Abstract
Glaucoma, the leading cause of visual impairment and irreversible blindness worldwide, is a multifactorial, progressive optic neuropathy characterized by loss of retinal ganglion cells, alterations of the optic nerve head, and specific visual field defects. Clinical evidence shows that intraocular pressure is the major risk factor of the treatable disease. However, in some patients, glaucoma develops and continues to progress despite normal intraocular pressure values, suggesting that other risk factors are involved in the disease. Consequently, neuroprotective treatments, focused on preventing retinal ganglion cells death by acting on different therapeutic strategies but not focused on intraocular pressure reduction, has therefore become of great interest. In this contest, coenzyme Q10, showing evidence in slowing or reversing pathological changes typical of the disease, has been proposed as a potential neuroprotective agent in glaucoma. In this review, we describe the possible mechanisms of action of coenzyme Q10 and the recent evidence in literature regarding the neuroprotective activity of the molecule.
Keywords: glaucoma, neuroprotection, retinal ganglion cells, coenzyme Q10, intraocular pressure, mitochondrion, oral administration, neurodegenerative diseases
Glaucoma, a leading cause of visual impairment and irreversible blindness worldwide, is a progressive and multifactorial optic neuropathy characterized by loss of retinal ganglion cells (RGCs) which is associated with specific deficits of the visual field consequent to typical alterations of the optic nerve. International clinical studies have shown that RGCs loss is commonly related to increased intraocular pressure (IOP). However, a significant reduction in the IOP does not always stops the onset and progression of the disease (Heijl et al., 2002). Although the IOP reduction strategies have been proved not always to be able to stop the onset or the progression of the disease (Heijl et al., 2002), till now they remained the only therapeutic strategy in the hands of clinicians. It is therefore imperative to identify new therapies not only based on IOP reduction, but also based on neuroprotection (Nucci et al., 2016, 2018). We have performed a PubMed literature search of articles published in the period of January 2003 - July 2018 on the use of coenzyme Q10 (CoQ10) in the treatment of glaucoma and on oral CoQ10.
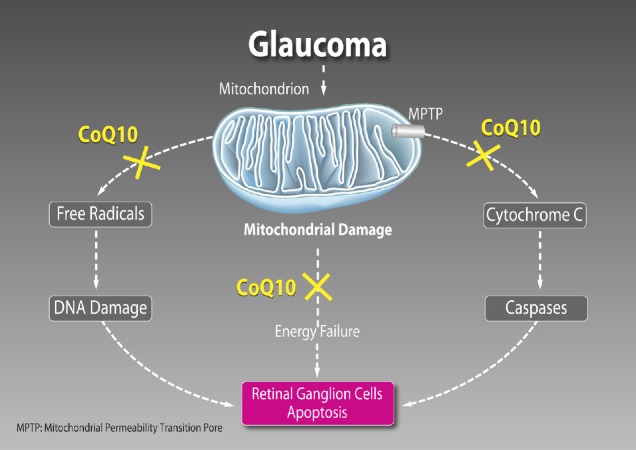
It has been hypothesized that mechanism causing cell loss involves overactivation of N-methyl-D-aspartate (NMDA) and non-NMDA subtypes of glutamate receptors. In this regard, CoQ10 reduces the harmful action of ischemia/reperfusion on mitochondrial energy metabolism and, subsequently, on the function of glutamate transporters, thus restraining accumulation of extracellular glutamate and preventing apoptotic death of RGC in the rat (Nucci et al., 2007). Excessive activation of glutamate receptors via the excitotoxic cascade leads to the mitochondrial permeability transition pore (MPTP) formation. Independently of its free radical scavenging property, CoQ10 has a specific antiapoptotic activity by directly maintaining MPTP in the closed conformation and preventing cytochrome c release and caspase 9 activation in response to free radical unrelated apoptotic stimuli (Papucci et al., 2003) (Figure 1).
Figure 1.
Main mechanisms of action of coenzyme Q10 (CoQ10).
It has free radical scavenging property that prevents deoxyribonucleic acid (DNA) damage, has a fundamental role in cellular bioenergetics and a specific antiapoptotic activity by directly maintaining mitochondrial permeability transition pore (MPTP) in the closed conformation.
