Subarachnoid hemorrhage by a ruptured cerebral aneurysm remains the most devastating cerebrovascular disorders with high morbidity and mortality (Suzuki et al., 2017), which are mainly caused by early brain injury and delayed cerebral ischemia. Despite many experimental and clinical research efforts, the mechanisms of early brain injury as well as delayed cerebral ischemia remain unclarified. However, recent works have suggested that tenascin-C, which is a special type of secreted extracellular matrix proteins, is promising as a novel therapeutic target against post-subarachnoid hemorrhage early brain injury and delayed cerebral ischemia.
Neurovascular events after subarachnoid hemorrhage: Delayed cerebral ischemia is an important post-subarachnoid hemorrhage neurovascular event, because delayed cerebral ischemia potentially can be prevented or treated. Classically, research efforts have focused on cerebral vasospasm, the delayed narrowing of large-capacitance arteries at the base of the brain, as a main cause of delayed cerebral ischemia. However, after some clinical trials targeting anti-vasospasm therapies failed to improve post-subarachnoid hemorrhage outcome, microcirculatory disturbance has attracted subarachnoid hemorrhage researchers’ attention to explore the mechanisms of delayed cerebral ischemia (Suzuki et al., 2018c). Delayed microcirculatory disturbance is considered to occur secondary to early brain injury that is characterized by neuronal apoptosis and global cerebral edema at the onset to during pre-vasospasm period post-subarachnoid hemorrhage (Suzuki et al., 2018c). Early brain injury consists of brain capillary endothelial injury, blood-brain barrier disruption, neuroinflammation, cortical spreading depolarization, loss of autoregulation, and others (Suzuki et al., 2018c). An aneurysmal rupture produces blood-derived substances (heme, fibrinogen, and so on), released intracellular components associated with tissue injuries, and the resultant inflammation-related proteins in the subarachnoid space or brain, which activate Toll-like receptor 4 (TLR4)-mediated signaling cascades and then upregulate proinflammatory mediators and tenascin-C, causing early brain injury and delayed cerebral ischemia (Okada and Suzuki, 2017).
Tenascin-C and subarachnoid hemorrhage: Tenascin-C is one of pleiotropic matricellular proteins that are barely expressed in healthy adult tissues, but transiently upregulated in response to inflammatory reactions or at tissue injuries (Liu et al., 2018). Tenascin-C has been extensively studied in the central nervous system: tenascin-C is expressed in radial glial cells and plays a crucial role in normal brain development (Song and Dityatev, 2018). Tenascin-C expression is decreased in brain 2–3 weeks after birth, but tenascin-C is still important to hippocampal synaptic plasticity and synchronized neural network activities by controlling postsynaptic L-type Ca2+ channels in mature brain (Song and Dityatev, 2018). In addition, even after brain maturation, tenascin-C is highly upregulated in reactive astrocytes, injured neurons and brain capillary endothelial cells in pathological conditions, and binds to receptors and other extracellular proteins, modulating signal transduction including proapoptotic and proinflammatory pathways (Liu et al., 2018; Song and Dityatev, 2018; Suzuki et al., 2018c).
In experimental subarachnoid hemorrhage studies, because platelet-derived growth factor is known as a strong inducer of tenascin-C, a selective platelet-derived growth factor receptor inhibitor of imatinib was used and demonstrated to prevent post-subarachnoid hemorrhage tenascin-C induction in brains and cerebral arteries (Shiba et al., 2014; Suzuki et al., 2018c). Post-subarachnoid hemorrhage tenascin-C downregulation inactivated mitogen-activated protein kinases (MAPKs), while an intracisternal administration of tenascin-C activated MAPKs in post-subarachnoid hemorrhage brains and cerebral arteries: neuronal apoptosis and cerebral vasospasm developed associated with MAPK activation (Shiba et al., 2014; Suzuki et al., 2018c). Therefore, it is suggested that tenascin-C is induced by platelet-derived growth factor after subarachnoid hemorrhage and causes early brain injury in terms of neuronal apoptosis, and cerebral vasospasm via MAPK-mediated signaling pathways.
