Keywords: nerve regeneration, spinal cord injury, walking, gait quality, plantar pressure system, plantar pressure distribution, speed, plantar pressure, impulse, contact area, symmetry index, neural regeneration
Abstract
The main goal of spinal cord rehabilitation is to restore walking ability and improve walking quality after spinal cord injury (SCI). The spatiotemporal parameters of walking and the parameters of plantar pressure can be obtained using a plantar pressure analysis system. Previous studies have reported step asymmetry in patients with bilateral SCI. However, the asymmetry of other parameters in patients with SCI has not been reported. This was a prospective, cross-sectional study, which included 23 patients with SCI, aged 48.1 ± 14.5 years, and 28 healthy subjects, aged 47.1 ± 9.8 years. All subjects underwent bare foot walking on a plantar pressure measurement device to measure walking speed and spatiotemporal parameters. Compared with healthy subjects, SCI patients had slower walking speed, longer stride time and stance time, larger stance phase percentage, and shorter stride length. The peak pressures under the metatarsal heads and toe were lower in SCI patients than in healthy subjects. In the heel, regional impulse and the contact area percentage in SCI patients were higher than those in healthy subjects. The symmetry indexes of stance time, step length, maximum force, impulse and contact area were increased in SCI patients, indicating a decline in symmetry. The results confirm that the gait quality, including spatiotemporal variables and plantar pressure parameters, and symmetry index were lower in SCI patients compared with healthy subjects. Plantar pressure parameters and symmetry index could be sensitive quantitative parameters to improve gait quality of SCI patients. The protocols were approved by the Clinical Research Ethics Committee of Shengjing Hospital of China Medical University (approval No. 2015PS54J) on August 13, 2015. This trial was registered in the ISRCTN Registry (ISRCTN42544587) on August 22, 2018. Protocol version 1.0.
Chinese Library Classification No. R493; R741
Introduction
Severe injury to the spinal cord, accompanied by neurological and sensorimotor disorders, severely limits mobility (Lucareli et al., 2011; Hasegawa et al., 2014; Wu et al., 2015; Narin et al., 2017; Takazawa et al., 2018). In the majority of patients who retain some walking ability, movements are limited to the confines of their homes or to short distances, and patients are dependent upon the assistance of mechanical devices or caregivers (Hardin et al., 2013; Shank et al., 2018). Hence, one central rehabilitative objective shared by these patients is the recovery and enhanced quality of ambulation (Lapointe et al., 2001; Sohn et al., 2018).
To obtain clinical data on the walking capacity of patients with injuries to the spinal cord, quantitative measures are needed that assess spatiotemporal, kinematic and kinetic parameters to reveal the fundamental impairments involved, which can be compared with healthy individuals (Arazpour et al., 2014; Yang et al., 2015). Tools that provide dependable and reproducible measurements in the diagnosis and developmental monitoring of various conditions make it possible for the identification of minor discrepancies that differentiate between healthy and pathological patterns and to highlight specific responses to treatment (Krizsan-Agbas et al., 2014; Safayi et al., 2015). The plantar pressure system focuses on the performance of the feet in response to the ground reaction forces experienced during everyday activities as the feet provide the support base for the body (Li et al., 2016). This system makes it possible to obtain quantitative data related to the walking pattern, including spatiotemporal parameters and pressure distribution (Nsenga Leunkeu et al., 2014; Lim et al., 2016; Zhang et al., 2016). By examination of pressure distribution, the interface pressure between the sole and foot plantar surface can be identified. There have been many plantar pressure-related studies since the 1950s investigating flat foot, cavus foot, diabetes mellitus patients, pressure ulcer, stroke patients, and obese subjects (Thijs et al., 2007; Razak et al., 2012; Chiu et al., 2013; Yan et al., 2013; Fernandez-Seguin et al., 2014; Brown et al., 2016; Mohd Said et al., 2016; Song-Hua et al., 2017), but previous studies have rarely assessed plantar pressure for spinal cord injury (SCI) patients (Beseler et al., 2012). Most of the studies measuring the gait of SCI patients have used a digital camera system to measure the spatiotemporal variables (Tamburella et al., 2013; Pramodhyakul et al., 2014; Arazpour et al., 2017; Quarrington et al., 2018). Spatiotemporal variables can also be determined by a plantar pressure plate system (Beseler et al., 2012; Greenhalgh et al., 2014), and studies of the static or dynamic plantar pressure measurements can highlight a range of typical attributes of plantar pressure distribution by which normal and impaired patterns can be compared, thus assisting in the recognition of underlying causes and consequent development of pathological gaits (Razak et al., 2012; Lim et al., 2016). To our knowledge, no studies have used this method to describe and compare the plantar pressure distribution between SCI patients and normal subjects.
