Abstract
Background
Clinicians often use population-based reference intervals (RIs) when interpreting patient results. However, this method can present problems if the analyte in question has wide variability from person to person.
Methods
We examined the biological variation of routine hematologic markers in 82 white non-Hispanic men every 6 months during a 30-year period, to determine the usefulness of population-based RIs and age-related decline of hematological markers.
Results
Many of these markers showed significant person-to-person differences (index of individuality <1.4 in 10/11 markers) and change over time with a decrease in mean for white blood cells (WBCs), red blood cells (RBCs), hemoglobin, hematocrit, platelets, and neutrophils. The mean increased for monocytes, mean corpuscular volume (MCV), and mean corpuscular hemoglobin (MCH) (all P <.05).
Conclusion
Longitudinal analysis demonstrated significant decline in hematologic marker counts, with the exception of MCV and MCH. Establishment of a personalized baseline for hematologic assessments may be more useful to clinicians than previous methods.
Keywords: CBC, WBC, RBC, lymphocyte, personalized reference intervals, intra- and interindividual coefficients of variation
Analyte components of blood can vary during the course of the lifespan. Some of these variations are caused by predicable biological cycles or rhythms that all individuals share in common, whereas other sources of variation may be due to differences between individuals. By comparing the test results from a patient with certain reference population values, it is possible to detect clinically relevant changes in blood biomarkers that can be useful for prevention, diagnosis, and treatment of various diseases.
Numerous factors can contribute to variations in blood markers. Some of the variables are controllable (blood collection technique), whereas other characteristics, such as ethnicity, sex, and age, are not subject to manipulation. Often, biological factors are the most important source of variation over time for certain analytes. For example, marked changes can occur during the neonatal period, childhood, puberty, menopause, and aging. Also, certain analytes have biological rhythms that can vary diurnally, monthly, or seasonally.1
Interpretation of test results from a patient using the reference values of the testing laboratory is, thus far, the only valid tool available to clinicians when the biological variability of the population reference values and of the target population are comparable.2 Population-based reference values, despite their limitations, remain the most commonly used interpretative tool for clinicians to date.
Reference populations commonly use convenience sampling from readily available and cooperative groups, such as blood donors, laboratory personnel, or medical students. This practice is problematic for several reasons. First, there may be a selection bias in instances in which the reference population does not adequately represent the population ranges for that analyte. For example, the chosen reference population may be extremely healthy or have a higher prevalence of some pathological condition, which will result in a reference interval (RI) that is biased relative to the truly “healthy” range. Conclusions based on these biased intervals may result in increased false-positive or false-negative rates, respectively. Second, certain analytes display a high degree of interindividual variation as a result of widely ranging homeostatic set points, even within a population that is considered “healthy.” This marked individuality makes it difficult to construct a single RI that represents a “healthy” range for all individuals because many subjects will present with values that are highly unique for them but are still within population-based reference value ranges.3–10
Repeated measurements of an analyte obtained by a longitudinal study of an individual may be preferable to use of a single measure in conjunction with population-based references. In the case of repeat measures, the patients have their own reference for each biomarker, and changes between results in consecutive tests may indicate illness.2 Longitudinal assessment requires knowledge of the within-subject variability. Sufficient hematological or chemistry values in published data during a long period of time (many years) are not available for most laboratory analytes.
The aim of our study was to assess the effect of aging on commonly requested hematological parameters during a 30-year period while also examining the inter- and intraindividual biological variation for hematological laboratory markers in a population of white non-Hispanic males to evaluate the appropriateness of RIs in clinical settings.
We suggest, in some cases, that personalized baseline laboratory tests may be preferable to the population-based reference values for monitoring and follow-up of a patient. In this study, we explored the biological variation of white blood cells (WBCs), lymphocytes, monocytes, neutrophils, red blood cells (RBCs), hemoglobin (Hg), hematocrit (Hct), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), and platelets (PLT).
