 1.
Limitations of previous studies of microRNAs in Alzheimer’s disease (AD) as indicated in four recent reviews

There is range of disease states from mild dementia to severe impairment within AD patients. The extent of cognitive decline is commonly estimated by the Mini-Mental State Examination (MMSE) and scores range from 0–30. Probable AD patients can be classified as having mild (MMSE scores 21–24), moderate (MMSE scores 10–20), or severe dementia (MMSE scores 0–9), while normal subjects have MMSE scores 25–30 (Galasko, 1998). The MMSE does not have sufficient sensitivity to detect cognitive decline at the MCI stage (MMSE mean score 27.0, reported by Nasreddine et al., 2005), and other cognitive assessment examinations have to be used. In the Montreal Cognitive Assessment (MoCA) examination, scores range from 0–30 and a score of ≥ 27 is considered normal. In a study by Nasreddine et al. (2005) normal subjects had a mean MoCA score of 27.4, MCI individuals a mean MoCA score 22.1 (range 17–23), and probable AD patients a mean MoCA score 16.2 (range 0–24). Identifying biomarkers capable of distinguishing MCI from the normal elderly and probable AD cases, as well the transition between the stages of mild, moderate, and severe cognitive impairment in AD, is an important need in indicating dysregulated signaling pathways and suggesting possible potential treatment strategies.
The goal is to identify microRNA biomarkers of high sensitivity and specificity in early-stage AD, and paired microRNA analysis or using a panel of microRNAs may be an important method to accomplish this. We have performed a PubMed literature search of articles published in the period January 2016–June 2018 on microRNAs in AD and also MCI when included as prodomal or early-stage AD. This is to provide further information on microRNA dysregulation in AD and to assess whether the weaknesses or limitations identified in previous studies have been taken into account or overcome.
Braak NFT staging of AD pathology
Histomicroscopic evaluation of AD-related pathology is based on the deposition of hyperphosphorylated tau protein within select neuronal types in specific nuclei or areas. The staging of AD-related neurofibrillary pathology using silver-stained sections 100 µm thickness has been described (Braak and Braak, 1991). A more recent description employs sections 5–15 µm thickness and immunostained for hyperphosphorylated tau protein with monoclonal antibody AT8 (Braak et al., 2006). Six stages can be recognized in the progression of AD and they are usually grouped under stages I–II, III–IV, V–VI with the major characteristics being as follows. In stage I, lesions develop in the transentorhinal region. Subcortical nuclei (viz. locus coeruleus, magnocellular nuclei of the basal forebrain) occasionally exhibit the earliest changes in the absence of cortical involvement. The transentorhinal region is the first site in the cerebral cortex to be involved. The entorhinal region proper is not or minimally involved. In stage II, lesions extend into the entorhinal region, while in stage III, lesions extend from the transentorhinal region to the neocortex of the fusiform and lingual gyri and then diminish markedy beyond this region. In stage IV, lesions progress more widely into neocortical association areas. Stage V is characterized by lesions appearing in previously uninvolved areas and extending widely into the first temporal convolution, and into high order association areas of frontal, parietal, and occipital neocortex (peristriate region). For stage VI, lesions reach the secondary and primary neocortical areas and extend into the striate region of the occipital lobe.
MicroRNAs in AD and MCI
A total of nineteen research articles were found in the PubMed search of which two had used blood, one PBMNs, four serum, four plasma, five CSF, two brain tissue, and one olfactory mucosa samples (Table 2). While most had used a validation method, which was usually real-time quantitative polymerase chain reaction (RT-qPCR), there were several studies where this was not performed. This is especially important as in many cases the validation findings were not in complete agrement with the screening results. Also when reported, all the studies had included both male and female subjects for AD, MCI and NC, but often there was a disparity in the proportions of the two genders in the different groups and also in their mean ages. In nearly all the studies there was no follow-up of MCI and NC individuals. Moreover, while several of the studies had involved quite large cohorts of AD and NC patients, the majority were performed with much smaller cohorts. Only a few studies had reported on ethnicity or medications being used by the patients. Inclusion and exclusion criteria were not reported in many of the studies. These are all important limitations as identified in the earlier reviews (Table 1) and many of these constitute confounding factors. Not all of the studies had used receiver operating characteristics (ROC) analysis with area under curve (AUC) values to establish which microRNAs are good or fair tests to distinguish AD from NC or MCI from NC patients.
Table 2.
MicroRNAs in human patients with Alzheimer’s disease (AD) or mild cognitive impairment (MCI)
The imporant findings from the research articles in the PubMed search are summarized as follows.
Blood samples
Chang et al. (2017) employed integrated analysis of microarray datasets of blood of AD and NC subjects and identified differentially expressed genes in AD that were regulated by miR-26b-5p, -103a-3p, -107, -26a-5p. However, using bloods from 3 AD and 3 NC patients, the upregulated expression of miR-26b-5p and miR-26a-5p by RT-qPCR was inconsistent with the integrated analysis results. In a study with large-sized groups, Keller et al. (2016) combined a screening/discovery set (USA) and a next-generation sequencing (NGS) validation study set (Germany, with 49 AD and 55 NC) to show upregulated expression of miR-151a-3p and downregulated expression of miR-17-3p for AD compared with NC subjects. ROC analysis indicated miR-151a-3p was a fair test to distinguish AD from NC (AUC 0.74). A marked upregulation of microRNAs was found for AD compared with MCI with miR-30c-5p having the lowest P value. This study had included Aβ42, t-tau and p-tau markers to aid confirmation of AD.
PBMN samples
By qPCR validation, Ren et al. (2016) showed miR-339 and miR-425 were upregulated and miR-639 was downregulated in AD PBMNs compared with NC PBMNs. ROC indicated miR-339 as a fair test to distinguish AD PBMNs from NC PBMNs (AUC 0.768), and miR-425 to be a good test to distinguish AD PBMNs from NC PBMNs (AUC 0.868). MiR-639 was a poor test to distinguish AD PBMNs from NC PBMNs (AUC 0.287). The β-amyloid precursor protein β-secretase BACE1 might be a target of miR-339 and miR-425.