Good walking quality entails not only walking fast (Aguirre-Guemez et al., 2017), but also walking symmetrically. In stroke patients and amputees, walking symmetry is an important variable to evaluate walking quality (Hsu et al., 2003; Plotnik et al., 2013; Yu et al., 2014). A recent study has reported step length asymmetry in SCI patients (Kumprou et al., 2017). However, it remains unclear if there is asymmetry of other walking variables in SCI patients, which could provide sensitive parameters to indicate the improvement of gait quality.
The purpose of this study is to determine the difference of gait variables, plantar pressure parameters and symmetry index between SCI patients and healthy subjects, and to identify sensitive quantitative parameters.
Participants and Methods
Design
This study was a prospective, cross-sectional design.
Participants
A total of 51 subjects (23 SCI patients and 28 healthy subjects) were recruited from the Rehabilitation Department of Shengjing Hospital of China Medical University, China. All subjects signed the informed consent. The protocols were approved by the Clinical Research Ethics Committee of Shengjing Hospital of China Medical University (approval No. 2015PS54J) on August 13, 2015.
Inclusion criteria of SCI patients
(1) Incomplete SCI, diagnosed with the American Spinal Cord Injury Association (ASIA) Impairment Scale (AIS) D (Armstrong et al., 2017; Yugue et al., 2018);
(2) The capacity for independent walking (with or without mechanical support) for a minimum of 15 minutes (Pramodhyakul et al., 2014; Guan et al., 2017).
Exclusion criteria of SCI patients
Indications or manifestations with the potential to impact upon mobility were employed as exclusion criteria:
-
(1)
Severe spasticity of the lower-extremity muscles rated as greater than or equal to 2 on the Modified Ashworth Scale;
-
(2)
Muscle or joint pain with an intensity rating greater than 5 out of 10 on a numerical scale;
-
(3)
The presence of cognitive or behavioral disorders;
-
(4)
Spinal or lower extremity deformities, such as scoliosis, kyphosis and equinovarus;
-
(5)
Clinical instability (Amatachaya et al., 2013; Guan et al., 2017).
Inclusion criteria of healthy subjects
For comparison with SCI patients, 28 healthy subjects of matching age (± 5 years) and sex who displayed none of the aforementioned conditions were recruited.
Exclusion criteria of healthy subjects
Healthy subjects with one or more of the following conditions were excluded: muscle or joint pain of lower extremity or spine, the presence of cognitive disorders, flat foot or cavus foot, or abnormal gait (Plotnik et al., 2013). Subjects were consulted and evaluated according to demographic factors, including age, height, weight, and body mass index.
Acquirement of walking pattern data
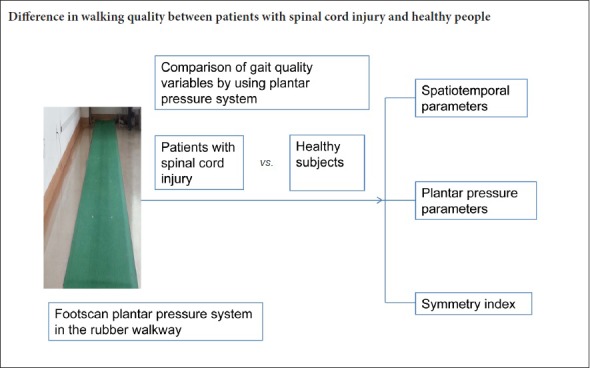
Walking pattern data were acquired using a 2-m long footscan plantar pressure plate system with 8192 resistive sensors, giving a resolution of 4 sensors per cm2 (RSscan International, Olen, Belgium) (Additional Figure 1 (173.1KB, tif) ). The platform was mounted on a secure flat surface, level with and centered upon a 10-m long rubber walkway. To prevent participants from adapting their walking pattern and aiming for the plate, the platform surface was covered with an overlayer of ethylene-vinyl acetate (Shore hardness A 70) (Li et al., 2016).
Footscan plantar pressure system in the rubber walkway.
Each subject undertook at least two practice walks along the plate walkway to familiarize them with, and hence feel at ease with, the experimental process. Following an adequate recuperation period, each subject was asked to walk in bare feet upon the footscan platform at their comfortable speed for at least three successful trials. Subjects reporting fatigue were permitted to rest for a minimum of 2 minutes between trials.
Data analysis
The footscan plate system partitioned the foot into the following ten anatomical regions: (i) heel lateral (HL), (ii) heel medial (HM), (iii) midfoot (MF), (iv) metatarsal 5 (M5), (v) metatarsal 4 (M4), (vi) metatarsal 3 (M3), (vii) metatarsal 2 (M2), (viii) metatarsal 1 (M1), (ix) toes 2 to 5 (T2–5), and (x) toe 1 (T1) (Yan et al., 2013).
Subjects’ spatiotemporal parameters, including walking speed, stride time, stance time, stride length, and step length, and pressure parameters, including peak planter pressure, impulse, maximum force (Max F) and contact area of each foot were extracted. The relative short step length side was defined as the weak side, and the other side was defined as the strong side. The average gait parameters were calculated.
