Abstract
Purpose
The objective of the present review is to provide an overview of the available clinical and preclinical data supporting the existence of an “inflammatory penumbra” in ischemic stroke.
Findings
Recent data from clinical trials suggest the existence of an inflammatory area at risk, surrounding the initial ischemic lesion and secondarily infiltrated by lymphocytes, that is ultimately recruited by the ischemic core: called the “inflammatory penumbra.” Experimental results support this concept. Lymphocytes, especially T-cells, enter the brain in the perilesional area in a vascular-cell adhesion molecule-1 dependent manner and participate in delayed neuronal cell death.
Methods
For writing this review, we used the more recent publications in the field, including the preclinical and clinical studies. We have also used our own experise in the field of in vivo imaging of inflammatory processes.
Discussion
Consequently, the intensity of the inflammatory reaction and the size of the inflammatory penumbra may vary considerably in patients, as it is the case in experimental stroke models in mice. By analogy with the ischemic penumbra of the acute phase of stroke, this secondary inflammatory penumbra represents a therapeutic opportunity during the subacute phase of stroke. Large clinical trials that target lymphocyte trafficking are currently taking place. However, to improve the benefit of such therapeutic strategies, adequate patient selection may be mandatory.
Conclusion
In this context, innovative imaging methods including magnetic resonance imaging of adhesion molecules may contribute to noninvasively detect this inflammatory penumbra and thus to select patients eligible for such therapy.
Keywords: ischemic stroke, inflammation, in vivo imaging
Introduction
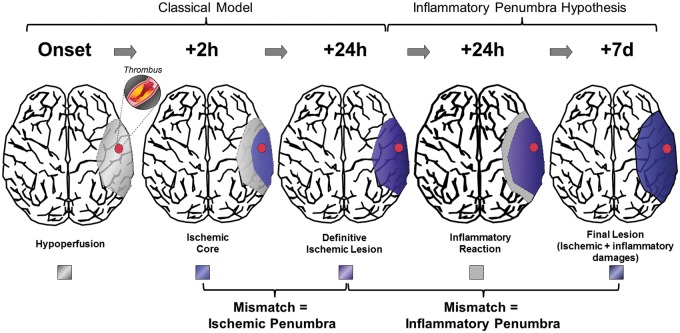
Acute ischemic stroke is caused by the sudden occlusion of a cerebral artery, leading to the progressive infarction of the brain and subsequent neurological deficits.1 During the first hours after ischemic onset, the affected brain tissue is classically divided in three regions: (i) an ischemic core, where the brain is definitively infarcted; (ii) a region at risk, called the “ischemic penumbra,” which will be ultimately recruited by the ischemic core if no reperfusion occurs; (iii) a region of oligemia, which presents a reduction in blood flow that is not sufficient to result in brain infarction.2 Along with the elapsed time after stroke onset, the ischemic core will grow and progressively recruit the ischemic penumbra, leading to the concept of “time is brain”: the sooner arterial recanalization is obtained, the smaller is the volume of definitively infarcted brain tissue and the better is the neurological outcome.3 Thus, a few hours after stroke (<24 h), it is currently considered that the therapeutic window for reperfusion therapy is over since the whole ischemic penumbra has been recruited by the ischemic core.
Interestingly, longitudinal evaluation of ischemic lesion size demonstrated significant lesion growth occurring between 24 h and seven days poststroke onset in both preclinical studies and humans.4–7 Notably, unlike the ischemic penumbra that is rescued by a rapid reperfusion, the secondary lesion growth can be prevented by immunomodulatory treatments. Indeed, a growing body of evidence suggests that the effectors of this secondary brain damage are cytotoxic T-cells.8,9 Therefore, the brain region recruited by the ischemic core in the subacute phase (>24 h) of ischemic stroke may represent a secondary inflammatory area, potentially salvaged by blockade of lymphocyte entry into the brain: the “inflammatory penumbra.”5,10 This observation offers new opportunities for therapeutic intervention aiming at improving ischemic stroke outcome. Thus, in analogy with the ischemic penumbra defined by a hypoperfused area potentially salvageable, the objective of the present review is to provide an overview of the current clinical and preclinical data supporting the existence of this “inflammatory penumbra” in acute stroke (Figure 1), salvageable with anti-inflammatory strategies.