Blood serum
Wu et al. (2017) using RT-qPCR validation with serum samples from 65 AD and 50 NC patients showed that expression levels of miR-146a-5p, -106b-3p, -195-5p, -20b-5p, -497-5p were higher, while those of miR-125b-3p, -29c-3p, -93-5p, -19b-3p were lower in AD than in NC subjects. Computational analysis predicted that 3’-UTR of STAT 3 mRNA was a target of both miR-29c-3p and miR-19b-3p. A large-scale study by Jia et al. (2016) with serum from 84 AD and 62 NC individuals used PCR screening to show a significant decrease in miR-29, -125b, -223 and an increase in miR-519 in AD compared with NC. Serum miR-223 was positively correlated with MMSE scores and might be a biomarker of AD severity. ROC indicated miR-223 to be a fair test to distinguish AD from NC (AUC 0.786). Also the combination miR-223 and miR-125b gave improved sensitivity/specificity (AUC 0.879) than miR-223 or miR-125b alone. Hara et al. (2017) found using a NGS validation set of serum samples from 35 AD and 22 NC patients that miR-501-3p was decreased in AD compared with NC and correlated with MMSE scores. In brains of patients in the screening/discovery set, miR-501-3p was markedly upregulated in AD compared with NC and positively correlated with disease progression as indicated by Braak staging. ROC showed serum miR-501-3p was a good test to distinguish AD from NC (AUC 0.82, sensitivity 0.53, specificity 1.00). A smaller-scale study by Kumar et al. (2017) with serum from 12 AD, 20 MCI and 18 NC subjects using RT-qPCR validation showed a significant upregulation of miR-455-3p in AD compared with MCI and NC. Upregulation of miR-4668-5p occurred for MCI compared with NC but not AD. Using frontal cortex of brain postmortem samples, a significant upregulation of miR-455-3p and miR-3613-3p was observed for AD Braak stage V compared with NC, and also of miR-4674 in AD Braak stage VI compared with NC. ROC indicated serum miR-455-3p to be a fair test to distinguish AD from NC (AUC 0.79), and miR-455-3p in postmortem brain to be a good test to distinguish AD from NC (AUC 0.86).
Blood plasma
Zirnheld et al. (2016) analysed blood plasma from 20 moderate-severe AD, 16 mild AD, 34 MCI and 37 NC subjects by RT-qPCR validation and found miR-34c was more highly expressed in moderate-severe and mild AD than in MCI or NC, but with no significant difference beween moderate-severe and mild AD or between MCI and NC. In addition, miR-181c was more abundant in moderate-severe and mild AD than MCI or NC, but no difference between moderate-severe and mild AD or between MCI and NC. MiR-411 was more highly expressed in moderate-severe and mild AD than in MCI or NC, but no difference between moderate-severe and mild AD or between MCI and NC. ROC indicated miR-34c to be a fair test to differentiate moderate-severe AD from MCI (AUC 0.78), a good test to distinguish moderate-severe AD from NC (AUC 0.82), and a poor test to distingush MCI from NC (AUC 0.68). Similarly, miR-181c differentiated moderate-severe AD from both MCI and NC (AUC 0.86, 0.79, respectively) but was a poor test to distingiush between mild AD and MCI or NC (AUC 0.63, 0.60, respectively). MiR-411 was a good test to distinguish moderate-severe AD from MCI and NC (AUC 0.92, 0.99, respectively, and sensitivity 0.77, 0.77 respectively) and for distinguishing mild AD from MCI and NC (AUC 0.93, 0.98, respectively, and sensitivity 0.79, 0.93, respectively). Hence miR-411 was a more powerful biomarker than miR-34c or miR-181c to distinguish MCI from mild, moderate and severe AD. Kayano et al. (2016) used RT-qPCR screening of microRNAs in blood plasma of 23 MCI and 30 NC subjects. Differential corelation analysis identified 20 pairs of microRNAs that distinguish MCI from NC. ROC showed that two pairs miR-191/miR-101 and miR-103/miR-222 had the highest AUC 0.962, and were good tests to distinguish MCI from NC. Other microRNA pairs that included miR-191 and miR-125b and miR-590-5p also had high AUC > 0.95. Differential correlation analysis detected much different and more sensitive biomarkers for MCI than t-test (mean AUC 0.800). Nagaraj et al. (2017) performed RT-qPCR validation with blood plasma from 20 AD, 15 MCI and 15 NC and found that increased levels of miR-486-5p and miR-483-5p were the most significant indicators of MCI and AD. In addition, there was upregulation of miR-502-3p and miR-200a-3p in MCI and AD compared with NC. ROC indicated that miR-483-5p and miR-502-3p were good tests to distinguish AD from NC, and MCI from NC (AUC > 0.9, specificity and sensitivity > 0.80). A set of 6 microRNAs was selected for distingushing MCI from NC and comprised 4 upregulated microRNAs (483-5p, 486-5p, 200a-3p, 502-3p) and 2 downregulated microRNAs (30b-5p, 142-3p). For MCI and AD patients, diagnoses were confirmed by CSF biomarkers. Mendes-Silva et al. (2017) performed microRNA screening of blood plasma from 13 MDD, 11 MDD with MCI and 19 NC individuals using ion proton sequencing. Ten microRNAs were differentially expressed between MDD and NC, among which miR-184 and miR-1-3p were differentially expressed between MDD and MDD with MCI.
CSF
Lusardi et al. (2017) performed RT-qPCR validation of microRNAs in CSF from 50 AD and 49 NC subjects and found that replicated microRNAs increased in AD were miR-378a-3p, -1291, -597-5p, and those decreased in AD were miR-143-3p, -142-3p, -328-3p,-193a-5p, -30a-3p, -19b-3p, -30d-5p, -340-5p, -140-5p, -125b-5p, -26b-5p, -16-5p, -146a-5p, -29a-3p, -195-5p, -15b-5p, -223-3p. Candidate miRNAs not retested increased in AD were miR-520b, -603, -202-3p, -519b-3p, -484, while those decreased in AD were miR-584-5p, -145-3p, -24-3p, -532-5p, -28-3p, -146b-5p, -27b-3p, -331-3p, -145-5p, 590-5p, -365a-3p. ROC indicated that the top performing linear combinations of 3 or 4 microRNAs were a fair test to distinguish AD from NC (AUC 0.80–0.82). Muller et al. (2016b) also used large group sizes of 57 AD, 37 MCI and 40 NC individuals to perform qPCR screening of microRNAs in CSF samples collected at three different centers. Importantly, no differences in microRNAs levels were found between AD, MCI and NC after correcting for confounding factors that included age, gender, sample storage time, centrifugation status. In a separate study, Muller et al. (2016a) showed using qPCR screening of microRNAs in CSF samples from 18 AD and 20 NC subjects with low concentrations of erythrocytes and leukocytes that miR-29a was upregulated in AD compared with NC and differentiated AD from NC with a sensitivity of 0.78 and specificity of 0.60. Riancho et al. (2017) performed RT-qPCR validation of microRNAs in raw and exosome-enriched CSF samples from 28 AD and 28 NC individuals. AD patients had clinical presentation consistent with AD and positive CSF biomarkers Aβ and tau for AD. NC subjects had no memory impairments and negative CSF biomarkers Aβ and tau for AD. MiR-9-5p was not detected in AD but was present in 50% of NC samples. Similarly, miR-598 was absent in all AD but present in 72% of NC samples. Liu et al. (2018) collected CSF from 17 AD, 36 MCI and 41 SMC (subjective memory complaints) patients and which was centrifuged and cell pellet sorted into lymphocyte populations by flow cytometry. RT-PCR screening of microRNAs showed the relative expression of let-7b in CSF cells was significantly increased in AD and MCI compared with SMC. Total number of CSF lymphocytes and ratio of CD4+ lymphocytes in AD and MCI were significantly higher than in SMC. Let-7b expression in CD4+ lymphocytes from AD and MCI was significantly higher than SMC, while let-7b expression in CD8+ T lymphocytes, B lymphocytes and NK cells were not significantly different. Adding let-7b to Aβ40 and Aβ42 as predictive parameters increased the probability of predicting AD to 89.7% (AUC 0.93).