To make the parameters comparable, some parameters were standardized as the percentage of partial parameter value to the sum of the whole parameter value.
The percentage of stance time to stride time was calculated as stance phase percentage:
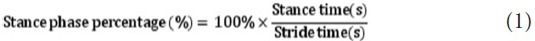
Percentage of regional impulse of the 10-distributional region to the sum of the whole region was calculated as regional impulse percentage:
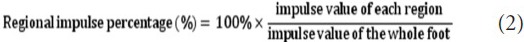
Percentage of regional contact area of the 10-distributional region to the sum of the whole foot contact area was calculated as regional contact area percentage:
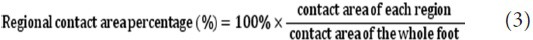
The following formula was used for computing the symmetry index (SI) of the gait variables (Hsu et al., 2003; Tyrell et al., 2011; Kumprou et al., 2017). Stance time SI, step length SI, Max F SI, impulse SI, and contact area SI were calculated separately. An SI value of 0 indicated the best symmetry.

Statistical analysis
The data were expressed as the mean ± SD. SPSS 16.0 software (SPSS, Chicago, IL, USA) was used for data analysis. The baseline demographics of the participants, along with the experimental outcomes, were interpreted by the application of descriptive statistics. The independent-sample t-test was used for intergroup analysis to compare the gait parameters of healthy and SCI subjects. Values with P < 0.05 were considered as statistically significant.
Results
Baseline demographics
Data of 51 subjects, including 28 healthy and 23 SCI subjects, were analyzed. The study did not detect any significant difference in the baseline demographics between the healthy and SCI groups (P > 0.05; Table 1).
Table 1.
Demographics of subjects with spinal cord injury (SCI) and healthy subjects

Spatiotemporal variables in SCI and healthy groups
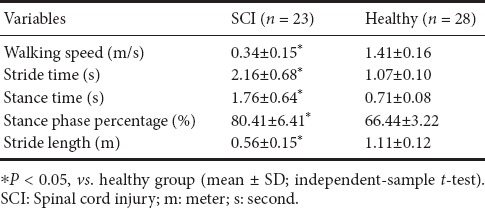
The walking speed was significantly slower in the SCI group (0.34 ± 0.15 m/s) than in the healthy group (1.41 ± 0.16 m/s) (P < 0.05; Table 2). The stride time was significantly longer and the stance time was significantly larger in the SCI group than in the healthy group (P < 0.05; Table 2). The stance phase percentage was also significantly larger in the SCI group than in the healthy group (80.41 ± 6.41%). Stride length was significantly shorter in the SCI group than in the healthy group (P < 0.05; Table 2).
Table 2.
Spatiotemporal variables in the SCI group and healthy group
Plantar pressure distribution in the SCI and healthy groups
The relative short step length side was defined as the weak side, and the other side was defined as the strong side. The parameters of the strong side and the weak side of the SCI group were compared with those in the healthy group separately.
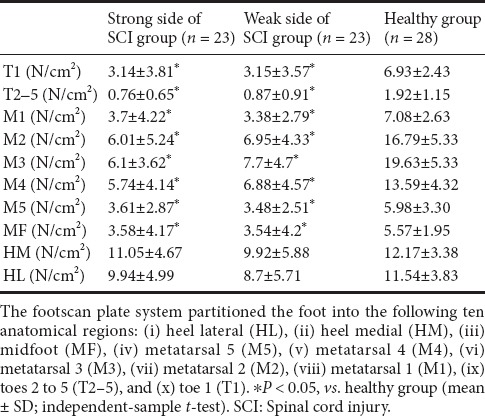
The highest peak plantar pressure in both sides was located in the heel (HM and HL) of SCI patients, and in the third metatarsal region (M3) of healthy subjects. The peak plantar pressure in each region was lower in SCI patients than in healthy subjects, and the difference was significant in the meta and toe regions (P < 0.05; Table 3).
Table 3.
Plantar pressure distribution in SCI group and healthy group
Regional impulse percentage and contact area percentage in different regions of SCI and healthy groups
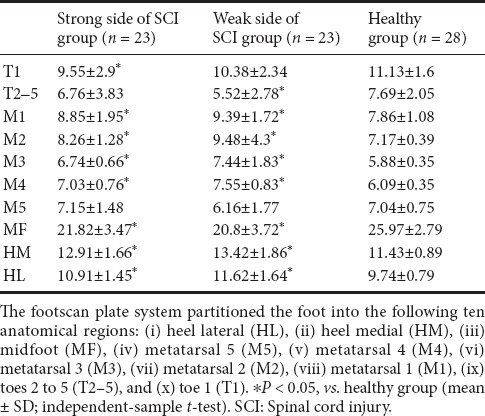
Bilateral heel (HM and HL) impulse percentage in SCI patients was significantly increased compared with that in healthy subjects. In T1, M3, midfoot of both sides and M2 of the strong side, the impulse percentage was significantly decreased in the SCI group compared with the healthy group (P < 0.05; Table 4).