Figure 1.
The inflammatory penumbra concept after ischemic stroke.
Clinical trial evidence for a beneficial effect of lymphocyte blockade in ischemic stroke
Studies on the role of inflammation in acute ischemic stroke were initially focused on the reperfusion phase. Inflammatory cells, such as neutrophils, monocytes, and lymphocytes, are able to interact with brain endothelial cells after reperfusion by binding to adhesion molecules, leading to brain inflammation and subsequent neuronal damage. However, initial attempts to use anti-inflammatory treatments in acute ischemic stroke failed.11 One of the first trial of antileukocyte treatment to blunt inflammation-related neuronal damage was the enlimomab trial, which evaluated the use of an anti-ICAM-1 (intercellular adhesion molecule-1) antibody to improve stroke outcome.12 Within 6 h of stroke onset, 625 patients with ischemic stroke were randomized to receive either enlimomab (n = 317) or placebo (n = 308) over five days. At day 90, the neurological outcome was worse in patients treated with enlimomab compared to placebo. Of note, there were significantly more adverse events, especially infections and fever, with enlimomab than with placebo, suggesting off-target effects of enlimomab that may have blunted the putative beneficial effects of ICAM-1 blockade in this trial (possibly related to the murine nature of the antibody).
More recently, the immunomodulatory drug fingolimod was evaluated in two small stroke trials.6,7 Fingolimod acts via sequestration of circulating mature lymphocytes in the lymph nodes.13 In an open-label, evaluator-blinded, parallel-group clinical pilot trial, 22 patients were recruited and placed into two groups: control group (standard treatment adhering to current American Heart Association guidelines) or fingolimod group (standard treatment plus fingolimod).7 Patients in each group were matched according to clinical and imaging characteristics. In the fingolimod group, each patient received 0.5 mg of the drug orally, once daily, for three consecutive days. The onset to treatment time was larger than in most recent clinical trials and patients were included up to 72 h after symptoms onset. One of the most interesting findings of this small trial was that the enlargement of the lesion size, as assessed by magnetic resonance imaging (MRI), was more restrained between baseline and day 7 in fingolimod-treated patients than in controls (+9 ml versus +27 ml). The authors also monitored the dynamics of lymphocyte subsets in the blood during fingolimod treatment. Interestingly, statistically significant effects of fingolimod on blood lymphocyte counts were mostly obtained after three days of treatment, suggesting that its beneficial effects are not obtained during the acute phase (0–24 h), but rather during the subacute phase of ischemic stroke (24 h to seven days).
The second trial investigating fingolimod as an ischemic stroke treatment was a randomized, open-label, evaluator-blind, multicenter pilot trial performed between 2013 and 2015 at three stroke centers in China.6 All patients included in this trial benefited from intravenous (IV) thrombolysis using tissue-type plasminogen activator (tPA). Forty-seven patients were randomized to receive either tPA or tPA + fingolimod in the first 4.5 h after stroke onset. All patients had intracranial occlusion in the anterior cerebral or middle cerebral arteries, as detected by MRI. In line with the previous trial results, fingolimod-treated patients presented smaller infarct volume expansion, smaller hemorrhage volume, and greater clinical improvement over the course of the study. In particular, a remarkable restriction in lesion growth between 24 h and seven days was observed in patients who received fingolimod, compared to control patients (−2.3 ml versus +12.1 ml).
These recent studies provide evidence that the lesion size after ischemic stroke increases during at least seven days after symptom onset, and that fingolimod treatment prevents this secondary infarct growth. The reduced circulating lymphocyte count in fingolimod-treated patients suggests that fingolimod reduces secondary infarct expansion by preventing lymphocyte entry in the injured brain.