Brain tissue and olfactory mucosa
Kumar and Reddy (2018) using RT-PCR screening of microRNAs in frontal cortex Broadmann’s Area 10 of postmortem brains of 27 AD and 15 NC individuals found that the expression of miR-455-3p was significantly increased in AD compared with NC. Also using banked skin fibroblasts and lymphocytes from AD and NC individuals, expression of miR-455-3p was significantly higher for AD compared with NC. ROC indicated that frontal cortex miR-455-3p was a fair test to distinguish AD from NC (AUC 0.792, sensitivity 0.889, specificity 0.667). Weinberg et al. (2015) performed qPCR validation of microRNAs in frontal and inferior temporal cortex of postmortem brains of 10 AD, 10 MCI and 12 NC subjects. In frontal cortex, miR-498 and miR-150 were significantly upregulated in AD, and miR-150 was upregulated in MCI, compared with NC. Several microRNAs were significantly downregulated in AD including miR-886-3p, -132, -21, -23a, -140-3p, -212, -23b, let-7d, -181a, -498, -150. Two distinct clusters miR-212/miR-132 and miR-23a/miR-23b were significantly downregulated in MCI. For temporal cortex, miR-212 and miR-132 expression levels were decreased only in AD, with miR-23a and miR-23b being unchanged across the groups. Moon et al. (2016) used RT-PCR screening of microRNAs in olfactory mucosa of subjects categorized on clinical dementia rating (CDR) score, ADAS-Cog-K score and Beck Depression Inventory II score. The groupings were 11 CDR 1, 13 CDR 0.5, and 9 CDR 0. Olfactory mucosa miR-206 was increased in CDR 0.5 and CDR 1 compared with CDR 0 and increased with progression of dementia. ROC indicated miR-206 was a good test to distinguish CDR 0.5 from CDR 0 (AUC 0.942, sensitivity 0.875, specificity 0.942). Also it was a good test to distinguish CDR 1 from CDR 0 (AUC 0.976, sensitivity 0.909, specificity 0.933).
The microRNAs indicated to have upregulated or downregulated expression in blood serum, blood plasma, CSF of AD and MCI patients are shown in Figure 1.
Figure 1.
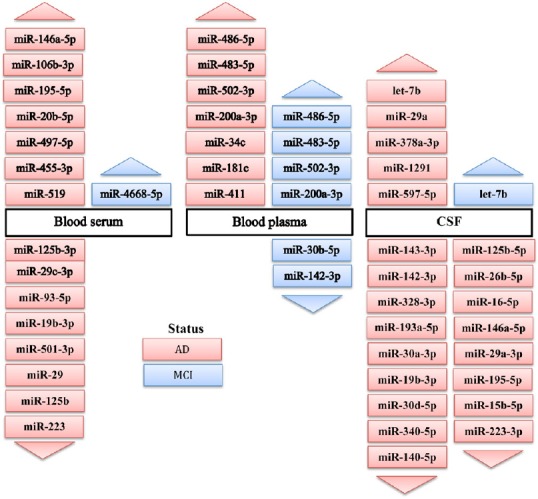
Changes in microRNA expression in blood serum, blood plasma, and cerebrospinal fluid (CSF) of Alzheimer’s disease (AD) and mild cognitive impairment (MCI) patients compared to normal controls as indicated by polymerase chain reaction/real-time polymerase chain reaction (PCR/RT-PCR) screening and validation assays in the articles reviewed.
Increased or decreased expression is indicated by arrows pointing upwards or downwards, respectively. Color code is AD (red) and MCI (blue).
Future Perspectives
The development of reliable, minimally or non-invasive methods for diagnosing early AD in MCI patients is imperative for increased efficiency of existing therapies, as well as for recruiting patients for clinical trials of new drugs, monitoring disease progression and response to treatment. At present the most reliable validation of blood or CSF microRNAs as biomarkers for AD is by histopathologic examination of postmortem brain sections. No methods are available to evaluate the expression of a specific microRNA in the brain of a living person. The olfactory epithelium has been shown to reflect brain Aβ and tau pathology in the majority of cases with pathologically verified AD (Arnold et al., 2010). Measurement of microRNAs in olfactory epithelium could be performed in patients with CSF levels of Aβ and tau indicative of AD as a possible way of validating changes in blood microRNA levels. In this regard, the olfactory epithelium miR-206 level was significantly correlated with cognitive assessment score in non-depressed subjects with cognitive impairment and could serve as a diagnostic marker of early AD (Moon et al., 2016).
Histomicroscopic examination of brain tissue specimens enables classification into early-, mid- and late-stages of AD. Brains stored in brain banks could be used for this purpose and blood collected from patients shortly before their death could be examined at early-stage AD (Braak NFT stages I and II) for diagnostic biomarkers. In addition progression of the disease could be monitored through biomarker analysis using blood samples from patients at mid-stage AD (Braak NFT stages III and IV) and late-stage AD (Braak NFT stages V and VI). Kumar et al. (2017) showed a gradual increase or decrease in several chosen serum microRNAs for controls, MCI and AD patients and could be used to monitor disease progression to AD. Also Hara et al. (2017) found in brains of patients in the screening/discovery set, miR-501-3p was markedly upregulated in AD compared with NC and positively correlated with disease progression as indicated by Braak staging. Keller et al. (2016) replicated and validated previous findings in a US study of blood microRNAs in AD patients compared to controls with a new cohort of subjects in Germany using next-generation sequencing. Included in the validation cohort were MCI patients and a substantial dysregulation of microRNAs in AD patients was found compared to MCI with 119 being upregulated and 29 downregulated in AD compared to MCI. Upregulated expression of miR-151a-3p and downregulated expression of miR-17-3p was found for AD compared with NC. In addition, blood samples of AD patients with disease duration < 2 years (early-stage) were compared to those of patients with longer disease duration (mid-/late-stage) and no significant differences in microRNAs were observed. (For time frame of the stages in AD progression see Ellis and Higuera, 2016). These findings warant further replication using larger-sized cohorts and RT-qPCR validation.
