Abstract
Purpose
The importance of troponin elevation at stroke presentation remains uncertain. We aimed to assess whether baseline ultrasensitive Troponin I (hs-TnI) predicts cardiac complications and outcome in acute stroke patients.
Method
Stroke patients admitted within 6 h were consecutively enrolled from May 2013 to March 2014. Blood samples were taken at admission to determine hs-TnI by chemiluminescent microparticle immunoassay. hs-TnI > 34.2 pg/ml (male) and >15.6 pg/ml (female) were considered elevated. Complications during in-hospital stay and outcome at 90 days were prospectively recorded. Independent predictors of cardiac complications (heart failure and acute coronary syndrome) and mortality were determined by logistic regression. The additional predictive value of hs-TnI was evaluated by integrated discrimination improvement index. A subanalysis was performed after excluding patients with previous cardiac diseases.
Findings
From 174 patients, 39(22%) had elevated hs-TnI, having these patients higher incidence of cardiac complications (57% versus 19%, p = 0.004). hs-TnI was an independent predictor of cardiac complications (OR = 16.1 (1.7–150.3)) together with diastolic blood pressure (OR = 0.92 (0.86–0.99)). Addition of hs-TnI to clinical variables significantly improved discrimination (IDI = 15.2% (7.8–22.7)). Subanalysis in patients without previous cardiac diseases showed similar results. Elevated hs-TnI was independently associated with 90 days mortality (OR = 3.6 (1.3–9.4)), but addition of hs-TnI to clinical data did not result in an increased discrimination.
Discussion
The present study confers hs-TnI a 2b level of evidence as a diagnostic tool to predict cardiac complications in stroke. Absence of serial hs-TnI measurements and limited sample size are the main weaknesses of the study.
Conclusion
Patients with elevated baseline hs-TnI showed a higher frequency of cardiac complications and a higher mortality. Measurement of hs-TnI in acute stroke might be useful to identify patients at a high risk of cardiac complications and death.
Keywords: Stroke, outcome, troponin-I, cardiac complications, acute coronary syndrome, heart failure
Introduction
Stroke represents currently the fifth cause of death worldwide, and in-hospital mortality rates for ischemic stroke have been estimated between 11 and 15%, increasing with age, being both genders equally affected.1,2 Approximately two-thirds of early death and poor outcome in acute stroke have been attributed to nonmodifiable predictors, such as advanced age or stroke severity, whereas the remaining third is integrated by modifiable factors such as early complications, which represent an opportunity to further improve the prognosis of acute stroke.3,4
Cardiac complications, such as acute coronary syndrome (ACS) or acute heart failure (AHF) account for 2–6% mortality within the first three months, being this risk higher within the first two weeks after the event.5–7 Several factors have been reported as predictors of cardiac complications in stroke patients. In fact, a score integrated by history of heart failure, diabetes, baseline creatinine levels, severe stroke, and a long QTc or ventricular extrasystole on baseline electrocardiogram (ECG) was reported to give a 10-fold higher risk of any serious cardiac event, defined as nonfatal ventricular tachycardia/fibrillation, acute myocardial infarction, pulmonary edema/moderate–severe cardiac failure, or cardiac death, in the presence of the four predictors.8 Identification of patients at the highest risk for cardiac events could be of interest as they may benefit from a closer monitoring and treatment, or even from prevention strategies.
Troponin (Tn) is a sensitive biomarker, widely used for diagnosis and risk stratification in ACS. A significant percentage of stroke patients have been reported to present raised Tn levels, which could be as high as 60% with the use of ultrasensitive assays.9 Although Tn measurement in acute ischemic stroke patients is recommended by the American Heart Association Guidelines,10 the clinical significance of these raised levels remains still unclear. Several studies have tried to assess this question, by evaluating whether Tn values could be predictors of poor outcome and/or indicative of concurrent ACS in stroke patients.11–16 Most of these studies evaluated Tn as a dichotomous variable (normal versus elevated), and most of them used conventional rather than ultrasensitive assays. These results were synthesized in a systematic review and meta-analysis, which found that patients with elevated Tn levels were more likely to have ECG changes suggestive of myocardial ischemia, being also Tn levels an independent predictor of mortality.17
In this prospective study, we aimed to test whether a single baseline Tn measurement is able to predict the occurrence of cardiac complications and death in acute stroke patients.