Preclinical trial evidence for a beneficial effect of lymphocyte blockade in ischemic stroke
In preclinical studies, more specific tools than fingolimod treatment can be used to investigate the role of lymphocytes during the subacute stage of ischemic stroke. Indeed, lymphocyte entry in the injured brain is dependent on the interaction between CD49d, an integrin expressed by circulating lymphocytes, and vascular cell adhesion molecule 1 (VCAM-1), an adhesion molecule expressed by activated endothelial cells.14 Accordingly, blockade of CD49d/VCAM-1 interaction by natalizumab, an anti-CD49d monoclonal antibody, has been shown to reduce relapse rate in multiple sclerosis patients by blocking lymphocyte trafficking into the brain.15 Therefore, blockade of CD49d offers an interesting tool to study the role of infiltrated lymphocytes on neuronal death during ischemic stroke.
Several studies have investigated the use of anti-CD49d antibodies (natalizumab-like) in experimental models of acute ischemic stroke and demonstrated beneficial effects on lesion size and neurological outcome at early time points (24 h poststroke onset). However, these beneficial effects failed to be reproduced in more recent preclinical studies.16,17 This lack of reproducibility of preclinical results dramatically reduces the predictive value of preclinical data, although it is not restricted to the stroke field.18 To improve the value of preclinical research, a recent call was made to perform phase III preclinical trials, involving large number of animals and a controlled, randomized, blinded, multicenter trial design.19 Recently, the first phase III preclinical trial has been published.17 Of note, this trial investigated the efficiency of CD49d blockade on stroke outcome in mice.
In this phase III preclinical trial, two different models of ischemic stroke were used to address potential pathophysiological differences among commonly used stroke models: transient middle cerebral artery occlusion (tMCAo) using transient mechanical vascular occlusion (TMVO) and permanent middle cerebral artery occlusion (pMCAo). The primary outcome was ischemic lesion size at day 4 (tMCAo) or day 7 (pMCAo). Randomized mice received either a monoclonal anti-CD49d antibody (300 µg, 3 h after stroke onset) or an isotype-matched, control immunoglobulin. In the pMCAo model, anti-CD49d treatment resulted in 19% smaller infarct volumes seven days after stroke onset. These results, obtained during a study with clinical trial-like standards, confirm the deleterious role of infiltrated lymphocytes in the first seven days after stoke onset. In contrast, no statistically significant difference in lesion size was observed in the tMCAo model, despite the use of ∼50 animals per group. This discrepancy between the two models may be explained by the stronger inflammatory reaction taking place in the brain after pMCAo than after tMCAo.20 Moreover, recent evidence suggests that TMVO models (such as the mouse tMCAo model) display a pathophysiology different from pMCAo models.21,22
Overall, this phase III preclinical trial provides strong evidence in mice that blockade of lymphocyte entry into the brain prevents ischemic lesion growth after stroke induced by pMCAo.
The inflammatory penumbra: A plausible hypothesis?
