Abstract
The devastating losses following traumatic spinal cord injury (SCI) encompass the motor, sensory and autonomic nervous systems. Neurogenic bowel is a slow transit colonic dysfunction marked by constipation, rectal evacuation difficulties, decreased anorectal sensation, fecal incontinence or some combination thereof. Furthermore, neurogenic bowel is one of the most prevalent comorbidities of SCI and is recognized by afflicted individuals and caregivers as a lifelong physical and psychological challenge that profoundly affects quality of life. The restoration of post-injury control of movement has received considerable scientific scrutiny yet the daily necessity of voiding the bowel and bladder remains critically under-investigated. Subsequently, physicians and caregivers are rarely presented with consistent, evidence-based strategies to successfully address the consequences of dysregulated voiding reflexes. Neurogenic bowel is commonly believed to result from the interruption of the supraspinal control of the spinal autonomic circuits regulating the colon. In this mini-review, we discuss the clinical challenges presented by neurogenic bowel and emerging pre-clinical evidence that is revealing that SCI also initiates functional remodeling of the colonic wall concurrent with a decrease in local enteric neurons. Since the enteric input to the colonic smooth muscle is the final common pathway for functional contractions of the colon, changes to the neuromuscular interface must first be understood in order to maximize the efficacy of therapeutic interventions targeting colonic dysfunction following SCI.
Keywords: colon, enteric nervous system, parasympathetic, sympathetic, autonomic nervous system, defecation reflexes, gastrointestinal, inflammation, constipation, incontinence
Neurogenic Bowel is A Multifactorial Consequence of Spinal Cord Injury
Colonic dysfunction following spinal cord injury (SCI), often referred to as neurogenic bowel, is one of the most prevalent and clinically recognized autonomic nervous system comorbidities of spinal trauma and disease (Coggrave and Norton, 2013). Epidemiological reports place the yearly incidence of SCI at approximately 17,000 annually and over 250,000 persons living with chronic injury (National Spinal Cord Injury Statistical Center, 2016) and estimates regarding the prevalence of neurogenic bowel range from 20–60% in the SCI population (Lynch et al., 2001; Coggrave and Norton, 2013).
Depending upon the injury level, neurogenic bowel is manifested as diminished colonic transit, constipation, evacuation dyssynergy, and the potential for overflow incontinence if the lesion is above the second sacral level. Lesions of this nature are commonly referred to as an upper motor neuron lesion. The polysymptomatic nature of neurogenic bowel stems from the fact that the functions and neural circuits innervating the colon are heterogeneous in order to achieve the diverse processes necessary for the final stages of digestion (Callaghan et al., 2018). In health, normal bowel function is the result of coordinated actions between the enteric, sympathetic and parasympathetic nervous systems. At the local level, enteric control is comprised of a polysynaptic reflex circuit (Figure 1) consisting of sensory neurons, interneurons and motoneurons governing secretory processes of the luminal epithelium as well as smooth muscle-mediated propulsion and segmentation (Furness et al., 2014). Even ex-vivo studies employing whole-tissue organ baths demonstrate that the enteric neurocircuitry of the muscularis propria is sufficient to achieve reflexive propulsion of colonic contents. At the segmental level, the colon receives sympathetic innervation from the hypogastric nerve (spinal level T12–L1) and parasympathetic innervation from the pelvic nerve (spinal level S2–4). The parasympathetic neurons, both preganglionic and postganglionic, are cholinergic and exert excitatory effects on enteric neurons. The parasympathetic system functions to modulate the tone and compliance of the muscles of the colonic wall allowing for the transport of colonic contents and promoting peristalsis. However, the parasympathetic innervation of the internal anal sphincter (IAS) through the pelvic nerves conveys both cholinergic and non-cholinergic excitatory, as well as non-adrenergic, non-cholinergic inhibitory input to the rectum and IAS. As such this confers a more nuanced role (and effect) of the parasympathetic nervous system. Specifically, experimental data indicates that stimulation of the pelvic or rectal nerves induces either (i) an increase in motility through the cholinergic pathways, or (ii) an inhibition of motility, via activation of an atropine-insensitive, nonadrenergic noncholinergic pathway that involves release of nitric oxide and/or purines [reviewed in Furness et al. (2014)]. These effects reflect the mixed excitatory and inhibitory nature of the enteric neuronal output. Conversely, the sympathetic innervation decreases overall motility by relaxing the muscles within the colonic wall (de Groat and Krier, 1978; Krier, 1989). Finally, defecation has both voluntary and reflexive components. The pudendal nerve (spinal level S2–4) provides somatic innervation to the muscles of the pelvic floor that reduce the sigmoid flexure that is associated with the bipedal anatomy of humans as well as external anal sphincter tone that can be relaxed voluntarily when voiding is appropriate (Dubrovsky and Filipini, 1990). Damage to the sacral region affects the somatic and preganglionic parasympathetic circuits and is considered a lower motor neuron lesion. Injury at this level is frequently accompanied by incontinence due to the damage to the motor neurons innervating the striated external anal sphincter.