The retina is one of the most metabolically active tissue in the body and requires an accurate regulation of the energy production. In this regard, mitochondria play a central role providing energy supply through oxidative phosphorylation. Adenosine triphosphate is generated by the electron transport chain complexes located within the cristae folds of the inner mitochondrial membrane. Moreover, mitochondria regulate a number of essential processes for neuronal functions including metabolic balance, intracellular calcium homeostasis, production of reactive oxygen species, and apoptotic signalling. Experimental studies have shown that RGCs death in glaucoma is an extremely complex process triggered by different molecular mechanisms (Nucci et al., 2018). Due to peculiar structural and energetic request, it is not surprising that RGCs are acutely vulnerable to mitochondrial dysfunction (Lee et al., 2011; Ito and Di Polo, 2017), which may increase their susceptibility to glaucoma-driven pathological factors (Figure 1).
The specific loss of RGCs is a common feature of mitochondrial diseases (Lee et al., 2011). Indeed, inherited mitochondrial defects are associated with a number of optic neuropathies including Leber’s hereditary optic neuropathy and autosomal dominant optic atrophy, but also with more severe central nervous system (CNS) involvement in many other syndromic mitochondrial diseases, which are characterized by selective RGC death (Carelli et al., 2009; Lee et al., 2011; Ito and Di Polo, 2017).
The selective loss of neuronal populations and the cell damage pattern in glaucoma also resemble those of other neurodegenerative diseases (Nucci et al., 2016, 2018; Mancino et al., 2018a, b) and increasing evidence supports a causative role of the glutamate-induced excitotoxic mechanism in RGCs loss in in vitro and in vivo experimental conditions (Nucci et al., 2007). Several free radical scavengers and/or agents, that ameliorate mitochondrial function, have been candidate as treating agents to prevent cell death in various neurodegenerative conditions, such as Alzheimer’s disease and Parkinson, and glaucoma (Spindler et al., 2009; Ahmadinejad et al., 2017). CoQ10 is both a ubiquitous free radical scavenger and a recognised electron transporter in complexes I, II, and III of mitochondrial respiratory chain. CoQ10 is an important antioxidant and has a fundamental role in cellular bioenergetics. This led to consider glaucoma as a neurodegenerative disease and promoted clinical studies on new neuroprotective strategies not only targeted to IOP reduction (Nucci et al., 2016, 2018).
Interestingly, increasing evidence indicates that age-related mitochondrial defects play a central role in the pathogenesis of glaucoma (Nucci et al., 2007; Russo et al., 2008, 2009; Lee et al., 2014). Levels of CoQ10 in the human retina have been reported to declines with age (Qu et al., 2009). In this regard, it is well known the existence of a link between older age and the prevalence of glaucoma, thus suggesting a possible increased vulnerability of RGCs in glaucomatous neurodegeneration due to a lack of CoQ10 in older age (Bhagavan and Chopra, 2006; Lee et al., 2014).
This opens new opportunities of investigation for the development of novel neurotherapeutic agents for the treatment of glaucoma and other major retinal pathologies (Russo et al., 2008, 2009; Zhang et al., 2017).
CoQ10 poor aqueous solubility (Fato et al., 2010) and low bioavailability, partially due to the interactions with the multi-drug efflux pump P-glycoprotein expressed in both corneal epithelial cells and RGCs, have limited the development of topically active formulations of this molecule (Davis et al., 2017). To enhance the topical delivery and pharmacological effects of CoQ10, the co-administration with a P-glycoprotein inhibitor, such as D-α-Tocopherol polyethylene glycol 1000 succinate (TPGS), has been proposed (Davis et al., 2017). Interestingly, it has been successfully demonstrated both in vitro and in vivo mitochondrial-mediated neurotoxicity models that twice daily topical instillation of CoQ10/TPGS micelles was found to be significantly neuroprotective against RGCs loss than TPGS alone. The findings, in agreement with previous work, also suggest that the antioxidant activity of TPGS alone was insufficient to protect an immortalised neuronal cell line from insults generating mitochondrial oxidative stress, such as dimethyl sulfoxide and paraquat (Davis et al., 2017).