The pathophysiological basis supporting the use of anti-CD49d antibodies to prevent delayed lesion growth after ischemic stroke came from a study published by Liesz and coworkers in 2011.5 The authors observed in a pMCAo model in mice that blockade of lymphocyte entry into the brain using anti-CD49d antibodies allows reducing the lesion size seven days after stroke onset. Interestingly, the lesion size did not differ between anti-CD49d treated and control mice at 24 h after stroke onset, suggesting that the beneficial effects of lymphocyte diapedesis blockade are only evident during the subacute phase of ischemic stroke. This delayed deleterious role of lymphocytes (especially T-cells) has to be distinguished from their early deleterious role described mostly in TMVO models and related to thrombo-inflammation.23
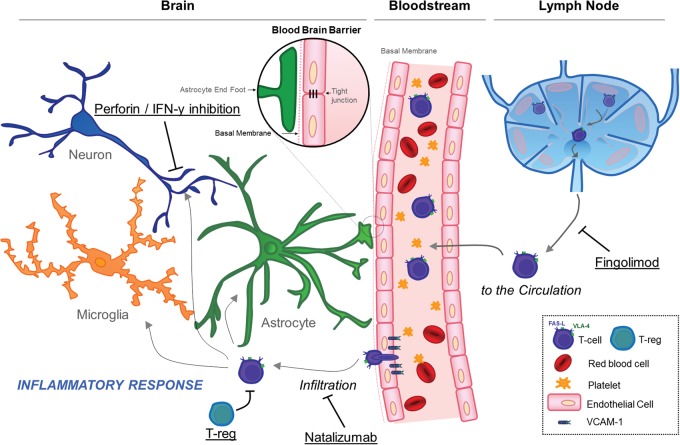
As assessed by immunohistochemistry, the localization of T-cells in the brain after pMCAo was rather surprising: they were predominantly located in the peri-infarct zone, where viable cells (including neurons) were still present. Therefore, these data suggested that T-cells, in the subacute phase of ischemic stroke, are able to infiltrate the peri-infarct regions and trigger a delayed and inflammation-related expansion of the initial lesion. To further test this hypothesis, Liesz and coworkers performed the same pMCAo model but this time, using mice with perforin-deficient T-cells (perforin is one of the key effector mechanism of T-cells to trigger cell death). Interestingly, these transgenic mice presented smaller lesion size at day 7 after stroke onset and did not benefit anymore from anti-CD49d treatment. The same results were obtained in mice in which interferon gamma (IFN-γ, another T-cell effector) was neutralized by injection of specific monoclonal antibodies in the cerebrospinal fluid. The role of Fas/Fas ligand, another key T-cell effector, remains to be investigated.
Instead of directly acting on T-cells, several studies suggested that their deleterious effects can be modulated by targeting regulatory T-cells, a subpopulation of T-cells which has anti-inflammatory properties. For instance, mice with depleted regulatory T-cells presented a dramatically larger lesion growth between 24 h and seven days poststroke onset than control mice.4 Conversely, amplification of regulatory T-cells using a CD28 superagonist reduces brain damage after ischemic stroke in mice (pMCAo).24 Therefore, the fate of the inflammatory penumbra seems to be dependent on a balance between T-cells and their regulatory counterparts, the regulatory T-cells.
Altogether, these experimental results support the existence of an inflammatory penumbra that is set up during the subacute stage of ischemic stroke, allowing the diapedesis of T-cells in intact brain regions, where they trigger neuronal cell death by at least to different mechanisms: direct cytotoxicity by perforin secretion and IFN-γ secretion. Regulatory T-cells seem to play an important role in this process by partially preventing T-cell-mediated damages (Figure 2).
Figure 2.
Potential therapeutic targets to prevent inflammatory penumbra recruitment by the ischemic core.
Is it possible to image the inflammatory penumbra?
According to the inflammatory penumbra hypothesis, there are intact brain areas in the subacute phase of ischemic stroke that support T-cell entry into the brain. Therefore, these areas present all the machinery necessary for T-cell diapedesis, including the expression of adhesion molecules by brain endothelial cells. The ability to noninvasively detect endothelial cell-associated VCAM-1, which is key for T-cell diapedesis, could therefore be a mean to measure the inflammatory penumbra: since the inflammatory penumbra presents intact brain tissue, there should be a mismatch between the infarcted area (easily detectable by T2-weighted or diffusion-weighted MRI) and VCAM-1 positive area. This mismatch should reveal the inflammatory penumbra.