Figure 1.
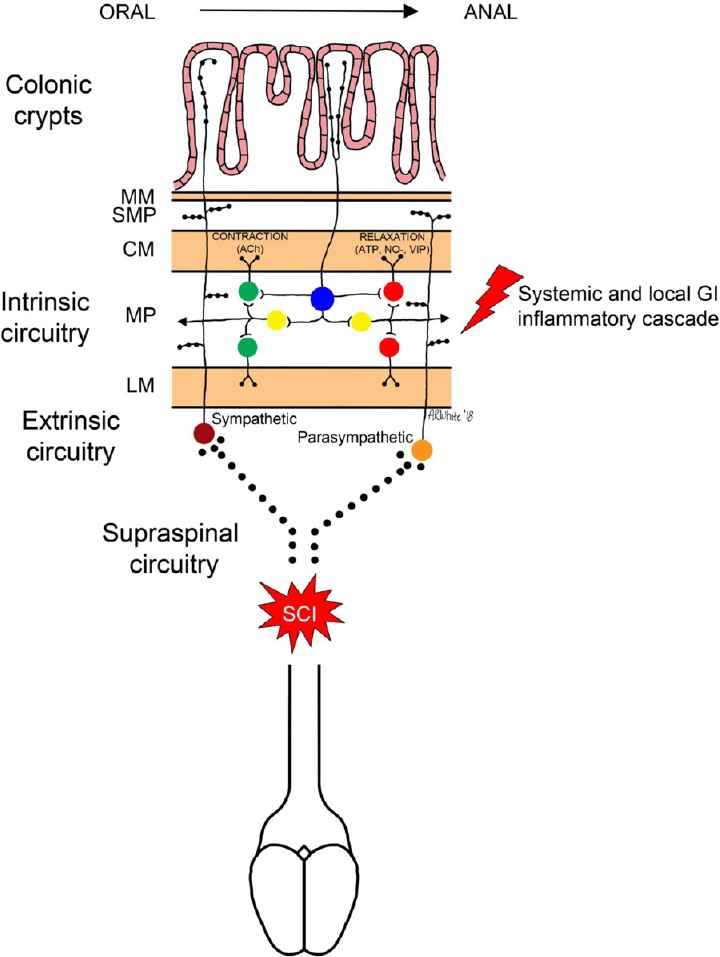
Intrinsic neural circuits for colonic propulsion.
Simplified schematic of the enteric neural circuitry involved in colonic contractions. Intrinsic primary afferent neurons (IPANs; blue) within the myenteric plexus (MP) transduce mechanical (stretch) or chemical stimuli from the colonic lumen. IPANs synapse upon interneurons (yellow) that project proximally (orad) and distally (caudad). Orad-projecting interneurons synapse onto excitatory motor neurons (green) that utilize acetylcholine (ACh) as a primary neurotransmitter to provoke contraction of circular and longitudinal smooth muscle (CM and LM, respectively). Caudad-projecting interneurons synapse onto inhibitory motor neurons (red) that utilize fast, purinergic (ATP), neurotransmission or slower nitrergic (NO–) neurotransmission to provoke relaxation of circular and longitudinal smooth muscle; thus generating a pressure gradient for movement of colonic contents. Vasoactive intestinal peptide (VIP) is also implicated in inhibitory neurotransmission. These intrinsic neural circuits are modulated by extrinsic innervation by the sympathetic and parasympathetic nervous system fibers that also project into the submucosal plexus (SMP) adjoining the muscularis mucosae (MM). Neurogenic bowel is likely a result of the effects of interruption of the supraspinal inputs onto extrinsic autonomic preganglionic neurons coupled with damage to intrinsic circuits by local and systemic inflammation.