Tenascin-C knockout and post-subarachnoid hemorrhage brain injuries: Recently, tenascin-C knockout mice have been used to demonstrate the relationships between tenascin-C and early brain injury or cerebral vasospasm. As with other matricellular proteins, tenascin-C knockout mice develop normally and show no abnormal reactions in steady-state condition, but differently react to pathological stimuli (Suzuki et al., 2018a). In experimental subarachnoid hemorrhage, tenascin-C knockout was demonstrated to prevent blood-brain barrier disruption and brain edema formation by inhibiting MAPK-mediated matrix metalloproteinase-9 activation in brain capillary endothelial cells, whereas the protective effects of tenascin-C knockout were reversed by exogenous tenascin-C administration (Suzuki et al., 2018c). Tenascin-C knockout also suppressed post-subarachnoid hemorrhage induction of another matricellular protein periostin, which was upregulated in brain capillary endothelial cells and neurons (Liu et al., 2017). Tenascin-C and periostin directly induced and positively fed back each other after subarachnoid hemorrhage, and caused and aggravated post-subarachnoid hemorrhage early brain injury at least in terms of blood-brain barrier disruption, of which the mechanisms consisted of MAPK-mediated matrix metalloproteinase-9 activation (Liu et al., 2017). In another study, tenascin-C knockout prevented not only post-subarachnoid hemorrhage neurological impairment, but also neuroinflammation and caspase-dependent neuronal apoptosis, which was at least partly mediated by the signaling cascades consisting of upregulation of TLR4, phosphorylation of nuclear factor-κB, and induction of proinflammatory cytokines in neurons (Liu et al., 2018). Tenascin-C is known to activate TLR4 and then to induce upregulation and phosphorylation of nuclear factor-κB, which in turn upregulates interleukins-1β and -6 (Okada and Suzuki, 2017). Overexpressed interleukins-1β and/or -6 cause apoptosis by triggering caspase cascade reactions (Okada and Suzuki, 2017). In another recent study, effects of tenascin-C knockout on cerebral vasospasm were examined in mice: tenascin-C was strongly induced in the periarterial inflammatory cells, as well as in spastic cerebral artery walls after subarachnoid hemorrhage (Fujimoto et al., 2018). Tenascin-C knockout suppressed post-subarachnoid hemorrhage periarterial inflammatory cell infiltration, and activation of MAPKs in cerebral arterial smooth muscle cells, leading to better neurobehavioral function and less severe cerebral vasospasm (Fujimoto et al., 2018).
Above experimental studies using tenascin-C-knockout subarachnoid hemorrhage mice consistently indicated that tenascin-C caused neuroinflammation, blood-brain barrier disruption, neuronal apoptosis, and cerebral vasospasm, and that tenascin-C knockout always exerted protective effects against early brain injury and cerebral vasospasm. Although the mechanisms that tenascin-C induces various types of deleterious effects after subarachnoid hemorrhage are not exactly clarified and need further studies, TLR4-mediated MAPK and nuclear factor-κB pathways may be important (Okada and Suzuki, 2017). A better understanding of the role of tenascin-C will provide valuable insights into the pathogenesis of neurovascular events after subarachnoid hemorrhage, and elucidating the pathogenesis of tenascin-C-mediated early brain injury and cerebral vasospasm may lead to the development of clinically effective therapy and the improvement of therapeutic outcomes (Figure 1).
Figure 1.
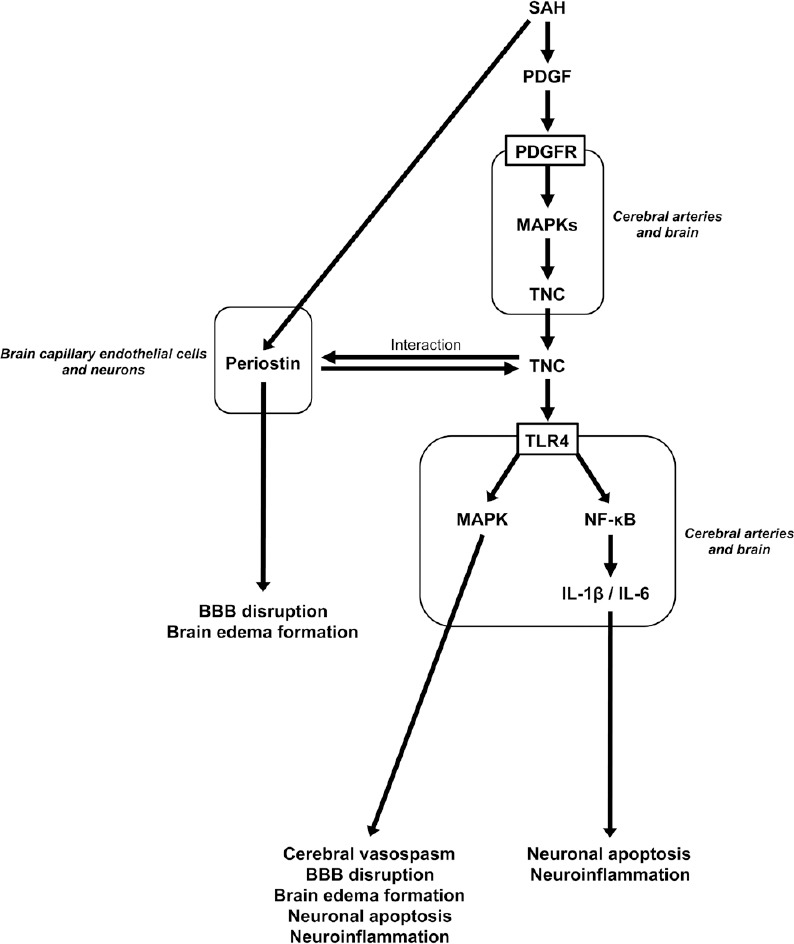
Possible molecular mechanisms for tenascin-C (TNC) to induce pathological conditions underlying delayed cerebral ischemia after subarachnoid hemorrhage (SAH).