Topical ocular administration, in a model of high IOP–induced transient ischemia in rat, of CoQ10 0.1% + vitamin E (Vit E) 0.5% showed the ability to minimize DNA fragmentation and retinal cell apoptosis (Nucci et al., 2007) (Figure 1). This study confirmed, for the first time, that, during the reperfusion phase, the ischemic insult induces a significant increase of glutamate with consequent RGCs apoptosis. Thus, providing evidence of the usefulness of CoQ10 as a neuroprotective agent. In these conditions, administration of CoQ10 prevents glutamate increase, minimizing RGCs death in rats. It is plausible that the CoQ10 free radical scavenging mechanism may have a minor role in this process and that CoQ10 ability to restrains extracellular glutamate accumulation, may reduce the harmful effect of ischemia/reperfusion on mitochondrial energy metabolism and, accordingly, on the glutamate transporters function, preventing RGC apoptosis in the rats (Nucci et al., 2007). Excessive activation of glutamate receptors via the excitotoxic cascade leads to the MPTP formation and release of a proapoptotic factor, the cytochrome C, from the mitochondrial intermembrane space into the cytosol. Remarkably, CoQ10 inhibits this cascade by maintaining MPTP in the closed conformation, preventing apoptosis (Papucci et al., 2003).
The main concern about the topical administration was the concentration of CoQ10 at the retinal and vitreal level reached after the instillation of the eye drops. In this regard, it has been reported that when CoQ10 in association with Vit E eye drops are topically applied to the cornea, CoQ10 reaches the retina, substantially increasing local CoQ10 concentration and protecting retinal layers from apoptosis, in a mouse model of kainate-induced retinal damage. In addition, patients undergoing pars plana vitrectomy, who were administered CoQ10 in association with Vit E eye drops 1 hour before surgery, showed the presence of CoQ10 in the collected vitreous samples, thus confirming the ability of CoQ10 to reach the posterior ocular tissues (Fato et al., 2010; Lulli et al., 2012).
Oral administration of CoQ10 has also been reported to be neuroprotective in neurodegenerative diseases, as well as in cardiovascular diseases. CoQ10 supplementation has been reported to increase plasma CoQ10 concentrations, and the safety of high doses of orally-ingested CoQ10 over long periods has been well documented also in humans (Bhagavan and Chopra, 2006). Interestingly, it has been reported that CoQ10 is taken up by all tissues, including heart and brain mitochondria. This finding, together with growing evidence indicating that CoQ10 is neuroprotective in RGCs against IOP in vivo and in vitro, as well as against oxidative stress and excitotoxicity, suggests that CoQ10 could also be taken up by the retina and lead to a beneficial effect in glaucomatous retina (Lee et al., 2014). In a recent study on preglaucomatous DBA/2J and age-matched control DBA/2J-Gpnmb+ mice, diet supplementation with CoQ10 for 6 months was tested to assess the effects on glutamate excitotoxicity and oxidative stress-mediated RGC degeneration (Lee et al., 2014). Intriguingly, CoQ10 endorsed RGC survival, preserved the axons in the optic nerve head, and inhibited astroglial activation by reducing glial fibrillary acidic protein expression in the retina and optic nerve head of glaucomatous DBA/2J mice. Interestingly, CoQ10 significantly blocked the upregulation of N-methyl-D-aspartate receptor 1 and 2A, as well as of superoxide dismutase-2, heme oxygenase-1 protein expression in the retina of glaucomatous DBA/2J mice. Moreover, CoQ10 was able to prevent cell apoptosis by reducing Bax protein expression or by enhancing phosphorylated Bad protein expression. mtDNA content and mitochondrial transcription factor A/oxidative phosphorylation complex IV expression in the retina of glaucomatous DBA/2J mice were also preserved by CoQ10 supplementation. This suggest that CoQ10 may have a beneficial potential for ameliorating glutamate excitotoxicity and oxidative stress mediated glaucomatous neurodegeneration in the retina (Lee et al., 2014).