Clinical Consequences and the Patient Experience
Neurogenic bowel is an unpredictable and recurring problem that does not resolve over time and may worsen with age. Upon transitioning to the rehabilitative phase following injury, individuals with SCI are commonly offered limited, sometimes invasive, interventions to manage long-term gastrointestinal symptoms (Multidisciplinary Association of Spinal Cord Injured Professionals, 2012; Wheeler et al., 2018). While conservative bowel programs can manage neurogenic bowel, an increasing incidence of clinical complications are reported to accompany aging with injury (Lynch et al., 2000; Nielsen et al., 2017; Wheeler et al., 2018). Subsequently, bowel dysfunction is a major physical and psychological challenge that reduces quality of life. The reported quality of life challenges associated with the inordinate amount of time devoted to bowel care is compounded by the necessity of tetraplegics to rely upon assistance from care-givers for bowel management. Broader clinical consequences of neurogenic bowel are reflected in the fact that gastrointestinal complications are reported to be responsible for roughly 11% of hospitalizations in the SCI population (Middleton et al., 2004; Jaglal et al., 2009; Hammond et al., 2013; Gabbe and Nunn, 2016). The side effects of long-term neurogenic bowel include impaction, megacolon, hemorrhoids, rectal bleeding, prolapse, formation of anal fissures in addition to chronic constipation and fecal incontinence (Lynch et al., 2001).
Pathophysiological remodeling of the spinal neural circuits that provide the diffuse innervation of the viscera (Gebhart and Bielefeldt, 2016) may lead to the presentation of referred pain symptoms. Perhaps the most urgent co-morbidity involving these heightened nociceptive processes involves the combined cardiovascular remodeling that occurs following SCI (Maiorov et al., 1998; Furlan et al., 2003; Wan and Krassioukov, 2013). Individuals with a SCI above the level of T6, have a greater risk of experiencing a phenomenon commonly referred to as autonomic dysreflexia. It is well established that during an instance of autonomic dysreflexia, a noxious stimulus is accompanied by a substantial increase in sympathetic discharge below the injury level [reviewed in Al Dera and Brock (2018)]. In rodent models of SCI, this episodic sympathetic discharge begins within 2 weeks following complete SCI (Rummery et al., 2010). Autonomic dysreflexia is a potentially life threatening medical emergency and it is an indicator of over-activity of the autonomic nervous system typically in response to a noxious or irritating stimulus, such as colon distension, below the level of injury. Lastly, the specter of social embarrassment associated with bowel incontinence contributes to the incidence of anxiety and depression in SCI individuals (Ng et al., 2005).
Subsequently, surveys among the SCI population often rank colorectal, bladder and sexual dysfunction as significant obstacles to daily life pursuits and prioritize recovery of bowel function above the ability to walk (Lynch et al., 2001; Anderson, 2004; Simpson et al., 2012). Despite these consequences, pre-clinical investigation of bowel dysfunction is profoundly lacking and substantial knowledge gaps persist between the neurotrauma and gastroenterological fields in understanding neurogenic bowel.
Pre-Clinical Evidence and Focus
The association of SCI with storage and evacuation deficits promotes an inherent tendency to focus upon the loss of supraspinal regulation of somatic (Holmes et al., 1998, 2005; Callaghan et al., 2018) and autonomic circuitry of the spinal cord (Chung and Emmanuel, 2005; Ferens et al., 2011; Callaghan et al., 2018). Hyperreflexic contractions of the external anal sphincter following experimental SCI (Holmes et al., 1998) or targeted supraspinal lesions (Holmes et al., 2002) suggest dysregulation of voiding reflexes that may be analogous to bladder smooth muscle and striated sphincter dyssynergy following injury. Recently, pre-clinical models of SCI have investigated the efficacy of the endogenous prokinetic ghrelin to act directly upon the segmental lumbosacral defecation reflex circuits (Ferens et al., 2011) while derangements in the prokinetic signaling mediated by acetylcholine have also been explored (Joo et al., 2011). Prokinetic effects of neurokinin receptor agonists are offering promising results for colon and bladder dysfunction with attention also directed at simultaneous cardiovascular stability (Kullmann et al., 2017; Marson et al., 2018). Finally, the anecdotal reports of improvements in bladder and bowel function following treadmill training or epidural stimulation have generated interest in the development of intermediate animal models to test colorectal pressure responses (Guiho et al., 2016, 2017).