However, high resolution molecular MRI requires contrast agents with very good sensitivity. The development of such imaging methods benefited from the discovery of a new family of contrast agents, called microsized particles of iron oxide (MPIO, presenting diameters of 1–4 µm), that present the particularity of conveying a very large payload of superparamagnetic atoms (iron oxide).25 Thanks to this high iron oxide content, MPIOs appear as large signal voids (diameter of ∼50 µm) on T2*-weighted MRI, a commonly used MRI sequence to detect hemorrhages.26 Using appropriate MPIO conjugation methods, it is possible to target them to specific molecules using antibodies, peptides, proteins, or any other targeting moiety. We demonstrated the ability of MPIO directed against VCAM-1 (MPIO-αVCAM-1) to reveal areas of brain inflammation in a number of experimental models, including experimental autoimmune encephalomyelitis, sepsis, intra-striatal lipopolysaccharide (LPS) injection, diabetes mellitus, aging, and Alzheimer disease with an unprecedented sensitivity.10,27,28
Moreover, we performed molecular imaging of VCAM-1 using MPIO-αVCAM-1 in ischemic stroke models in mice.10 In two pMCAo models (electrocoagulation and ferric-chloride-induced thrombosis of the middle cerebral artery), 24 h after ischemic onset, we demonstrated that the peri-infarct areas (as visualized on T2-weighted images) present high levels of VCAM-1 expression. In line with the inflammatory penumbra hypothesis, these VCAM-1+ areas were progressively recruited by the ischemic core between 24 h and seven days poststroke onset, as assessed by longitudinal MRI. Interestingly, whereas this VCAM-1 overexpression was strong and sustained in pMCAo models, it was milder and transient in two tMCAo stroke models (thrombin-induced tMCAo and transient mechanical compression of the middle cerebral artery). In fact, there was no inflammatory penumbra in tMCAo model, contrasting with the common view that reperfusion potentiates brain inflammation. Accordingly, there was no secondary lesion growth during the subacute phase in these models. These results support the findings of the phase III preclinical trial where anti-CD49d treatment failed to influence lesion size at seven days in a tMCAo model.17 Moreover, they support the use of molecular imaging strategies to select individuals with stroke that may benefit from immunomodulatory treatment.
Importantly, inflammation-directed therapy may have the ability to impact the size of the inflammatory penumbra, as revealed by VCAM-1 molecular imaging. For instance, high doses of statins (atorvastatin, 80 mg/kg) reduced VCAM-1 expression as assessed by molecular MRI and partially prevented delayed lesion growth in a pMCAo model.10 The same effect was observed when mice were treated with a Cox-2 inhibitor (celecoxib).10 Interestingly, thrombolysis also influences VCAM-1 expression after stroke: in the thrombin-induced tMCAO model, early treatment with tPA (10 mg/kg) was able to reduce both ischemic lesion size and VCAM-1 expression at 24 h poststroke onset. However, the effect of late thrombolysis on VCAM-1 expression remains to be investigated, especially since tPA displays pro-inflammatory properties when injected beyond its ideal therapeutic window.29
The downside of immunomodulation in ischemic stroke
Although blockade of lymphocyte entry into the brain and immunomodulatory treatments may improve the fate of the affected brain tissue after ischemic stroke, it also carries the risk of promoting infections. Among the most feared infections after stroke is pneumonia, which is promoted by poststroke immunodepression. The mechanisms driving immunodepression after brain injury are yet not fully understood.30,31 Notably, the occurrence of pneumonia after stroke deteriorates clinical outcome, arguing for caution in the use of immunomodulatory treatments in the acute and subacute phases.32
Regarding the effect of fingolimod on poststroke infections, an experimental study suggested that fingolimod does not significantly increase the risk of pneumonia, as assessed by measuring bacterial colonization in the lung of ischemic stroke mice after fingolimod treatment.33 Anti-CD49d antibodies (natalizumab in humans) have been more widely studied on this regard, especially in multiple sclerosis patients.34 Chronic natalizumab administration is associated with an increased rate of urinary and pulmonary infections, as well as a low risk of progressive multifocal leukoencephalopathy, a rare and potentially fatal neurologic disease caused by reactivation of JC virus infection. In the context of an acute treatment for ischemic stroke, it is however likely that the risk of infection-related side effects will be lower than in chronically treated patients.