Table 4.
Regional impulse percentage (%) in different regions of SCI and healthy groups

Significantly increased regional contact area percentage under the heel (HM and HL) and decreased regional contact area percentage under the midfoot were found in SCI patients. Under bilateral M1 to M4, the contact area percentage was significantly increased; under T2–5 on the weak side in SCI patients, the contact area percentage was significantly decreased (P < 0.05; Table 5).
Table 5.
Regional contact area percentage (%) in different regions of SCI and healthy groups
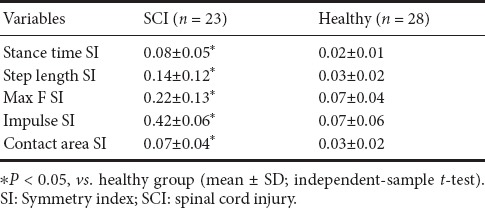
Symmetry index of SCI and healthy groups
When comparing the symmetry between SCI and healthy groups, the SI of the stance time and the step length were significantly larger in the SCI group than in the healthy group (P < 0.05; Table 6). The symmetry of whole foot plantar Max F, impulse, and contact area were compared between the healthy and SCI groups. In the SCI group, the SI of Max F, impulse and contact area were all significantly larger compared with those in the healthy group (P < 0.05; Table 6).
Table 6.
Comparison of SI between SCI and healthy groups
Discussion
Walking quality enhancement is one of the central rehabilitative objectives for SCI patients (Knippenberg et al., 2017; Gollie, 2018). In the present study, spatiotemporal variables and plantar pressure data were measured simultaneously. Compared with healthy subjects, SCI patients had slower walking speed, larger stance phase percentage, shorter stride length, and larger SI of stance time and step length. The data for plantar pressure indicated that the peak pressures under the metatarsal heads and toe were lower, regional impulse and the contact area percentage in the heel (HM and HL) were higher, and the SI of Max F, impulse and contact area were all significantly larger in SCI patients.
To perform exercises in daily life, adequate walking speed is a key determinant (Guo et al., 2012; Pramodhyakul et al., 2014). A study by Pramodhyakul et al. (2014) using a digital camera has shown that the subjects with incomplete SCI have slower walking speed, and lower stride length and cadence compared with healthy subjects (Pramodhyakul et al., 2014). A study by Tamburella et al. included 15 patients with chronic or subacute SCI, level D at the ASIA and 15 healthy controls (Tamburella et al., 2013). The motion analysis using the KineView Motion System revealed that the speed of SCI patients is slower than that in healthy controls. Their results indicate that the stride length is shorter, gait cycle time is longer, and stance phase is larger in SCI patients compared with healthy controls. Our results were in line with the previous studies. In the present study using the plantar pressure system, walking speed was slower in the SCI group (0.34 ± 0.15 m/s) than in the healthy group (1.41 ± 0.16 m/s). The stride time and the stance time were longer and the stance phase percentage was larger in the SCI group than in the healthy group, indicating that the propel swing phase was short. In SCI subjects, the activation of muscle is delayed and the increasing motor control instability may lead to the quick swings and reduce step length (Wang et al., 2013).
Previous studies exploring foot pressure patterns during gait when walking barefoot have shown that the highest mean pressure occurs under the third metatarsal head, and the second highest pressure region occurs under the heel (Soames, 1985; Razak et al., 2012; Yan et al., 2013). This is consistent with the present study’s results of healthy subjects, however the highest peak plantar pressure in patients with SCI was located in the heel (HM and HL), and the peak plantar pressure in each region was lower, especially in meta and toe regions. In the heel (HM and HL), the regional percentage parameters of regional impulse and the contact area in SCI patients were higher than those in healthy subjects. There is no comparable study on the pressure distribution of SCI subjects. A study of patients with multiple sclerosis reported similar results; it suggested that the decrease of plantar flexion and of force propulsion in the swing phase (Rusu et al., 2014), and the increase of the contact area was essential because of the loss of motor control in the limb at heel strike (Neamtu et al., 2012). However, the results should be interpreted carefully because of the small sample size used in the current study.