Several of the reviewed studies showed that microRNAs could distinguish MCI from NC subjects. For example, Kumar et al. (2017) observed an upregulation of miR-4668-5p in blood serum for MCI compared with NC but not AD patients. Kayano et al. (2016) using differential corelation analysis identified 20 pairs of microRNAs in blood plasma that distinguish MCI from NC, and these included the two pairs miR-191/miR-101 and miR-103/miR-222. Differential correlation analysis detected much different and more sensitive MCI biomarkers compared to t-test. Also the highest AUC value of any four microRNAs was less than the highest in the two-pair approach. Nagaraj et al. (2017) reported a set of 6 microRNAs in blood plasma that distingushed MCI from NC, and which was comprised of 4 upregulated microRNAs (483-5p, 486-5p, 200a-3p, 502-3p) and 2 downregulated microRNAs (30b-5p, 142-3p). Liu et al. (2018) showed that let-7b expression in CD4+ lymphocytes isolated from CSF cell pellets of AD and MCI individuals was significantly higher than for SMC (subjective memory complaints) patients.
It is recognized that MCI patients are the most promising group of patients for whom therapy could be initiated to delay the onset of AD (Michael-Titus et al., 2010). Identification of microRNAs that differentiate MCI or AD from NC with high sensitivity and specificity also provides a feasible therapeutic strategy. MicroRNA mimics or agomirs could be administered to increase the levels of specific microRNAs that are downregulated, while microRNA inhibitors or antagomirs could be given to lower the levels of those microRNAs that are upregulated, in MCI or AD compared to NC. These agents should be tested in future clinical trials. An increased risk of developing AD has been reported for females compared with males at age of 65–75 years (Neu et al., 2017). Many of the studies presented in this review had considerable disparity in the numbers and proportions of male and female subjects in the AD, MCI and NC groups, as well as significant variation in age and extent of disease progression.
A panel of microRNAs has a higher AUC value than a single microRNA for distinguishing AD from NC (Jia et al., 2016). Nagaraj et al. (2017) reported a 6 microRNA panel in blood plasma which distinguished non-demented control subjects from MCI patients diagnosed with probable early AD by CSF assay of Aβ42, total tau and phosphorylated tau. These 6 microRNAs were functionally mapped to proteins involved in AD pathology by searches in databases containing predictive and validated microRNA targets including β-secretase 1 (BACE1), BACE2, microtubule-associated protein/tau, and presenilin 2 (PSEN2). It was suggested that the 6 microRNA panel might serve as a possible replacement of invasive CSF biomarkers to identify early AD (Najaraj et al., 2017). Such a microRNA panel together with correlation analysis of specific paired microRNAs would provide a very good test for differentiaing early AD from NC. The amyloid precursor protein (APP) cleavage enzyme BACE1 was also a possible direct target of miR-425-5p and miR-339-5p which were upregulated in PBMNs from AD compared to NC group. Overexpression of miR-425-5p decreased BACE1 protein levels (Ren et al. 2016).
A number of differentially expressed genes were found to be regulated by microRNAs. These included PLCB2, CDK5R1, LRP1, NDUFA4, DLG4 which were regulated by miR-26b-5p, -103a-3p, -107. -26a-5p and these microRNAs had increased expression levels in blood samples of AD compared with NC (Chang et al., 2017). Also 14 validated target genes were found of at least 5 of 33 microRNAs overalapping in screening and validation studies of blood samples of AD compared with NC and were VEGFA, DICER1, AGO1, PTEN, CDKN1A, APP, RBI, CCND1, CCND2, WEE1, IL13, HMGA2, TNFRSF1OB, MYC (Keller et al., 2016). Many of these have key roles in AD and microRNAs might regulate the genes involved in signaling pathways. For example, low serum levels of vascular endothelial growth factor (VEGF) were associated with AD (Mateo et al., 2007) and VEGF was found to be expressed in the brains of AD patients and to increase with AD severity (Thomas et al., 2015). Also the tumor-suppressor phosphatase and tensin homolog (PTEN) was found to accumulate in NFTs (Sonoda et al., 2010). PTEN affects tau phosphorylation, binding to microtubules and formation of aggregates and neurite outgrowth (Zhang et al., 2006). PTEN is a negative regulator of PI3 kinase and the predominant effects on tau appeared to be medited by reducing ERK1/2 activity (Kerr et al., 2006). Furthermore, the expression of miR-455-3p was upregulated in blood serum of AD compared to NC and shown to have a relationship with 11 biological pathways and associated genes. The most important signaling pathways were extracellular matrix (ECM)-receptor interaction, adherence junction, transforming growth factor-β (TGF-β) signaling pathway, hippo signaling pathway, cell cycle pathway, and the regulation of the actin cytoskeleton. The upregulation of miR-455-3p in AD development might be associated with these signaling pathways and through altered expression of HSPG2, THBS1, COL3A1, COL6A1, TNC, MYC, Smad2, RAN, PLK1, TP73, ACTN1 and IQGAP1 genes (Kumar et al., 2017). Computational analysis predicted that the 3’-UTR of signal transduction and activator of transcription 3 (STAT3) mRNA to be a target of miR-29c-3p and miR-19b-3p, both of which had lower serum expression levels in AD than in NC subjects. A regulatory network of microRNAs and target genes was identified and contained miR-29c-3p and miR-19b-3p, 4 AD virulence genes, and STAT3 (Wu et al., 2017). Several studies have suggested that STAT3 activation can promote glial differentiation from neural progenitor cells and inhibit neuronal differentiation of neural progenitor cells (Choi et al., 2003; Sriram et al., 2004; Okada et al., 2006). STAT3 can cause excessive gliosis (Kwak et al., 2010; Tsuda et al., 2011) which is often found in AD patients. Interestingly, miR-501-3p had lower serum expression level in AD compared to NC but was upregulated in the brains of AD patients (Hara et al., 2017). It is possible that miR-501-3p upregulation could cause alterations in the cell cyle of AD brains. Inappropriate cell cycle re-entry in postmitotic neurons, which leads to apoptotic death, is an early sign that preceeds the formation of amyloid plaques and NFTs (Kruman et al., 2004; Borda et al., 2010; Swerdlow, 2012). Alternatively, miR-501-3p could mediate the activity-dependent regulation of the AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazole-propionate) receptor subunit GluA1 in dendrites, suggesting that it contributes to synaptic plasticity related to cognitive functions, including learning and memory (Hu et al., 2015). In the differential correlation analysis study, miR-125b was one of the plasma microRNAs found in several pairs having a high AUC value (≥ 0.95) for MCI detection (Keller et al., 2016). Also miR-125b was dowregulated in serum of AD compared to NC (Wu et al., 2017). MiR-125b was shown to bind to 3′-UTR of p53 mRNA and worked as a negative regulator of p53 (Le et al., 2009). p53 has been explored as a tumor supressor but other aspects have been reported including to control diseases, aging and metabolism (Vousden and Lane, 2007). p53 promotes apoptosis and has important implications for the brain during neurodegeneration in AD (Perluigi et al., 2016). MAPK, TGF-β and neurotrophin signaling pathways were indicated as characteristic in MCI. Similarly to p53 signaling, these pathways have common