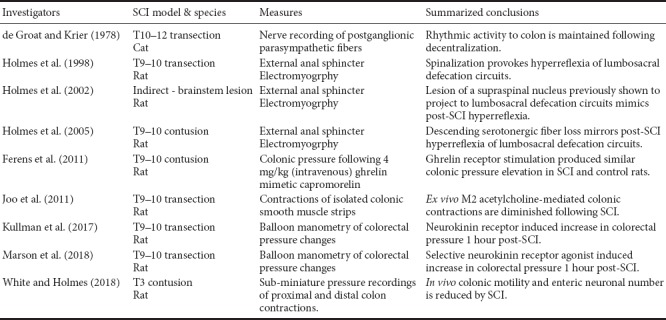
Clearly, data collected in neurally-intact organisms provides abundant evident that somatic motoneurons and autonomic preganglionic neurons are ultimately modulated by the central nervous system in the form of a complex network of brainstem, limbic, and cortical inputs [see Furness et al. (2014)]. The multiple levels of colonic neural regulation (enteric, lumbosacral segmental reflexes, and modulation by the descending supraspinal inputs) demonstrate that neurogenic bowel is more nuanced than what is sometimes considered in pre-clinical and clinical research. We conducted a literature search (PubMed, Web of Science) to identify published pre-clinical studies of neurogenic bowel following SCI (summarized in Table 1) (Krogh et al., 2001). Our analysis reveals the paucity of pre-clinical research addressing colonic and defecation abnormalities after SCI. Interpretation of this limited pre-clinical data is further compounded by the lack of standardized outcome measures these studies employed for assessing neurogenic bowel experimentally.
Table 1.
Pre-clinical studies of neurogenic bowel following traumatic spinal cord injury (SCI)
Local and Systemic Inflammatory Responses following SCI
While the interruption of supraspinal inputs onto spinal GI neural circuits following SCI is self-evident, emerging evidence is expanding our understanding of the early inflammatory responses that likely affect gastrointestinal (GI) health. Proper GI function requires adequate blood flow at the local organ level to a degree that is second only to central nervous system tissue. Reduction or loss of GI vascular supply provokes localized inflammatory responses and pathophysiological changes to GI function. Spinal cord injury brings about diminished sympathetic tone as a result of the interruption descending brainstem presympathetic vasomotor neurons that is accompanied by a brief inflammatory bout and a reduction in GI vascular tone and reflexes (Besecker et al., 2017). The local inflammatory response in GI tissues is superimposed by a systemic inflammatory cascade that can induce multi-organ dysfunction known as systemic inflammatory response syndrome [SIRS; see Anthony and Couch (2014)]. Generally, SIRS is associated with hepatic, renal and pulmonary damage and the relative contributions of circulating inflammatory mediators upon GI function remains as unclear as the widespread loss of the spinal integrative control upon digestive system physiology. Finally, it is increasingly evident that there is bidirectional communication between gut microbiota and the nervous system by way of neural, endocrine and metabolic pathways (Bonaz et al., 2018). These interrelationships are proposed to alter the anti-inflammatory properties in response to vagal nerve activity; in addition to the normal physiological regulation of digestive reflexes within the GI tract by the vagus nerve. Furthermore, in addition to shifts in the diversity of gut microbiota, the loss of GI mucosal barrier integrity following SCI is well recognized and bacterial translocation of some or all of the constituents of the microbial flora is also emerging as a significant co-morbidity of SCI.