Methods
We conducted an observational, prospective study, to assess the value of a single measurement of blood biomarkers for the prediction of stroke associated complications. The study was conducted at University Hospital Vall d'Hebron. Patients or relatives signed the informed consent and the study protocol was approved by the local Ethics Committee (PR_AG_157-2011). Primary endpoint was the development of cardiac complications (ACS or AHF) during hospital admission. A subanalysis of this primary endpoint was carried out after exclusion of patients with previous structural cardiopathies, such as coronary artery disease (CAD) or congestive heart failure (CHF). Secondary endpoints were poor outcome, defined as a modified Rankin score >2, and all-cause mortality at 90 days after stroke.
Patients
From May 2013 to March 2014, patients with stroke suspicion and stroke code activation (usually candidates to reperfusion therapies, <6 h from symptom onset or unknown origin) were prospectively recruited at hospital arrival. A detailed medical history of vascular risk factors and cardiologic comorbidities was obtained from each patient at hospital admission. Stroke diagnosis was made according to the World Health Organization criteria18 and confirmed by neuroimaging. All patients underwent a computer tomography or magnetic resonance imaging at hospital admission. Hemorrhagic strokes and stroke-mimicking conditions, as well as patients with more than 6 h from the last time known to be asymptomatic were excluded. Transient ischemic attack (TIA) was defined as a transient episode of neurologic dysfunction caused by focal brain, spinal cord, or retinal ischemia, without acute infarction. Neurological severity was assessed by the National Institutes of Health Stroke Scale (NIHSS). Stroke clinical syndrome was classified according to the Oxfordshire Stroke Project Classification (OCSP), as total (TACI) or partial anterior circulation infarcts, posterior circulation infarcts and lacunar infarcts. After routine clinical workup, stroke etiology was classified with the Trial of Org 10172 in Acute Stroke Treatment. In-hospital complications were recorded according to treating physician diagnosis, normally based on AHA guidelines.19 Therefore, ACS was considered if typical chest pain and/or ECG abnormalities (ST-segment elevation or new left bundle-branch block on the ECG) and/or enzymatic alterations (elevated Tn and/or creatin kinase MB levels) were present. AHF was diagnosed as new-onset dyspnea and/or fatigue, together with fluid retention, which may lead to pulmonary and/or splanchnic congestion and/or peripheral edema, in the absence of other documented causes of dyspnea.
At 90 days after stroke, vital status and functional outcome were assessed by a certified physician with the mRS. Whenever possible, follow-up visits were performed by face-to-face visits and by a phone interview otherwise. Outcome assessment and collection of clinical information was performed blinded to biomarker results.
Samples and high-sensitivity troponin I measurement
Blood samples were collected at hospital admission at the emergency department, before any treatment. After centrifugation at 1500g for 15 min at 4℃, serum was frozen at −80℃ until biomarker measurement, so ultrasensitive Troponin I (hs-TnI) values were not available to treating physicians and therefore, had no role on patients' clinical management. Therefore, serial measurements in patients with baseline elevated hs-TnI were not possible. hs-TnI was measured using chemiluminescent microparticle immunoassay with the ARCHITECT STAT High Sensitive Troponin-I assay (Abbott Laboratories, Abbott Park, IL), by single measurements. Levels were expressed in pg/ml. Under local laboratory conditions, the interassay coefficient of variation was less than 10% at 50 ng/l, and this concentration was used as the diagnostic threshold. The diagnostic threshold for the assay was based on a standard assessment of precision using pooled serum at a range of low concentrations across multiple platforms and reagent lots. According to manufacturer's instructions, levels > 34.2 pg/ml in male and >15.6 pg/ml in female were considered elevated (indicated as the 99th percentile of an apparently healthy population). Biomarker measurement was done blinded to clinical information.
Statistical analysis
Statistical analysis was performed using SPSS statistical package, version 17.0. hs-TnI was analyzed as a dichotomous variable (normal or abnormal) according to the above-mentioned values. Factors associated with elevated hs-TnI were assessed using the Chi-squared test for categorical variables and Student's t-test or Mann–Whitney U tests for continuous variables. Univariate analyses were conducted for the primary and secondary endpoints. Logistic regression analyses were conducted by the forward-stepwise method, including at the first step those variables associated with the endpoint in the univariate analysis, considering a threshold of p < 0.1. hs-TnI was added to the model at the last step, by the enter method. The models were further tested for relevant interactions. Predictive logistic regression models, with and without hs-TnI, were compared by the C-statistics using DeLong's method20 and by integrated discrimination improvement (IDI) index.21 A subanalysis for cardiac complications was performed after excluding patients with previous structural cardiopathies, following the same method.