Nowadays, the major translational problem in monitoring new drug efficacy in glaucoma patients is the lack of objective diagnostic instruments to diagnose and monitor glaucoma progression since the early stage of the disease. Clinicians have to rely on visual field test, which is not particularly sensitive and accurate, and need a long period of follow-up. On the contrary, monitoring RGC apoptosis, which is an early event in glaucoma, could have a fundamental role. DARC (detection of apoptosing retinal cells) is a novel imaging technology able to detect RGC apoptosis in vivo (Davis et al., 2017). In a recent study by Davis et al. (2017) CoQ10/TPGS micelles, but not TPGS alone, resulted neuroprotective against mitochondrial targeted cytotoxic insults in mixed murine retinal cultures containing RGCs. Pre-treatment of this cell cultures with CoQ10/TPGS micelles significantly reduced their vulnerability to dimethyl sulfoxide and paraquat induced cytotoxicity. Moreover, CoQ10/TPGS micelles exhibited a significant neuroprotective effect in the Morrison’s ocular hypertension model. In this regard, DARC displayed fewer apoptotic retinal cells in CoQ10/TPGS treated Adult Dark Agouti rats compared to eyes receiving TPGS only micelles or ocular hypertension only eyes. For this reason, the Authors suggested an effectiveness of CoQ10/TPGS treatment in patients at risk of IOP spikes such as those who undergoes posterior capsulotomy or in pigment dispersion and Posner-Schlossman syndromes (Davis et al., 2017).
The effect of administration of CoQ10 in association with Vit E in eye drops formula on pattern-evoked retinal and cortical responses using pattern electroretinogram and visual-evoked potential, respectively, has been evaluated in open angle glaucoma patients, at baseline and after 6 and 12 months of instillation. Forty-three open angle glaucoma patients were included in the study, 22 of which were treated with CoQ10 in association with Vit E eye drops in addition to beta-blocker monotherapy, and 21 were treated only with beta-blockers. Remarkably, patients undergoing CoQ10 in association with Vit E additional treatment experimented a beneficial effect on the inner retinal function, showing pattern electroretinogram improvement, and consequent enhancement of the visual cortical responses, highlighted by visual-evoked potential enhancement, compared to those only treated with beta-blocker (Parisi et al., 2014).
Another possible application of CoQ10 emerged from a randomized clinical study on pseudo-exfoliative glaucoma patients who underwent phacoemulsification and intraocular lens implantation surgery. Patients were randomly divided into two groups. The first group topically received CoQ10 and Vit-E TPGS eye drops twice daily for one month preoperatively in addition to a prostaglandin agent, the second only received a prostaglandin agent. Interestingly, superoxide dismutase level, which is and oxidative stress markers, was significantly lower in the group treated with CoQ10 and Vit-E than in the other group, who had not received topical CoQ10 in association with Vit E during 1-month follow-up period. This result may indicate possible helpful effect of topical CoQ10 in association with Vit E on superoxide dismutase level (Ozates et al., 2018).
In conclusion, there is an increasing body of evidence supporting the potential beneficial effect of CoQ10 in protecting neuroretinal cells from oxidative damage. Interestingly, the efficacy of an ophthalmic solution of CoQ10 in association with Vit E added to the pharmacological treatment with prostaglandin analogue monotherapy on visual fields progression is under assessment in an ongoing randomized, double blind, controlled trial evaluating patients affected by primary open angle glaucoma. The study is sponsored by the Italian “Istituto di Ricerche Farmacologiche Mario Negri” and involves 17 ophthalmological centers in different parts of Italy. The study aims to enroll 612 primary open angle glaucoma patients to evaluate, as primary endpoint, the time to progression, defined as the time between baseline visit and the visit with the first evidence of progression in either eye is detected. As secondary points, velocity of visual field loss, determined by guided progression analysis software, variations of retinal nerve fiber layer thickness, by means of optical coherence tomography, and IOP and best corrected visual acuity changes from baseline will be assessed. These data will shed new light on CoQ10 in association with Vit E eye drops as neuroprotective approach to reduce the progression of ocular damage induced by glaucoma.
Overall, preliminary results in literature on CoQ10 are promising in glaucoma, as well as in other neurodegenerative conditions, thus endorsing the potential utility of CoQ10 in the treatment of glaucoma.
Footnotes
Financial support: None.