Materials and Methods
We investigated the routine hematological markers of 82 participants with no diagnosis of major illness, such as cancer, hepatitis B and C, kidney problems, or diabetes, during a 30-year period. These individuals were men who have sex with men (MSM) participating in the Multicenter AIDS Cohort Study (MACS), who were documented to have human immunodeficiency virus (HIV)–1 seronegativity at every study visit and self-reported as white, non-Hispanic.11 Each follow-up visit of each participant was monitored by reviewing his health questionnaire form, physical examination form, and laboratory test reports. Based on this information, if there was evidence of any acute illness, the blood-marker data for that participant for that visit was excluded from the data analysis.
The participants ranged from ages 20 to 49 years, with a mean age of 33 years and median age of 32 years at entry into the study in 1984 and 1985, and aged 50 to 79 years, with a mean of age of 63 years at the last recorded visit, through March 2015. The average length of time between the first and last visits for a participant was 30 years. Of the 82 cohort individuals, 31 were nonsmokers, 12 had smoked before the enrollment, 29 formerly smoked (13 had quit in the 1980s, 8 had quit in the 1990s, and 8 had quit in the 2000s). Also, 5 participants reported that they currently smoke, and 5 others reported that they smoked intermittently during the study period.
The institutional review board (IRB) for human studies at UCLA (University of California, Los Angeles) approved the protocols. After informed consent, blood specimens were obtained every 6 months from each subject, collected in a 4-mL prelabeled tube with ethylenediaminetetraacetic acid (EDTA) anticoagulant (VACUTAINER Systems; Becton, Dickinson and Company) between 8:00 AM and 12:00 PM and sent at ambient temperature for analysis to the local clinical laboratory. Complete blood count (CBC) assessments with 3-part differential and platelet count were performed with automated hematology analyzers by Clinical Laboratory Improvement Amendments (CLIA)–certified clinical reference laboratories (MetPath Central from 1984 through October 1987, SmithKline Bio-Science from November 1987 through August 1988, MetPath from October 1988 through October 1989, MetWest Clinical Laboratories from February 1990 through March 1993, UNILAB from April 1993 through December 2006, and Quest Diagnostics from January 2007 through the present time); those laboratories have used various brands of automated analyzers for CBC analysis in the course of 4 decades. Quest Diagnostics acquired MetPath, SmithKline Bio-Science, and UNILAB laboratories; no information is available regarding the hematology analyzers brand and type used by those laboratories. Quest Diagnostics has been using the Sysmex-XN series (Sysmex Corporation).
The lower reference level (LRL) and upper reference level (URL) in Figure 1 and Figure 2 are the calculated weighted mean of the LRL and URL of the aforementioned laboratories. CBC data were available for analysis from 4759 of 4920 (96.7%) of the overall expected individual visits.
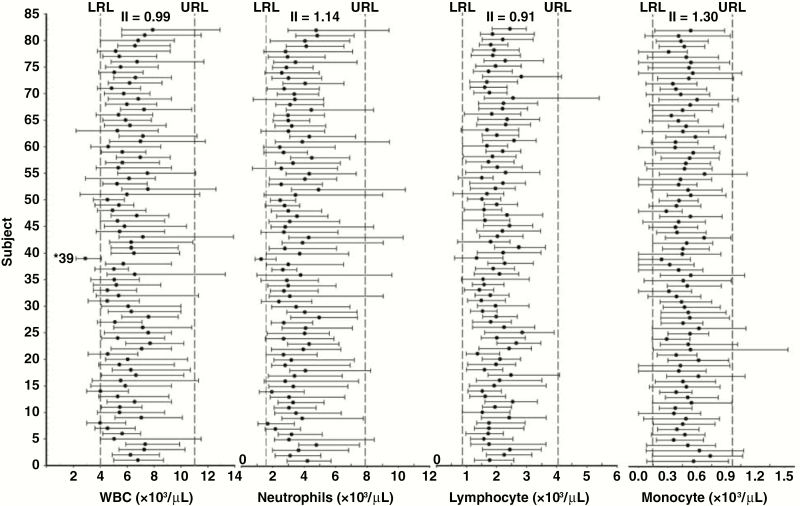
Figure 1.
Data points for study participants regarding white blood cells (WBCs), lymphocytes, monocytes, and neutrophils, including lower and upper reference levels, as well as means and absolute ranges, for each individual during the 30-year study period.