Results
During the study period, 295 patients were admitted to our emergency department, of which 11 did not agree to participate in the study. Stroke mimics (N = 35), hemorrhagic strokes (N = 44), and patients with uncertain diagnosis (N = 5) were excluded. From the remaining 200 with ischemic stroke, 24 were excluded because of unknown time from stroke onset and last time known to be asymptomatic >6 h. hs-TnI measurement was not possible in two cases due to insufficient serum sample. Finally, 174 patients (156 ischemic strokes and 18 TIAs) were included in the analyses. Median age was 79 (66–84) years and 95 patients (54.6%) were male.
Cardiac complications were observed in 14 patients (8%), including two cases of ACS and 12 cases of AHF. Median time from admission to the occurrence of complication was three (2–8) days. These cardiac complications resulted in death in seven cases (50%). A description of the patients experiencing cardiac complications is provided on online supplementary Table 1. Overall, those patients developing cardiac complications were more frequently TACIs, with higher stroke severity at baseline and lower admission diastolic blood pressure (DBP). Occurrence of cardiac complications was associated with three-month outcome and mortality (Table 1).
Table 1.
Baseline characteristics of the studied cohort and univariate analysis for cardiac complications.
| All (N=174) | Cardiac complications (N=14) | No cardiac complications (N=160) | p-value | |
|---|---|---|---|---|
| Age | 79 (66–84) | 80 (67–83) | 79 (66–84) | 0.342 |
| Gender (male) | 95 (54.6%) | 7 (50%) | 88 (55.3%) | 0.700 |
| Arterial hypertension | 123 (70.7%) | 9 (64.3%) | 114 (71.3%) | 0.557 |
| Diabetes mellitus | 43 (24.7%) | 5 (35.7%) | 38 (23.9%) | 0.341 |
| Dyslipidemiaa | 83 (47.7%) | 5 (35.7%) | 78 (49.4%) | 0.339 |
| Previous stroke | 35 (20.1%) | 4 (28.6%) | 31 (19.5%) | 0.486 |
| Previous disability | 27 (15.5%) | 4 (28.6%) | 23 (14.5%) | 0.239 |
| Atrial fibrillationa | 49 (28.2%) | 5 (35.7%) | 44 (29.1%) | 0.760 |
| CADa | 27 (15.6%) | 3 (21.4%) | 24 (15.2%) | 0.464 |
| Previous congestive heart failure | 15 (8.6%) | 1 (7.1%) | 14 (8.8%) | 0.999 |
| OCSPa | ||||
| TACI | 54 (35.3%) | 11 (78.6%) | 43 (30.9%) | 0.005 |
| PACI | 66 (43.1%) | 3 (21.4%) | 63 (45.3%) | |
| POCI | 16 (10.5%) | 0 (0%) | 16 (11.5%) | |
| LACI | 17 (11.1%) | 0 (0%) | 17 (12.2%) | |
| TOASTa | ||||
| LAA | 30 (17.3%) | 6 (42.9%) | 24 (15.1%) | 0.071 |
| CE | 73 (42.2%) | 6 (42.9%) | 67 (42.1%) | |
| LAC | 18 (10.4%) | 0 (0%) | 18 (11.3%) | |
| UND | 47 (27.1%) | 2 (14.3%) | 45 (28.3%) | |
| Laterality (right)a | 78 (44.8%) | 5 (41.7%) | 31 (39.2%) | 0.999 |
| Baseline NIHSS | 7.5 (3–12.5) | 12 (7–17) | 7 (3–12) | 0.001 |
| SBP (mmHg) | 150.1 ± 16.2 | 131.7 ± 27 | 150.3 ± 31.4 | 0.148 |
| DBP (mmHg) | 78.5 (70–86.5) | 61.5 (54–75) | 79 (70–87) | 0.018 |
| Glycemia (mg/dl) | 122.5 (106–152) | 139 (129–189) | 121 (106–152) | 0.979 |
| Creatinine (mg/dl) | 0.96 (0.78–1.16) | 0.97 (0.82–1.12) | 0.95 (0.77–1.18) | 0.863 |
| Elevated hs-TnI | 39 (22%) | 8 (57.1%) | 31 (19.5%) | 0.004 |
| Poor outcomea | 73/151 (48.3%) | 13/14 (92.9%) | 60/137 (43.8%) | 0.001 |
| Mortalitya | 28/151 (18.5%) | 9/14 (64.3%) | 19/137 (13.9%) | <0.0001 |
CAD: coronary artery disease; CE: cardioembolic; DBP: diastolic blood pressure; hs-TnI: ultrasensitive Troponin I; LAA: large artery atherothrombotic; LAC: lacunar; LACI: lacunar infarct; NIHSS: National Institutes of Health Stroke Scale; OCSP: Oxfordshire Stroke Project classification; PACI: partial anterior circulation infarct; POCI: posterior arterial circulation infarct; SBP: systolic blood pressure; TACI: total anterior circulation infarct; TOAST: Trial of Org 10172 in Acute Stroke Treatment; UND: undetermined.