biological functions such as cell survival, cell cycle and apoptosis (Kayano et al., 2016). MAPK pathway is also known to be involved in aberrant cellular signaling in AD pathology (Schnoder et al., 2016). Finally, animal models of AD and MCI have been described and could be used to examine and verify microRNA profiles in blood, blood serum, blood plasma and brain tissues and to trial microRNA-based therapies. Transgenic mice overproducing mutant APP were found to develop extracellular Aβ plaques which was age-dependent, only occurring in mid to late adulthood in most of the animals (LaFeria and Green, 2012). While these mice did not develop NFTs, many exhibited increased tau hyperphosphorylation (Gotz J et al., 2007). These animals develop marked cognitive decline and undergo subtle alterations in tau that resemble the precursors to NFTs in the human brain (LaFeria and Green, 2012). Transgenic mice that express further gene alterations in addition to mutated APP such as mutated human tau (Lewis et al., 2001; Oddo et al., 2003) or removal of nitric oxide synthase 2 (Wilcock et al., 2008) develop NFTs similar to those in the human AD brain. One of the main considerations is that AD mouse models do not show the extensive neuronal loss found in the brains of AD patients. Most of the AD patients at clinical diagnosis already have reached Braak stage V or VI with marked synaptic and neuronal loss. Moreover, the loss of synapses is the best correlate of cognitive impairment in patients with AD (DeKosky et al., 1996). The synapse loss, which occurs before neuronal death in humans, is present in most of the mouse models and gives rise to the memory deficits seen in behavioral tasks for testing memory function. The memory deficits can be associated with neuropathological alterations (Pepeu, 2004). Hence, therapies for reversing memory deficits in AD mouse models might aid in treating the memory decline in patients with MCI (Cuadrado-Tejedor and Garcia-Osta, 2014). The appearance of amyloid plaques and synapse loss appears in some mouse AD models even at 2 to 4 months of age. Aging is the most important risk factor for AD and despite being such an important risk factor it is often absent in studies with animal models. Therefore the use of late-plaque models for preclinical studies (e.g., Tg2576, PDAPP, TgAPP23) could be more appropriate than using early-plaque models (Lee and Han, 2013). In addition, late-plaque and early-plaque models may provide complementary data necessary to decipher the role of microRNAs as diagnostic and therapeutic tools for AD. In summary, considerable advances have been made in the recent studies included in this review with regard to distinguishing MCI and AD from NC by analyzing microRNAs in blood serum, blood plasma and CSF (Figure 1), and have included individual and combinations of microRNAs as well as differential correlation of paired microRNA testing. Limitations that were identified in previous studies (Table 1) included small group sizes, and marked disparity of individuals in the AD, MCI and NC groups including age, gender, number, ethnicity, stage of disease progression, screening and validation methods, data processing and normalization, statistical analysis. These have been taken into consideration in many of these recent studies, but some concerns still remain regarding recruitment of patients including numbers, gender, inclusion and exclusion criteria, medications taken by the patients, most appropriate validation methods, normalization and statistical analysis of data. It is hoped that future studies will continue to address these concerns in the planning and implementing of such studies so that a sensitive and specific, minimally invasive test can be developed for identifying patients with MCI (early AD) and therapy initiated to slow the memory decline and progression to AD.
Additional file: Open peer review report 1 (99.2KB, pdf) .
Footnotes
Conflicts of interest: None declared.
Financial support: None.
Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Alain Buisson, Université Joseph Fourier Grenoble, France.
P-Reviewer: Buisso A; C-Editors: Zhao M, Yu J; T-Editor: Liu XL
References
- 1.Aasebø E, Opsahl JA, Bjørlykke Y, Myhr KM, Kroksveen AC, Berven FS. Effects of blood contamination and the rostro-caudal gradient on the human cerebrospinal fluid proteome. PLoS One. 2014;9:e90429. doi: 10.1371/journal.pone.0090429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnold SE, Lee EB, Moberg PJ, Stutzbach L, Kazi H, Han LY, Lee VM, Trojanowski JQ. Olfactory epithelium amyloid-beta and paired helical filament-tau pathology in Alzheimer disease. Ann Neurol. 2010;67:462–469. doi: 10.1002/ana.21910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bali J, Gheinani HA, Zurbriggen S, Rajendran L. Role of genes linked to sporadic Alzheimer's disease risk in the production of β-amyloid peptides. Proc Nat Acad Sci U S A. 2012;109:15307–15311. doi: 10.1073/pnas.1201632109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barber RC. The genetics of Alzheimer's disease. Scientifica 2012. 2012:246210. doi: 10.6064/2012/246210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Batistela MS, Josviak ND, Sulzbach CD, de Souza RL. An overview of circulating cell-free microRNAs as putative biomarkers in Alzheimer's and Parkinson's diseases. Int J Neurosci. 2017;127:547–558. doi: 10.1080/00207454.2016.1209754. [DOI] [PubMed] [Google Scholar]
- 6.Bjerke M, Portelius E, Minthon L, Wallin A, Anckarsäter H, Anckarsäter R, Andreasen N, Zetterberg H, Andreasson U, Blennow K. Confounding factors influencing amyloid Beta concentration in cerebrospinal fluid. Int J Alzheimers Dis 2010. 2010:986310. doi: 10.4061/2010/986310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borda DJ, Lee HP, Kudo W, Zhu X, Smith MA, Lee HG. Pathological implications of cell cycle re-entry in Alzheimer disease. Expert Rev Mol Med. 2010;12:e19. doi: 10.1017/S146239941000150X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 9.Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112:389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer's disease. Alzheimers Dement. 2007;3:186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 11.Chang WS, Wang YH, Zhu XT, Wu CJ. Genome-wide profiling of miRNA and mRNA expression in Alzheimer's disease. Med Sci Monit. 2017;23:2721–2731. doi: 10.12659/MSM.905064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi JS, Kim SY, Cha JH, Choi YS, Sung KW, Oh ST, Kim ON, Chung JW, Chun MH, Lee SB, Lee MY. Upregulation of gp130 and STAT3 activation in the rat hippocampus following transient forebrain ischemia. Glia. 2003;41:237–246. doi: 10.1002/glia.10186. [DOI] [PubMed] [Google Scholar]
- 13.Cuadrado-Tejedor, Garcia-Osta A. Curent animal models of Alzheimer's disease: challenges in translational research. Front Neurol. 2014;5:182. doi: 10.3389/fneur.2014.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeKosky ST, Scheff SW, Styren SD. Structural correlates of cognition in dementia: quantification and assessment of synapse change. Neurodegeneration. 1996;5:417–421. doi: 10.1006/neur.1996.0056. [DOI] [PubMed] [Google Scholar]
- 15.Dorszewska J, Prendecki M, Oczkowska A, Dezor M, Kozubski W. Molecular basis of familial and sporadic Alzheimer's disease. Curr Alzheimer Res. 2016;13:952–963. doi: 10.2174/1567205013666160314150501. [DOI] [PubMed] [Google Scholar]
- 16.Ellis ME, Higuera V. What are the stages of Alzheimer's disease? 2016 [Google Scholar]
- 17. [Accessed 16 June 2018]. www.healthline.com/health/stages-progression-alzheimers .