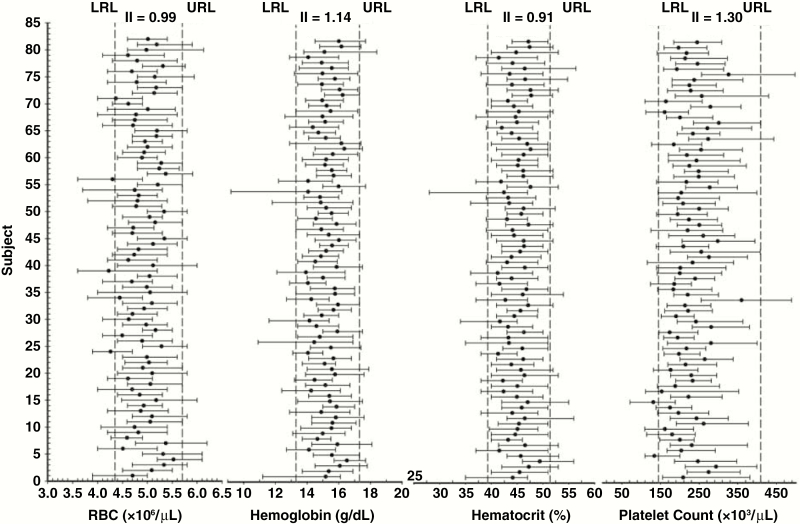
Figure 2.
Data points for study participants regarding red blood cells (RBCs), hemoglobin (Hg), hematocrit (Hct), and platelets (PLT), including lower and upper reference levels, as well as means and absolute ranges, for each individual during the 30-year study period.
Statistical Analysis
Descriptive statistics for the 11 hematological markers that comprised the complete blood count (absolute cell counts for WBC, RBC, PLT, lymphocytes, monocytes, neutrophils; Hb, Hct, MCV, MCH, and MCHC) were generated for mean and absolute ranges for CVG and CVI. Mixed models were used for repeated-measures analyses to identify trends during a 30-year period for each marker and the cross-sectional effect of age on blood markers; the nested analysis of variance was used for calculation of coefficients of variation for CVI and CVG. CVI and CVG were calculated according to the approach used by Fraser and Harris.1,12 Because the precision of the measurement tools was high and the reliability of replicate measures was not of interest to us, we omitted measures of analytic variation from these calculations.
The index of individuality (II) is the simple ratio of the 2 biological components of variation, namely, intraindividual to interindividual, and is calculated using the formula CVI/CVG.1 The II, as defined by Harris,12 assesses the usefulness of population-based reference values for interpretation of laboratory tests: if the II of a given analyte is greater than 1.4, population-based intervals are appropriate; an II below 1.4 indicates decreased usefulness of population-based RIs. Analytes with a II less than 0.6 demonstrate a high degree of individuality, making individual-based RIs more useful.
All analysis was performed using SAS software, version 9.4 (SAS Institute, Inc). Graphs were created by using SigmaPlot software, version 13 (Systat Software, Inc).
Results
The CVI, CVG, and II, along with the overall means of hematologic parameters for 1 year (baseline, visit 1, and visit 2), 10 years (baseline to visit 19), 20 years (baseline to visit 39), and 30 years (baseline to visit 59) of follow-up, are presented in Table 1. As described in the Materials and Methods section, it is considered appropriate to use population-based reference ranges when the II of a given analyte is greater than 1.4.12 With the exception of MCHC at all 4 time intervals and monocytes at 10 years and 20 years of follow-up, the IIs in our study for the routine hematological markers were less than 1.4.
Table 1.