Another concern regarding lymphocyte blockade is the potential beneficial role of leukocytes on poststroke rehabilitation, which could be reduced by inhibiting their entry into the brain. For instance, there is experimental evidence that after the acute inflammatory phase, macrophages function as beneficial scavengers of necrotic cell and brain tissue debris.35 These macrophages also produce trophic factors that are involved in tissue repair and neural cell regeneration. Therefore, the timing and duration of immunomodulation and/or lymphocyte trafficking blockade should be carefully selected, since they may also influence the distribution of other leukocyte subtypes and thus, alter the poststroke regeneration phase.
What can we learn from ongoing trials and new imaging developments?
The main question that remains unanswered to date is whether targeting T-cells improves neurological outcome in all ischemic stroke patients. Only clinical trials in small series of patients have been completed so far and large, randomized, controlled phase III trials are needed to draw a conclusion on this matter. A first answer will be given by the “Effect of Natalizumab on Infarct Volume in Acute Ischemic Stroke” (ACTION) completed trial, that investigated whether one 300 mg dose of IV natalizumab reduces change in infarct volume from baseline to day 5 on MRI in participants with acute ischemic stroke. The still recruiting “Efficacy and Safety of FTY720 for Acute Stroke” trial, that investigates the effect of fingolimod on stroke outcome using a randomized design, may also provide additional clues on whether T-cell-directed therapies are worth pursuing as ischemic stroke treatments. If positive, these clinical trials will also constitute strong arguments supporting the existence of an inflammatory penumbra in ischemic stroke.
However, to improve the benefit of such therapeutic strategies, adequate patient selection may be mandatory. Indeed, the way how the poststroke inflammation develops depends most probably on the patient natural history, the undertaken treatments and other, yet unknown, clinical and biological factors. Consequently, the intensity of the inflammatory reaction and the size of the inflammatory penumbra may vary considerably in patients, as it is the case in experimental stroke models in mice. As reperfusion therapy appears futile in patients without an ischemic penumbra on acute imaging, T-cell-targeted treatments may be inappropriate (and even dangerous) in patients without an inflammatory penumbra. Innovative imaging strategies, such as MPIO-αVCAM-1, should be developed to select the patients who will benefit the most from immunomodulatory therapies. Since currently used MPIO for preclinical studies are not biodegradable, a concerted effort between chemists, neuroscientists, and radiologists will probably be necessary to overcome the translational roadblock of molecular imaging.26
Interestingly, the inflammatory penumbra concept may not be restricted to ischemic stroke but could also be considered in intracranial hemorrhage. Indeed, using MPIO-αVCAM-1 enhanced imaging, we demonstrated that an area of intense VCAM-1 overexpression is present around the hematoma in a collagenase-induced intracranial hemorrhage model in mice.10 Moreover, fingolimod treatment has been shown to reduce lymphocyte infiltration in and around the hematoma in two murine experimental models of intracranial hemorrhage.36 In a phase II clinical trial involving 23 patients presenting small- to moderate-sized deep primary supratentorial hemorrhage, the individuals that received fingolimod once daily for three consecutive days presented reduced perihematomal edema, attenuated neurologic deficits, and an improved recovery.37 These findings warrant confirmation in a larger clinical trial.
Conclusion
The accumulating evidence from experimental and clinical studies supports the existence of an inflammatory area at risk surrounding the initial lesion in the subacute phase of ischemic stroke: the “inflammatory penumbra.” Unlike the mechanisms taking place in the ischemic penumbra at the acute phase, cellular death occurring in this inflammatory penumbra seems to be triggered by cytotoxic T-cells and occurs during the subacute stage of stroke. Numerous actors involved in T-cells trafficking and activity therefore appear as potential therapeutic targets to improve stroke outcome. Ongoing clinical trials of fingolimod and natalizumab in ischemic stroke patients, as well as innovations in molecular imaging of brain inflammation, will bring further and hopefully definitive answers to the existence and the nature of this inflammatory penumbra.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the INSERM (French National Institute for Health and Medical Research), Equipe FRM N°DEQ20140329555 and the Regional Council of Lower Normandy.