Data are expressed as N (%), median (interquartile range), or mean ± standard deviation.
denotes existence of missing values.
Regarding hs-TnI, 39 patients (22%) had elevated baseline levels. Overall, those patients with elevated hs-TnI were most frequently women, older and previously disabled (mRS>2). Baseline creatinine levels were higher in patients with elevated hsTnI. Hypertension was also more frequent in the group of elevated levels. Patients with abnormal levels of hs-TnI had a higher incidence of cardiac complications (57.1% versus 19.5%, p = 0.004) (Table 2).
Table 2.
Comparison of baseline characteristics between patients with normal or elevated hs-TnI levels.
| Elevated (N=39) | Normal (N=135) | p | Elevated (N=39) | Normal (N=135) | p | ||||
|---|---|---|---|---|---|---|---|---|---|
| Age | 82 (75–85) | 77 (64–84) | 0.015 | TOASTa | LAA | 10 (25.6%) | 20 (14.9%) | 0.155 | |
| Gender (male) | 12 (30.8%) | 83 (61.5%) | 0.001 | CE | 18 (46.2%) | 55 (41%) | |||
| Arterial hypertension | 34 (87.2%) | 89 (65.9%) | 0.010 | LAC | 1 (5.6%) | 17 (12.7%) | |||
| Diabetes mellitus | 11 (28.2%) | 32 (23.7%) | 0.566 | UND | 10 (25.6%) | 37 (27.6%) | |||
| Dyslipidemiaa | 19 (48.7%) | 64 (48.1%) | 0.948 | Other | 0 (0%) | 5 (3.7) | |||
| Previous stroke | 7 (17.9%) | 28 (20.7%) | 0.702 | Laterality (right)a | 9 (47.4%) | 27 (37%) | 0.409 | ||
| Atrial fibrillationa | 14 (37.8%) | 35 (27.1%) | 0.208 | Baseline NIHSS score | 10 (4–17) | 7 (2–11) | 0.112 | ||
| CADa | 7 (17.9%) | 20 (14.9%) | 0.647 | SBP (mmHg) | 147 (126–162) | 148 (127–171) | 0.876 | ||
| Previous CHF | 9 (40.9%) | 30 (19.7%) | 0.051 | DBP (mmHg) | 75 (61–82) | 80 (70–89) | 0.068 | ||
| Previous disability | 11 (28.2%) | 16 (11.9%) | 0.013 | Glycemia (mg/dl) | 132 (112–157) | 121 (104–150) | 0.310 | ||
| OCSPa | TACI | 13 (38.2%) | 41 (34.5%) | 0.719 | Creatinine (mg/dl) | 1.12 (0.85–1.18) | 0.93 (0.77–1.1) | 0.002 | |
| PACI | 16 (47.1%) | 50 (42.0%) | Cardiac complications | 8 (57.1%) | 6 (4.5%) | 0.004 | |||
| POCI | 2 (5.9%) | 14 (11.8%) | Poor outcomea | 21/37 (56.8%) | 52/114 (45.6%) | 0.239 | |||
| LACI | 3 (8.8%) | 14 (11.8%) | Mortalitya | 13/37 (35.1%) | 15/114 (13.2%) | 0.003 | |||
CAD: coronary artery disease; CE: cardioembolic; CHF: congestive heart failure; DBP: diastolic blood pressure; hs-TnI: ultrasensitive Troponin I; LAA: large artery atherothrombotic; LAC: lacunar; LACI: lacunar infarct; NIHSS: National Institutes of Health Stroke Scale; OCSP: Oxfordshire Stroke Project classification; PACI: partial anterior circulation infarct; POCI: posterior arterial circulation infarct; SBP: systolic blood pressure; TACI: total anterior circulation infarct; TOAST: Trial of Org 10172 in Acute Stroke Treatment; UND: undetermined.
Data are expressed as N (%), median (interquartile range) or mean ± standard deviation.
Denotes existence of missing values.