Mean Values, CVI, CVG, and II of Each Tested Hematological Marker for 82 Subjects at Intervals from 1–30 Years
| Length of Interval | 1st Year | 10 Years | 20 Years | 30 Years |
|---|---|---|---|---|
| Obs/total obsa | 237/246 | 1553/1640 | 3176/3280 | 4759/4920 |
| WBCs | ||||
| Mean (×103/μL) | 6.29 | 6.08 | 5.96 | 5.95 |
| CVI (%) | 15.4 | 17.0 | 16.8 | 16.5 |
| CVG (%) | 19.1 | 17.6 | 17.1 | 16.7 |
| II | 0.80 | 0.97 | 0.98 | 0.99 |
| Neutrophils | ||||
| Mean (×103/μL) | 3.67 | 3.49 | 3.32 | 3.33 |
| CVI (%) | 22.3 | 25.7 | 26.2 | 25.6 |
| CVG (%) | 22.8 | 22.8 | 22.7 | 22.4 |
| II | 0.98 | 1.13 | 1.15 | 1.14 |
| Lymphocyte | ||||
| Mean (×103/μL) | 1.96 | 2.01 | 2.00 | 1.98 |
| CVI (%) | 20.7 | 17.5 | 16.9 | 16.5 |
| CVG (%) | 20.1 | 17.6 | 17.5 | 18.1 |
| II | 1.03 | 0.99 | 0.97 | 0.91 |
| Monocyte | ||||
| Mean (×103/μL) | 0.49 | 0.42 | 0.47 | 0.47 |
| CVI (%) | 24.8 | 33.0 | 28.0 | 26.0 |
| CVG (%) | 26.2 | 20.3 | 19.5 | 20.0 |
| II | 0.95 | 1.63 | 1.44 | 1.30 |
| RBCs | ||||
| Mean (×106/μL) | 5.09 | 5.02 | 4.96 | 4.92 |
| CVI (%) | 3.9 | 4.3 | 4.5 | 4.8 |
| CVG (%) | 6.0 | 5.4 | 5.5 | 5.7 |
| II | 0.64 | 0.79 | 0.81 | 0.84 |
| Hg | ||||
| Mean (g/dL) | 15.6 | 15.4 | 15.3 | 15.2 |
| CVI (%) | 4.0 | 4.0 | 4.0 | 4.4 |
| CVG (%) | 4.8 | 4.2 | 4.0 | 4.2 |
| II | 0.84 | 0.96 | 1.00 | 1.04 |
| Hct | ||||
| Mean (%) | 46.7 | 45.5 | 45.0 | 44.9 |
| CVI (%) | 4.8 | 4.7 | 4.7 | 4.9 |
| CVG (%) | 4.4 | 4.0 | 3.9 | 4.1 |
| II | 1.09 | 1.18 | 1.21 | 1.20 |
| MCV | ||||
| Mean (fL) | 92.0 | 90.6 | 90.8 | 91.3 |
| CVI (%) | 2.2 | 2.5 | 2.3 | 2.6 |
| CVG (%) | 3.6 | 3.5 | 3.5 | 3.6 |
| II | 0.64 | 0.72 | 0.66 | 0.73 |
| MCH | ||||
| Mean (pg) | 30.8 | 30.8 | 31.0 | 31.1 |
| CVI (%) | 2.8 | 2.7 | 2.7 | 2.9 |
| CVG (%) | 4.0 | 3.8 | 3.7 | 3.8 |
| II | 0.71 | 0.71 | 0.72 | 0.77 |
| MCHC | ||||
| Mean (g/dL) | 33.4 | 33.9 | 34.1 | 34.0 |
| CVI (%) | 3.4 | 2.8 | 2.6 | 2.7 |
| CVG (%) | N/Ab | 1.1 | 1.0 | 1.1 |
| II | N/A | 2.58 | 2.61 | 2.55 |
| PLT | ||||
| Mean (×103/μL) | 271 | 241 | 230 | 225 |
| CVI (%) | 12.4 | 13.6 | 13.7 | 13.8 |
| CVG (%) | 18.4 | 19.1 | 18.7 | 18.5 |
| II | 0.67 | 0.71 | 0.73 | 0.74 |
CVI, intraindividual; CVG, interindividual; II, index of individuality; obs, observations; WBCs, white blood cells; RBCs, red blood cells; Hg, hemoglobin; MCV, mean corpuscular volume; Hct, hematocrit; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; PLT, platelets.
aNo. of obs from the total ob available for analyses.
bis not calculable.
The means and absolute ranges (minimum-maximum values) during all study visits for each individual (n = 82) are shown graphically for WBCs, lymphocytes, monocytes, and neutrophils (Figure 1), as well as for RBCs, Hb, Hct, and PLT (Figure 2). Visual assessment of these figures shows that although the mean values of the same analyte for individuals are clearly different from each other, most of the mean values lie within the RI. Looking at the data ranges, much of the data fall within the RIs; however, certain individuals have some data points outside the upper or lower reference limits (the laboratory reference values) or both. In 1 case, nearly all the data points for a participant were outside the RIs (ie, WBC and neutrophils; Figure 1, subject 39).