Ethical approval
N/A
Informed consent
N/A
Guarantor
MG
Contributorship
MG, SMDL and DV wrote the review.
References
- 1.Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci 2003; 4: 399–415. [DOI] [PubMed] [Google Scholar]
- 2.Manning NW, Campbell BC, Oxley TJ, et al. Acute ischemic stroke: time, penumbra, and reperfusion. Stroke 2014; 45: 640–644. [DOI] [PubMed] [Google Scholar]
- 3.Saver JL. Time is brain—quantified. Stroke 2006; 37: 263–266. [DOI] [PubMed] [Google Scholar]
- 4.Liesz A, Suri-Payer E, Veltkamp C, et al. Regulatory t cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med 2009; 15: 192–199. [DOI] [PubMed] [Google Scholar]
- 5.Liesz A, Zhou W, Mracsko E, et al. Inhibition of lymphocyte trafficking shields the brain against deleterious neuroinflammation after stroke. Brain 2011; 134: 704–720. [DOI] [PubMed] [Google Scholar]
- 6.Zhu Z, Fu Y, Tian D, et al. Combination of the immune modulator fingolimod with alteplase in acute ischemic stroke: a pilot trial. Circulation 2015; 132: 1104–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fu Y, Zhang N, Ren L, et al. Impact of an immune modulator fingolimod on acute ischemic stroke. Proc Natl Acad Sci USA 2014; 111: 18315–18320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chamorro A, Meisel A, Planas AM, et al. The immunology of acute stroke. Nat Rev Neurol 2012; 8: 401–410. [DOI] [PubMed] [Google Scholar]
- 9.Brait VH, Arumugam TV, Drummond GR, et al. Importance of t lymphocytes in brain injury, immunodeficiency, and recovery after cerebral ischemia. J Cereb Blood Flow Metab 2012; 32: 598–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gauberti M, Montagne A, Marcos-Contreras OA, et al. Ultra-sensitive molecular mri of vascular cell adhesion molecule-1 reveals a dynamic inflammatory penumbra after strokes. Stroke 2013; 44: 1988–1996. [DOI] [PubMed] [Google Scholar]
- 11.del Zoppo GJ. Acute anti-inflammatory approaches to ischemic stroke. Ann NY Acad Sci 2010; 1207: 143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Enlimomab Acute Stroke Trial Investigators. Use of anti-icam-1 therapy in ischemic stroke: results of the enlimomab acute stroke trial. Neurology 2001; 57: 1428–1434. [DOI] [PubMed]
- 13.Brunkhorst R, Vutukuri R, Pfeilschifter W. Fingolimod for the treatment of neurological diseases—state of play and future perspectives. Front Cell Neurosci 2014; 8: 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med 2011; 17: 796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller DH, Khan OA, Sheremata WA, et al. A controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 2003; 348: 15–23. [DOI] [PubMed] [Google Scholar]
- 16.Langhauser F, Kraft P, Gob E, et al. Blocking of alpha4 integrin does not protect from acute ischemic stroke in mice. Stroke 2014; 45: 1799–1806. [DOI] [PubMed] [Google Scholar]
- 17.Llovera G, Hofmann K, Roth S, et al. Results of a preclinical randomized controlled multicenter trial (prct): anti-cd49d treatment for acute brain ischemia. Sci Transl Med 2015; 7: 299ra121. [DOI] [PubMed] [Google Scholar]
- 18.Bolli R. Reflections on the irreproducibility of scientific papers. Circ Res 2015; 117: 665–666. [DOI] [PubMed] [Google Scholar]
- 19.Boltze J, Ayata C, Wagner DC, et al. Preclinical phase iii trials in translational stroke research: call for collective design of framework and guidelines. Stroke 2014; 45: 357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou W, Liesz A, Bauer H, et al. Postischemic brain infiltration of leukocyte subpopulations differs among murine permanent and transient focal cerebral ischemia models. Brain Pathol (Zurich, Switzerland) 2013; 23: 34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gauberti M, Martinez de Lizarrondo S, Orset C, et al. Lack of secondary microthrombosis after thrombin-induced stroke in mice and non-human primates. J Thromb Haemost 2014; 12: 409–414. [DOI] [PubMed] [Google Scholar]