The present study simultaneously measured the spatial, temporal and plantar pressure valuables. To evaluate walking quality, speed alone is not sufficient. Recently, symmetry variances were emphasized as a critical index for walking ability (Tyrell et al., 2011). The gait pattern of healthy individuals is symmetric with respect to spatiotemporal parameters and muscle activation by normal motor control (Kawashima et al., 2006; Plotnik et al., 2013; Ogawa et al., 2015). However, in patients with unilateral impairments due to stroke and amputation, walking asymmetry is considered as an important variable to evaluate walking quality (Hsu et al., 2003; Tyrell et al., 2011; Savin et al., 2014; Yu et al., 2014; Awad et al., 2015). Recently, Kumprou et al. (2017) have shown that there is walking asymmetry in ambulatory patients with SCI (Kumprou et al., 2017). In the study, in the patients with SCI in level D, the median walking step length symmetry was 92% (interquartile range was 83–97%). In the present study, the formula was the same as that in the previous study for patients with stroke and SCI, where a smaller SI value indicates better symmetry. The result indicated that the patients with SCI in level D not only had space asymmetry (step length SI), but also had temporal asymmetry (stance time SI), plantar force asymmetry (Max F SI, impulse SI), and contact area asymmetry. When individuals were walking, the leg in the stance phase was providing support and control for the other leg, which was in the swing phase for propulsion. The reason for the asymmetry could be that there is disrupted control and functional asymmetry to properly support weight in the support leg, which results in dysfunction in the other leg’s swing phase (Kamibayashi et al., 2010; Rusu et al., 2014). The SI, as a variable to reveal control discrepancy and walking quality, could be a potential variable for future research to improve walking quality.
The present study explored gait quality parameters by using a footscan plantar pressure plate system. The data collecting process was relatively easy and convenient, and the quantity of gait-related information was reliable. However, there were also limitations in the study. First, the sample size was small, and it was difficult to do a stratified analysis. Second, only patients with ASIA level D injury were included in this study because the walking speed in patients with SCI below level C was too slow to analyze the spatiotemporal data. However, it would still be possible to analyze the plantar pressure data and SI, so future studies should include patients with SCI in level C.
In conclusion, the present study used the plantar pressure system to confirm the spatiotemporal difference of gait variables between SCI patients and healthy subjects. The results showed that the highest peak pressure was located in the heel (HM and HL) of SCI patients, rather than in meta region, as in healthy subjects. Regional impulse percentage and regional contact area percentage of the heel (HM and HL) were larger in SCI patients than in healthy subjects. The SI was larger in the SCI group. The variables extracted from the plantar pressure system and SI could provide sensitive quantitative parameters for future interventional study to improve gait quality of SCI patients.
Additional files:
Additional figure 1 (173.1KB, tif) : Footscan plantar pressure system in the rubber walkway.
Additional file 1: Open peer review report 1 (106.2KB, pdf) .
OPEN PEER REVIEW REPORT 1
Acknowledgments:
The authors are grateful for the support by staff from Department of Rehabilitation, Shengjing Hospital, China Medical University, China. The authors are grateful to Jun Na, Shu-Xian Li and Rui Wang for the technical assistance.
Footnotes
Conflicts of interest: There are not any conflicts of interest to declare.
Financial support: This study was supported by the New Technique Project of Shengjing Hospital of China Medical University, No. 2015-117 (to XNY). The funding body played no role in the study design, in the collection, analysis and interpretation of data, in the writing of the paper, and in the decision to submit the paper for publication.
Institutional review board statement: The study protocol was approved by the Clinical Research Ethics Committee of Shengjing Hospital of China Medical University (approval No. 2015PS54J) on August 13, 2015. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a prior approval by the institution’s human research committee.
Declaration of participant consent: The authors certify that they have obtained all appropriate participant consent forms. In the form the participants have given their consent for participants’ images and other clinical information to be reported in the journal. The participants understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Reporting statement: This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.
Biostatistics statement: The statistical methods of this study were reviewed by the biostatistician of Shengjing Hospital of China Medical University.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Individual participant data that underlie the results reported in this article, after deidentification (text, tables, figures, and appendices) will be in particular shared. Study protocol form will be available. The data will be available immediately following publication without end date. Anonymized trial data will be available indefinitely at www.figshare.com.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Hao Chen, Shanghai Sixth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, China.
P-Reviewer: Chen H; C-Editor: Zhao M; S-Editors: Wang J, Li CH; L-Editors: McCollum L, de Souza M, Qiu Y, Song LP; T-Editor: Liu XL
Funding: This study was supported by the New Technique Project of Shengjing Hospital of China Medical University, China, No. 2015-117 (to XNY).