To evaluate direction and magnitude of changes in each marker from baseline during the 30-year follow-up period, we used mixed modeling via SAS software to estimate the average changes in values per year or interval and the corresponding P value (Table 2). The longitudinal analysis during the course of 30 years showed a statistically significant decrease for the directly measured hematologic markers WBCs, RBCs, Hg, and PLT. Other markers that also showed significant decreases were Hct (which is calculated using the equation RBC × MCV) and absolute neutrophil count (calculated using the equation WBC × % neutrophils in automated 3-part WBC differential).
Table 2.
Average Change per Year or Interval in Hematological Markers during the 30-Year Follow-Up Period
| Marker (Unit) | Measurement or Calculation | Changea | P Value |
|---|---|---|---|
| WBCs (103 cells/μL) | Direct | 0.0084↓ | .04 |
| RBCs (106 cells/μL) | Direct | 0.0088↓ | <.001 |
| Hg (g/dL) | Direct | 0.0183↓ | <.001 |
| PLT (103 count/μL) | Direct | 1.6766↓ | <.001 |
| Hct (%) | RBC × MCV | 0.0532↓ | <.001 |
| Neutrophil (103 cell/μL) | WBC × % neutrophilsb | 0.0075↓ | .01 |
| MCV (fL) | Direct | 0.0410↑ | <.001 |
| MCH (pg) | Hb/RBC | 0.0192↑ | <.001 |
| Monocyte (103 cell/μL) | WBC × % monocytes | 0.0014↑ | .001 |
| MCHC (g/dL) | Hb/RBC × MCV | 0.0027↔ | .18 |
| Lymphocyte (103 cell/μL) | WBC × % lymphocytes | 0.0021↔ | .14 |
WBC, white blood cells; ↓, decrease; RBCs, red blood cells; Hg, hemoglobin; PLT, platelets; Hct, hematocrit; MCV, mean corpuscular volume; ↑, increase; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; ↔ no statistically significant change.
aPopulation average changes in marker values per year.
bNeutrophil also reported as polymorphonuclear cells, PMNs (polymorphonuclear neutrophils), polys, and granulocytes.
We observed an increase for MCV, which is directly measured in automated hematology analyzers. This finding suggests that the decreases in Hct were due to a relatively greater decrease in RBC count, compared with the increases in MCV. Similarly, MCH (calculated from Hb/RBC) showed an increase, suggesting that the RBC count was also relatively more decreased than the Hg concentration. The absolute monocyte count (WBC × % monocytes) also increased, despite the concomitant decrease in total WBC count, which suggests that the percentage of monocytes was increasing even more than was evident from the absolute numbers. We observed no significant change over time for MCHC (Hb/RBC × MCV), which is calculated from 3 different RBC-related measurements moving in different directions, nor any for absolute lymphocyte count (WBC × % lymphocytes). There were no cross-sectional effects of age on the tested blood markers (P> .05).
Discussion
Clinical laboratory tests and population-based RIs are used for interpreting laboratory test results for diagnosis, case finding, screening, and monitoring the health of patients during treatment. Also, serial testing of patients can assist clinicians in detecting changes over time.
Ideally, a dedicated hematology analyzer and a single laboratory would be used for blood analysis for the entire length of the study (30 years); however, this desired end was unattainable. A limitation in our study is the use of different laboratories (due to certain laboratories having been bought out by competing laboratories) or different instruments (due to upgraded models and technology advances) for measurement of blood markers during the 30-year course of the study. Based on our knowledge of the comparability of blood counts between the Coulter Ac•T Diff (Beckman Coulter, Inc) and Sysmex XT-1800i (Sysmex Corporation) hematology analyzers, as well as CLIA restrictions and requirements on clinical laboratories, the switching of clinical laboratories or hematological instruments during the course of the study should have had a minimal, but not significant, impact on the final analyses of our data.