Conflicts of interest: Dr. Martucci is consultant for Visufarma S.p.A. Professor Nucci has no conflict of interest.
Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
C-Editors: Zhao M, Yu J; T-Editor: Liu XL
References
- 1.Ahmadinejad F, Geir Moller S, Hashemzadeh-Chaleshtori M, Bidkhori G, Jami MS. Molecular mechanisms behind free radical scavengers function against oxidative stress. Antioxidants (Basel) 2017;6:E51. doi: 10.3390/antiox6030051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhagavan HN, Chopra RK. Coenzyme Q10: absorption, tissue uptake, metabolism and pharmacokinetics. Free Radic Res. 2006;40:445–453. doi: 10.1080/10715760600617843. [DOI] [PubMed] [Google Scholar]
- 3.Carelli V, La Morgia C, Valentino ML, Barboni P, Ross-Cisneros FN, Sadun AA. Retinal ganglion cell neurodegeneration in mitochondrial inherited disorders. Biochim Biophys Acta. 2009;1787:518–528. doi: 10.1016/j.bbabio.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 4.Davis BM, Tian K, Pahlitzsch M, Brenton J, Ravindran N, Butt G, Malaguarnera G, Normando EM, Guo L, Cordeiro MF. Topical coenzyme Q10 demonstrates mitochondrial-mediated neuroprotection in a rodent model of ocular hypertension. Mitochondrion. 2017;36:114–123. doi: 10.1016/j.mito.2017.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fato R, Bergamini C, Leoni S, Pinna A, Carta F, Cardascia N, Ferrari TM, Sborgia C, Lenaz G. Coenzyme Q10 vitreous levels after administration of coenzyme Q10 eyedrops in patients undergoing vitrectomy. Acta Ophthalmol. 2010;88:e150–151. doi: 10.1111/j.1755-3768.2009.01632.x. [DOI] [PubMed] [Google Scholar]
- 6.Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M Early Manifest Glaucoma Trial Group. Reduction of intraocular pressure and glaucoma progression: results from the early manifest glaucoma trial. Arch Ophthalmol. 2002;120:1268–1279. doi: 10.1001/archopht.120.10.1268. [DOI] [PubMed] [Google Scholar]
- 7.Ito YA, Di Polo A. Mitochondrial dynamics, transport, and quality control: A bottleneck for retinal ganglion cell viability in optic neuropathies. Mitochondrion. 2017;36:186–192. doi: 10.1016/j.mito.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 8.Lee D, Shim MS, Kim KY, Noh YH, Kim H, Kim SY, Weinreb RN, Ju WK. Coenzyme Q10 inhibits glutamate excitotoxicity and oxidative stress-mediated mitochondrial alteration in a mouse model of glaucoma. Invest Ophthalmol Vis Sci. 2014;55:993–1005. doi: 10.1167/iovs.13-12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee S, Van Bergen NJ, Kong GY, Chrysostomou V, Waugh HS, O’Neill EC, Crowston JG, Trounce IA. Mitochondrial dysfunction in glaucoma and emerging bioenergetic therapies. Exp Eye Res. 2011;93:204–212. doi: 10.1016/j.exer.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 10.Lulli M, Witort E, Papucci L, Torre E, Schipani C, Bergamini C, Dal Monte M, Capaccioli S. Coenzyme Q10 instilled as eye drops on the cornea reaches the retina and protects retinal layers from apoptosis in a mouse model of kainate-induced retinal damage. Invest Ophthalmol Vis Sci. 2012;53:8295–8302. doi: 10.1167/iovs.12-10374. [DOI] [PubMed] [Google Scholar]
- 11.Mancino R, Martucci A, Cesareo M, Giannini C, Corasaniti MT, Bagetta G, Nucci C. Glaucoma and Alzheimer disease: one age-related neurodegenerative disease of the brain. Curr Neuropharmacol. 2018a;16:971–977. doi: 10.2174/1570159X16666171206144045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mancino R, Cesareo M, Martucci A, Di Carlo E, Ciuffoletti E, Giannini C, Morrone LA, Nucci C, Garaci F. Neurodegenerative process linking the eye and the brain. Curr Med Chem. 2018b doi: 10.2174/0929867325666180307114332. doi: 10.2174/0929867325666180307114332. [DOI] [PubMed] [Google Scholar]