- 18.Fagan AM, Perrin RJ. Upcoming candidate cerebrospinal fluid biomarkers of Alzheimer's disease. Biomark Med. 2012;6:455–476. doi: 10.2217/bmm.12.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galasko D. An integrated approach to the management of Alzheimer's disease: assessing cognition, function and behavior. Eur J Neurol. 1998;5:S9–S17. [Google Scholar]
- 20.Garcia-Ptacek S, Eriksdotter M, Jelic V, Porta-Etessam J, Kåreholt I, Manzano Palomo S. Subjective cognittive impairment: towards early identification of Alzheimer's disease. Neurologia. 2016;31:562–571. doi: 10.1016/j.nrl.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 21.Götz J, Deters N, Doldissen A, Bokhari L, Ke Y, Wiesner A, Schonrock N, Ittner LM. A decade of tau transgenic animal models and beyond. Brain Pathol. 2007;17:91–103. doi: 10.1111/j.1750-3639.2007.00051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo H, Ingolia NT, Weissman JS, Bartel DP. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010;466:835–840. doi: 10.1038/nature09267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hara N, Kikuchi M, Miyashita A, Hatsuta H, Saito Y, Kasuga K, Murayama S, Ikeuchi T, Kuwano R. Serum microRNA miR-501-3p as a potential biomarker related to the progression of Alzheimer's disease. Acta Neuropathol Commun. 2017;5:10. doi: 10.1186/s40478-017-0414-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holzman DM. CSF biomarkers for Alzheimer's disease: current utility and potential future use. Neurobiol Aging. 2011;32:S4–S9. doi: 10.1016/j.neurobiolaging.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu Z, Zhao J, Hu T, Luo Y, Zhu J, Li Z. miR-501-3p mediates the activity-dependent regulation of the expression of AMPA receptor subunit GluA1. J Cell Biol. 2015;208:949–959. doi: 10.1083/jcb.201404092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jia LH, Liu YN. Downregulated serum miR-223 serves as biomarker in Alzheimer's disease. Cell Biochem Funct. 2016;34:233–237. doi: 10.1002/cbf.3184. [DOI] [PubMed] [Google Scholar]
- 27.Kayano M, Higaki S, Satoh JI, Matsumoto K, Matsubara E, Takikawa O, Niida S. Plasma microRNA biomarker detection for mild cognitive impairment using differential correlation analysis. Biomark Res. 2016;4:22. doi: 10.1186/s40364-016-0076-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keller A, Backes C, Haas J, Leidinger P, Maetzler W, Deuschle C, Berg D, Ruschil C, Galata V, Ruprecht K, Stähler C, Würstle M, Sickert D, Gogol M, Meder B, Meese E. Validating Alzheimer's disease micro RNAs using next-generation sequencing. Alzheimers Dement. 2016;12:565–576. doi: 10.1016/j.jalz.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 29.Kerr F, Rickle A, Nayeem N, Brandner S, Cowburn RF, Lovestone S. PTEN, a negative regulator of PI3 kinase signalling, alters tau phophorylation in cells by mechanisms independent of GSK-3. FEBS Lett. 2006;580:3121–3128. doi: 10.1016/j.febslet.2006.04.064. [DOI] [PubMed] [Google Scholar]
- 30.Kruman II, Wersto RP, Cardozo-Pelaez F, Smilenov L, Chan SL, Chrest FJ, Emokpae R, Jr, Gorospe M, Mattson MP. Cell cycle activation linked to neuronal cell death initiated by DNA damage. Neuron. 2004;41:549–561. doi: 10.1016/s0896-6273(04)00017-0. [DOI] [PubMed] [Google Scholar]
- 31.Kumar S, Vijayan M, Reddy PH. MicroRNA-455-3p as a potential peripheral biomarker for Alzheimer's disease. Hum Mol Genet. 2017;26:3808–3822. doi: 10.1093/hmg/ddx267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar S, Reddy PH. MicroRNA-455-3p as a potential biomarker for Alzheimer's disease: An update. Front Aging Neurosci. 2018;10:41. doi: 10.3389/fnagi.2018.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwak YD, Dantuma E, Merchant SM, Bushnev S, Sugaya K. Amyloid-βprecursor protein induces glial differentiation of neural progenitor cells by activation of the IL-6/gp130 signaling pathway. Neurotox Res. 2010;18:328–338. doi: 10.1007/s12640-010-9170-6. [DOI] [PubMed] [Google Scholar]
- 34.LaFeria FM, Green KN. Animal models of Alzheimer disease. Cold Spring Harb Pespect Med. 2012;2:a006320. doi: 10.1101/cshperspect.a006320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le MT, Teh C, Shyh-Chang N, Xie H, Zhou B, Korzh V, Lodish HF, Lim B. MicroRNA-125b is a novel negative regulator of p53. Genes Dev. 2009;23:862–876. doi: 10.1101/gad.1767609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee JE, Han PL. An update of animal models of Alzheimer disease with a reevaluation of plaque depositions. Exp Neurobiol. 2013;2:84–95. doi: 10.5607/en.2013.22.2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lewis J, Dickson DW, Lin WL, Chisholm L, Corral A, Jones G, Yen SH, Sahara N, Skipper L, Yager D, Eckman C, Hardy J, Hutton M, McGowan E. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science. 2001;293:1487–1491. doi: 10.1126/science.1058189. [DOI] [PubMed] [Google Scholar]
- 38.Liu Y, He X, Li Y, Wang T. Cerebrospinal fluid CD4+T lymphocytes-derived miRNA-let-7b can enhances the diagnostic performance of Alzheimer's disease biomarkers. Biochem Biophys Res Commun. 2018;495:1144–1150. doi: 10.1016/j.bbrc.2017.11.122. [DOI] [PubMed] [Google Scholar]
- 39.Lusardi TA, Phillips JI, Wiedrick JT, Harrington CA, Lind B, Lapidus JA, Quinn JF, Saugstad JA. MicroRNAs in human cerebrospinal fluid as biomarkers for Alzheimer's disease. J Alzheimers Dis. 2017;55:1223–1233. doi: 10.3233/JAD-160835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mateo I, Llorca J, Infante J, Rodriguez-Rodriguez E, Fernandez-Vladero C, Pefia N, Berciano J, Combarros O. Low serum VEGF levels are associated with Alzheimer's disease. Acta Neurol Scand. 2007;116:56–58. doi: 10.1111/j.1600-0404.2006.00775.x. [DOI] [PubMed] [Google Scholar]