We have longitudinally examined the extent of biological variation in routine circulating blood markers measured in a relatively homogeneous cohort of men as they aged during a 30-year period and assessed data for a number of hematological parameters in the serial results of individuals. The ability to evaluate as many as 60 observations per person during the 30-year interval provided us a unique opportunity to generate individual mean and ranges of blood markers (Figures 1 and 2). These figures show many occurrences in which the value of a test result may be very unusual for a particular individual (ie, extends the minimum or maximum of the range dramatically, relative to the mean) but is still within the conventional population-based RI. In these circumstances, in which a value is clearly out of the ordinary for a specific individual but is not flagged as “abnormal” according to population-based reference ranges, the reference values may become unreliable and may mask biologically or clinically important changes.
We recognize that our study population may limit the generalizability of our observations. However, a strength of our study is that it includes a single-sex, single-ethnicity population, which maximizes our ability to evaluate biological variation over time.
Numerous other investigators have studied the source of biological variation of hematologic markers on a daily, weekly, or monthly basis and, in one instance, for 3 visits during a 10-year period.13 We are unaware, however, of any published study that is as intensive (twice a year), extended (during 3 decades), and longitudinal as the study we have presented herein.
We expect to observe disagreement in variation values due to differences in participant population restrictions, along with frequency and duration of follow-up. With the exception of MCHC, monocyte, and neutrophils, our data show some similarity with published data, such as lower CVI and higher CVG.1,3
As early as 1935, Miller et al14 observed a decrease in RBC count and Hg and no changes for WBC and differential counts (cross-sectional) in a population of elderly individuals, compared with younger adults. Our longitudinal data showed similar statistically significant decreases in RBC count and Hg (g/dL), as well as Hct (%) but, in contrast with the results of earlier studies, a decrease in the total WBC and neutrophils, as participants aged during a 30-year period. It is impossible to determine whether these differences are attributable to study design (cross-sectional vs longitudinal), study populations, and/or the technology in use at the time.
Statland et al15studied physiologic variation of hour-to-hour, day-to-day, and week-to week changes of hematological parameters during a 4-week period. Comparing their week-to-week data to ours, the CVI reported in the Statland study findings is smaller than ours, which may be attributed to the shorter (4 weeks for Statland et al) versus longer (30 years for our study) follow-up period. In contrast, their CVG data values were greater, compared with those from our study results, which could be due to differences in sex and ethnicity among their 20 subjects versus the 82 white non-Hispanic male participants in our study.
In a cross-sectional study, MacKinney16 observed a significant age-dependent decline in absolute lymphocyte count. This decline appeared to be in 3 phases, namely, a precipitous drop from birth to age 20 years, a stationary phase for 3 decades (ages 20–50 years), and accelerating decline up to age 90 years.
Sparrow et al13 longitudinally evaluated peripheral lymphocyte count in men of 3 age groups (23–44 years, 45–54 years, and 55+ years) for 3 visits during an average period of 10 years. The researchers found that the 3 age cohorts did not have significantly different lymphocyte counts; when those groups were followed during a 10-year period (the older participant was 70 years), no significant differences were observed.
When we applied the mixed model for each marker, we found that with the exception of MCHC and lymphocytes, there are longitudinal effects of aging (P < .05); however, there was no cross-sectional effect of age on any of the hematologic markers. In practical terms, this means that age is not predictive of blood-marker level; rather, knowing baseline blood-marker level and collection time of baseline can help predict current blood-marker levels.
Costongs et al2 studied the variation of blood cells and the differential leukocyte count during a period of 6 months (month to month); the 6-month CVI that they reported was lower by approximately 50%, compared with our data. Fraser et al17 studied the biological variation of common hematologic markers in healthy elderly adults (age 70 years or older) for a period of 20 weeks; their subjects had lower CVI and higher CVG values (with the exception of monocytes), compared with our data.