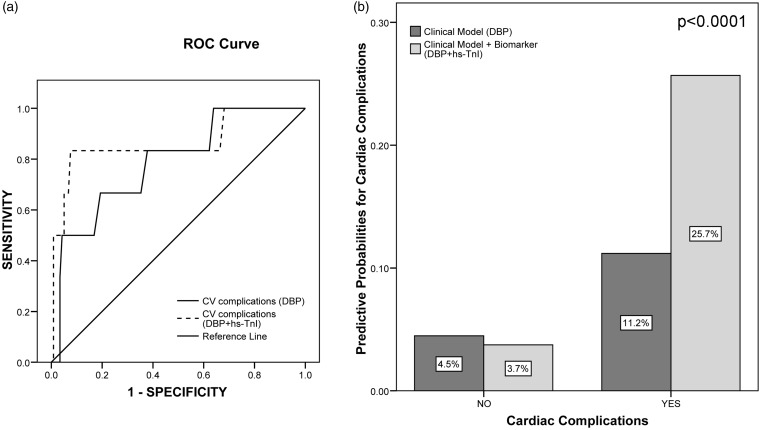
The cut-off for elevated levels of hs-TnI had 57.1% sensitivity and 80.5% specificity for the prediction of cardiac complications. Logistic regression analysis, including in the first step baseline NIHSS score, OCSP and DBP showed that just a low admission DBP was an independent predictor of cardiac complications (OR = 0.92 (0.86–0.99), p = 0.020, for each reduction on DBP in 1 mmHg). When hs-TnI was added to this model it remained as an independent predictor of cardiac complications, having patients with elevated hs-TnI a 16-fold increased risk of cardiac complications (OR = 16.1(1.71–150.3), p = 0.015). There was no significant interaction between DBP and hs-TnI. The addition of hs-TnI to the model significantly improved its discrimination (IDI = 15.2% (7.8–22.7), p < 0.0001) and trended to improve its accuracy (from 0.786 to 0.863, p = 0.134) (Figure 1).
Figure 1.
Comparison between predictive models for cardiac complications, including or not the biomarker ultrasensitive Troponin I (hs-TnI). (a) Comparison of the predictive accuracy (area under the ROC curve) of both models. The continuous line represents the predictive model constructed just with clinical variables (diastolic blood pressure, DBP), and the discontinuous line represents the predictive model constructed with the previous one plus hs-TnI as a dichotomous variable (normal versus elevated levels according to manufacturer's instructions, >34.2 pg/ml male, >15.6 pg/ml female). The addition of hs-TnI trended to improve the accuracy of the model (area under curve (AUC) from 0.786 to 0.863, p = 0.134). (b) Integrated discrimination improvement (IDI) index: the bars represent the same models than (a) (dark bars, clinical model and light bars, the same model plus hs-TnI). The predictive probabilities of events (cardiovascular complications) or no events for each model are expressed as %. The IDI value (15.2% (7.8–22.7%), p < 0.0001) results from the sum of the differences in predictive probabilities for events and no events.
When patients with previous structural cardiopathy (27 patients with CAD and 15 patients with CHF, N = 39 patients for overlapping) were excluded, the incidence of elevated hs-TnI was 21.5%. As well as in the whole cohort, patients with elevated hs-TnI levels had higher risk for development of cardiac complications (24.1% versus 3.8%, p = 0.002) than those with normal levels. In logistic regression analysis (clinical variables included in the first step: baseline NIHSS, OCSP, DBP), elevated hs-TnI remained as an independent predictor of cardiac complications (OR = 15.3 (1.49–156.2), p = 0.021) together with admission DBP.
Three months after stroke, outcome data were available for 151 patients (87% of the cohort). A total of 73 patients had poor outcome (mRS>2), and 90-day all-cause mortality was 18.5%. hs-TnI levels were not related with poor outcome. Patients who died had higher hs-TnI levels (21.6 (10.9–59.1) pg/ml versus 10 (10–16.6) pg/ml, p < 0.0001), and the probability of dying within three months after stroke was higher in those patients with elevated hs-TnI (35% versus 13%, p = 0.003). After adjustment for univariate mortality predictors (Supplementary Table 2), hs-TnI remained an independent predictor of three-month mortality (OR = 3.01 (1.14–7.92), p = 0.026), together with previous CHF (OR = 4.99 (1.30–19.16), p = 0.019) and admission NIHSS (OR = 1.15 (1.07–1.24), p < 0.0001). There was no significant interaction between previous CHF and hs-TnI. However, the addition of hs-TnI to the model did not improve its accuracy (from 0.777 to 0.803, p = 0.286), neither discrimination (IDI = 3.55% (-0.7 to 7.8), p = 0.107) (Supplementary Figure 1).