- 13.Nucci C, Martucci A, Giannini C, Morrone LA, Bagetta G, Mancino R. Neuroprotective agents in the management of glaucoma. Eye (London, England) 2018;32:938–945. doi: 10.1038/s41433-018-0050-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nucci C, Russo R, Martucci A, Giannini C, Garaci F, Floris R, Bagetta G, Morrone LA. New strategies for neuroprotection in glaucoma, a disease that affects the central nervous system. Eur J Pharmacol. 2016;787:119–126. doi: 10.1016/j.ejphar.2016.04.030. [DOI] [PubMed] [Google Scholar]
- 15.Nucci C, Tartaglione R, Cerulli A, Mancino R, Spano A, Cavaliere F, Rombola L, Bagetta G, Corasaniti MT, Morrone LA. Retinal damage caused by high intraocular pressure-induced transient ischemia is prevented by coenzyme Q10 in rat. Int Rev Neurobiol. 2007;82:397–406. doi: 10.1016/S0074-7742(07)82022-8. [DOI] [PubMed] [Google Scholar]
- 16.Ozates S, Elgin KU, Yilmaz NS, Demirel OO, Sen E, Yilmazbas P. Evaluation of oxidative stress in pseudo-exfoliative glaucoma patients treated with and without topical coenzyme Q10 and vitamin E. Eur J Ophthalmol. 2018 doi: 10.1177/1120672118779486. doi: 10.1177/1120672118779486. [DOI] [PubMed] [Google Scholar]
- 17.Papucci L, Schiavone N, Witort E, Donnini M, Lapucci A, Tempestini A, Formigli L, Zecchi-Orlandini S, Orlandini G, Carella G, Brancato R, Capaccioli S. Coenzyme q10 prevents apoptosis by inhibiting mitochondrial depolarization independently of its free radical scavenging property. J Biol Chem. 2003;278:28220–28228. doi: 10.1074/jbc.M302297200. [DOI] [PubMed] [Google Scholar]
- 18.Parisi V, Centofanti M, Gandolfi S, Marangoni D, Rossetti L, Tanga L, Tardini M, Traina S, Ungaro N, Vetrugno M, Falsini B. Effects of coenzyme Q10 in conjunction with vitamin E on retinal-evoked and cortical-evoked responses in patients with open-angle glaucoma. J Glaucoma. 2014;23:391–404. doi: 10.1097/IJG.0b013e318279b836. [DOI] [PubMed] [Google Scholar]
- 19.Qu J, Kaufman Y, Washington I. Coenzyme Q10 in the human retina. Invest Ophthalmol Vis Sci. 2009;50:1814–1818. doi: 10.1167/iovs.08-2656. [DOI] [PubMed] [Google Scholar]
- 20.Russo R, Rotiroti D, Tassorelli C, Nucci C, Bagetta G, Bucci MG, Corasaniti MT, Morrone LA. Identification of novel pharmacological targets to minimize excitotoxic retinal damage. Int Rev Neurobiol. 2009;85:407–423. doi: 10.1016/S0074-7742(09)85028-9. [DOI] [PubMed] [Google Scholar]
- 21.Russo R, Cavaliere F, Rombola L, Gliozzi M, Cerulli A, Nucci C, Fazzi E, Bagetta G, Corasaniti MT, Morrone LA. Rational basis for the development of coenzyme Q10 as a neurotherapeutic agent for retinal protection. Prog Brain Res. 2008;173:575–582. doi: 10.1016/S0079-6123(08)01139-4. [DOI] [PubMed] [Google Scholar]
- 22.Spindler M, Beal MF, Henchcliffe C. Coenzyme Q10 effects in neurodegenerative disease. Neuropsychiatr Dis Treat. 2009;5:597–610. doi: 10.2147/ndt.s5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X, Tohari AM, Marcheggiani F, Zhou X, Reilly J, Tiano L, Shu X. Therapeutic potential of co-enzyme Q10 in retinal diseases. Curr Med Chem. 2017;24:4329–4339. doi: 10.2174/0929867324666170801100516. [DOI] [PubMed] [Google Scholar]