- 41.Mattsson N, Zetterberg H, Hansson O, Andreasen N, Parnetti L, Jonsson M, Herukka SK, van der Flier WM, Blankenstein MA, Ewers M, Rich K, Kaiser E, Verbeek M, Tsolaki M, Mulugeta E, Rosén E, Aarsland D, Visser PJ, Schröder J, Marcusson J, de Leon M, Hampel H, Scheltens P, Pirttilä T, Wallin A, Jönhagen ME, Minthon L, Winblad B, Blennow K. CSF biomarkers and incident Alzheimer's disease in patients with mild cognitive impairment. JAMA. 2009;302:385–393. doi: 10.1001/jama.2009.1064. [DOI] [PubMed] [Google Scholar]
- 42.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NICDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 43.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging: Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mendes-Silva AP, Pereira KS, Tolentino-Araujo GT, Nicolau Ede S, Silva-Ferreira CM, Teixeira AL, Diniz BS. Shared biologic pathways between Alzheimer's disease and major depression: A systematic review of microRNA expression studies. Am J Geriatr Psychiatry. 2016;24:903–912. doi: 10.1016/j.jagp.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 45.Mendes-Silva AP, Diniz BS, Araujo GT, de Souza Nicolau E, Pereira KS, Ferreira CM, Barroso LS. MiRNAs and their role in the correlation between major depressive disorder, mild cognitive impairment and Alzheimer's disease. Alzheimers Dement. 2017;13(Suppl):P1017–P1018. [Google Scholar]
- 46.Michael-Titus A, Revest P, Shortland P. The Nervous System. 2nd ed. Churchill Livingstone; 2010. Dementia; pp. 251–266. [Google Scholar]
- 47.Moon J, Lee ST, Kong IG, Byun JI, Sunwoo JS, Shin JW, Shim JY, Park JH, Jeon D, Jung KH, Jung KY, Kim DY, Lee SK, Kim M, Chu K. Early diagnosis of Alzheimer's disease from elevated olfactory mucosal miR-206 level. Sci Rep. 2016;6:20364. doi: 10.1038/srep20364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morris JC, Storandt M, Miller JP, McKeel DW, Price JL, Rubin EH, Berg L. Mild cognitive impairment represents early-stage Alzheimer's disease. Arch Neurol. 2001;58:397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- 49.Morris JC, Cummings J. Mild cognitive impairment (MCI) represents early-stage Alzheimer's disease. J Alzheimers Dis. 2005;7:235–239. doi: 10.3233/jad-2005-7306. [DOI] [PubMed] [Google Scholar]
- 50.Müller M, Jäkel L, Bruinsma IB, Claassen JA, Kuiperij HB, Verbeek MM. MicroRNA-29a is a candidate biomarker for Alzheimer's disease in cell-free cerebrospinal fluid. Mol Neurobiol. 2016a;53:2894–2899. doi: 10.1007/s12035-015-9156-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Müller M, Kuiperij HB, Versleijen AA, Chiasserini D, Farotti L, Baschieri F, Parnetti L, Struyfs H, De Roeck N, Luyckx J, Engelborghs S, Claassen JA, Verbeek MM. Validation of microRNAs in cerebrospinal fluid as biomarkers for different forms of dementia in a multicenter study. J Alzheimers Dis. 2016b;52:1321–1333. doi: 10.3233/JAD-160038. [DOI] [PubMed] [Google Scholar]
- 52.Najaraj S, Laskowska-Kaszub K, Dębski KJ, Wojsiat J, Dąbrowski M, Gabryelewicz T, Kuźnicki J, Wojda U. Profile of 6 microRNA in blood plasma distinguish early stage Alzheimer's disease patients from non-demented subjects. Oncotarget. 2017;8:16122–16143. doi: 10.18632/oncotarget.15109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 54.Neu SC, Pa J, Kukull W, Beekly D, Kuzma A, Gangadharan P, Wang LS, Romero K, Arneric SP, Redolfi A, Orlandi D, Frisoni GB, Au R, Devine S, Auerbach S, Espinosa A, Boada M, Ruiz A, Johnson SC, Koscik R, Wang JJ, Hsu WC, Chen YL, Toga AW. Apolipoprotein E genotype and sex risk factors for Alzheimer disease: A meta-analysis. JAMA Neurol. 2017;74:1178–1189. doi: 10.1001/jamaneurol.2017.2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 56.Okada S, Nakamura M, Katoh H, Miyao T, Shimazaki T, Ishii K, Yamane J, Yoshimura A, Iwamoto Y, Toyama Y, Okano H. Conditional ablation of Stat3 or Socs discloses a dual role for astrocytes after spinal cord injury. Nat Med. 2006;12:829–834. doi: 10.1038/nm1425. [DOI] [PubMed] [Google Scholar]
- 57.Pepeu G. Mild cognitive impairment: animal models. Dialogues Clin Neurosci. 2004;6:369–377. doi: 10.31887/DCNS.2004.6.4/gpepeu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Perluigi M, Barone E, Di Domenico F, Butterfield DA. Aberrant protein phosphorylation in Alzheimer disease brain disturbs pro-survival and cell death pathways. Biochim Biophys Acta. 2016;1862:1871–1882. doi: 10.1016/j.bbadis.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 59.Piaceri I, Nacmias B, Sorbi S. Genetics of familial and sporadic Alzheimer's disease. Front Biosci (Elite Ed) 2013;5:167–177. doi: 10.2741/e605. [DOI] [PubMed] [Google Scholar]
- 60.Querfurth HW, LaFerla FM. Alzheimer's disease. N Eng J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 61.Reddy PH, Tonk S, Kumar S, Vijayan M, Kandimalla R, Kuruva CS, Reddy AP. A critical evaluation of neuroprotective and neurodegenerative microRNAs in Alzheimer's disease. Biochem Biophys Res Commun. 2017;483:1156–1165. doi: 10.1016/j.bbrc.2016.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ren RJ, Zhang YF, Dammer EB, Zhou Y, Wang LL, Liu XH, Feng BL, Jiang GX, Chen SD, Wang G, Cheng Q. Peripheral blood microRNA expression profiles in Alzheimer's disease: Screening, validation, association with clinical phenotype and implications for molecular mechanism. Mol Neurobiol. 2016;53:5772–5781. doi: 10.1007/s12035-015-9484-8. [DOI] [PubMed] [Google Scholar]