Discussion
In this study, we found that baseline elevated hs-TnI measurements have prognostic information that could be applied for acute stroke management. In fact, these elevated levels were independent predictors of subsequent cardiac complications, and its addition to clinical information resulted in an improvement of the discrimination of these cardiac events and trended to improve its accuracy. In this study we also found the presence of low admission DBP as an independent predictor of cardiac complications. Similar data were found in the study of Prosser et al.,8 with lower baseline SBP levels in patients suffering any serious cardiac adverse events, although this variable was not present in the final score.
In contrast with other poststroke complications such as those related with reperfusion therapies,22 or poststroke infections,23 not many studies have looked for biomarkers predicting cardiac complications in stroke patients. In a group of 72 patients with ischemic stroke, hemorrhagic stroke and subarachnoid hemorrhage, Koening et al. found that N-terminal pro-B-type nautriuretic peptide (NT-proBNP) was not an independent predictor of AHF. However, a cut-off of 900 pg/ml raised a high sensitivity (94%) and negative predictive value (88%) for AHF, despite a lack of specificity.24 Elevated Tn has been associated with concomitant ACS or ECG ischemic changes in stroke patients, especially when serial changes are observed.25 In fact, in the study of Anders et al., relevant cases of ACS were just diagnosed in patients with a dynamic hs-TnI profile, defined as a 30% rise or fall at 3 h.26 However, our study is the first, to our knowledge, that assessed the predictive value of a single baseline hs-TnI measurement to predict further cardiac complications developed in the acute and subacute stages of stroke. In these stages, patients are still in the stroke unit and some measures could be done for the prediction of cardiac complications in these high-risk patients. If our data were confirmed in further studies, hs-TnI could be used to indicate comprehensive cardiologic workups or closer monitoring in patients with elevated levels. Some recent data from the TRELAS study27 support that ACS is not the single cause of Tn elevation in acute stroke and, therefore, other cardiac disorders such as atrial fibrillation, heart failure, or Takotsubo cardiomyopathy should be suspected and evaluated in stroke patients with elevated Tn.
Being Tn a fast and widely available tool in emergency rooms and stroke units, implementation of this measure should not be neither difficult nor expensive. In fact, Tn measurement in acute stroke patients is currently recommended by the American Heart Association Guidelines,10 although no specific indications are given. In the recent review of Scheitz et al.,9 a new algorithm on how to deal with an elevated Tn is proposed, based first on the static or dynamic pattern of the elevation, and second, in the features of the cardiologic workup. Based on our results, we might recommend longer monitoring, serial measurements, and a deeper cardiological workup in patients with raised Tn levels at admission. In this sense, the results of our separated subanalysis in patients without previous cardiac comorbidities, which have been reported to increase the hazard of cardiac complications,8 might complement this algorithm. This analysis rule out its role as a confounder, as these comorbidities may influence both occurrence of cardiac complications and baseline Tn levels.28,29 Results from this substudy were similar to the analysis including the entire cohort, which reinforces our results and indicates that stroke patients presenting with an elevated hs-TnI have increased risk of cardiologic complications, even in the absence of structural cardiopathies.
Our study also showed that a baseline Tn measurement could be of interest in prediction of stroke mortality, being an independent predictor. Some studies previously found a significant association between troponin levels and mortality,11,13 being this finding clarified in a systematic review and meta-analysis.17 More controversial is the association with poor functional outcome, with some studies reporting the association of elevated Tn levels with poor outcome,12,16 and others reporting opposite findings.14,15 In our opinion, these issues are of less interest for clinicians because, even when some decisions could be taken based on an accurate prediction of mortality (withdrawal of care, aggressive measures), the prediction of specific complications such as cardiac is easier to be translated into clinical orders that might prevent or early detect these complications.