Role of Inflammatory Processes in Colonic Dysmotility
With such a severe knowledge gap pertaining to neurogenic bowel, it is tempting to draw upon other disorders, such as inflammatory bowel diseases (IBDs) that share similar abnormalities in colonic motility, to elucidate possible underlying mechanisms. Specific IBDs, such as Crohn’s disease and ulcerative colitis, are distinguished by their clinical manifestation, sites of inflammation, immunologic profiles, as well as marked abnormalities in colonic motility such as abnormal transit and decreased contractility of colonic smooth muscle. Experimental models of IBD have demonstrated pathophysiological remodeling of the neuromuscular compartment associated with dysmotility. Physical evidence of IBD oxidative damage and inflammation include architectural changes to the colonic mucosa, increased collagen deposition, thickening of the colonic smooth muscle, as well as a loss of enteric neurons. The effect of increased collagen deposition serves to reduce the compliancy of the colon by making the colonic wall stiffer and ultimately reducing propulsive capacity. Myenteric alterations are also frequently noted and may explain the disrupted motor activity that persists long after resolution of the inflammatory bout [reviewed in Mawe (2015)]. Specifically, it is the reduction of inhibitory neurotransmission involving the loss of enteric neurons that frequently affects colonic motility. Receptive relaxation of distal colonic segments is as necessary as propulsive contractions to prevent delayed colonic transit, constipation, and impaction.
Due to the profound, and persistent, physiological changes following SCI, comparisons regarding parallel mechanistic changes with IBD must be made cautiously. An integrated assessment of the colonic pathophysiology associated with SCI in humans has recently received systematic, though semiquantitative, investigation. Analysis of archival samples identified an increase in collagen within the longitudinal muscle layer as well as a general reduction in myenteric neuronal density in individuals with chronic SCI (den Braber-Ymker et al., 2017). Similar changes have been observed in rats with acute (3 day) and semi-chronic (3 week) experimental SCI. Specifically, the proximal and distal colons from rats that displayed in vivo reductions in colonic contractions also demonstrated increased collagen content and muscle thickness along with a progressive reduction myenteric neuronal density (White and Holmes, 2018). The progressive reduction in the myenteric neurons that ultimately drive peristalsis (refer to Figure 1) suggests that neurogenic bowel includes remodeling of the enteric neuromuscular control of the colon.
Potential Implications of Colonic Neuromuscular Remodeling upon Therapeutic Strategies
Current bowel management programs frequently range from overly conservative and noninvasive strategies to surgical treatments (Coggrave and Norton, 2013). Unfortunately, many of these bowel management strategies offered to the SCI individual or caretakers are based upon anecdotal reports or trial and error in the absence of evidence-based support. Lifestyle strategies, digital rectal stimulation and multiple pharmacological approaches with osmotic and stimulant laxatives (poly-pharmacy) are the primary means for symptom management of neurogenic bowel. Only as conservative symptom management deteriorates are many individuals willing to consider invasive surgical interventions or implanted neuromodulatory devices. Therapies designed to promote the restoration of descending inputs to the lumbosacral autonomic and somatic circuits innervating the colon may achieve greater success than those targeting locomotion due to the relative simplicity of visceral reflexes. However, failure to take into consideration the remodeling of the enteric neuromuscular compartment may profoundly limit the efficacy of such approaches.
Conclusions
The intrinsic (e.g., enteric) nervous control of the colon combined with the extrinsic (sympathetic and parasympathetic) modulation of the enteric neural circuitry demonstrates that there are multiple underlying factors that contribute to the development of neurogenic bowel. While each level requires greater research focus in order to elucidate the post-SCI changes to colonic function, the enteric neuromuscular junction is the final common pathway for the extrinsic regulation of the colon by segmental reflex arcs and serves as a logical starting point for investigation. The secondary organ dysfunction that results from the local and systemic inflammatory cascade immediately following a SCI lesion suggests that neurogenic bowel is not merely a lack of descending neural control and greater emphasis must be placed on the potential post-inflammatory remodeling of the critical enteric neuromuscular interface. Both the local and systemic inflammatory response can incite elevated levels of oxidative stress within multiple tissues and the therapeutic targeting of this process in the broader context of neural protection in the brain or spinal cord may also offer a common element for protection of the enteric nervous system.
Additional file: Open peer review report 1 (92.2KB, pdf) .