BBB: Blood-brain barrier; IL: interleukin; MAPK: mitogen-activated protein kinase; NF-κB: nuclear factor-kappa B; PDGF: platelet-derived growth factor; PDGFR: PDGF receptor; TLR4: Toll-like receptor 4.
Clinical translation of experimental tenascin-C research in subarachnoid hemorrhage: As described above, experimental studies suggest that tenascin-C is a causative factor, and can be a therapeutic target for early brain injury, cerebral vasospasm, and delayed cerebral ischemia. Tenascin-C is induced preceding the development of the pathophysiological events, and secreted into peripheral blood and cerebrospinal fluid (Suzuki et al., 2018a). Moreover, tenascin-C concentrations in blood or cerebrospinal fluid can be easily measured using an enzyme-linked immunosorbent assay test (Suzuki et al., 2018a). Thus, at the present time, the most practical clinical application is possibly to use tenascin-C as a biomarker.
If it is true that early brain injury causes delayed cerebral ischemia consisting of cerebral vasospasm and/or delayed microcirculatory disturbance, the discovery of reliable biomarkers of early brain injury is very useful for clinicians, neurosurgeons or intensivists. Early brain injury is a concept based on basic experiments, and means any brain pathophysiology except for iatrogenic brain injuries that occurs before post-subarachnoid hemorrhage delayed cerebral ischemia development. In a clinical setting, it is impossible to diagnose early brain injury exactly, and therefore loss of consciousness at ictus, poor initial clinical grades, more massive subarachnoid hemorrhage and intraventricular hematoma, global cerebral edema, or inflammatory mediators may be used as a surrogate marker of early brain injury (Suzuki et al., 2018c). However, these clinical markers are somewhat subjective, or not so specific to early brain injury (Suzuki et al., 2018a). Therefore, highly specific biomarkers that are increased in blood or cerebrospinal fluid reflecting early brain injury at days 1–3 or at latest before the development of delayed cerebral ischemia may allow monitoring of the response to treatment for early brain injury and facilitate earlier diagnosis of delayed cerebral ischemia. We chronologically measured peripheral blood levels of an isoform of tenascin-C with an extra alternatively spliced fibronectin type III domain termed C (tenascin-C-C) in aneurysmal subarachnoid hemorrhage patients, and revealed that the plasma tenascin-C-C levels peaked at days 4–6, a few days before the development of delayed cerebral ischemia: at the time, the tenascin-C-C levels were significantly higher in patients with subsequent development of angiographic vasospasm and delayed cerebral ischemia compared with those without (Suzuki et al., 2018b). On the other hand, tenascin-C-C levels in the cerebrospinal fluid peaked at days 1–3 post-subarachnoid hemorrhage possibly reflecting the severity of early brain injury, and then decreased as time passed (Suzuki et al., 2018a). Although it remains unknown why the time course of tenascin-C-C levels after subarachnoid hemorrhage is different between peripheral blood and cerebrospinal fluid, tenascin-C-C in both peripheral blood and cerebrospinal fluid could be used to predict or timely diagnose the development of angiographic vasospasm and delayed cerebral ischemia in a clinical setting (Suzuki et al., 2018a, b). As tenascin-C has many different isoforms with different functions (Suzuki et al., 2018c), the measurements of specific isoforms other than tenascin-C-C may be useful for diagnosing individual pathological conditions underlying delayed cerebral ischemia in the future.