- 63.Riancho J, Vázquez-Higuera JL, Pozueta A, Lage C, Kazimierczak M, Bravo M, Calero M, Gonalezález A, Rodríguez E, Lleó A, Sánchez-Juan P. MicroRNA profiles in patients with Alzheimer's disease: Analysis of miR-9-5p and miR-598 in raw and exosome enriched cerebrospinal fluid samples. J Alzheimers Dis. 2017;57:483–491. doi: 10.3233/JAD-161179. [DOI] [PubMed] [Google Scholar]
- 64.Rodrigues R, Petersen RB, Perry G. Parallels between major depressive disorder and Alzheimer's disease: role of oxidative stress and genetic vulnerability. Cell Mol Neurobiol. 2014;34:925–949. doi: 10.1007/s10571-014-0074-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schnoder L, Hao W, Qin Y, Liu S, Tomic I, Liu X, Fassbender K, Liu Y. Deficiency of neuronal p38αMAPK attenuates amyloid pathology in Alzheimer disease mouse and cell models through facilitating lysosomal degradation of BACE1. J Biol Chem. 2016;291:2067–2079. doi: 10.1074/jbc.M115.695916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT. Neuropathological alterations in Alzheimer's disease. Cold Spring Harb Perspect Med. 2011;1:a006189. doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sewell MC, Vigario A, Sano M. Neuropsychology in the diagnosis and treatment of dementia. In: Fillit HM, Rockwood K, Woodhouse K, editors. Brocklehurst's Textbook of Geriatric Medicine and Gerontology. 7th ed. Elsevier; 2010. pp. 402–410. [Google Scholar]
- 68.Sheinerman KS, Tsivinsky VG, Crawford F, Mullan MJ, Abdullah L, Umansky SR. Plasma microRNA biomarkers for detection of mild cognitive impairment. Aging. 2012;4:590–605. doi: 10.18632/aging.100486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sonoda Y, Mukai H, Matsuo K, Takahashi M, Ono Y, Maeda K, Akiyama H, Kawamata T. Accumulation of tumor-suppressor PTEN in Alzheimer neurofibrillary tangles. Neurosci Lett. 2010;471:20–24. doi: 10.1016/j.neulet.2009.12.078. [DOI] [PubMed] [Google Scholar]
- 70.Sriram K, Benkovic SA, Hebert MA, Miller DB, O’Callaghan JP. Induction of gp130-related cytokines and activation of JAK2/STAT3 pathway in astrocytes precedes up-regulation of glial fibrillary acidic protein in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of neurodegeneration: key signaling pathway for astrogliosis in vivo? J Biol Chem. 2004;279:19936–19947. doi: 10.1074/jbc.M309304200. [DOI] [PubMed] [Google Scholar]
- 71.Swerdlow RH. Alzheimer's disease pathologic cascades: who comes first, what drives what? Neurotox Res. 2012;22:182–194. doi: 10.1007/s12640-011-9272-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thies W, Bleiler L. 2011 Alzheimer's disease facts and figures. Alzheimers Dement. 2011;7:208–244. doi: 10.1016/j.jalz.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 73.Thomas T, Miners S, Love S. Post-mortem assessment of hypoperfusion of cerebral cortex in Alzheimer's disease and vascular dementia. Brain. 2015;138:1059–1069. doi: 10.1093/brain/awv025. [DOI] [PubMed] [Google Scholar]
- 74.Tsuda M, Kohro Y, Yano T, Tsujikawa T, Kitano J, Tozaki-Saitoh H, Koyanagi S, Ohdo S, Ji RR, Salter MW, Inoue K. JAK-STAT3 pathway regulates spinal astrocyte proliferation and neuropathic pain maintenance in rats. Brain. 2011;134:1127–1139. doi: 10.1093/brain/awr025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Van Giau V, An SS. Emergence of exosomal miRNAs as a diagnostic biomarker for Alzheimer's disease. J Neurol Sci. 2016;360:141–152. doi: 10.1016/j.jns.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 76.Vlassenko AG, Benzinger TL, Morris JC. PET amyloid-beta imaging in preclinical Alzheimer's disease. Biochim Biophys Acta. 2012;1822:370–379. doi: 10.1016/j.bbadis.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vousden KH, Lane DP. p53 in health and disease. Nat Rev Mol Cell Biol. 2007;8:275–283. doi: 10.1038/nrm2147. [DOI] [PubMed] [Google Scholar]
- 78.Weinberg RB, Mufson EJ, Counts SE. Evidence for a neuroprotective microRNA pathway in amnestic mild cognitive impairment. Front Neurosci. 2015;9:430. doi: 10.3389/fnins.2015.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wilcock DM, Lewis MR, Van Nostrand WE, Davis J, Previti ML, Gharkholonarehe N, Vitek MP, Colton CA. Progression of amyloid pathology to Alzheimer's disease pathology in an amyloid precursor protein transgenic mouse mod. el by removal of nitric oxide synthase 2. J Neurosci. 2008;28:1537–1545. doi: 10.1523/JNEUROSCI.5066-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu HZ, Ong KL, Seeher K, Armstrong NJ, Thalamuthu A, Brodaty H, Sachdev P, Mather K. Circulating microRNAs as biomarkers of Alzheimer's disease: A systematic review. J Alzheimers Dis. 2016;49:755–766. doi: 10.3233/JAD-150619. [DOI] [PubMed] [Google Scholar]
- 81.Wu Y, Xu J, Xu J, Cheng J, Jiao D, Zhou C, Dai Y, Chen Q. Lower serum levels of miR-29c-3p and miR-19b-3p as biomarkers for Alzheimer's disease. Toihoku J Exp Med. 2017;242:129–136. doi: 10.1620/tjem.242.129. [DOI] [PubMed] [Google Scholar]
- 82.Zirnheld AL, Shetty V, Chertkow H, Schipper HM, Wang E. Distinguishing mild cognitive impairment from Alzheimer's disease by increased expression of key circulating microRNAs. Curr Neurobiol. 2016;7:38–50. [Google Scholar]
- 83.Zhang X, Li F, Bulloj A, Zhang YW, Tong G, Zhang Z, Liao FF, Xu H. Tumor-suppressor PTEN affects tau phosphorylation, aggregation and binding to microtubules. FASEB J. 2006;20:1272–1274. doi: 10.1096/fj.06-5721fje. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.