References
- 1.Aguirre-Guemez AV, Perez-Sanpablo AI, Quinzanos-Fresnedo J, Perez-Zavala R, Barrera-Ortiz A. Walking speed is not the best outcome to evaluate the effect of robotic assisted gait training in people with motor incomplete spinal cord injury: a systematic review with meta-analysis. J Spinal Cord Med. 2017:1–13. doi: 10.1080/10790268.2017.1390644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amatachaya S, Amatachaya P, Keawsutthi M, Siritaratiwat W. External cues benefit walking ability of ambulatory patients with spinal cord injury. J Spinal Cord Med. 2013;36:638–644. doi: 10.1179/2045772312Y.0000000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arazpour M, Gholami M, Bahramizadeh M, Sharifi G, Bani MA. Influence of reciprocating link when using an isocentric reciprocating gait orthosis (IRGO) on walking in patients with spinal cord injury: a pilot study. Top Spinal Cord Inj Rehabil. 2017;23:256–262. doi: 10.1310/sci16-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arazpour M, Mehrpour SR, Bani MA, Hutchins SW, Bahramizadeh M, Rahgozar M. Comparison of gait between healthy participants and persons with spinal cord injury when using a powered gait orthosis-a pilot study. Spinal Cord. 2014;52:44–48. doi: 10.1038/sc.2013.139. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong AJ, Clark JM, Ho DT, Payne CJ, Nolan S, Goodes LM, Harvey LA, Marshall R, Galea MP, Dunlop SA. Achieving assessor accuracy on the international standards for neurological classification of spinal cord injury. Spinal Cord. 2017;55:994–1001. doi: 10.1038/sc.2017.67. [DOI] [PubMed] [Google Scholar]
- 6.Awad LN, Palmer JA, Pohlig RT, Binder-Macleod SA, Reisman DS. Walking speed and step length asymmetry modify the energy cost of walking after stroke. Neurorehabil Neural Repair. 2015;29:416–423. doi: 10.1177/1545968314552528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beseler MR, Grao CM, Gil A, Martinez Lozano MD. Walking assessment with instrumented insoles in patients with lower limb spasticity after botulinum toxin infiltration. Neurologia (Barcelona, Spain) 2012;27:519–530. doi: 10.1016/j.nrl.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Brown S, Boulton A, Bowling F, Reeves N. Benefits, challenges, and potential utility of a gait database for diabetes patients. J Diabet Sci Tech. 2016;10:1065–1072. doi: 10.1177/1932296816640290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiu MC, Wu HC, Chang LY. Gait speed and gender effects on center of pressure progression during normal walking. Gait Posture. 2013;37:43–48. doi: 10.1016/j.gaitpost.2012.05.030. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez-Seguin LM, Diaz Mancha JA, Sanchez Rodriguez R, Escamilla Martinez E, Gomez Martin B, Ramos Ortega J. Comparison of plantar pressures and contact area between normal and cavus foot. Gait Posture. 2014;39:789–792. doi: 10.1016/j.gaitpost.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 11.Gollie JM. Fatigability during volitional walking in incomplete spinal cord injury: cardiorespiratory and motor performance considerations. Neural Regen Res. 2018;13:786–790. doi: 10.4103/1673-5374.232461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenhalgh A, Taylor PJ, Sinclair J. The influence of different force and pressure measuring transducers on lower extremity kinematics measured during walking. Gait Posture. 2014;40:476–479. doi: 10.1016/j.gaitpost.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Guan X, Kuai S, Ji L, Wang R, Ji R. Trunk muscle activity patterns and motion patterns of patients with motor complete spinal cord injury at T8 and T10 walking with different un-powered exoskeletons. J Spinal Cord Med. 2017;40:463–470. doi: 10.1080/10790268.2017.1319033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo SM, Li JM, Wu QW, Shen HT. Effect of gait training and assessment system of Lokomat automatic robot on walking ability of patients with incomplete spinal cord injury. Zhongguo Zuzhi Gongcheng Yanjiu. 2012;16:2324–2327. [Google Scholar]
- 15.Hardin EC, Kobetic R, Triolo RJ. Ambulation and spinal cord injury. Phys Med Rehabil Clin N Am. 2013;24:355–370. doi: 10.1016/j.pmr.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Hasegawa T, Uchiyama Y, Uemura K, Harada Y, Sugiyama M, Tanaka H. Physical impairment and walking function required for community ambulation in patients with cervical incomplete spinal cord injury. Spinal Cord. 2014;52:396–399. doi: 10.1038/sc.2014.18. [DOI] [PubMed] [Google Scholar]
- 17.Hsu AL, Tang PF, Jan MH. Analysis of impairments influencing gait velocity and asymmetry of hemiplegic patients after mild to moderate stroke. Arch Phys Med Rehabil. 2003;84:1185–1193. doi: 10.1016/s0003-9993(03)00030-3. [DOI] [PubMed] [Google Scholar]