Our study presents some limitations. First, the absence of serial hs-TnI measurements remains a limitation, as it is known from the cardiology field that a temporal profile is more precise for the diagnosis of ACS than a single measurement. Further studies could assess this issue, to better clarify if these raised levels at baseline are predictive of cardiac complication just with dynamic patterns, as described for ACS, or a single elevated measure is still valuable. Second, the lack of a comprehensive ECG assessment did not allow us to test the additional predictive value over the only published risk score for prediction of cardiovascular complications,8 question that remains to be answered by future studies. Third, although some cardiologic conditions that could elevate Tn have been excluded in the correspondent subanalysis, other diseases such as chronic kidney disease were not considered. However, even when creatinine levels were correlated with hs-TnI, the lack of association of creatinine and cardiac complications in our cohort makes any effect of kidney disease in our results very unlikely. Fourth, the limited sample size and, specially, the relatively low rates of cardiac complications and Tn elevation did not allow us to perform secondary analysis to quantify whether mild versus high elevations could be more predictive or specific for any type of cardiac complication. Finally, from a clinical point of view, the rates of sensitivity–specificity (57.1 and 80.5%) and discrimination (15.2%) are maybe insufficient to be translated into clinical decision, and these numbers could be improved by the addition of other biomarkers in a panel or with a better patient's selection.
In conclusion, our study shows that a single hs-TnI measurement in the acute phase of stroke could be useful in the identification of patients at highest risk for cardiac complications, who are at a high risk of morbidity and mortality and may benefit from a better management like extensive cardiologic workup or closer monitoring. Our study confers hs-TnI a 2b level of evidence as a diagnostic tool to predict cardiac complications in stroke. Future studies should address the usefulness of serial measurements in this indication as well as the addition of other markers.
Supplementary Material
Acknowledgements
Neurovascular Research Laboratory takes part in the Spanish stroke research network INVICTUS (RD12/0014/0005). The kits for hs-TnI measurement were kindly provided by Abbott Laboratories within an unrestricted research grant. Abbott Laboratories had no role on study design, data collection, data analysis, data interpretation, or writing the report.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by FIS PI15/354, cofinanced by the European Regional Development Fund (FEDER). A.B. is supported by a Rio Hortega contract CM/00265 from the Instituto de Salud Carlos III.
Ethical approval
The ethics committee of Hospital Vall d'Hebron approved this study (REC number: PR_AG_157-2011).
Informed consent
Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
Guarantor
JM.
Contributorship
AB designed the study, obtained data, performed and interpreted statistical analysis and drafted the manuscript; BD-F obtained data, and drafted the manuscript; JP and MR enrolled patients, obtained data, and critically reviewed the manuscript; AB-G and AP coordinated sample collection, processing and analysis, and critically reviewed the manuscript; TG-B performed and interpreted statistical analysis and critically reviewed the manuscript; JM conceived and designed the study and critically reviewed the manuscript. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation 2016; 133: e38–e360. [DOI] [PubMed] [Google Scholar]
- 2.Johnston SC, Mendis S, Mathers CD. Global variation in stroke burden and mortality: estimates from monitoring, surveillance, and modelling. Lancet Neurol 2009; 8: 345–354. [DOI] [PubMed] [Google Scholar]
- 3.Koennecke HC, Belz W, Berfelde D, et al. Factors influencing in-hospital mortality and morbidity in patients treated on a stroke unit. Neurology 2011; 77: 965–972. [DOI] [PubMed] [Google Scholar]
- 4.Bustamante A, García-Berrocoso T, Rodriguez N, et al. Ischemic stroke outcome: a review of the influence of post-stroke complications within the different scenarios of stroke care. Eur J Int Med 2016; 29: 9–21. [DOI] [PubMed] [Google Scholar]
- 5.Silver FL, Norris JW, Lewis AJ, et al. Early mortality following stroke: a prospective review. Stroke 1984; 15: 492–496. [DOI] [PubMed] [Google Scholar]
- 6.Adams RJ, Chimowitz MI, Alpert JS, et al. Coronary risk evaluation in patients with transient ischemic attack and ischemic stroke: a scientific statement for healthcare professionals from the Stroke Council and the Council on Clinical Cardiology of the American Heart Association/Am Stroke Association. Stroke 2003; 34: 2310–2322. [DOI] [PubMed] [Google Scholar]