- 22.Hossmann KA. The two pathophysiologies of focal brain ischemia: implications for translational stroke research. J Cereb Blood Flow Metab 2012; 32: 1310–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gauberti M, Vivien D. Letter by gauberti and vivien regarding article, “amplification of regulatory t cells using a cd28 superagonist reduces brain damage after ischemic stroke in mice.”. Stroke 2015; 46: e50–51. [DOI] [PubMed] [Google Scholar]
- 24.Na SY, Mracsko E, Liesz A, et al. Amplification of regulatory t cells using a cd28 superagonist reduces brain damage after ischemic stroke in mice. Stroke 2015; 46: 212–220. [DOI] [PubMed] [Google Scholar]
- 25.McAteer MA, Sibson NR, von Zur Muhlen C, et al. In vivo magnetic resonance imaging of acute brain inflammation using microparticles of iron oxide. Nat Med 2007; 13: 1253–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gauberti M, Montagne A, Quenault A, et al. Molecular magnetic resonance imaging of brain-immune interactions. Front Cell Neurosci 2014; 8: 389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montagne A, Gauberti M, Macrez R, et al. Ultra-sensitive molecular mri of cerebrovascular cell activation enables early detection of chronic central nervous system disorders. NeuroImage 2012; 63: 760–770. [DOI] [PubMed] [Google Scholar]
- 28.Belliere J, Martinez de Lizarrondo S, Choudhury RP, et al. Unmasking silent endothelial activation in the cardiovascular system using molecular magnetic resonance imaging. Theranostics 2015; 5: 1187–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vivien D, Gauberti M, Montagne A, et al. Impact of tissue plasminogen activator on the neurovascular unit: from clinical data to experimental evidence. J Cereb Blood Flow Metab 2011; 31: 2119–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dirnagl U, Klehmet J, Braun JS, et al. Stroke-induced immunodepression: experimental evidence and clinical relevance. Stroke 2007; 38: 770–773. [DOI] [PubMed] [Google Scholar]
- 31.Engel O, Akyuz L, da Costa Goncalves AC, et al. Cholinergic pathway suppresses pulmonary innate immunity facilitating pneumonia after stroke. Stroke 2015; 46: 3232–3240. [DOI] [PubMed] [Google Scholar]
- 32.Hilker R, Poetter C, Findeisen N, et al. Nosocomial pneumonia after acute stroke: implications for neurological intensive care medicine. Stroke 2003; 34: 975–981. [DOI] [PubMed] [Google Scholar]
- 33.Pfeilschifter W, Czech-Zechmeister B, Sujak M, et al. Activation of sphingosine kinase 2 is an endogenous protective mechanism in cerebral ischemia. Biochem Biophys Res Commun 2011; 413: 212–217. [DOI] [PubMed] [Google Scholar]
- 34.McCormack PL. Natalizumab: a review of its use in the management of relapsing-remitting multiple sclerosis. Drugs 2013; 73: 1463–1481. [DOI] [PubMed] [Google Scholar]
- 35.Shichita T, Ito M, Yoshimura A. Post-ischemic inflammation regulates neural damage and protection. Front Cell Neurosci 2014; 8: 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rolland WB, Lekic T, Krafft PR, et al. Fingolimod reduces cerebral lymphocyte infiltration in experimental models of rodent intracerebral hemorrhage. Exp Neurol 2013; 241: 45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fu Y, Hao J, Zhang N, et al. Fingolimod for the treatment of intracerebral hemorrhage: a 2-arm proof-of-concept study. JAMA Neurol 2014; 71: 1092–1101. [DOI] [PubMed] [Google Scholar]