As to the treatment, cilostazol, an antiplatelet agent, has been tested in clinical subarachnoid hemorrhage, because it inhibits tenascin-C expression at the transcriptional level possibly through the activation of cyclic adenosine monophosphate–protein kinase A signaling pathway, which inactivates MAPK pathway (Suzuki et al., 2018b). In our recent clinical study, cilostazol treatment suppressed plasma tenascin-C-C levels at least from days 1–3 to days 10–12 post-subarachnoid hemorrhage, and showed dose-dependent effects against delayed cerebral ischemia, leading to improved outcome (Suzuki et al., 2018b). Multivariate analyses revealed that the highest tested dosage of 300 mg/day cilostazol treatment was an independent determinant against poor outcomes post-subarachnoid hemorrhage (Suzuki et al., 2018b). Because of its availability and safety, non-specific inhibition of tenascin-C by high-dose cilostazol would be an effective therapeutic choice for improving outcome after subarachnoid hemorrhage at this time. However, some new therapies have been developed to directly, specifically and selectively inhibit tenascin-C (Suzuki et al., 2018c). In the near future, such a selective tenascin-C inhibition may prove to be a novel approach for the prevention and treatment of delayed cerebral ischemia, resulting in the improvement of outcomes in subarachnoid hemorrhage patients.
Further studies focusing on tenascin-C will provide more valuable information that tenascin-C potentially plays pivotal roles in neurovascular events after subarachnoid hemorrhage. It is to be hoped that molecular target drugs for tenascin-C will be developed and established in the setting of aneurysmal subarachnoid hemorrhage.
This work was funded by a grant-in-aid for Scientific Research from Japan Society for the Promotion of Science, No. 17K10825 (to HS) and 17K16640 (to MS).
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
C-Editors: Zhao M, Yu J; T-Editor: Liu XL
References
- 1.Fujimoto M, Shiba M, Kawakita F, Liu L, Shimojo N, Imanaka-Yoshida K, Yoshida T, Suzuki H. Effects of tenascin-C knockout on cerebral vasospasm after experimental subarachnoid hemorrhage in mice. Mol Neurobiol. 2018;55:1951–1958. doi: 10.1007/s12035-017-0466-x. [DOI] [PubMed] [Google Scholar]
- 2.Liu L, Kawakita F, Fujimoto M, Nakano F, Imanaka-Yoshida K, Yoshida T, Suzuki H. Role of periostin in early brain injury after subarachnoid hemorrhage in mice. Stroke. 2017;48:1108–1111. doi: 10.1161/STROKEAHA.117.016629. [DOI] [PubMed] [Google Scholar]
- 3.Liu L, Fujimoto M, Nakano F, Nishikawa H, Okada T, Kawakita F, Imanaka-Yoshida K, Yoshida T, Suzuki H. Deficiency of tenascin-C alleviates neuronal apoptosis and neuroinflammation after experimental subarachnoid hemorrhage in mice. Mol Neurobiol. 2018;55:8346–8354. doi: 10.1007/s12035-018-1006-z. [DOI] [PubMed] [Google Scholar]
- 4.Okada T, Suzuki H. Toll-like receptor 4 as a possible therapeutic target for delayed brain injuries after aneurysmal subarachnoid hemorrhage. Neural Regen Res. 2017;12:193–196. doi: 10.4103/1673-5374.200795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shiba M, Fujimoto M, Imanaka-Yoshida K, Yoshida T, Taki W, Suzuki H. Tenascin-C causes neuronal apoptosis after subarachnoid hemorrhage in rats. Transl Stroke Res. 2014;5:238–247. doi: 10.1007/s12975-014-0333-2. [DOI] [PubMed] [Google Scholar]
- 6.Song I, Dityatev A. Crosstalk between glia, extracellular matrix and neurons. Brain Res Bull. 2018;136:101–108. doi: 10.1016/j.brainresbull.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki H, Nishikawa H, Kawakita F. Matricellular proteins as possible biomarkers for early brain injury after aneurysmal subarachnoid hemorrhage. Neural Regen Res. 2018a;13:1175–1178. doi: 10.4103/1673-5374.235022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suzuki H, Shiba M, Nakatsuka Y, Nakano F, Nishikawa H. Higher cerebrospinal fluid pH may contribute to the development of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Transl Stroke Res. 2017;8:165–173. doi: 10.1007/s12975-016-0500-8. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki H, Nakatsuka Y, Yasuda R, Shiba M, Miura Y, Terashima M, Suzuki Y, Hakozaki K, Goto F, Toma N. Dose-dependent inhibitory effects of cilostazol on delayed cerebral infarction after aneurysmal subarachnoid hemorrhage. Transl Stroke Res. 2018b doi: 10.1007/s12975-018-0650-y. doi: 10.1007/s12975-018-0650-y. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki H, Fujimoto M, Kawakita F, Liu L, Nakatsuka Y, Nakano F, Nishikawa H, Okada T, Kanamaru H, Imanaka-Yoshida K, Yoshida T, Shiba M. Tenascin-C in brain injuries and edema after subarachnoid hemorrhage: Findings from basic and clinical studies. J Neurosci Res. 2018c doi: 10.1002/jnr.24330. doi: 10.1002/jnr.24330. [DOI] [PubMed] [Google Scholar]