These differences may be due to the variability of sex and ethnicity, along with shorter follow-up versus white non-Hispanic men and longer follow-up and more data points than in our study. Hematological markers showed significant individuality; as a consequence, the conventional population-based reference values are of limited usefulness. Also, screening using reference limits will not detect latent or early diseases in many subjects.17
The limitation of the II on population-based reference values is restricted to a situation in which a conclusion is drawn based on changes in a single sample. When the II is low, it is important to stratify the population,18 to obtain a separate RI for subpopulations, and to collect data from specimens from the same individual.19 When the laboratory values for a specimen are outside an RI and the result is verified by a repeat test, a high II has considerable influence on the repeated result, facilitating the decision-making process. However, a low II on repeated testing will be close to the original result and give no new information.20
Conclusions
We observed a decline in RBC, Hg (g/dL), Hct (%), WBC, neutrophil counts, and PLT and a corresponding increase in MCV (fL), MCH (pg), and monocyte counts; no statistically significant changes were observed in MCHC (g/dL) and lymphocyte counts during the 30 years in which we followed up with the cohort individuals (Table 2). The percentage of CVI of RBC, MCV, MCH, WBC, lymphocytes (except in the first year), and PLT were smaller than the percentage of CVG; CVI was greater than CVG in Hg (except at the 20-year mark, at which it was the same and bigger at 30 year mark), Hct, MCHC, monocytes (except in the first year), and neutrophils (except in the first year) (Table 1).
The conventional population-based reference value has its own problems. Blood cells and markers are not always constants that can be measured once in a single reference sample group and made applicable in all situations. Inherent biological variation (BV) affects all biomarkers tested in clinical laboratories and must be taken into consideration in the generation and application of reference values.8 Stratification of reference values by sex, age, and clinical indication, or even individual-based reference values used in a longitudinal approach, can significantly improve clinical decision making21 and are often advantageous, compared with using only population-based reference values.
Personalized baseline values for laboratory-test results may be able to resolve the issue of unreliability of RIs, although at the present time, using patient data to derive baseline and reference values may not be supported by the International Committee for Standardization in Hematology or the World Health Organization (WHO). However, with advances in laboratory technologies, nationwide electronic access to test results, and review of the data presented in this study, we could advocate for establishment of an adult personalized baseline laboratory test. These tests would be performed to determine levels of common blood markers and be performed at the start of each decade of adult life by the family physician. That baseline could be used as an interpretation tool or reference value for future repetition of those tests, to monitor the health of the patient during that decade. LM
Funding
This work was supported by grants from the National Institutes of Health (U01-A1-35042). The MACS is funded by the National Institute of Allergy and Infectious Diseases, with additional supplemental funding from the National Cancer Institute (U01-AI35042, 5-M01-RR00722 [GCRC], U01-A135043, U01-AI37984, U01-A135039, U01-AI35040, U01-AI37613, and U01-AI35041). This project has been funded entirely or partly with federal funding from the National Cancer Institute, National Institutes of Health, under contract no. N01-CO56000.
Acknowledgments
We thank the men who participated in the Multicenter AIDS Cohort Study (MACS); their participation makes this and many other studies possible. We also thank Daniel Chang, BS, May Htike, MD, MPH, Carlos Aquino, BA, Ray Mercado, BA, and Eduardo Mercado, BA, for recruitment coordination; Cindy Chang, MS, for statistical analysis; and Timothy Ryner, BS, for manuscript help.