- 7.Touzé E, Varenne O, Chatellier G, et al. Risk of myocardial infarction and vascular death after transient ischemic attack and ischemic stroke: a systematic review and meta-analysis. Stroke 2005; 36: 2748–2755. [DOI] [PubMed] [Google Scholar]
- 8.Prosser J, MacGregor L, Lees KR, et al. Predictors of early cardiac morbidity and mortality after ischemic stroke. Stroke 2007; 38: 2295–2302. [DOI] [PubMed] [Google Scholar]
- 9.Scheitz JF, Nolte CH, Laufs U, et al. Application and interpretation of high-sensitivity cardiac troponin assays in patients with acute ischemic stroke. Stroke 2015; 46: 1132–1140. [DOI] [PubMed] [Google Scholar]
- 10.Jauch EC, Saver JL, Adams HP Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013; 44: 870–947. [DOI] [PubMed] [Google Scholar]
- 11.Di Angelantonio E, Fiorelli M, Toni D, et al. Prognostic significance of admission levels of troponin I in patients with acute ischaemic stroke. J Neurol Neurosurg Psychiatry 2005; 76: 76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fure B, Bruun Wyller T, Thommessen B. Electrocardiographic and troponin T changes in acute ischaemic stroke. J Intern Med 2006; 259: 592–597. [DOI] [PubMed] [Google Scholar]
- 13.Jensen JK, Kristensen RS, Bak S, et al. Frequency and significance of troponin T elevation in acute ischemic stroke. Am J Cardiol 2007; 99: 108–112. [DOI] [PubMed] [Google Scholar]
- 14.Barber M, Morton JJ, Macfarlane PW, et al. Elevated troponin levels are associated with sympathoadrenal activation in acute ischaemic stroke. Cerebrovasc Dis 2007; 23: 260–266. [DOI] [PubMed] [Google Scholar]
- 15.Raza F, Alkhouli M, Sandhu P, et al. Elevated cardiac troponin in acute stroke without acute coronary syndrome predicts long-term adverse cardiovascular outcomes. Stroke Res Treat 2014; 2014: 621650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scheitz JF, Mochmann HC, Erdur H, et al. Prognostic relevance of cardiac troponin T levels and their dynamic changes measured with a high-sensitivity assay in acute ischaemic stroke: analyses from the TRELAS cohort. Int J Cardiol 2014; 177: 886–893. [DOI] [PubMed] [Google Scholar]
- 17.Kerr G, Ray G, Wu O, et al. Elevated troponin after stroke: a systematic review. Cerebrovasc Dis 2009; 28: 220–226. [DOI] [PubMed] [Google Scholar]
- 18.Hatano S. Experience from a multicentre stroke register: a preliminary report. Bull World Health Organ 1976; 54: 541–553. [PMC free article] [PubMed] [Google Scholar]
- 19.Amsterdam EA, Wenger NK, Brindis RG, et al. Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 130: e344–e426. [DOI] [PubMed] [Google Scholar]
- 20.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988; 44: 837–845. [PubMed] [Google Scholar]
- 21.Pencina MJ, D'Agostino RB, Vasan RS. Statistical methods for assessment of added usefulness of new biomarkers. Clin Chem Lab Med 2010; 48: 1703–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montaner J. Blood biomarkers to guide stroke thrombolysis. Front Biosci 2009; 1: 200–208. [DOI] [PubMed] [Google Scholar]
- 23.Meisel A, Meisel C, Harms H, et al. Predicting post-stroke infections and outcome with blood-based immune and stress markers. Cerebrovasc Dis 2012; 33: 580–588. [DOI] [PubMed] [Google Scholar]
- 24.Koenig MA, Puttgen HA, Prabhakaran V, et al. B-type natriuretic peptide as a marker for heart failure in patients with acute stroke. Intensive Care Med 2007; 33: 1587–1593. [DOI] [PubMed] [Google Scholar]
- 25.Apak I, Iltumer K, Tamam Y, et al. Serum cardiac troponin T levels as an indicator of myocardial injury in ischemic and hemorrhagic stroke patients. Tohoku J Exp Med 2005; 205: 93–101. [DOI] [PubMed] [Google Scholar]
- 26.Anders B, Alonso A, Artemis D, et al. What does elevated high-sensitive troponin I in stroke patients mean: concomitant acute myocardial infarction or a marker for high-risk patients?. Cerebrovasc Dis 2013; 36: 211–217. [DOI] [PubMed] [Google Scholar]
- 27.Mochmann HC, Scheitz JF, Petzold GC, et al. Coronary angiographic findings in acute ischemic stroke patients with elevated cardiac troponin: The TRoponin ELevation in Acute Ischemic Stroke (TRELAS) Study. Circulation 2016; 133: 1264–1271. . [DOI] [PubMed] [Google Scholar]
- 28.Bakshi TK, Choo MK, Edwards CC, et al. Causes of elevated troponin I with a normal coronary angiogram. Intern Med J 2002; 32: 520–525. [DOI] [PubMed] [Google Scholar]
- 29.Tanindi A, Cemri M. Troponin elevation in conditions other than acute coronary syndromes. Vasc Health Risk Manag 2011; 7: 597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.