- 18.Kamibayashi K, Nakajima T, Fujita M, Takahashi M, Ogawa T, Akai M, Nakazawa K. Effect of sensory inputs on the soleus H-reflex amplitude during robotic passive stepping in humans. Exp Brain Res. 2010;202:385–395. doi: 10.1007/s00221-009-2145-2. [DOI] [PubMed] [Google Scholar]
- 19.Kawashima N, Yano H, Ohta Y, Nakazawa K. Stretch reflex modulation during imposed static and dynamic hip movements in standing humans. Exp Brain Res. 2006;174:342–350. doi: 10.1007/s00221-006-0470-2. [DOI] [PubMed] [Google Scholar]
- 20.Knippenberg E, Verbrugghe J, Lamers I, Palmaers S, Timmermans A, Spooren A. Markerless motion capture systems as training device in neurological rehabilitation: a systematic review of their use, application, target population and efficacy. Arch Phys Med Rehabil. 2017;14:61. doi: 10.1186/s12984-017-0270-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krizsan-Agbas D, Winter MK, Eggimann LS, Meriwether J, Berman NE, Smith PG, McCarson KE. Gait analysis at multiple speeds reveals differential functional and structural outcomes in response to graded spinal cord injury. J Neurotrauma. 2014;31:846–856. doi: 10.1089/neu.2013.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumprou M, Amatachaya P, Sooknuan T, Thaweewannakij T, Mato L, Amatachaya S. Do ambulatory patients with spinal cord injury walk symmetrically? Spinal Cord. 2017;55:204–207. doi: 10.1038/sc.2016.149. [DOI] [PubMed] [Google Scholar]
- 23.Lapointe R, Lajoie Y, Serresse O, Barbeau H. Functional community ambulation requirements in incomplete spinal cord injured subjects. Spinal Cord. 2001;39:327–335. doi: 10.1038/sj.sc.3101167. [DOI] [PubMed] [Google Scholar]
- 24.Li X, Huang H, Wang J, Yu Y, Ao Y. The analysis of plantar pressure data based on multimodel method in patients with anterior cruciate ligament deficiency during walking. Biomed Res Int 2016. 2016:7891407. doi: 10.1155/2016/7891407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lim BO, O’Sullivan D, Choi BG, Kim MY. Comparative gait analysis between children with autism and age-matched controls: analysis with temporal-spatial and foot pressure variables. J Phys Ther Sci. 2016;28:286–292. doi: 10.1589/jpts.28.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lucareli PR, Lima MO, Lima FP, de Almeida JG, Brech GC, D’Andrea Greve JM. Gait analysis following treadmill training with body weight support versus conventional physical therapy: a prospective randomized controlled single blind study. Spinal Cord. 2011;49:1001–1007. doi: 10.1038/sc.2011.37. [DOI] [PubMed] [Google Scholar]
- 27.Mohd Said A, Justine M, Manaf H. Plantar pressure distribution among older persons with different types of foot and its correlation with functional reach distance. Scientifica 2016. 2016:8564020. doi: 10.1155/2016/8564020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Narin F, Hanalioglu S, Ustun H, Kilinc K, Bilginer B. Topiramate as a neuroprotective agent in a rat model of spinal cord injury. Neural Regen Res. 2017;12:2071–2076. doi: 10.4103/1673-5374.221164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neamtu MC, Rusu L, Marin M, Avramescu T, Rusu PF, Neamtu OM, Iancau M. Morphofunctional aspects of lower limb in multiple sclerosis. Rom J Morphol Embryol. 2012;53:117–120. [PubMed] [Google Scholar]
- 30.Nsenga Leunkeu A, Lelard T, Shephard RJ, Doutrellot PL, Ahmaidi S. Gait cycle and plantar pressure distribution in children with cerebral palsy: clinically useful outcome measures for a management and rehabilitation. NeuroRehabilitation. 2014;35:657–663. doi: 10.3233/NRE-141163. [DOI] [PubMed] [Google Scholar]
- 31.Ogawa T, Kawashima N, Obata H, Kanosue K, Nakazawa K. Mode-dependent control of human walking and running as revealed by split-belt locomotor adaptation. J Exp Biol. 2015;218:3192–3198. doi: 10.1242/jeb.120865. [DOI] [PubMed] [Google Scholar]
- 32.Plotnik M, Bartsch RP, Zeev A, Giladi N, Hausdorff JM. Effects of walking speed on asymmetry and bilateral coordination of gait. Gait Posture. 2013;38:864–869. doi: 10.1016/j.gaitpost.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pramodhyakul N, Amatachaya P, Sooknuan T, Arayawichanon P, Amatachaya S. Effects of a visuotemporal cue on walking ability of independent ambulatory subjects with spinal cord injury as compared with healthy subjects. Spinal Cord. 2014;52:220–224. doi: 10.1038/sc.2013.160. [DOI] [PubMed] [Google Scholar]
- 34.Quarrington RD, Costi JJ, Freeman BJC, Jones CF. Quantitative evaluation of facet deflection, stiffness, strain and failure load during simulated cervical spine trauma. J Biomech. 2018;72:116–124. doi: 10.1016/j.jbiomech.2018.02.036. [DOI] [PubMed] [Google Scholar]