Abbreviations
- RI
reference interval
- WBCs
white blood cells
- RBCs
red blood cells
- Hg
hemoglobin
- Hct
hematocrit
- MCV
mean corpuscular volume
- MCH
mean corpuscular hemoglobin
- MCHC
mean corpuscular hemoglobin concentration
- PLT
platelets
- MSM
men who have sex with men
- MACS
Multicenter AIDS Cohort Study
- HIV
human immunodeficiency virus
- IRB
institutional review board
- UCLA
University of California, Los Angeles
- EDTA
ethylenediaminetetraacetic acid
- CBC
complete blood count
- CLIA
Clinical Laboratory Improvement Amendments
- LRL
lower reference level
- URL
upper reference level
- II
index of individuality
- BV
biological variation
- WHO
World Health Organization
- obs
observations
- ↓
decrease
- ↑
increase
- ↔
no statistically significant change
- PMNs
polymorphonuclear neutrophils
References
- 1. Fraser CG. Biological Variation: From Principles to Practice. Washington, DC: AACC Press; 2001. [Google Scholar]
- 2. Costongs GM, Janson PC, Bas BM, Hermans J, Brombacher PJ, van Wersch JW. Short-term and long-term intra-individual variations and critical differences of haematological laboratory parameters. J Clin Chem Clin Biochem. 1985;23(2):69–76. [PubMed] [Google Scholar]
- 3. Ricós C, Alvarez V, Cava F, et al. Current databases on biological variation: pros, cons and progress. Scand J Clin Lab Invest. 1999;59(7):491–500. [DOI] [PubMed] [Google Scholar]
- 4. Ricós C, Álvarez V, Perich C, et al. Rationale for using data on biological variation. Clin Chem Lab Med. 2015;53(6):863–870. [DOI] [PubMed] [Google Scholar]
- 5. Ricós C, Carmen P, Michaela J, et al. Application of biological variation—a review. Biochemia Medica. 2009;19(3) 250–259. [Google Scholar]
- 6. Lacher DA, Barletta J, Hughes JP. Biological variation of hematology tests based on the 1999–2002 National Health and Nutrition Examination Survey. Natl Health Stat Report. 2012;(54):1–10. [PubMed] [Google Scholar]
- 7. Ceriotti F, Hinzmann R, Panteghini M. Reference intervals: the way forward. Ann Clin Biochem. 2009;46(Pt 1):8–17. [DOI] [PubMed] [Google Scholar]
- 8. Fraser CG. Inherent biological variation and reference values. Clin Chem Lab Med. 2004;42(7):758–764. [DOI] [PubMed] [Google Scholar]
- 9. Díaz-Jauanen E, Strickland RG, Williams RC Jr. Studies of human lymphocytes in the newborn and the aged. Am J Med. 1975;58(5):620–628. [DOI] [PubMed] [Google Scholar]
- 10. Ferguson T, Crichton DN, Price WH. Lyphocyte-counts in relation to age. Lancet. 1977;310(8027):35. [DOI] [PubMed] [Google Scholar]
- 11. Detels R, Jacobson L, Margolick J, et al. The multicenter AIDS Cohort Study, 1983 to …. Public Health. 2012;126(3):196–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harris EK. Effects of intra- and interindividual variation on the appropriate use of normal ranges. Clin Chem. 1974;20(12):1535–1542. [PubMed] [Google Scholar]
- 13. Sparrow D, Silbert JE, Rowe JW. The influence of age on peripheral lymphocyte count in men: a cross-sectional and longitudinal study. J Gerontol. 1980;35(2):163–166. [DOI] [PubMed] [Google Scholar]
- 14. Miller I. Normal hematologic Standards in the aged. J Lab Clin Med. 1939;28:1172–1176. [Google Scholar]
- 15. Statland BE, Winkel P, Harris SC, Burdsall MJ, Saunders AM. Evaluation of biologic sources of variation of leukocyte counts and other hematologic quantities using very precise automated analyzers. Am J Clin Pathol. 1978;69(1):48–54. [DOI] [PubMed] [Google Scholar]
- 16. MacKinney AA., Jr Effect of aging on the peripheral blood lymphocyte count. J Gerontol. 1978;33(2):213–216. [DOI] [PubMed] [Google Scholar]
- 17. Fraser CG, Wilkinson SP, Neville RG, Knox JD, King JF, MacWalter RS. Biologic variation of common hematologic laboratory quantities in the elderly. Am J Clin Pathol. 1989;92(4):465–470. [DOI] [PubMed] [Google Scholar]
- 18. Harris EK, Boyd JC. On dividing reference data into subgroups to produce separate reference ranges. Clin Chem. 1990;36(2):265–270. [PubMed] [Google Scholar]
- 19. Harris EK, Yasaka T, eds. Maintaining a Healthy State Within the Individual. Amsterdam, Netherlands: Elsevier; 1987. [Google Scholar]
- 20. Petersen PH, Fraser CG, Sandberg S, Goldschmidt H. The index of individuality is often a misinterpreted quantity characteristic. Clin Chem Lab Med. 1999;37(6):655–661. [DOI] [PubMed] [Google Scholar]
- 21. Sottas PE, Kapke GF, Vesterqvist O, Leroux J-M. Patient-specific measures of a biomarker for the generation of individual reference intervals: hemoglobin as example. Transl Res. 2011;158(6):360–368. [DOI] [PubMed] [Google Scholar]