Footnotes
Conflicts of interest: No competing financial interests exist.
Financial support: This work was supported by grants from the National Institutes of Health, No. NINDS 49177 (to GMN) and Craig H. Neilsen Foundation Senior Research award, No. 295319 (to GMN).
Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Lukas Grassner, Trauma Center Murnau, Germany.
P-Reviewer: Grassner L; C-Editors: Zhao M, Yu J; T-Editor: Liu XL
Funding: This work was supported by grants from the National Institutes of Health, No. NINDS 49177 (to GMH) and Craig H. Neilsen Foundation Senior Research award, No. 295319 (to GMH).
References
- 1.Al Dera H, Brock JA. Changes in sympathetic neurovascular function following spinal cord injury. Auton Neurosci. 2018;209:25–36. doi: 10.1016/j.autneu.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Anderson KD. Targeting recovery: Priorities of the spinal cord injured population. J Neurotrauma. 2004;21:1371–1383. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- 3.Anthony DC, Couch Y. The systemic response to CNS injury. Exp Neurol. 2014;258:105–111. doi: 10.1016/j.expneurol.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 4.Besecker EM, Deiter GM, Pironi N, Cooper TK, Holmes GM. Mesenteric vascular dysregulation and intestinal inflammation accompanies experimental spinal cord injury. Am J Physiol Regul Integr Comp Physiol. 2017;312:146–156. doi: 10.1152/ajpregu.00347.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonaz B, Bazin T, Pellissier S. The vagus nerve at the interface of the microbiota-gut-brain axis. Front Neurosci. 2018;12:49. doi: 10.3389/fnins.2018.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callaghan B, Furness JB, Pustovit RV. Neural pathways for colorectal control, relevance to spinal cord injury and treatment: a narrative review. Spinal Cord. 2018;56:199–205. doi: 10.1038/s41393-017-0026-2. [DOI] [PubMed] [Google Scholar]
- 7.Chung EA, Emmanuel AV. Gastrointestinal symptoms related to autonomic dysfunction following spinal cord injury. Prog Brain Res. 2005;152:317–333. doi: 10.1016/S0079-6123(05)52021-1. [DOI] [PubMed] [Google Scholar]
- 8.Coggrave M, Norton C. Management of faecal incontinence and constipation in adults with central neurological diseases. Cochrane Database Syst Rev. 2013;12:CD002115. doi: 10.1002/14651858.CD002115.pub4. [DOI] [PubMed] [Google Scholar]
- 9.de Groat WC, Krier J. The sacral parasympathetic reflex pathway regulating colonic motility and defaecation in the cat. J Physiol. 1978;276:481–500. doi: 10.1113/jphysiol.1978.sp012248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.den Braber-Ymker M, Lammens M, van Putten MJAM, Nagtegaal ID. The enteric nervous system and the musculature of the colon are altered in patients with spina bifida and spinal cord injury. Virchows Arch. 2017;470:175–184. doi: 10.1007/s00428-016-2060-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubrovsky B, Filipini D. Neurobiological aspects of the pelvic floor muscles involved in defecation. Neurosci Biobehav Rev. 1990;14:157–168. doi: 10.1016/s0149-7634(05)80216-7. [DOI] [PubMed] [Google Scholar]
- 12.Ferens DM, Habgood MD, Saunders NR, Tan YH, Brown DJ, Brock JA, Furness JB. Stimulation of defecation in spinal cord-injured rats by a centrally acting ghrelin receptor agonist. Spinal Cord. 2011;49:1036–1041. doi: 10.1038/sc.2011.60. [DOI] [PubMed] [Google Scholar]
- 13.Furlan JC, Fehlings MG, Shannon P, Norenberg MD, Krassioukov AV. Descending vasomotor pathways in humans: correlation between axonal preservation and cardiovascular dysfunction after spinal cord injury. J Neurotrauma. 2003;20:1351–1363. doi: 10.1089/089771503322686148. [DOI] [PubMed] [Google Scholar]