- 35.Razak AH, Zayegh A, Begg RK, Wahab Y. Foot plantar pressure measurement system: a review. Sensors. 2012;12:9884–9912. doi: 10.3390/s120709884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rusu L, Neamtu MC, Rosulescu E, Cosma G, Dragomir M, Marin MI. Analysis of foot and ankle disorders and prediction of gait in multiple sclerosis rehabilitation. Eur J Med Res. 2014;19:73. doi: 10.1186/s40001-014-0073-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Safayi S, Jeffery ND, Shivapour SK, Zamanighomi M, Zylstra TJ, Bratsch-Prince J, Wilson S, Reddy CG, Fredericks DC, Gillies GT, Howard MA., 3rd Kinematic analysis of the gait of adult sheep during treadmill locomotion: parameter values, allowable total error, and potential for use in evaluating spinal cord injury. J Neurol Sci. 2015;358:107–112. doi: 10.1016/j.jns.2015.08.031. [DOI] [PubMed] [Google Scholar]
- 38.Savin DN, Morton SM, Whitall J. Generalization of improved step length symmetry from treadmill to overground walking in persons with stroke and hemiparesis. Clin Neurophysiol. 2014;125:1012–1020. doi: 10.1016/j.clinph.2013.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shank CD, Walters BC, Hadley MN. Current topics in the management of acute traumatic spinal cord injury. Neurocrit Care. 2018 doi: 10.1007/s12028-018-0537-5. DOI: 10.1007/s12028-018-0537-5. [DOI] [PubMed] [Google Scholar]
- 40.Soames RW. Foot pressure patterns during gait. J Biomed Eng. 1985;7:120–126. doi: 10.1016/0141-5425(85)90040-8. [DOI] [PubMed] [Google Scholar]
- 41.Sohn WJ, Tan AQ, Hayes HB, Pochiraju S, Deffeyes J, Trumbower RD. Variability of leg kinematics during overground walking in persons with chronic incomplete spinal cord injury. J Neurotrauma. 2018 doi: 10.1089/neu.2017.5538. DOI: 10.1089/neu.2017.5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song-Hua Y, Lu W, Kuan Z. Effects of different movement modes on plantar pressure distribution patterns in obese and non-obese Chinese children. Gait Posture. 2017;57:28–34. doi: 10.1016/j.gaitpost.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 43.Takazawa A, Kamei N, Adachi N, Ochi M. Endoplasmic reticulum stress transducer old astrocyte specifically induced substance contributes to astrogliosis after spinal cord injury. Neural Regen Res. 2018;13:536–540. doi: 10.4103/1673-5374.228759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tamburella F, Scivoletto G, Cosentino E, Molinari M. Walking in water and on land after an incomplete spinal cord injury. Am J Phys Med Rehabil. 2013;92:e4–e15. doi: 10.1097/PHM.0b013e3182a1e6c3. [DOI] [PubMed] [Google Scholar]
- 45.Thijs Y, Van Tiggelen D, Roosen P, De Clercq D, Witvrouw E. A prospective study on gait-related intrinsic risk factors for patellofemoral pain. Clin J Sport Med. 2007;17:437–445. doi: 10.1097/JSM.0b013e31815ac44f. [DOI] [PubMed] [Google Scholar]
- 46.Tyrell CM, Roos MA, Rudolph KS, Reisman DS. Influence of systematic increases in treadmill walking speed on gait kinematics after stroke. Phys Ther. 2011;91:392–403. doi: 10.2522/ptj.20090425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang P, Low KH, McGregor AH, Tow A. Detection of abnormal muscle activations during walking following spinal cord injury (SCI) Res Dev Disabil. 2013;34:1226–1235. doi: 10.1016/j.ridd.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 48.Wu MF, Zhang SQ, Liu JB, Li Y, Zhu QS, Gu R. Neuroprotective effects of electroacupuncture on early- and late-stage spinal cord injury. Neural Regen Res. 2015;10:1628–1634. doi: 10.4103/1673-5374.167762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yan SH, Zhang K, Tan GQ, Yang J, Liu ZC. Effects of obesity on dynamic plantar pressure distribution in Chinese prepubescent children during walking. Gait Posture. 2013;37:37–42. doi: 10.1016/j.gaitpost.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 50.Yang A, Asselin P, Knezevic S, Kornfeld S, Spungen AM. Assessment of in-hospital walking velocity and level of assistance in a powered exoskeleton in persons with spinal cord injury. Top Spinal Cord Inj Rehabil. 2015;21:100–109. doi: 10.1310/sci2102-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu CH, Hung YC, Lin YH, Chen GX, Wei SH, Huang CH, Chen CS. A 3D mathematical model to predict spinal joint and hip joint force for trans-tibial amputees with different SACH foot pylon adjustments. Gait Posture. 2014;40:545–548. doi: 10.1016/j.gaitpost.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 52.Yugue I, Okada S, Maeda T, Ueta T, Shiba K. Sensitivity and specificity of the ’knee-up test’for estimation of the American Spinal Injury Association Impairment Scale in patients with acute motor incomplete cervical spinal cord injury. Spinal Cord. 2018;56:347–354. doi: 10.1038/s41393-017-0046-y. [DOI] [PubMed] [Google Scholar]
- 53.Zhang Y, Liu H, Fu C, Ning Y, Zhang J, Zhou L, Li Z, Bai P. The biomechanical effect of acupuncture for poststroke cavovarus foot: study protocol for a randomized controlled pilot trial. Trials. 2016;17:146. doi: 10.1186/s13063-016-1264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Footscan plantar pressure system in the rubber walkway.
OPEN PEER REVIEW REPORT 1