- 14.Furness JB, Callaghan BP, Rivera LR, Cho HJ. The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv Exp Med Biol. 2014;817:39–71. doi: 10.1007/978-1-4939-0897-4_3. [DOI] [PubMed] [Google Scholar]
- 15.Gabbe BJ, Nunn A. Profile and costs of secondary conditions resulting in emergency department presentations and readmission to hospital following traumatic spinal cord injury. Injury. 2016;47:1847–1855. doi: 10.1016/j.injury.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 16.Gebhart GF, Bielefeldt K. Physiology of visceral pain. Compr Physiol. 2016;6:1609–1633. doi: 10.1002/cphy.c150049. [DOI] [PubMed] [Google Scholar]
- 17.Guiho T, Coste CA, Delleci C, Chenu JP, Vignes JR, Bauchet L, Guiraud D. An intermediate animal model of spinal cord stimulation. Eur J Transl Myol. 2016;26:6034. doi: 10.4081/ejtm.2016.6034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guiho T, Delleci C, Azevedo-Coste C, Fattal C, Guiraud D, Vignes JR, Bauchet L. Impact of direct epispinal stimulation on bladder and bowel functions in pigs: A feasibility study. Neurourol Urodyn. 2017;37:138–147. doi: 10.1002/nau.23325. [DOI] [PubMed] [Google Scholar]
- 19.Hammond FM, Horn SD, Smout RJ, Chen D, DeJong G, Scelza W, Jha A, Ballard PH, Bloomgarden J. Acute rehospitalizations during inpatient rehabilitation for spinal cord injury. Arch Phys Med Rehabil. 2013;94:S98–105. doi: 10.1016/j.apmr.2012.11.051. [DOI] [PubMed] [Google Scholar]
- 20.Holmes GM, Hermann GE, Rogers RC, Bresnahan JC, Beattie MS. Dissociation of the effects of nucleus raphe obscurus or rostral ventrolateral medullary lesions on eliminatory and sexual reflexes. Physiol Behav. 2002;75:49–55. doi: 10.1016/s0031-9384(01)00631-x. [DOI] [PubMed] [Google Scholar]
- 21.Holmes GM, Rogers RC, Bresnahan JC, Beattie MS. External anal sphincter hyper-reflexia following spinal transection in the rat. J Neurotrauma. 1998;451:451–457. doi: 10.1089/neu.1998.15.451. [DOI] [PubMed] [Google Scholar]
- 22.Holmes GM, Van Meter MJ, Bresnahan JC, Beattie MS. Serotonergic fiber sprouting to external anal sphincter motoneurons after spinal cord contusion. Exp Neurol. 2005;193:29–42. doi: 10.1016/j.expneurol.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 23.Jaglal SB, Munce SEP, Guilcher SJ, Couris CM, Fung K, Craven BC, Verrier M. Health system factors associated with rehospitalizations after traumatic spinal cord injury: a population-based study. Spinal Cord. 2009;47:604–609. doi: 10.1038/sc.2009.9. [DOI] [PubMed] [Google Scholar]
- 24.Joo MC, Kim YS, Choi ES, Oh JT, Park HJ, Lee MY. Changes in the muscarinic receptors on the colonic smooth muscles of rats with spinal cord injury. Ann Rehabil Med. 2011;35:589–598. doi: 10.5535/arm.2011.35.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krier J. Motor function of anorectum and pelvic floor musculature. In: Wood J, editor. Handbook of Physiology. Bethesda: American Physiological Society; 1989. pp. 1025–1053. [Google Scholar]
- 26.Krogh K, Christensen P, Laurberg S. Colorectal symptoms in patients with neurological diseases. Acta Neurol Scand. 2001;103:335–343. doi: 10.1034/j.1600-0404.2001.103006335.x. [DOI] [PubMed] [Google Scholar]
- 27.Kullmann FA, Katofiasc M, Thor KB, Marson L. Pharmacodynamic evaluation of Lys5 MeLeu9 Nle10-NKA(4-10) prokinetic effects on bladder and colon activity in acute spinal cord transected and spinally intact rats. Naunyn Schmiedebergs Arch Pharmacol. 2017;390:163–173. doi: 10.1007/s00210-016-1317-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lynch AC, Antony A, Dobbs BR, Frizelle FA. Bowel dysfunction following spinal cord injury. Spinal Cord. 2001;39:193–203. doi: 10.1038/sj.sc.3101119. [DOI] [PubMed] [Google Scholar]
- 29.Lynch AC, Wong C, Anthony A, Dobbs BR, Frizelle FA. Bowel dysfunction following spinal cord injury: a description of bowel function in a spinal cord-injured population and comparison with age and gender matched controls. Spinal Cord. 2000;38:717–723. doi: 10.1038/sj.sc.3101058. [DOI] [PubMed] [Google Scholar]
- 30.Maiorov DN, Fehlings MG, Krassioukov AV. Relationship between severity of spinal cord injury and abnormalities in neurogenic cardiovascular control in conscious rats. J Neurotrauma. 1998;15:365–374. doi: 10.1089/neu.1998.15.365. [DOI] [PubMed] [Google Scholar]
- 31.Marson L, Thor KB, Katofiasc M, Burgard EC, Rupniak NMJ. Prokinetic effects of neurokinin-2 receptor agonists on the bladder and rectum of rats with acute spinal cord transection. Eur J Pharmacol. 2018;819:261–269. doi: 10.1016/j.ejphar.2017.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mawe GM. Colitis-induced neuroplasticity disrupts motility in the inflamed and post-inflamed colon. J Clin Invest. 2015;125:949–955. doi: 10.1172/JCI76306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Middleton JW, Lim K, Taylor L, Soden R, Rutkowski S. Patterns of morbidity and rehospitalisation following spinal cord injury. Spinal Cord. 2004;42:359–367. doi: 10.1038/sj.sc.3101601. [DOI] [PubMed] [Google Scholar]
- 34.Multidisciplinary Association of Spinal Cord Injured Professionals. Guidelines for Management of Neurogenic Bowel Dysfunction in Individuals with Central Neurological Conditions. 2012. [Accessed 14 May 2017]. http://www.mascip.co.uk/wp-content/uploads/2015/02/CV653N-Neurogenic-Guidelines-Sept-2012.pdf .
- 35.National Spinal Cord Injury Statistical Center. SCI facts and figures 2016. J Spinal Cord Med. 2016;39:737–738. doi: 10.1080/10790268.2016.1253912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ng C, Prott G, Rutkowski S, Li Y, Hansen R, Kellow J, Malcolm A. Gastrointestinal symptoms in spinal cord injury: relationships with level of injury and psychologic factors. Dis Colon Rectum. 2005;48:1562–1568. doi: 10.1007/s10350-005-0061-5. [DOI] [PubMed] [Google Scholar]
- 37.Nielsen SD, Faaborg PM, Finnerup NB, Christensen P, Krogh K. Ageing with neurogenic bowel dysfunction. Spinal Cord. 2017;55:769. doi: 10.1038/sc.2017.22. [DOI] [PubMed] [Google Scholar]
- 38.Rummery N, Tripovic D, McLachlan EM, Brock JA. Sympathetic vasoconstriction is potentiated in arteries caudal but not rostral to a spinal cord transection in rats. J Neurotrauma. 2010;27:2077–2089. doi: 10.1089/neu.2010.1468. [DOI] [PubMed] [Google Scholar]
- 39.Simpson LA, Eng JJ, Hsieh JT, Wolfe DL Spinal Cord Injury Rehabilitation Evidence Scire Research Team. The health and life priorities of individuals with spinal cord injury: A systematic review. J Neurotrauma. 2012;29:1548–1555. doi: 10.1089/neu.2011.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wan D, Krassioukov AV. Life-threatening outcomes associated with autonomic dysreflexia: A clinical review. J Spinal Cord Med. 2013;37:2–10. doi: 10.1179/2045772313Y.0000000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bowel and Bladder Workshop Participants. Wheeler TL, de Groat W, Eisner K, Emmanuel A, French J, Grill W, Kennelly MJ, Krassioukov A, Gallo Santacruz B, Biering-Sørensen F, Kleitman N. Translating promising strategies for bowel and bladder management in spinal cord injury. Exp Neurol. 2018;306:169–176. doi: 10.1016/j.expneurol.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.White A, Holmes GM. Anatomical and functional changes to the colonic neuromuscular compartment after experimental spinal cord injury. J Neurotrauma. 2018;35:1079–1090. doi: 10.1089/neu.2017.5369. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


